Abstract
Objective: LGGs (low-grade gliomas) are sometimes encountered by chance during radiological examinations. These incidentally discovered LGGs (IDLGGs) were relatively under-studied in the literature. The purpose of current study is to review a cohort of patients with IDLGGs surgically treated in our institution for their clinical and histological aspects and determine their IDH1 and 1p19q status. Methods: All patients with hemispheric LGGs receiving operation in our institution between 2001 and 2004 were reviewed. Clinical, radiological and treatment data of the patients were collected and IDLGGs were retrieved and compared with symptomatic LGGs. Histological review was carried out and formalin-fixed paraffin embedded (FFPE) tissues of IDLGGs were examined for IDH1/IDH2 mutation and 1p/19q codeletion. Results: Twenty three IDLGGs (10.4%) were identified while 196 patients had symptomatic LGGs. The reasons for patients with IDLGGs having radiological examination included trauma (47.8%), dizziness (26.1%), unrelated headache (21.7%), and health checkup (4.4%). Clinically, patients with IDLGGs had higher preoperative KPS (P < 0.001), smaller tumor volume (P = 0.014), lower frequency of eloquent areas involvement (P < 0.001) and higher rate of complete resection (P = 0.037) comparing to those with symptomatic LGGs. Histologically, there is a preponderance of oligodendroglial differentiation with 6 oligodendrogliomas and 11 oligoastrocytomas but there were also 6 astrocytomas. IDH1 mutation and 1p/19q co-deletion were detected in 95.7% (22/23) and 69.6% (16/23) of IDLGGs, respectively. The latter encompassed all but one of the cases of oligodendroglial tumors. Patients with IDLGGs had longer overall survival than those with symptomatic LGGs (P = 0.027). Conclusions: We conclude that the majority of IDLGGs are IDH1 mutated and are predominantly oligodendroglial tumors. With a median follow-up of 9.3 years to our series, we conclude that patients with IDLGGs had better prognosis than those with symptomatic LGGs. The favorable prognosis of IDLGGs may be accounted by the higher practicability of extensive resection, non-eloquent tumor location and smaller tumor volume. Frequent IDH1 mutation and 1p/19q co-deletion in IDLGGs may also contribute to the favorable prognosis of this subgroup of patients.
Keywords: Low-grade glioma, incidental, surgery, pathology, prognosis
Introduction
According to The World Health Organization (WHO) classification of tumors of central nervous system, low-grade gliomas (LGGs) in adults comprise mainly astrocytoma (AII), oligodendroglioma (OII) and oligoastrocytoma (mixed glioma, OAII) [1]. Adults with LGGs most frequently present with seizures, and less commonly with symptoms of neurological deficits, increased intracranial pressure, etc. [2-4]. Some LGGs, however, are discovered by chance during radiological examinations for unrelated complaints or reasons [5-10]. These incidentally discovered LGGs (IDLGGs) were recently studied by several scholars since little was known about them and their clinical management remained controversial [5,6,11,12].
Previous retrospective studies revealed that 3.8% to 9.6% of supratentorial hemispheric LGGs were IDLGGs, which were associated with smaller tumor volumes, higher rate of complete resection and better prognosis comparing to symptomatic LGGs [5,6]. However, these studies mainly focused on differences of radiological features between IDLGGs and symptomatic LGGs and our knowledge in pathological features of IDLGGs is relatively limited [5,6]. Moreover, the follow-up duration of previous studies was not relatively long (median of 6.6 years in Pallud et al.; mean of 5.1 years in Potts et al.) [5,6] and in order to further clarify the prognosis of IDLGGs, study with long term follow-up data will be needed. We therefore conducted long-term follow-up in patients with IDLGGs surgically treated in our institution and analyzed these cases from both clinical and pathological perspectives. Our data demonstrated that IDLGGs are mostly oligodendroglial tumors with 1p/19q co-deletion and the vast majority of IDLGGs are IDH1 mutated. Patients with IDLGGs survived significantly longer than those with symptomatic LGGs.
Patients and methods
Selection criteria
The study was approved by the Huashan Ethics Committee, Fudan University. We reviewed a consecutive series of patients with LGGs who underwent initial surgery in the Department of Neurosurgery, Huashan Hospital between 2001 and 2004. Inclusion criteria of the series were: (1) adult patients (age ≥ 16 years); (2) supratentorial hemispheric location; (3) pathological diagnosis of LGGs according to 2007 WHO classification of tumors of central nervous system (astrocytoma, oligodendroglioma, and mixed glioma). In this series, an “incidentally discovered low-grade glioma (IDLGGs)” was defined as LGGs revealed by radiological images with a reason unrelated to the underlying tumor such as trauma, headache, dizziness and health check-up. For patients with headache or dizziness, we excluded those with severe headache/dizziness and with any sign of increased intracranial pressure.
Collection of clinical data and tissue samples
Clinical data were collected from medical charts and radiological films. Follow-up data were collected by telephone interviews or clinic follow-ups. These data included age, sex, neurological symptoms, neuroimaging findings, extent of resection, postoperative primary radiation therapy (RT), postoperative primary chemotherapy (CHT) and patients’ clinical outcomes. Tumor volume was calculated by ellipsoid approximation using three largest radii in axial, coronal and sagittal planes on T2-weighted/fluid attenuation inversion recovery (FLAIR) sequences demonstrating maximal abnormalities [15]. Extent of resection was determined based on postoperative MR or neurosurgeons’ intraoperative opinions. Histologic sections were independently reviewed by two neuropathologists (Y.W. and H.K.N.) according to the 2007 WHO classification of tumours of the central nervous system [1]. Formalin-fixed paraffin embedded (FFPE) tissues of IDLGGs were retrieved for molecular analyses.
