Abstract
Objective: While it’s widely accepted that the etiology of ulcerative colitis (UC) involves both genetic and environmental factors, the pathogenesis of ulcerative colitis is still poorly understood. Intestinal epithelial apoptosis is one of the most common histopathological changes of UC and the expression of a number of apoptosis genes may contribute to the progression of UC. MicroRNAs have recently emerged as powerful regulators of diverse cellular processes and have been shown to be involved in many immune-mediated disorders such as psoriasis, rheumatoid arthritis, lupus, and asthma. A unique microRNA expression profile has been identified in UC, suggesting that, microRNAs play an important role in the pathogenesis of UC. We investigated the role of miR-29a in intestinal epithelial apoptosis in UC. Methods: The expression of miR-29a and Mcl-1, an anti-apoptotic BCL-2 family member, was evaluated in both UC patients and UC mice model induced by dextran sodium sulfate (DSS). The apoptosis rate of intestinal epithelial cells was also evaluated. Results: In UC patients and DSS-induced UC in mice, the expression of miR-29a and Mcl-1, were up-regulated and down-regulated, respectively. We identified a miR-29a binding site (7 nucleotides) on the 3’UTR of mcl-1 and mutation in this binding site on the 3’UTR of mcl-1 led to mis-match between miR-29a and mcl-1. Knockout of Mcl-1 caused apoptosis of the colonic epithelial HT29 cells. In addition, miR-29a regulated intestinal epithelial apoptosis by down-regulating the expression of Mcl-1. Conclusion: miR-29a is involved in the pathogenesis of UC by regulating intestinal epithelial apoptosis via Mcl-1.
Keywords: MiR-29a, apoptosis, ulcerative colitis, Mcl-1
Introduction
Ulcerative colitis (UC), one of the two major forms of inflammatory bowel disease (IBD), is characterized by relapsing inflammation of the large bowel [1]. Clinical manifestations include rectal bleeding and diarrhea during periods of exacerbation [2]. UC represents an important public health problem, as it tends to afflict young people. Epidemiological studies demonstrated that the incidence and prevalence of UC are increasing around the world, indicating its emergence as a global disease [3,4]. It was proposed that, UC develops in a genetically predisposed host as a consequence of dis-regulated immune response to environmental, in particular, enteric antigens, resulting in continuous immune mediated inflammation.
While the pathogenesis of UC is still unclear, numerous studies support that UC occurs with aberrant and exaggerated immune response [1,2,5] in genetically susceptible individuals [6]. A number of studies have suggested the role of apoptosis in the intestinal epithelial cells, caused by increased cytokine production, such as TNF, IL, and interferon family members [7]. Increased apoptosis of intestinal epithelial cells has been detected at the acute inflammatory sites in UC [8,9]. Induction of apoptosis of intestinal epithelial cells has also been described in a number of studies using murine colitis models [10-12]. Apoptosis can disrupt intestinal mucosal integrity and barrier function eventually leading to inflammation. Anti-TNF therapies for treating IBD patients were found to inhibit apoptosis of intestinal epithelial cells [13,14]. However, the molecular basis of apoptosis of intestinal epithelial cells in response to intestinal inflammation remains poorly understood. In addition, it has been suggested that the epithelial cell apoptosis rate is primarily influenced by local inflammatory response and is the consequence of UC rather than the cause of UC [15].
MicroRNAs are a class of small, single-stranded non-coding RNAs that regulate gene expression by mediating mRNA cleavage, repressing mRNA translation, or causing mRNA destabilization [16]. They are approximately 20-22 nucleotide RNA sequences that bind to complementary sequences in the 3’ UTR of multiple target mRNAs, usually resulting in their silencing. MicroRNAs, which are abundantly present in human cells, target ~60% of all genes. Most of the miRNAs have multiple target genes and can often repress hundreds of target genes expression.
MicroRNAs play crucial roles in various biological processes including cellular proliferation and differentiation, development and apoptosis [17,18]. Understanding the function of microRNA provides new insights into the pathogenesis of many human diseases including cancer, diabetes, infectious diseases, and autoimmune diseases.
The mammalian inflammatory response is a rapid and complex physiological reaction to noxious stimuli including microbial pathogens. Although inflammation plays a valuable role in combating infection, its dysregulation often occurs in people and can cause a variety of pathologies, ranging from chronic inflammation, to autoimmunity, to cancer. In recent years, our understanding of both the cellular and molecular networks that regulate inflammation has improved dramatically. Although much of the focus has been on the study of protein regulators of inflammation, recent evidence also points to a critical role for microRNAs, in managing certain features of the inflammatory process. Unique miRNA expression profiles have recently been reported in intestinal epithelial cells of patients with UC and the results suggested that miRNAs may play a central role in the pathogenesis of UC [19]. Among these miRNAs, our previous study demonstrated that miR-29a contributed to the development of UC by regulating TNF-α [20].
