Abstract
Ki-67 expression is an important tool for distinguishing between malignant and benign tumors. It usually shows the nuclear staining. However, the cell membrane staining of MIB-1, which is one of the clones of Ki-67, in the hyalinizing trabecular adenoma of the thyroid gland and other tumors had also been reported. In our practice, we found that the 7B11 antibody could be immunoreactived with the adipose tissues inside or around tumors in the membrane. Thus, in this study, we determined if Ki-67 expression would be useful in recognizing the lipoblasts and adipocytes. The five clones of the Ki-67 antibody, namely, 7B11, K-2, SP5, MIB-1, and SP6 were selected. The adipocytes showed strong 7B11 staining in the cell membrane. The brown fat cells were strongly immunoreactive with 7B11 in the arachnoid layer of the cytoplasm. The adipocytes and brown fat cells showed positive, albeit weaker K-2 staining in the cell membrane and cytoplasm, respectively, compared to 7B11. The adipose tissues and brown fat cells were non-reactive to clones SP5, MIB-1, and SP6. All adipocytes in the lipomas, angiolipomas, uterine lipoleiomyomas, and angioleiomyolipomas showed diffusedly positive 7B11 and K-2 staining in the cell membrane, with stronger immunoreactivity to 7B11 compared with K-2. All hibernomas showed diffusedly cytoplasmic arachnoid staining of 7B11, but only focal to K-2. The lipoblasts in adipocytic tumors also showed positive 7B11 and K-2 staining; however, nearly all of the vacuolated lipoblasts showed strong 7B11 staining, only focal vacuolated lipoblasts in the adipocytic tumors were immunoreactive to K-2 positivity. All other components of the adipocytic tumors were non-reactive to 7B11, K-2, SP5, MIB-1, and SP6 in the cell membrane and cytoplasm. Our results showed that the 7B11 could well help to identify the lipoblasts, which would be useful to diagnose the malignant adipocytic tumors.
Keywords: Ki-67, adipocyte, lipoblast
Introduction
Adipocytic tumors represent the largest group of mesenchymal tumors, and liposarcomas are the single most common type of soft tissue sarcoma. In sometimes, the liposarcomas can be difficult to distinguish between dedifferentiated liposarcoma, myxoid liposarcoma, and pleomorphic liposarcoma myxofibrosarcoma, carcinoma, and other malignant tumors. Thus, immunochemistry has been a valuable tool to help differentiate various types of tumors. Specifically, S-100, CDK4, and MDM2 are immunohistochemical markers that are commonly used to differentiate liposarcomas from other malignant tumors [1]. However, these markers are not specific for the liposarcomas.
Ki-67 is a human nuclear protein that is present during all active phases of the cell cycle (G1, S, G2, and mitosis), but is absent from resting cells (G0) [2,3]. Therefore, it is an excellent tool for measuring the growth fraction of cells in human tumors. Ki-67, which is associated with tumor malignancy, has been widely applied in the studies of proliferative activity in various neoplasms, including salivary cancer [4], breast carcinoma [5-7], serous tubal intraepithelial carcinoma [8], squamous intraepithelial neoplasia [9,10], and goblet cell carcinoid [11].
Previously, the cell membrane or cytoplasmic MIB-1 staining has been successively described in sclerosing hemangioma of the lung [12], salivary gland pleomorphic adenoma [13], and hyalinizing trabecular adenoma of the thyroid [14,15].
Recently, we found that the Ki-67 antibody (clone 7B11) was immunoreactive with normal white adipose tissues, which are located inside or around tumors in a membranous pattern; however, clone MIB-1 of the Ki-67 antibody was non-reactive with adipose tissue. Therefore, in this study, we used five clones of Ki-67 (7B11, K-2, SP5, SP6 and MIB-1) to immunostain normal adipose tissues and adipocytic tumors.