Mutation analysis of IDH1 IDH2
Mutational hotspots of IDH1 at codon 132 and IDH2 at codon 172 were evaluated by direct sequencing as previously reported [29]. Briefly, tissues from representative tumor area scrapped off from dewaxed sections into microfuge tubes were resuspended in 10 mM Tris-HCl buffer, pH 8.5. Proteinase K was added to a final concentration of 2 g/l and the mixture was incubated at 55°C for 2 hours and then at 98°C for 10 minutes. PCR was conducted in 10 μl reaction mixture containing 1 μl of cell lysate, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, 0.2 mM of each deoxyribonucleoside triphosphate, 0.4 mM of each primer (IDH1-F: 5’-CGGTCTTCAGAGAAGCCATT-3’ and IDH1-R: 5’-CACATTATTGCCAACATGAC-3’; IDH2-F: 5’-AGCCCATCATCTGCAAAAAC-3’ and IDH2-R: 5’-CTAGGCGAGGAGCTCCAGT-3’) and 0.2 units of AmpliTaq Gold DNA polymerase (Applied Biosystems, Hong Kong). PCR was initiated at 95°C for 10 min, followed by 45cycles of 95°C for 20 sec, 60°C for 20 sec and 72°C for 30 sec, and a final extension step of 72°C for 3 min. PCR products were then treated with exonuclease I and alkaline phosphatase (TakaRa, Japan). Sequencing reaction was performed using BigDye Terminator Cycle Sequencing kit v1.1. The products were subsequently resolved in the Genetic Analyzer 3130xl and analyzed by Sequencing Analysis software. All base changes were confirmed by sequencing of a newly amplified fragment.
Immunohistochemistry of IDH1-R132H
Immunohistochemistry of IDH1-R132H was performed as reported previously [29]. In brief, 4 μm thick of FFPE tissue sections were deparaffinized in xylene and rehydrated in graded ethanol. Antigen retrieval was performed by treating the sections in 1mM ethylenediaminetetraacetic acid solution (pH 8.0) in a microwave oven. The sections were then processed by BenchMark XT automated tissue staining systems (Ventana Medical Systems, Inc., Tucson, U.S.A.). Sections were incubated with mouse monoclonal anti-IDH1-R132H antibody (1:50 dilution; Dianova, Hamburg, Germany) at 37°C for 32 minutes and followed by incubation with UltraView HRP-conjugated multimer antibody reagent (Ventana). Antigen detection was performed using UltraView diaminobenzidine chromogen step (Ventana). Tissues were counterstained with Mayer’s haematoxylin. The presence of cytoplasmic staining indicated IDH1-R132H positivity.
Mutation analysis of TERT promoter
Tissues from representative tumor area were scrapped off from dewaxed sections and treated with proteinase K at a final concentration of 2 μg/μl in 10 mM Tris-HCl buffer (pH 8.5) at 55°C for 2-18 hours and then at 98°C for 10 minutes. The crude cell lysate was centrifuged and supernatant was used for subsequent PCR analysis. The forward primer TERT-F (5’-GTCCTGCCCCTTCACCTT-3’) and reverse primer TERT-R (5’-CAGCGCTGCCTGAAACTC-3’) were used to amplify a 163bp fragment spanning the two mutational hotspots [chr5, 1,295,228 (C228T) and 1,295,250 (C250T)] in TERT promoter region. PCR was performed in 10 μl reaction mixture containing 1 μl of cell lysate, 0.3 mM of each dNTP, 2.5 mM MgCl2, 0.3 μM of each primer and 0.2 U of KAPA HiFi HotStart DNA Polymerase (Kapa Biosystems Inc., Wilmington, USA), and was initiated at 95°C for 5 min, followed by 40-45 cycles of 98°C for 20 sec, 68°C for 15 sec and 72°C for 30 sec, and a final extension of 72°C for 1 min. Products were then treated with exonuclease I and alkaline phosphatase (TakaRa, Japan). Sequencing was performed using BigDye Terminator Cycle Sequencing kit v1.1 (Life Technologies). The products were resolved in Genetic Analyzer 3130xl and analyzed by Sequencing Analysis software.
Chromosome 1p/19q status by fluorescence in situ hybridization
Chromosome 1p/19q status was examined by fluorescence in situ hybridization as reported previously [6]. Briefly, 5 μm thick FFPE sections were deparaffinized in xylene, treated with 1 M sodium thiocyanate at 80°C for 10 minutes, digested in pepsin solution at 37°C for 20 to 30 minutes, rinsed in milli-Q water and dehydrated. Bacterial artificial chromosome clones were used to prepare locus-specific probes using nick translation, in the presence of Spectrum Orange deoxyuridine triphosphate (dUTP) or Spectrum Green deoxyuridine triphosphate (dUTP). Cot-1 DNA (Life Technologies) were mixed with the labeled probes in Hybrisol VI solution (Appligene Oncor, Graffenstaden, France), applied to the section and denatured. Hybridization was carried out at 37°C overnight for 16 hours. Sections were washed in 1.5 M urea in 0.1X saline sodium citrate at 48°C for 30 minutes and then in 2X saline sodium citrate at 48°C for 5 min. The sections were then stained with Vectashield mounting medium containing 4’, 6-diamidino-2-phenylindole (DAPI; Vector Laboratories) and viewed under a Zeiss Axioplan fluorescence microscope (Carl Zeiss Microscopy LLC, NY, USA). Hybridizing signals in at least 100 non-overlapping nuclei were counted. The loci interrogated were 1p36.3 (RP11-62M23 labeled red)/1q25.3-q31.1 (RP11-162L13 labeled green) and 19q13.3 (CTD-2571L23 labeled red)/19p12 (RP11-420K14 labeled green). A sample was considered 1p or 19q deleted when more than 50% of counted nuclei exhibited one target (red) signal and two reference (green) signals.