Myeloid cell leukemia 1 (Mcl-1), an anti-apoptotic BCL-2 family member localized to the mitochondrial membrane, is essential for the survival of multiple cell lineages, and is characterized as the most highly amplified genes in cancer [21-23]. Studies demonstrated that Mcl-1 play a central role in determining the cell fate of cancer. Whether Mcl-1 is involved in the apoptosis and inflammation in UC is unknown. Recently, several observation suggest miR-29b regulates Mcl-1 expression. Mott et al. reported that mir-29b regulates Mcl-1 protein expression and apoptosis in H69 cells. The isoform of miR-29a and miR-29b share the first 9 nucleotides with minor divergence thereafter, it is suggested potentially target Mcl-1 [24]. In this study, we first measured the expression of Mcl-1 in UC patients and mice induced by DSS. In addition, we also investigated the possible role of miRNA, especially miR-29a in regulating Mcl-1 and its involvement in apoptosis and pathogenesis of UC.
Materials and methods
Ethics statement
All experiments involving human participants have been approved by the Medical Research Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine, and conducted according to the principles expressed in the Declaration of Helsinki. All participants involved in the study signed the informed consent forms and all animal experiments were conducted according to relevant national and international guidelines. This project was approved by the Medical Research Animal Ethics Committee of Guangzhou University of Chinese Medicine.
Human colon tissue collection and cell culture
Human colon tissue specimens from 10 normal volunteer donors (6 men and 4 women; mean age 32.4 years, range 21-48 years; have no previous history of chronic autoimmune diseases such as multiple sclerosis, autoimmune encephalitis, diabetes mellitus, systemic lupus erythematosus, rheumatoid arthritis, ankylosing spondylitis and asthma and no family history of cancer) and 24 patients (15 men and 9 women; mean age 36.5 years; range 19-51 years; inflammation grading: 9 mild and 15 moderate; clinical disease activity:19 initial onset and 5 recurrent; duration of disease: range from 0.1 to 12 years, mean 1.27 years; medical treatment: 18 treated with mesalamine, 2 with steroid and 4 with herbal medicine) with active UC were obtained from the First Affiliated Hospital of Guangzhou University of Chinese Medicine. Under endoscopic biopsy, tissue specimens were snap-frozen shortly after taken from flared sigmoid in UC patients and corresponding site in controls. For later histological research, specimens were sectioned into 5-10 mm thickness. Human intestinal epithelial HT29 cells (American Type Culture Collection, Rockville, MD, USA) were cultured in DMEM supplemented with 10% fetal bovine serum, penicillin, and streptomycin in a 5% CO2 incubator.
Induction of colitis in mice
Six- to 8-week-old male C57BL/6J mice were fed in the standard laboratory in an air-conditioned room equipped with a 12-hour light/12-hour dark cycle. For active colitis group, mice were orally administered of with 3.5% DSS (MP Biomedicals, CA, USA) in drinking water for up to 12-19 days. Control mice were administered with normal sterilized water. Data of body weight and pathological features were collected each day before and after the treatment of DSS. Body weight, stool consistency (scores: 0, normal stools; 1, soft stools; 2, liquid stools), hemoccult positivity and presence of gross blood (scores: 0, negative fecal occult blood; 1, positive fecal occult blood; 2, visible rectal bleeding) were assessed daily. After induction of colitis, mice were then scarified, the cecum was removed, and remained colon was divided into two distal halves. Tissues were frozen for further Western blot analysis and were fixed in 10% buffered formalin for immunohistochemistry.
Quantitative real-time RT-PCR (RT-qPCR)
Total RNA was isolated from tissue samples with Trizol (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA was reverse-transcribed by ReverTra Ace qPCR RT Kit (Toyobo Biochemicals, Osaka, Japan) according to the manufacturer’s protocol. For mcl-1 detection, total RNA was reversely transcribed into cDNA using RNA reverse transcription kits. Sequence-specific primers for U6 and miR-29a were purchased from company. (Bulge-LoopTM hsa-miR-29a-3p qRT-PCR Primer Set, Ruibo). Specific primers for Mcl-1 and GAPDH mRNA amplification were as follows, Mcl-F 5’-TCAAAGATGGCGTAACAAACTGG-3’, Mcl-R 5’-CCCGTTTCGTCCTTACAAGAAC-3’, GAPDH-F 5’-AGGTCGGTGTGAACGGATTTG-3’, 5’-GAPDH-R TGTAGACCATGTAGTTGAGGTCA-3’. Real-time PCR was performed with SsoFast EvaGreen Supermix Kit (Bio-Rad) on a Bio-Rad Q5 instrument (Bio-Rad Laboratories, Hercules, CA) using the SYBR Green detection protocol as outlined by the manufacturer. Briefly, the amplification mixture consisted of 0.4 μM primers, 10 μl of SsoFast EvaGreen supermix (Bio-Rad), and 2 μl template DNA in a total volume of 20 μl. Samples were amplified with the following program: initial denaturation at 98°C for 2 min, followed by 40 cycles of denaturation for 5 s at 98°C and annealing/elongation for 30 s at 57°C. All PCRs were run in triplicate, and control reactions without template were included.