Materials and methods
Tissue samples
The following cases were retrieved from the Department of Pathology at the People’s Liberation Army 152 Hospital and Yexian People’s Hospital (Henan, China) between 2000 and 2013: three cases of hibernomas, 35 cases of lipomas, 25 cases of angiolipomas, 10 cases of uterine lipoleiomyomas, 35 cases of atypical lipomatous tumors/well-differentiated liposarcomas, five cases of dedifferentiated liposarcomas, 12 cases of myxoid liposarcomas, seven cases of pleomorphic liposarcomas and four cases of mixed-type liposarcomas. Normal tissues were obtained from the People’s Liberation Army 152 Hospital, and included the appendix (Figure 1A), skin, parotid gland (Figure 1D), gastrointestinal tract, vocal cord, breast, and brown fat tissues (Figure 1G).
Figure 1.
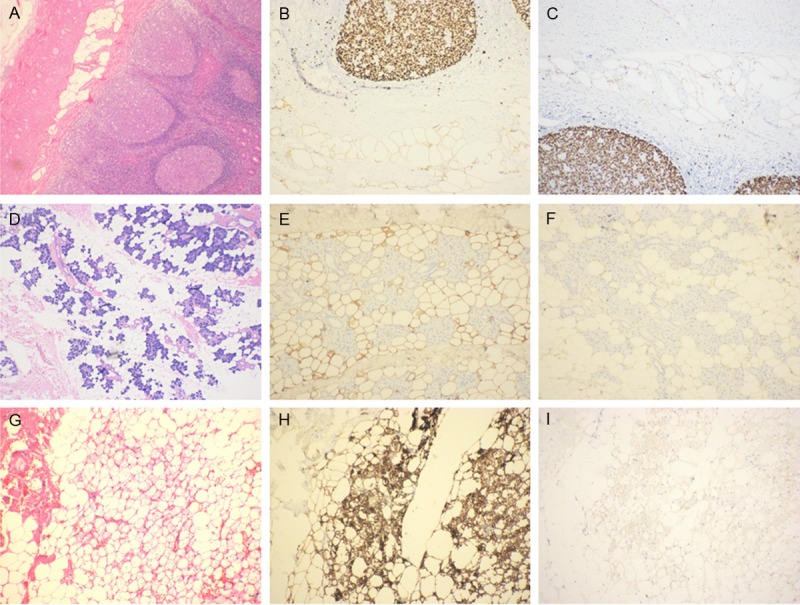
Immunostaining of normal adipose tissues with Ki-67 antibodies, clones 7B11 and K-2. A, D, G. H & E staining of normal appendix, parotid gland, and brown adipose tissues. B, E, H. Strong cytoplasmic staining of 7B11 in the adipocytes of the appendix, parotid gland, and brown adipose tissues. C, F, I. Weak cytoplasmic staining of K-2 in adipocytes of the appendix, parotid gland, and brown adipose tissues.
All specimens were fixed in 10% neutral buffered formalin, dehydrated through graded alcohol solutions, embedded in paraffin, cut into 4-μm-thick sections for hematoxylin and eosin (H & E) staining, and visualized by light microscopy.
Immunohistochemistry
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded tissue sections using the EnVision method. The primary Ki-67 antibodies used included five different clones; namely, 7B11, MIB-1, and SP6 from Shanghai Haojie Biological Technology Co. Ltd (Shanghai, China), and SP5 and K-2 from Beijing ZhongShan Golden Bridge Biological Technology Co. Ltd (Beijing, China). For 7B11, SP5, MIB-1, and SP6 antibody clones tested, antigen retrieval was based on three 5-minute passages in microwave oven at 750 W in 10 mM citrate buffer solution at pH 6.0. For K-2 antibody clone, antigen retrival was based on three 5-minute passages in microwave oven at 750 W in 10 mM EDTA solution at pH 9.0. The extent of Cytoplasmic and cell membrane staining was graded as follows: negative (0% to < 5%), focal (5% to 50%), and diffuse (> 50% to 100%).
Results
Differential immunoreactivity of Ki-67 antibodies in adipocytes
The Ki-67 antibody (clone 7B11) was immuoreactive with adipose tissues in the membrane in the appendix (Figure 1B), subcutis, parotid gland (Figure 1E), vocal cord, breast, and brown adipocytes (Figure 1H) in the neck area. The brown adipocytes were immunoreactive with 7B11, and showed a strong arachnoid staining pattern in the cytoplasm (Figure 1C). Adipose tissues and brown fat cells showed positive K-2 staining in the cell membrane and cytoplasm; however, the signal was weaker compared to staining with clone 7B11 (Figure 1F, 1I) (Tables 1, 2). There was no immnoreactivity in adipose tissues and brown fat cells with clones SP5, MIB-1, and SP6 (Table 1).