Statistical analysis
Comparisons between IDLGGs group and symptomatic LGGs group were conducted using chi-square or Fisher exact tests for categorical variables (sex proportion, tumor location, pathological diagnosis, extent of resection, etc.) and t-test for continuous variables (age, tumor volume, etc.). Overall survival (OS) was measured from the date of diagnosis to the date of death or last follow-up. Event-free survival (EFS) was measured from the date of diagnosis to the date of disease recurrence, death, or last follow-up. Survival curves were constructed using Kaplan-Meier methods. Differences in OS and EFS between subgroups of patients were analyzed using log-rank test (univariate analysis). Statistical significance was defined as p-value being less than 0.05. Statistical analysis was performed using software IBM SPSS Statistics 19 (IBM Corp., Armonk, NY, USA).
Results
Between Jan 2001 and Dec 2004, 248 adult patients receiving surgeries in our institution were pathologically confirmed the diagnosis of primary LGGs located in the supratentorial hemispheres. 29 patients (11.6%) were lost to follow-up and the remaining 219 patients were included in this study. 23 patients (10.4%) met the criteria for IDLGGs, and the other 196 patients represented the symptomatic group.
Comparison of clinical data demonstrated IDLGGs were significantly smaller and more likely to be completely resected than symptomatic LGGs
The clinical, radiological, surgical and pathological characteristics of the entire cohort were summarized (Table 1). The reasons for radiological examination in patients with IDLGGs were trauma (47.8%), followed by dizziness (26.1%), headache (21.7%), and health checkup (4.4%). Headache was described as “mild”, “migraine-like”, “little” in the medical records. The mean age at radiological diagnosis in the IDLGGs group was 41.9±2.6 years, which was similar to that in the symptomatic group (39.0±0.7 years). There was no significant difference in sex composition between the two groups. Preoperative KPS in the IDLGGs group was significantly higher than that of the symptomatic group (P < 0.001). At the time of operation, the mean tumor volume was significantly smaller in IDLGGs group than that in the symptomatic group (P = 0.014). IDLGGs were much less likely to involve eloquent areas such as cortices of motor, sensory or language areas as well as basal ganglia (P < 0.001) (Figure 1). The rate of complete resection in the IDLGGs group was significantly higher than that in the symptomatic group (P = 0.037) (Table 1).
Table 1.
Comparison of clinical, radiological, pathological and surgical characteristics between IDLGGs and Symptomatic LGGs
| IDLGGs | Symptomatic LGGs | P-value | |
|---|---|---|---|
| No. of cases | 23 | 196 | |
| Reasons for imaging in IDLGGs (%) | |||
| Trauma | 11 (47.8%) | - | |
| Dizziness | 6 (26.1%) | - | |
| Headache | 5 (21.7%) | - | |
| Health checkup | 1 (4.4%) | ||
| Clinical characteristics | |||
| Mean age at diagnosis (years) | 41.9±2.6 | 39.0±0.7 | 0.183 |
| Sex, Male/Female | 13/10 | 117/79 | 0.770 |
| Median preoperative KPS | 100 | 90 | < 0.001 |
| Radiological findings | |||
| Main tumor location | 0.360 | ||
| Frontal | 15 | 120 | |
| Temporal | 3 | 44 | |
| Parietal | 4 | 16 | |
| Occipital | 1 | 5 | |
| Insular | 0 | 11 | |
| Mean tumor volume (cm3) | 23.8±5.1 | 65.3±5.7 | 0.014 |
| Eloquent area involvement (Yes/No) | 2/21 | 112/84 | < 0.001 |
| Extent of resection | 0.037 | ||
| Gross total resection | 21 | 139 | |
| Subtotal or partial resection | 2 | 57 | |
| Pathological classification | < 0.001 | ||
| Grade II Astrocytoma | 6 | 128 | |
| Grade II Oligodendroglioma | 6 | 55 | |
| Grade II Oligoastrocytoma | 11 | 13 |
KPS = Karnofsky performance status.
Figure 1.
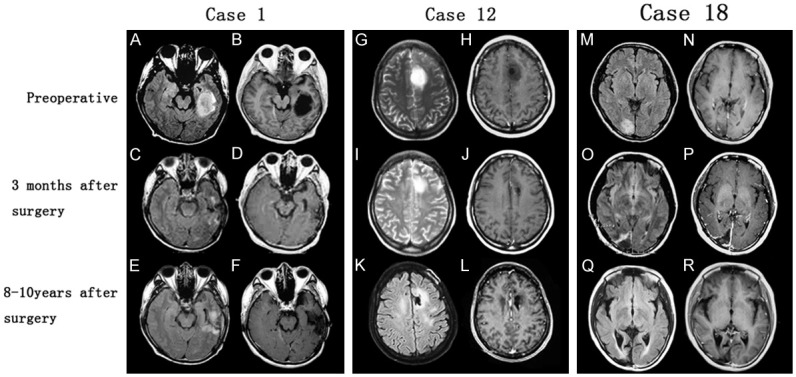
Radiological findings in three cases of IDLGGs. Preoperative MRs incidentally revealed left temporal lesion (Case 1), left frontal lesion (Case 2) and right occipital lesion (Case 18). Postoperative MRs 3 months after operations demonstrated surgical changes with no obvious tumor residual and latest follow-up MRs showed no signs of tumor recurrence. (A, C, E, M, O, Q: axial FLAIR sequences; G, I, K: axial T2-weighted sequences; B, D, F, H, J, L, N, P, R: axial T1-weighted sequences after contrast enhancement). FLAIR = fluid attenuation inversion recovery.