Western blot
Tissue lysates were separated using SDS-PAGE and electrophoretically transferred to a polyvinylidene difluoride membrane. Membranes were blocked in Tris-buffered saline with 5% milk and 0.05% Tween 20 and probed with primary antibodies at 4°C overnight. The following primary antibodies: anti-Mcl-1, anti-GAPDH and anti-caspase-3 [all from Santa Cruz Biotechnology, CA, USA]; and corresponding appropriate horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA) were used for detection with the ECL and ECL-plus systems (Forevergen Biosciences, Guangzhou, China).
Immunohistochemistry
Frozen colon tissue sections were fixed on glass slides by incubating in acetone for 10 min at 4°C. The slides were incubated with 3% H2O2 for 20 min at room temperature and indirectly immunolabeled using an ABC kit (Forevergen Biosciences) according to the manufacturer’s instructions. Slides were then blocked in donkey serum for 30 min at 37°C and incubated with a goat anti-mouse and anti-Mcl-1 (Santa Cruz Biotechnology) at 4°C overnight. For the negative controls, the primary antibody was replaced with phosphate buffered saline (PBS). This was followed by incubation with biotinylated anti-mouse or anti-human IgG in PBS for 2 h at room temperature. Sections were rinsed in PBS and then in distilled water. The slides were stained with 3,3’-diaminobenzidine and counterstained with hematoxylin.
Knockdown of Mcl-1 through small interfering RNA (siRNA)
Cells were plated at a density of 1.5 × 105 cells/well in 12-well plates 24 h before the first transfection. siRNA sequences targeting the human c-Myb cDNA were designed and synthesized by forevergen biosciences (Guangzhou, China). A scrambled siRNA that could not target human Mcl-1 cDNA was included as a negative control. siRNA sequences were as follows: Scramble: 5’-UGGUUUACAUGUCGACUAA-3’; si-Mcl-1-1: 5’-TCCAAGGCATGCTTCGGAA-3’; si-Mcl-1-2: 5’-CCAAGGCATGCTTCGGAAA-3’; siRNA was transfected into HT29 cells using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s instructions.
Apoptosis assays
HT29 cells were cultured in 12-wells plates and transfected with 100 pmol miR-29a precursors or siRNA of Mcl-1. Pre-miRNA control and scramble siRNA served as negative controls. Cells were cultured overnight with both serum-containing complete medium and serum-depleted medium; the attached cells and floating cells were then harvested. Flow cytometry analysis of apoptotic cells was carried out using an Annexin V-FITC/PI staining kit (BD Biosciences, CA, USA). After washing with cold PBS, the cells were resuspended in binding buffer (100 mM HEPES, pH 7.4, 100 mM NaCl, and 25 mM CaCl2), followed by staining with AnnexinV-FITC/PI at RT in darkness for 15 min. Apoptotic cells were then evaluated by gating PI and Annexin V positive cells on a FACSCalibur (BD, New Jersey, USA). All experiments were performed in triplicate.
Evaluation of UC severity based on the disease activity index (DAI) score
The DAI score that was first described by Murthy et al. has been widely used to evaluate the severity of UC in animal models [25]. Briefly, an investigator complying the protocol recorded and scored the changes in weight, hemoccult positivity or gross bleeding, and stool consistency according to the previous report. The DAI score was a combination of scores of all these parameters mentioned.
Statistical analysis
All images of western blots and immunohistochemistry are representative of at least three independent experiments. The data shown are presented as the mean ± standard derivation for at least three independent experiments. The groups were compared using Student’s t-test with control values. The statistical significance is indicated as follows: *P < 0.05; **P < 0.01.
Results
Down-regulation of Mcl-1 in human colon tissues with ulcerative colitis
It has been confirmed that Mcl-1, a Bcl-2 family member, plays a critical role in the pathogenesis of many cancers by regulating apoptosis. Abnormal apoptosis of the intestinal epithelial cells has been observed at the acute inflammatory sites in UC as well as in colitis animal models. Thus, we measured the expression of Mcl-1 in normal human colon tissues and colon tissues with active UC. Based on immunohistochemistry assay using Mcl-1 antibody, we observed that damaged structure, inflammatory cell infiltration, and reduced expression of Mcl-1 in the colon tissue of UC patients (Figure 1). Western blot was used to further confirm the decrease in Mcl-1 expression. As shown in Figure 1, four tissues with UC showed lower level of Mcl-1 expression compared to the four normal tissues, with GAPDH as loading controls. These data suggest that the expression of Mcl-1 is significantly decreased in human colon UC, which may cause the apoptosis in human colon tissues with ulcerative colitis.
Figure 1.