Table 1.
Different clones of Ki-67 expression in the normal adipose tissues and adipocytic tumors
| 7B11 | K-2 | SP5 | MIB-1 | SP6 | |
|---|---|---|---|---|---|
| The normal white adipose cells | + | + | - | - | - |
| The normal brown fat cells | + | + | - | - | - |
| The adipocytes in benign adipocytic tumors | + | + | - | - | - |
| The hibernoma | + | + | - | - | - |
| The the vacuolated lipoblasts in liposarcomas | + | + | - | - | - |
Table 2.
Comparison the extent and intensity of 7B11 and K-2 expression in the normal adipose tissues and adipocytic tumors
| 7B11 | K-2 | |||
|---|---|---|---|---|
|
| ||||
| Extent | Intensity | Extent | Intensity | |
| The normal white adipose cells | Diffuse | Strong | Moderate | diffuse |
| The normal brown fat cells | Diffuse | Strong | Moderate | diffuse |
| The adipocytes in benign adipocytic tumors | Diffuse | Strong | Mild | diffuse |
| The hibernoma | Diffuse | Strong | Weak | focal |
| The vacuolated lipoblasts in liposarcomas | Diffuse | Strong | Moderate | focal |
Differential immunoreactivity of Ki-67 antibodies in some adipocytic tumors
All adipocytes in the lipomas, angiolipomas, uterine lipoleiomyomas, angioleiomyolipomas, and lipomatosis of nerves showed positive 7B11 and K-2 staining in the cell membrane; however staining was stronger with clone 7B11 than K-2. All hibernomas showed cytoplasmic arachnoid staining with 7B11, but only focal and weak to K-2 (Tables 1, 2).
The lipoblasts in adipocytic tumors showed positive staining with 7B11 and K-2 (Figure 2A-F); however, nearly all vacuolated lipoblasts were strongly immunoreactive to 7B11, only focal were immunoreactive to clone K-2 (Figure 2B, 2C).
Figure 2.

Immunostaining of myxoid/round cell liposarcoma and pleomorphic liposarcoma with 7B11 and K-2. A, D. H & E staining of myxoid/round cell liposarcoma and pleomorphic liposarcoma. B, C. Membranous 7B11 and K-2 staining in the lipoblasts of myxoid/round cell liposarcoma. K-2-positive cell numbers were lower than 7B11-positive cell numbers. E. Cytoplasmic and membranous staining of 7B11 in pleomorphic liposarcoma. F. Immunoreactivity of 7B11 in lipoblasts at different stages. Red arrow indicates early-stage lipoblasts, green arrow indicates mid-stage lipoblasts, blue arrow indicates late-stage lipoblasts.
All other components of the adipocytic tumors were non-reactive to 7B11 and K-2 in the cell membrane and cytoplasm. In addition, adipocytic tumors were non-reactive to clones SP5, MIB-1, and SP6.
Discussion
Ki-67 is a large (~350 kDa) cell-cycle associated non-histone protein that is strictly associated with, and is apparently required for, cell proliferation [2,3]. It is present during all active phases of the cell cycle (G1, S, G2, mitosis), but is absent from resting cells (G0) [3]. A correlation has been demonstrated between Ki-67 index and the histopathological grade of neoplasms [4-8]. The assessment of Ki-67 expression in renal and ureter tumors showed a correlation between tumor proliferation and disease progression, thus making it possible to differentiate high-risk patients [11,16]. Therefore, Ki-67 expression may also prove to be important for distinguishing between malignant and benign tumors.
Cytoplasmic or cell membrane immunostaining with MIB-1 antibody in sarcomatous pleural mesothelioma, sclerosing hemangioma of the lung [12], salivary gland pleomorphic adenoma [13], hyalinizing trabecular adenoma of the thyroid [14,15], renal oncocytoma [17], and invasive breast carcinomas [17].
In our practice, we found that Ki-67 antibody (clone 7B11) was immunoreactive with adipose tissues, which are located inside or around tumors; however, clone MIB-1 was non-reactive with adipose tissue. To verify the diagnostic role of Ki67 membrane pattern, we selected the different clones of the Ki-67 to stain the adipose tissues and the adipose tumors.