Pathological analysis revealed IDLGGs were mostly oligodendroglioma and oligoastrocytoma, with high frequency of IDH1 mutation and 1p/19q co-deletion
Pathological diagnoses of the 23 IDLGGs comprised 6 WHO grade II astrocytomas, 6 WHO grade II oligodendrogliomas and 11 WHO grade II oligoastrocytomas. This predominance of oligo-component (17/23) in IDLGGs was significantly different from the proportion of oligo-component in symptomatic LGGs (68/196) (Table 1). Table 2 summarized additional pathological features in patients with IDLGGs. IDH1/IDH2 mutation analysis by direct sequencing and IDH1-R132H immunohistochemistry demonstrated 95.7% (22/23) of the IDLGGs harbored IDH1-R132H mutation. The result of IDH1-R132H immunohistochemistry was 100% concordant with the result of direct sequencing. TERT promoter mutations were detected in 43.5% (10/23) of the cohort. Chromosome 1p/19q co-deletion was detected in 69.6% (16/23) of the IDLGGs. One case exhibited chromosome 1p deletion and six cases had intact 1p and 19q. Relationships between IDH mutations, TERT promoter mutations, and 1p/19q codeletion in IDLGGs were analyzed (Figure 3). TERT promoter mutations and 1p/19q codeletion were both included in IDLGGs with IDH mutations. There were 9 cases with both TERT promoter mutations and 1p/19q codeletion, 1 case with TERT promoter mutations and IDH mutations, and 7 cases with 1p/19q codeletion and IDH mutations. 1 IDLGG was IDH wild-type, TERT promoter wild-type and 1p/19q intact.
Table 2.
Summary of pathological features and clinical courses in patients with IDLGGs (n = 23)
| Case No. | Symptoms | Sex | Age | Preop KPS | Resection extent | Pathology | IDH1/2 | IHC of IDHR132H | 1p19q status | TERT promoter | Postop KPS | RT | CHT | Recurrence | EFS (Years) | Death | OS (Years) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Trauma | M | 35 | 100 | CR | AII | Mut | Positive | intact | Wt | 100 | Y | Y | N | 9.2 | N | 9.2 |
| 2 | Trauma | M | 45 | 100 | CR | OAII | Mut | Positive | co-deleted | Mut | 100 | N | Y | N | 9.8 | N | 9.8 |
| 3 | Dizziness | M | 27 | 100 | ICR | OAII | Mut | Positive | co-deleted | Mut | 100 | Y | Y | N | 8.8 | N | 8.8 |
| 4 | Trauma | M | 34 | 100 | CR | OAII | Mut | Positive | co-deleted | Mut | 0 | Y | Y | Y | 9.0 | Y | 10.0 |
| 5 | Health checkup | M | 48 | 100 | CR | OII | Mut | Positive | co-deleted | Mut | 20 | Y | Y | Y | 9.0 | N | 10.3 |
| 6 | Headache | F | 42 | 90 | CR | OAII | Mut | Positive | co-deleted | Wt | 100 | Y | Y | N | 10.7 | N | 10.7 |
| 7 | Trauma | F | 60 | 100 | CR | AII | Mut | Positive | intact | Wt | 100 | Y | Y | N | 8.4 | N | 8.4 |
| 8 | Dizziness | F | 32 | 100 | CR | OII | Mut | Positive | intact | Mut | 100 | Y | Y | N | 12.3 | N | 12.3 |
| 9 | Dizziness | M | 37 | 100 | CR | OAII | Mut | Positive | co-deleted | Mut | 0 | Y | Y | Y | 5.7 | Y | 6.2 |
| 10 | Dizziness | M | 50 | 100 | CR | OAII | Mut | Positive | co-deleted | Wt | 100 | Y | N | N | 10.3 | N | 10.3 |
| 11 | Trauma | F | 37 | 100 | CR | AII | Mut | Positive | co-deleted | Wt | 0 | U | U | Y | 3.3 | Y | 3.9 |
| 12 | Trauma | F | 34 | 100 | CR | AII | Mut | Positive | intact | Wt | 100 | Y | Y | N | 10.4 | N | 10.4 |
| 13 | Headache | M | 37 | 90 | CR | OAII | Mut | Positive | co-deleted | Mut | 100 | Y | N | N | 7.7 | N | 7.7 |
| 14 | Dizziness | F | 55 | 100 | CR | OII | Mut | Positive | 1p loss | Wt | 0 | U | U | Y | 2.8 | Y | 3.3 |
| 15 | Trauma | M | 47 | 100 | CR | OII | Mut | Positive | co-deleted | Mut | 100 | Y | Y | N | 9.3 | N | 9.3 |
| 16 | Trauma | F | 35 | 100 | CR | OAII | Mut | Positive | co-deleted | Mut | 100 | Y | Y | N | 8.0 | N | 8.0 |
| 17 | Trauma | M | 79 | 100 | ICR | AII | Mut | Positive | intact | Wt | 0 | Y | Y | Y | 4.0 | Y | 4.4 |
| 18 | Headache | F | 40 | 100 | CR | OAII | Wt | Negative | intact | Wt | 100 | Y | Y | N | 10.0 | N | 10.0 |
| 19 | Headache | F | 42 | 100 | CR | OII | Mut | Positive | co-deleted | Wt | 100 | Y | Y | N | 10.0 | N | 10.0 |
| 20 | Trauma | M | 57 | 100 | CR | OAII | Mut | Positive | co-deleted | Wt | 100 | N | Y | N | 7.9 | N | 7.9 |
| 21 | Headache | F | 28 | 90 | CR | OII | Mut | Positive | co-deleted | Mut | 90 | Y | Y | N | 11.5 | N | 11.5 |
| 22 | Dizziness | M | 37 | 100 | CR | AII | Mut | Positive | co-deleted | Wt | 100 | Y | Y | N | 11.8 | N | 11.8 |
| 23 | Trauma | M | 26 | 100 | CR | OAII | Mut | Positive | co-deleted | Wt | 90 | Y | Y | N | 8.3 | N | 8.3 |
M = male; F = female; CR = complete resection; ICR = incomplete resection; AII = WHO grade II astrocytoma; OII = WHO grade II oligodendroglioma; OAII = WHO grade II Oligoastrocytoma; Mut = mutation; Wt = wile-type; IHC = immunohistochemistry; Y = yes; N = no; U = unknown; RT = radiation therapy; CHT = chemotherapy; EFS = event-free survival; OS = overall survival.