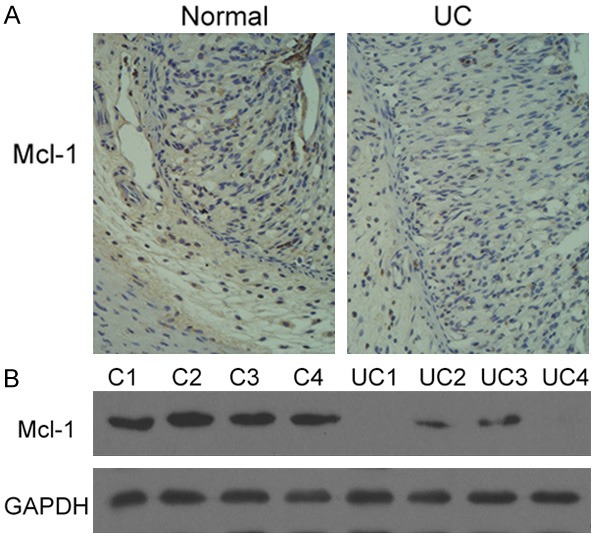
Down-regulation of Mcl-1 in human colon tissues with ulcerative colitis. A. Immunohistochemical staining of Mcl-1 in human colon tissues. Compared to normal human colon tissues, Mcl-1 expression was significantly decreased in human colon tissues with ulcerative colitis. B. Western blot analysis of Mcl-1 in human colon tissues. Compared to normal human colon tissues (C1, C2, C3 and C4), Mcl-1 expression was significantly decreased in human colon tissues with ulcerative colitis (UC1, UC2, UC3, and UC4).
Down-regulation of Mcl-1 in the DSS-induced mice experimental colitis
Next, we examined the expression of Mcl-1 in DSS-induced mice colitis. We found that DSS administration resulted in a significantly reduced expression of Mcl-1 with obvious unstructured colonic epithelia (Figure 2). We performed both quantitative real-time PCR and Western blot to detect the expression of mRNA and protein of Mcl-1 in DSS-induced mice colitis. Our results showed that both the expression of mRNA and protein of Mcl-1 in DSS-induced mice colitis was significantly down-regulated compared to the normal tissues (Figure 2). These results were consistent with the observations of down-regulation of Mcl-1 in human colon tissue with ulcerative colitis as mentioned above.
Figure 2.
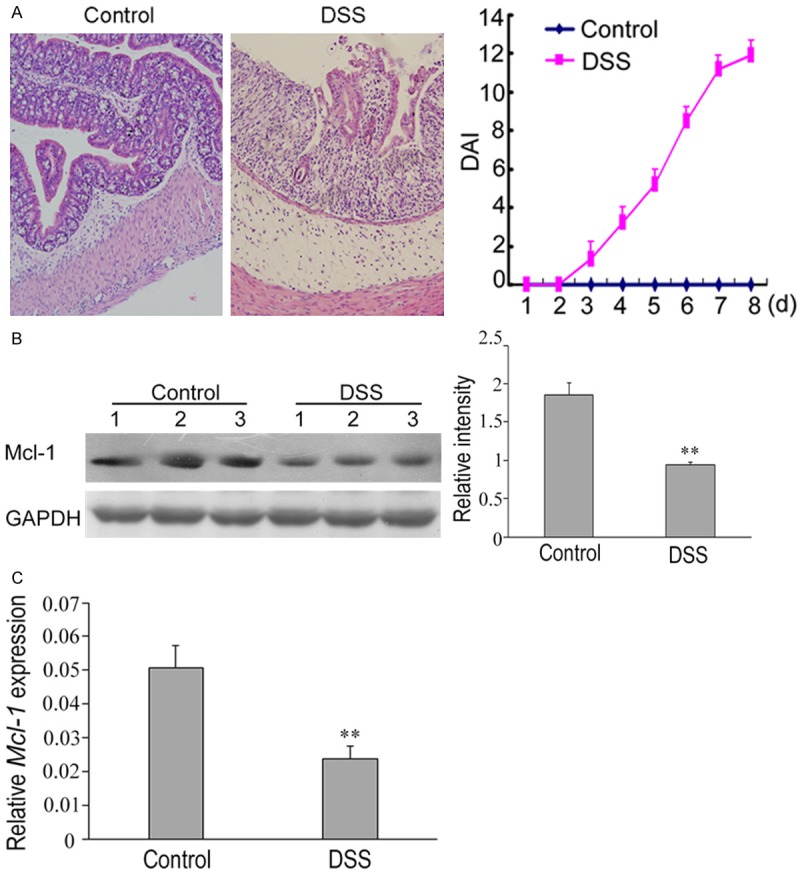
Down-regulation of Mcl-1 in mice with DSS-induced experimental colitis. A. Histopathological changes in colon tissues of mice with DSS-induced colitis. Compared to the normal colon tissues, the epithelial structure of the colon tissues of mice with DSS-induced colitis was destroyed and a large number of lymphocytic infiltration was observed in the mucosa and submucosa. The disease activity index (DAI) was significantly higher in mice with DSS-induced colitis than in the control animals (right). B. The expression levels of mcl-1 in each group. The expression of mcl-1 protein was significantly decreased in mice with DSS-induced colitis compared to the control animals. Western blot analyses of band intensity from three independent experiments are presented as the relative ratio of mcl-1 to β-actin. **P < 0.01 vs. control. C. RT-qPCR analysis of the relative expression of miR-29a in the control and DSS group. U6 served as an internal control. **P < 0.01 vs. control.