Our results indicated that white adipose tissues in the skin, breast, parotid gland, vocal cord, submucosa, and serosa of the gastrointestinal tract and vocal cords showed strong and diffusedly membranous immunoreactivity for 7B11. Brown fat tissues showed strong and diffusedly membranous and cytoplasmic immunoreactvity for 7B11 in a silk screen pattern. K-2 also immunoreacted with normal adipose tissues in a similar pattern as 7B11; however, the extent and intensity of immunostaining were less than that of 7B11. Clones SP5, MIB-1, SP6 were non-reactive with normal adipose tissues in both the membrane and cytoplasm. The normal gland tissue was non-reactive with 7B11, SP6, SP5, MIB-1, and K-2 in the membrane and cytoplasm. These results indicate that 7B11 and K-2 can be used to differentiate adipocytes from the sebaceous gland, eccrine sweat gland, apocrine glands, terminal tubular glands of the gastrointestinal tract, and terminal duct-lobular unit.
We also found that the 7B11 and K-2 antibodies were immunoreactive with adipocytes in lipomas, angiolipomas, uterine lipoleiomyomas, angioleiomyolipomas, and lipomatosis of nerves in a membranous pattern. The adipocytes in the atypical lipomatous tumors/well differentiated liposarcomas were also positive for the 7B11 and K-2. Most importantly, lipoblasts were immunoreactive with 7B11 and K-2 in the membrane and cytoplasm, but were non-reactive to SP5, SP6, and MIB-1. However, the number of cells that expressed the 7B11 was more than that expressed the K-2. The data from this study show that 7B11 and K-2 is a useful marker to detect the lipoblasts in the liposarcomas.
The mechanism underlying the 7B11 and K-2 cytoplasmic and membranous staining pattern in adipocytes and lipoblasts remains unclear. Some deemed that the transmigration of Ki-67 antigen from the nucleus to cytoplasm and cell membrane is impossible, so cell membrane expression of MIB-1 is an artifact [18]. But someone had the converse viewpoint. Jose′ A. Ortiz-Rey, et al found that this staining pattern did not depend on the visualization system, not related to a specific type of breast carcinoma or to apocrine differentiation, and positive cases were not correlative [19]. So they presumed that the staining is not am artifact and it depends on a cross reactivity of antibody with another epitope [19]. In our study, although, the heat retrieval was carried out on the 7B11, the antigen retrieval for K-2 was EDTA. So our results would be not relate to the antigen retrieval and we presume that 7B11 and K-2 expression in the cytoplasmic and membranous staining pattern in adipocytes and lipoblasts is not artifact, but a cross reactivity of 7B11 and K-2 to the unknown antigenic determinant in the membrane and cytoplasm.
Disclosure of conflict of interest
None.
References
- 1.Binh MB, Sastre-Garau X, Guillou L, de Pinieux G, Terrier P, Lagacé R, Aurias A, Hostein I, Coindre JM. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft tissue neoplasms with genetic data. Am J Surg Pathol. 2005;29:1340–1347. doi: 10.1097/01.pas.0000170343.09562.39. [DOI] [PubMed] [Google Scholar]
- 2.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 3.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Izhak O, Akrish S, Nagler RM. Ki67 and salivary cancer. Cancer Invest. 2008;26:1015–1023. doi: 10.1080/07357900802088968. [DOI] [PubMed] [Google Scholar]
- 5.Barbareschi M, Girlando S, Mauri FM, Forti S, Eccher C, Mauri FA, Togni R, Dalla Palma P, Doglioni C. Quantitative growth fraction evaluation with MIBI and Ki67 antibodies in breast carcinomas. Am J Clin Pathol. 1994;102:171–175. doi: 10.1093/ajcp/102.2.171. [DOI] [PubMed] [Google Scholar]