Figure 3.
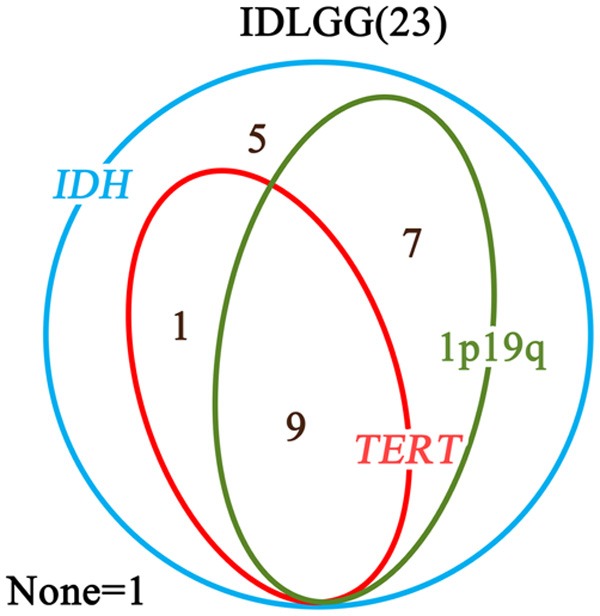
Relationships between IDH mutations, TERT promoter mutations, and 1p/19q codeletion in IDLGGs. The Venn diagram demonstrated that TERT promoter mutations and 1p/19q codeletion were both included in IDLGGs with IDH mutations. There were 9 cases with both TERT promoter mutations and 1p/19q codeletion, 1 case with TERT promoter mutations and IDH mutations, and 7 cases with 1p/19q codeletion and IDH mutations. 1 IDLGG was IDH wild-type, TERT promoter wild-type and 1p/19q intact.
Survival analysis showed patients with IDLGGs had much longer survival than those with symptomatic LGGs
The median follow-up was 9.3 years in the cohort of IDLGGs. Median survival time has not been reached due to insufficient number of death cases. The estimated 10-year OS and EFS of the IDLGGs group were 74.3% and 69.9%, respectively. In the univariate analysis of prognostic factors in 219 patients with supratentorial hemispheric LGGs, OS of IDLGGs group was significantly better than that of symptomatic LGGs group (P = 0.027) (Figure 2).
Figure 2.
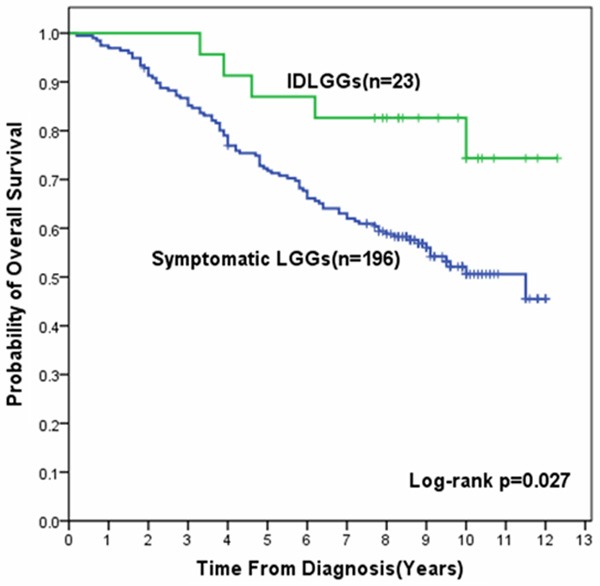
Kaplan-Meier survival curves for OS in patients with IDLGGs and Symptomatic LGGs. OS in patients with IDLGGs was significantly longer than that in patients with symptomatic LGGs (Log-rank P = 0.027).