Knockdown of Mcl-1 induced colonic epithelial HT29 cell apoptosis
Apoptosis is a complex biological activity in which many factors and molecules are involved. To further understand the role of Mcl-1 in the apoptosis of intestinal epithelial cells, we analyzed the apoptosis of colonic epithelial HT29 cell line with Mcl-1 knockdown of through small interfering RNA (siRNA). Based on Western blot, the expression of Mcl-1 in colonic epithelial HT29 cells with Mcl-1 knockdown was dramatically reduced compared to normal HT29 cells, suggesting that Mcl-1 was successfully inhibited by small interfering RNA (Figure 3). Based on flow cytometric analysis of apoptotic cells, we found that the apoptotic cells in colonic epithelial HT29 cells with Mcl-1 knockdown was significantly higher than the control cells without Mcl-1 knockdown. These results indicated that Mcl-1 inhibited the apoptosis of colonic epithelial HT29 cells. Taken together with the observations that Mcl-1 is down-regulated in human colon tissues with ulcerative colitis and in the DSS-induced mice experimental colitis, Mcl-1 is involved in the pathogenesis of UC by inhibiting apoptosis of intestinal epithelial cells. Based on these observations, we further explored the role of miRNA, miR-29a in the regulation of Mcl-1 in UC.
Figure 3.
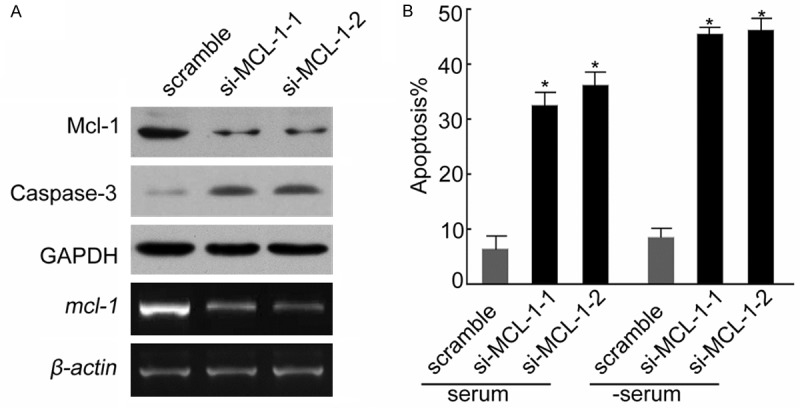
Knockdown of Mcl-1 induced colonic epithelial HT29 cell apoptosis. A. The mcl-1 gene was knocked down via small interfering RNA (siRN A). Based on RT-PCR assay, in the two mutants (si-MCL-1-1 and si-MCL-1-2) with mcl-1 knockdown, the mcl-1 mRNA level is significantly lower than wild-type HT29 cells (lower panel). The level of Mcl-1 protein in the mutants with mcl-1 knockdown was significantly lower than wild-type HT29 cells (upper panel) and the level of apoptosis protein Caspase-3 was significantly higher in the mutants with mcl-1 knockdown than wild-type HT29 cells based on Western blot analysis (upper panel). B. The apoptosis rate in the mutants with mcl-1 knockdown was significantly higher than wild-type HT29 cells. Data are expressed as the means ± SD (n = 3). *P < 0.05 vs. scramble (serum).
Up-regulation of miR-29a in DSS-induced mice experimental colitis and regulations of Mcl-1
Until now, there was no study illustrating the role of miR-29a in UC, hence we measured the expression level of miR-29a in the colon tissues of mice with DSS-induced colitis using quantified real-time PCR. As showed in Figure 4B, the expression of miR-29a was significantly increased in the colon tissues of mice with DSS-induced colitis (P < 0.05) compared to the controls. Whether miR-29a regulates the function of Mcl-1 and is involved in apoptosis of the intestinal epithelial cells are still unclear. The regulated genes of miRNA typically have sequence matches with miRNA at 3’-UTR that bind to and be regulated by miRNA. TargetScan and MicroCosm programs were used to search for potential sequence matches between miR-29a and Mcl-1. As shown in Figure 4A, both human and mouse miR-29a shared perfect sequence matches of seven nucleotides with the 3’-UTR of human and mouse Mcl-1.
Figure 4.