- 6.Tawfik K, Kimler BF, Davis MK, Fan F, Tawfik O. Ki-67 expression in axillary lymph node metastases in breast cancer is prognostically significant. Hum Pathol. 2013;44:39–46. doi: 10.1016/j.humpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Iwata H, Masuda N, Sagara Y, Kinoshita T, Nakamura S, Yanagita Y, Nishimura R, Iwase H, Kamigaki S, Takei H, Tsuda H, Hayashi N, Noguchi S. Analysis of Ki-67 expression with neoadjuvant anastrozole or tamoxifen in patients receiving goserelin for premenopausal breast cancer. Cancer. 2013;119:704–713. doi: 10.1002/cncr.27818. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn E, Kurman RJ, Sehdev AS, Shih IM. Ki-67 Labeling index as an adjunct in the diagnosis of serous tubal intraepithelial carcinoma. Int J Gynecol Pathol. 2012;31:416–422. doi: 10.1097/PGP.0b013e31824cbeb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang WC, Wu TT, Chandan VS, Lohse CM, Zhang LZ. Ki-67 and ProExC are useful immunohistochemical markers in esophageal squamous intraepithelial neoplasia. Human Pathology. 2011;42:1430–1437. doi: 10.1016/j.humpath.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Donà MG, Vocaturo A, Giuliani M, Ronchetti L, Rollo F, Pescarmona E, Carosi M, Vocaturo G, Benevolo M. p16/Ki-67 dual staining in cervico-vaginal cytology: correlation with histology, Human Papillomavirus detection and genotyping in women undergoing colposcopy. Gynecol Oncol. 2012;126:198–202. doi: 10.1016/j.ygyno.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Liu E, Telem DA, Warner RR, Dikman A, Divino CM. The role of Ki-67 in predicting biological behavior of goblet cell carcinoid tumor in appendix. Am J Surg. 2011;202:400–403. doi: 10.1016/j.amjsurg.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 12.Hattori H. Sclerosing haemangioma of the lung is positive for MIB-1 in cell membrane and cytoplasmic staining pattern. Histopathology. 2002;40:291–293. doi: 10.1046/j.1365-2559.2002.01330.x. [DOI] [PubMed] [Google Scholar]
- 13.Tashiro T, Hirokawa M, Harada H, Yokoyama S, Sano T. Cell membrane expression of MIB-1 in salivary gland pleomorphic adenoma. Histopathology. 2002;41:559–561. doi: 10.1046/j.1365-2559.2002.01457_1.x. [DOI] [PubMed] [Google Scholar]
- 14.Hirokawa M, Carney JA. Cell membrane and cytoplasmic staining for MIB-1 in hyalinizing trabecular adenoma of the thyroid gland. Am J Surg Pathol. 2000;24:575–578. doi: 10.1097/00000478-200004000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Casey MB, Sebo TJ, Carney JA. Hyalinizing trabecular adenoma of the thyroid gland. Identification through MIB-1 staining of fine-needle aspiration biopsy smears. Am J Clin Pathol. 2004;122:506–510. doi: 10.1309/5KJU-9LV8-LLA6-CL76. [DOI] [PubMed] [Google Scholar]
- 16.Masuda M, Iki M, Takano Y, Asakura T, Noguchi S, Ikeda I, Kubota Y, Hosaka M. Prognostic significance of Ki-67 labeling index in urothelial tumors of the renal pelvis and ureter. J Urol. 1996;1556:1877–1880. [PubMed] [Google Scholar]
- 17.Leonardo E, Volante M, Barbareschi M, Cavazza A, Dei Tos AP, Bussolati G, Papotti M. Cell membrane reactivity of MIB-1 antibody to Ki67 in human tumors: fact or artifact? Appl Immunohistochem Mol Morphol. 2007;15:220–223. doi: 10.1097/01.pai.0000213122.66096.f0. [DOI] [PubMed] [Google Scholar]
- 18.Sordo RD, Sidoni A. MIB-1 cell membrane reactivity: a finding that should be interpreted carefully. Appl Immunohistochem Mol Morphol. 2007;16:568. doi: 10.1097/PAI.0b013e31817af2cf. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz-Rey JA, Peteiro A, Ferna’ndez-Costas A, Gómez de María C, Antón I. Cell membrane and cytoplasmic immunoreactivity of MIB-1 in invasive breast carcinoma. Appl Immunohistochem Mol Morphol. 2009;17:563–564. doi: 10.1097/PAI.0b013e3181a75e83. [DOI] [PubMed] [Google Scholar]