Discussion
IDLGGs are commonly defined as low-grade gliomas detected by neuroimaging with unrelated complaints or purposes [5,6,11,12]. The incidence of IDLGGs was reported to range from 0.025% to 0.3% in health volunteers [6-9,12]. Pallud et al [5] reported 47 IDLGGs (3.8%) in 1296 cases of LGGs and the proportion of IDLGGs was 9.6% (35/364) in the series presented by Potts et al [6]. In our series, 10.4% of pathologically confirmed supratentorial LGGs were incidentally discovered. The rate of IDLGGs in our study was similar to that reported by Potts et al and higher than that in Pallud’s series. One of the possible reasons may be that Potts and our series were in a later time span in which modern brain imaging technology such as magnetic resonance imaging (MRI) was more available for examination of suspected cerebral lesions. Nevertheless, IDLGGs are occasionally encountered in current neurosurgical practice and our knowledge on this subgroup of LGGs is relatively limited.
Although IDLGGs are histologically in the scope of LGGs, they differ from symptomatic LGGs in several aspects. First, IDLGGs were found to be much smaller at diagnosis compared with symptomatic LGGs and the frequency of midline shift in neuroimaging of IDLGGs was significantly lower. Additionally, unlike symptomatic LGGs, IDLGGs were revealed rarely involving the functional areas of brain. These radiological features of IDLGGs (smaller tumor volume and non-eloquent locations) were confirmed by previous two series of IDLGGs as well as ours [5,6], and may be correlated with the absence of symptoms when the tumors were discovered. Another remarkable difference demonstrated by our data is that IDLGGs is much more amenable to be completely resected than symptomatic LGGs. This finding is not unexpected and is in accordance with the conclusions of Pallud et al and Potts et al [5,6].
The survival analysis based on long follow-up time (median duration of follow-up: 9.3 years) in our series of IDLGGs and symptomatic LGGs compensated the limitations on relatively short follow-up time in previous studies and reinforced the conclusions that patients with IDLGGs survived significantly longer than those with symptomatic LGGs if surgically treated [5,6]. Previous studies identified that absence of neurological deficits, higher preoperative KPS, smaller tumor volume, non-eloquent tumor location and more extensive surgical resection were favorable prognostic factors significantly influencing the survival of LGGs [3,4,13-15]. These factors mentioned above were all clinical characteristics of IDLGGs compared to symptomatic LGGs and therefore may contribute to the better survival of patients with IDLGGs. In the multivariate analysis of our LGGs cohort, however, IDLGGs were not identified as an independent prognostic marker. The effects of symptom, preoperative KPS, tumor volume, tumor location and extent of resection on the survival of patients with LGGs were possibly correlated and inTERTwined with each other and led to the loss of significance in the multivariate analysis. A series of LGGs with larger sample size may be able to identify the prognostic role of IDLGGs to a greater extent.
A primary finding of this study is that the majority of the IDLGGs were oligodendroglioma and oligoastrocytoma with 95.7% harboring IDH1 mutation and 69.6% containing 1p/19q co-deletion. These pathological results concerning IDLGGs have several important implications based on previous studies on molecular markers in LGGs. First, oligodendroglial histology, IDH1 mutation and 1p/19q co-deletion were demonstrated to be favorable prognostic factors impacting the overall survival of patients by several major retrospective studies on LGGs [16-19]. Therefore, the high proportion of oligodendroglioma and oligoastrocytoma, IDH1 mutation and 1p/19q co-deletion in IDLGGs may also explain the better prognosis of the cohort compared to symptomatic LGGs, in addition to the difference between extents of surgical resection in the two groups. Second, our data reinforced previous findings that IDH mutations are early genetic events occurred in the development of gliomas [20,21]. Statistics in a recent review on IDH mutations in glioma showed that these genetic events are found in 76% WHO Grade II astrocytoma, 78.8% WHO Grade II oligodendroglioma and 79.8% WHO Grade II oligoastrocytoma [22]. The high incidence of IDH mutations in LGGs raised the possibility that IDH mutations occurred early in the natural course of glioma development. The fact that 95.7% of IDLGGs harbored IDH1 mutations in our study suggested these genetic events probably occurred even earlier--when LGGs were asymptomatic and smaller in volume. Third, we consider IDLGGs not a subgroup largely different from other LGGs, at least in pathological perspective. Recent analysis on distribution of molecular features and clinical outcomes in LGGs showed a trend in distinction of three subgroups of the disease: LGGs with IDH mutations and 1p/19q co-deletion, in which the majorities are oligodendroglioma or oligoastrocytoma; LGGs with IDH mutations and without 1p/19q co-deletion, which are mainly astrocytoma; and LGGs without IDH mutations, in which different histological types exist and are characterized by worse survival [18,19]. Our cohort of IDLGGs fall into all three subgroups of LGGs and this may indicate that IDLGGs were not a unique entity discrepant from other LGGs. Nevertheless, our IDLGGs cohort showed frequent IDH1 mutation and 91.3% (21/23) of the cases received gross total resection. This data was consistent with the high grade counterparts which IDH1 mutated malignant gliomas were more amenable to surgical resection [2].