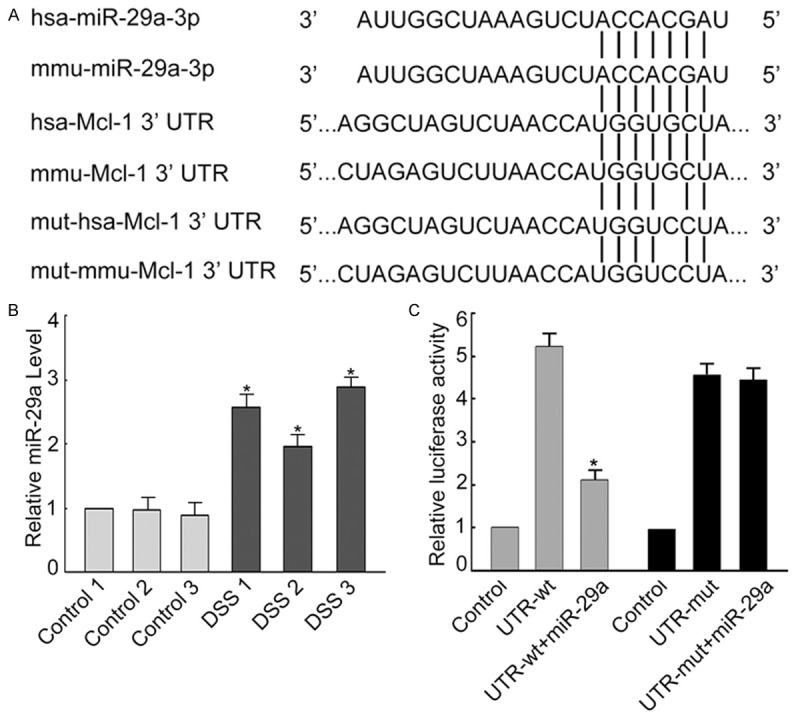
Up-regulated miR-29a in DSS-induced mice experimental colitis regulates Mcl-1. (A) Predicted duplex formation between human and mouse mcl-1 3’UTR (has-Mcl-1 3’UTR and mmu-Mcl-1 3’UTR, respectively) and human and mouse miR-29a (has-miR-29a-3p and mmu-miR-29a-3p, respectively). Vertical lines represent complementary matches between miR-29a and mcl-1 3’UTR. Point mutation (5th G -> C) on the binding site of human and mouse mcl-1 with miR-29a was introduced. Mutants (mut-has-Mcl-1 3’UTR and mut-mmu-Mcl-1 3’UTR) were transfected into colonic epithelial HT29 cells and the luciferase activity of reporter genes were measured and compared with negative control and positive control (UTR-wt). (B) The miR-29a level in mice with DSS-induced experimental colitis was significantly higher than in control animals. *P < 0.05 vs. control 1 (C). In colonic epithelial HT29 cells, mutation on the 3’UTR miR-29a binding site of mcl-1 caused significant up-regulation of the expression of reporter gene. When HT29 cells were co-transfected with 3’UTR binding site mutants of mcl-1 and miR-29a plasmid, the luciferase activity of reporter genes increased significantly. Data are expressed as the means ± SD (n = 3). *P < 0.05 vs. control.
The interaction between miR-29a and Mcl-1 was further investigated in colonic epithelial HT29 cells. We first constructed a luciferase reporter plasmid (UTR-WT) containing the human Mcl-1 3’-UTR region and a mutant plasmid (UTR-mut) containing the same region but with a SNP at the miR-29a complementary sequence. The UTR-WT and UTR-mut plasmids were transfected into colonic epithelial HT29 cells, measured the relative luciferase activities, respectively, and compared with the relative luciferase activities when miR-29a was co-transfected with either UTR-WT or UTR-mut plasmid. We found that miR-29a transfection can suppress the luciferase signal of UTR-WT, but not the UTR-mut with mutation at the complementary sequence to miR-29a (Figure 4C). These results suggest that miR-29a can directly regulate Mcl-1 expression in colonic epithelial HT29 cells. Similar results were observed when mouse miR-29a and mouse Mcl-1 were used (Figure 4C).
miR-29a induced apoptosis of colonic epithelial HT29 cell by down-regulating Mcl-1
To further investigate the interaction of miR-29a and Mcl-1 and the role of miR-29a in intestinal epithelial apoptosis, miR-29a plasmid was transfected into colonic epithelial HT29 cell and the level of mcl-1 and apoptosis gene Caspase-3 mRNA was measured using RT-PCR. As shown in Figure 5A, the level of mcl-1 and apoptosis gene Caspase-3 mRNA was significantly reduced and increased, respectively by miR-29a, suggesting that miR-29a inhibited Mcl-1 and activated Caspase-3 in HT29 cells. In addition, the apoptosis of HT29 cells increased significantly with the transfection of miR-29a plasmid (Figure 5B). Taken together, these results demonstrated that miR-29a induced apoptosis by down-regulating Mcl-1 and activating Caspase-3.
Figure 5.
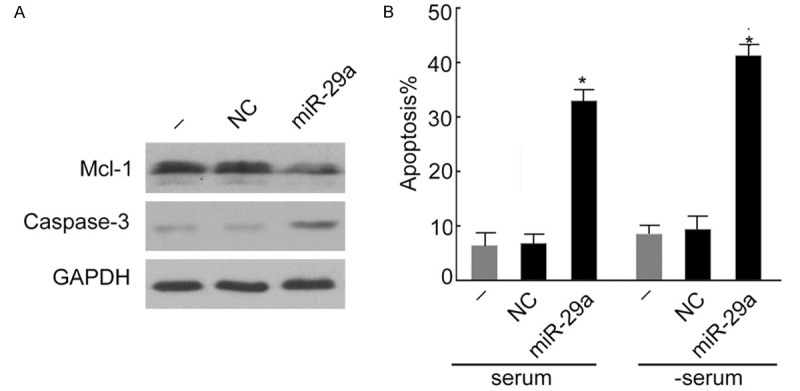
miR-29a induced colonic epithelial HT29 cell apoptosis by down-regulating Mcl-1. A. miR-29a down-regulated mcl-1 in colonic epithelial HT29 cells but the expression of apoptosis protein Caspase-3 was increased based on Western blot analysis. GAPDH was loaded as internal control. B. The apoptosis rate of HT29 cells was significantly higher with the transfection of miR-19a plasmid. Data are expressed as the means ± SD (n = 3). *P < 0.05 vs. HT29 cells supplemented with serum (-).