Due to the rarity of IDLGGs and difficulty in long-term follow-up, there lack prospective and retrospective studies clarifying the questions on the treatment of the disease. Thus, managements of IDLGGs, which included “Watch and Wait” strategy (including biopsy) and early surgical resection, were controversially discussed in former articles focusing on the entities [5,6,11,12]. In our opinion, patients with suspected IDLGGs are recommended to receive surgical resection early for the following reasons. Firstly, IDLGGs provide neurosurgeons an opportunity to resect tumors when they were in the smallest volume at diagnosis. Tumor volume was demonstrated to relate to extent of resection in LGGs [13], and data in previous studies and ours all confirmed about the significantly smaller tumor volume and greater extent of resection in IDLGGs [5,6]. More extensive resection was independently associated with favorable OS in LGGs [13] and therefore may contribute to better prognosis in IDLGGs. Secondly, although deferring surgery may be suitable in managing some incidentally discovered lesions of brain, this strategy may run the risk of malignant transformation in IDLGGs in our experience (Supplementary Figure 1). Thirdly, radiological and pathological analyses in previous studies and ours suggested that IDLGGs were proliferative tumors with genetic events (IDH mutations and 1p/19q co-deletion) commonly occurred in LGGs [5,6]. Thus, IDLGGs are likely to share the same histopathological nature as their symptomatic counterparts and treatment strategies on LGGs are probably applicable to the scenarios of IDLGGs. Although “Watch and Wait” strategy until progression of LGGs was claimed to be secure by some studies [23,24], a recent controlled research on LGGs demonstrated significantly better OS and lower malignant transformation rate in the group favoring early surgical resection than the group favoring “Watch and Wait” strategy [25]. Another point worth noting is that nearly 70% of IDLGGs in our study harbored IDH1 mutation and 1p/19q co-deletion. IDH1 mutation and 1p/19q deletion were suggested as favorable markers for sensitivity to adjuvant genotoxic treatment (radiation therapy and chemotherapy) in LGGs [18,26,27]. IDLGGs therefore may be a subgroup of LGGs that benefit more in adjuvant radiation therapy and chemotherapy. This perspective seems to support a strategy consisting surgical resection, which diminishes tumor cells to a great extent, and subsequent adjuvant radiation therapy and chemotherapy.
To our knowledge, IDLGGs are the early stages in the development of LGGs [15], and are valuable for investigating genetic abnormalities occurred in the initial periods of glioma development. Our results (Figure 3) suggested that, in IDH mutated IDLGGs, IDH mutations may be the first step that occurred, followed by mutations in TERT promoter and codeletion of chromosome 1p/19q. The chronological orders of TERT promoter mutations and chromosome 1p/19q codeletion remains undetermined. This hypothesis on the chronological orders of the three genetic changes (IDH mutations, TERT promoter mutations and 1p/19q codeletion) in the development of gliomas is consistent with the model proposed by Arita H et al [31].
In conclusion, we conclude that the rare group of IDLGGs is mostly oligodendroglial tumors with 1p19q co-deletion and the vast majority possesses IDH1 mutation. Patients with IDLGGs survived significantly longer than those with symptomatic LGGs. The reasons for better prognosis of IDLGGs may include clinical factors such as more extensive resection, non-eloquent tumor location and smaller tumor volume, as well as pathological factors such as preponderance of oligodendroglioma and oligoastrocytoma, high frequency of IDH1 mutation and 1p/19q co-deletion. Although the question of how to manage IDLGGs requires definitive data from future prospective or at least retrospective controlled studies focusing on these entities, this study with long-term follow-up data further demonstrates the safety and utility of early surgical resection in treating patients with IDLGGs.
Acknowledgements
This study was supported by Natural Science Foundation Grant 81172412, the Research Special Fund for Public Welfare Industry of Health (201402008), the Project for Science and Technology Commission of Shanghai Municipality Grant (13JC1408000), National key clinical specialist construction Programs of China and The major research and development project of innovative drugs, Ministry of Science and Technology (2012ZX09303004001).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang EF, Potts MB, Keles GE, Lamborn KR, Chang SM, Barbaro NM, Berger MS. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108:227–235. doi: 10.3171/JNS/2008/108/2/0227. [DOI] [PubMed] [Google Scholar]
- 3.Cavaliere R, Lopes MB, Schiff D. Low-grade gliomas: an update on pathology and therapy. Lancet Neurol. 2005;4:760–770. doi: 10.1016/S1474-4422(05)70222-2. [DOI] [PubMed] [Google Scholar]
- 4.Pignatti F, van den Bent M, Curran D, Debruyne C, Sylvester R, Therasse P, Afra D, Cornu P, Bolla M, Vecht C, Karim AB. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J. Clin. Oncol. 2002;20:2076–2084. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 5.Pallud J, Fontaine D, Duffau H, Mandonnet E, Sanai N, Taillandier L, Peruzzi P, Guillevin R, Bauchet L, Bernier V, Baron MH, Guyotat J, Capelle L. Natural history of incidental World Health Organization grade II gliomas. Ann Neurol. 2010;68:727–733. doi: 10.1002/ana.22106. [DOI] [PubMed] [Google Scholar]
- 6.Potts MB, Smith JS, Molinaro AM, Berger MS. Natural history and surgical management of incidentally discovered low-grade gliomas. J Neurosurg. 2012;116:365–372. doi: 10.3171/2011.9.JNS111068. [DOI] [PubMed] [Google Scholar]