Discussion
Aberrant expression of miRNAs is frequently associated with the initiation and progression of many cancers as well as the pathogenesis of inflammation-related diseases [18,25]. Increasing evidence supports that miRNAs were differentially expressed and were involved in the pathogenesis of UC [18,19,26-28]. Koukos et al. have observed that the level of miR-124 decreased in colon tissues of children with active UC and reduced miR-124 up-regulated the activity of STAT3 to promote inflammation and the pathogenesis of UC in children [27].
In this study, we found that miR-29a, a member of the miRNA-29 family, was highly expressed in colon tissues of UC patients and mice with DSS-induced experimental colitis. The miRNA-29 family miRNAs, including miR-29a, miR-29b and miR-29c, were recently reported to be aberrantly expressed in multiple cancers. Increasing evidence shows that the abnormal expression of miR-29 family is associated with tumorigenesis and cancer progression, making miR-29a well-analyzed group of miRNAs in cancer research [29,30]. Cushing et al. also showed that miR-29 is a major regulator of genes associated with pulmonary fibrosis [31]. Wei et al. demonstrated that miR-29 targets Akt3 to reduce proliferation and facilitate differentiation of myoblasts in skeletal muscle development [32].
A few studies investigated the role of miR-29a in UC and related diseases. Wu et al. found that three miRNAs including miR-192, miR-375, and miR-422b were down-regulated; and 8 miRNAs including miR-16, miR-21, miR-23a, miR-24, miR-29a, miR-126, miR-195, and Let-7f were significantly up-regulated in active UC tissues as compared with healthy control tissues [19]. Our observation of increased expression of miR-29a in colon tissues in UC patients and mice of DSS-induced experimental colitis was consistent with the results conducted by Wu et al [19]. In addition, we further investigated the role of increased expression of miR-29a in intestinal epithelial apoptosis and the interaction between miR-29a and Mcl-1.
The mechanism of miRNAs involved in the pathogenesis of UC was largely unknown. Zhou et al. have observed that the expression of miR-29a was increased in the colon tissues of irritable bowel syndrome (IBS) patients with increased intestinal membrane permeability, which may be caused by the regulated expression of GLUL gene by miR-29a [33]. Here, we found that, miR-29a is also involved in the Mcl-1 pathway to control the apoptosis of intestinal epithelial cells. Thus, miR-29a is involved in multiple pathways mediating the integrity and growth of intestinal epithelium. It has been shown that miRNA-29b regulated Mcl-1 protein expression and apoptosis in H69 cholangiocyte and malignant KMCH cholangiocarcinoma cell lines [24]. Our study further provided evidence that another member of the miRNA-29 family, miR-29a also regulated the expression of Mcl-1 protein and apoptosis in colonic epithelial HT29 cells.
Ulcerative colitis (UC) is an immune disorder of the large intestine, which is characterized by contiguous inflammation of the colonic lamina propria. It has been suggested that apoptosis promoted the development of UC, and was one of the major processes involved in the pathogenesis of UC [15,34]. Qiu et al. found that, p53-upregulated modulator of apoptosis (PUMA), a p53 target and proapoptotic BH3-only protein, was involved in the pathogenesis of UC by promoting intestinal epithelial apoptosis [34]. Herein, we found that miR-29a promoted intestinal epithelial apoptosis by down-regulating Mcl-1. We speculated that the intestinal epithelial apoptosis in UC is controlled by multiple pathways including miR-29a/Mcl-1 and the PUMA pathways as previously identified. Whether these two pathways have cross-talks between each other and whether more pathways are involved in intestinal epithelial apoptosis in UC need to be further investigated.
While the etiology of UC is still unclear, some previous studies have suggested that genetic, immunological, and environmental factors are involved in the pathogenesis of UC. It has been proposed that UC is trigged by environmental factors in genetically susceptible individuals. Exaggerated and aberrant immune responses can be observed in the large intestine, in which distinct immune cells and cytokines such as TNF-α, IL-6, and IL-13, are involved [5,35-37].
MiRNAs have been shown to be involved in a wide range of biological processes such as cell cycle control, apoptosis and cell differentiation. By regulating the cytokine expression, miRNAs are involved in inflammation and eventually, contributed to the pathogenesis of many inflammation-related diseases [26,38,39]. It has been reported that miR-29a regulated pro-inflammatory cytokine secretion and scavenger receptor expression by targeting LPL in ox LDL-stimulated dendritic cells [40]. We speculate that miR-29a regulate other molecules including other cytokines and contribute to the pathogenesis of UC.