- 7.Morris Z, Whiteley WN, Longstreth WJ, Weber F, Lee YC, Tsushima Y, Alphs H, Ladd SC, Warlow C, Wardlaw JM, Al-Shahi SR. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. doi: 10.1136/bmj.b3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eskandary H, Sabba M, Khajehpour F, Eskandari M. Incidental findings in brain computed tomography scans of 3000 head trauma patients. Surg Neurol. 2005;63:550–553. doi: 10.1016/j.surneu.2004.07.049. [DOI] [PubMed] [Google Scholar]
- 9.Katzman GL, Dagher AP, Patronas NJ. Incidental findings on brain magnetic resonance imaging from 1000 asymptomatic volunteers. JAMA. 1999;282:36–39. doi: 10.1001/jama.282.1.36. [DOI] [PubMed] [Google Scholar]
- 10.Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, Niessen WJ, Breteler MM, van der Lugt A. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357:1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 11.Shah AH, Madhavan K, Sastry A, Komotar RJ. Managing Intracranial Incidental Findings Suggestive of Low-Grade Glioma: Learning from Experience. World Neurosurg. 2013;80:e75–7. doi: 10.1016/j.wneu.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Yao Y, Zhou LF. Treatment of Incidentally Discovered Low-Grade Gliomas: “Watch-and-Wait” or Not? World Neurosurg. 2013;80:e121–2. doi: 10.1016/j.wneu.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW, Berger MS. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J. Clin. Oncol. 2008;26:1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 14.Chang EF, Clark A, Jensen RL, Bernstein M, Guha A, Carrabba G, Mukhopadhyay D, Kim W, Liau LM, Chang SM, Smith JS, Berger MS, McDermott MW. Multiinstitutional validation of the University of California at San Francisco Low-Grade Glioma Prognostic Scoring System. Clinical article. J Neurosurg. 2009;111:203–210. doi: 10.3171/2009.2.JNS081101. [DOI] [PubMed] [Google Scholar]
- 15.Schomas DA, Laack NN, Rao RD, Meyer FB, Shaw EG, O'Neill BP, Giannini C, Brown PD. Intracranial low-grade gliomas in adults: 30-year experience with long-term follow-up at Mayo Clinic. Neuro Oncol. 2009;11:437–445. doi: 10.1215/15228517-2008-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houillier C, Wang X, Kaloshi G, Mokhtari K, Guillevin R, Laffaire J, Paris S, Boisselier B, Idbaih A, Laigle-Donadey F, Hoang-Xuan K, Sanson M, Delattre JY. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75:1560–1566. doi: 10.1212/WNL.0b013e3181f96282. [DOI] [PubMed] [Google Scholar]
- 17.Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, El HS, Boisselier B, Mokhtari K, Hoang-Xuan K, Delattre JY. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J. Clin. Oncol. 2009;27:4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann C, Hentschel B, Tatagiba M, Schramm J, Schnell O, Seidel C, Stein R, Reifenberger G, Pietsch T, von Deimling A, Loeffler M, Weller M. Molecular markers in low-grade gliomas: predictive or prognostic? Clin Cancer Res. 2011;17:4588–4599. doi: 10.1158/1078-0432.CCR-10-3194. [DOI] [PubMed] [Google Scholar]
- 19.Weiler M, Wick W. Molecular predictors of outcome in low-grade glioma. Curr Opin Neurol. 2012;25:767–773. doi: 10.1097/WCO.0b013e32835a0217. [DOI] [PubMed] [Google Scholar]
- 20.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn GP, Andronesi OC, Cahill DP. From genomics to the clinic: biological and translational insights of mutant IDH1/2 in glioma. Neurosurg Focus. 2013;34:E2. doi: 10.3171/2012.12.FOCUS12355. [DOI] [PubMed] [Google Scholar]
- 23.Recht LD, Lew R, Smith TW. Suspected low-grade glioma: is deferring treatment safe? Ann Neurol. 1992;31:431–436. doi: 10.1002/ana.410310413. [DOI] [PubMed] [Google Scholar]
- 24.Reijneveld JC, Sitskoorn MM, Klein M, Nuyen J, Taphoorn MJ. Cognitive status and quality of life in patients with suspected versus proven low-grade gliomas. Neurology. 2001;56:618–623. doi: 10.1212/wnl.56.5.618. [DOI] [PubMed] [Google Scholar]
- 25.Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgard G, Solheim O. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308:1881–1888. doi: 10.1001/jama.2012.12807. [DOI] [PubMed] [Google Scholar]
- 26.Bauman GS, Ino Y, Ueki K, Zlatescu MC, Fisher BJ, Macdonald DR, Stitt L, Louis DN, Cairncross JG. Allelic loss of chromosome 1p and radiotherapy plus chemotherapy in patients with oligodendrogliomas. Int J Radiat Oncol Biol Phys. 2000;48:825–830. doi: 10.1016/s0360-3016(00)00703-3. [DOI] [PubMed] [Google Scholar]
- 27.Weller M, Berger H, Hartmann C, Schramm J, Westphal M, Simon M, Goldbrunner R, Krex D, Steinbach JP, Ostertag CB, Loeffler M, Pietsch T, von Deimling A. Combined 1p/19q loss in oligodendroglial tumors: predictive or prognostic biomarker? Clin Cancer Res. 2007;13:6933–6937. doi: 10.1158/1078-0432.CCR-07-0573. [DOI] [PubMed] [Google Scholar]
- 28.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao Y, Chan AK, Qin ZY, Chen LC, Zhang X, Pang JC, Li HM, Wang Y, Mao Y, Ng HK, Zhou LF. Mutation analysis of IDH1 in paired gliomas revealed IDH1 mutation was not associated with malignant progression but predicted longer survival. PLoS One. 2013;8:e67421. doi: 10.1371/journal.pone.0067421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao Y, Zhou LF. Treatment of Incidentally Discovered Low-Grade Gliomas: “Watch-and-Wait” or Not? World Neurosurg. 2012;80:e121–122. doi: 10.1016/j.wneu.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 31.Arita H, Narita Y, Fukushima S, Tateishi K, Matsushita Y, Yoshida A, Miyakita Y, Ohno M, Collins VP, Kawahara N, Shibui S, Ichimura K. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013;126:267–276. doi: 10.1007/s00401-013-1141-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