In summary, we found in the present study that miR-29a is highly expressed in the colon tissues of UC patients and mice of DSS-induced experimental colitis. We also successfully demonstrated that miR-29a targeted the 3’UTR of Mcl-1 gene and down-regulated the expression of Mcl-1 in the colon tissues of UC patients and mice of DSS-induced experimental colitis. In addition, we observed that knockdown of Mcl-1 induced apoptosis of the colonic epithelial HT29 cells. Our studies suggest that Mcl-1 and miR-29a may be useful targets for effective treatment of UC.
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (No. 81001506), Science and technology planning project of Guangzhou. 2012B031800483 and National Key Specialty Construction Programs.
Disclosure of conflict of interest
None.
References
- 1.Fries W, Comunale S. Ulcerative colitis: pathogenesis. Curr Drug Targets. 2011;12:1373–1382. doi: 10.2174/138945011796818261. [DOI] [PubMed] [Google Scholar]
- 2.Griffel LH, Das KM. Ulcerative colitis: pathogenesis, diagnosis, and current treatment. J Assoc Acad Minor Phys. 1996;7:63–69. [PubMed] [Google Scholar]
- 3.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Lakatos L, Mester G, Erdelyi Z, Balogh M, Szipocs I, Kamaras G, Lakatos PL. Striking elevation in incidence and prevalence of inflammatory bowel disease in a province of western Hungary between 1977-2001. World J Gastroenterol. 2004;10:404–409. doi: 10.3748/wjg.v10.i3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funakoshi K, Sugimura K, Sasakawa T, Bannai H, Anezaki K, Ishizuka K, Yoshida K, Narisawa R, Asakura H. Study of cytokines in ulcerative colitis. J Gastroenterol. 1995;30(Suppl 8):61–63. [PubMed] [Google Scholar]
- 6.Cavanaugh JA, Pavli P. Ulcerative colitis: a genetic disease? Baillieres Clin Gastroenterol. 1997;11:1–15. doi: 10.1016/s0950-3528(97)90050-6. [DOI] [PubMed] [Google Scholar]
- 7.Edelblum KL, Yan F, Yamaoka T, Polk DB. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflamm Bowel Dis. 2006;12:413–424. doi: 10.1097/01.MIB.0000217334.30689.3e. [DOI] [PubMed] [Google Scholar]
- 8.Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK. Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol. 1996;180:152–159. doi: 10.1002/(SICI)1096-9896(199610)180:2<152::AID-PATH649>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 9.Di Sabatino A, Ciccocioppo R, Luinetti O, Ricevuti L, Morera R, Cifone MG, Solcia E, Corazza GR. Increased enterocyte apoptosis in inflamed areas of Crohn’s disease. Dis Colon Rectum. 2003;46:1498–1507. doi: 10.1007/s10350-004-6802-z. [DOI] [PubMed] [Google Scholar]
- 10.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, Gumucio D, Neurath MF, Pasparakis M. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 11.Eckmann L, Nebelsiek T, Fingerle AA, Dann SM, Mages J, Lang R, Robine S, Kagnoff MF, Schmid RM, Karin M, Arkan MC, Greten FR. Opposing functions of IKKbeta during acute and chronic intestinal inflammation. Proc Natl Acad Sci U S A. 2008;105:15058–15063. doi: 10.1073/pnas.0808216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frey MR, Edelblum KL, Mullane MT, Liang D, Polk DB. The ErbB4 growth factor receptor is required for colon epithelial cell survival in the presence of TNF. Gastroenterology. 2009;136:217–226. doi: 10.1053/j.gastro.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeissig S, Bojarski C, Buergel N, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–1302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marini M, Bamias G, Rivera-Nieves J, Moskaluk CA, Hoang SB, Ross WG, Pizarro TT, Cominelli F. TNF-alpha neutralization ameliorates the severity of murine Crohn’s-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc Natl Acad Sci U S A. 2003;100:8366–8371. doi: 10.1073/pnas.1432897100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seidelin JB, Nielsen OH. Epithelial apoptosis: cause or consequence of ulcerative colitis? Scand J Gastroenterol. 2009;44:1429–1434. doi: 10.3109/00365520903301212. [DOI] [PubMed] [Google Scholar]
- 16.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 17.Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91:827–887. doi: 10.1152/physrev.00006.2010. [DOI] [PubMed] [Google Scholar]
- 18.Raisch J, Darfeuille-Michaud A, Nguyen HT. Role of microRNAs in the immune system, inflammation and cancer. World J Gastroenterol. 2013;19:2985–2996. doi: 10.3748/wjg.v19.i20.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Brant SR, Chakravarti S, Kwon JH. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624–1635. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 20.Chen B, She S, Li D, Liu Z, Yang X, Zeng Z, Liu F. Role of miR-19a targeting TNF-alpha in mediating ulcerative colitis. Scand J Gastroenterol. 2013;48:815–824. doi: 10.3109/00365521.2013.800991. [DOI] [PubMed] [Google Scholar]
- 21.Perciavalle RM, Opferman JT. Delving deeper: MCL-1’s contributions to normal and cancer biology. Trends Cell Biol. 2013;23:22–29. doi: 10.1016/j.tcb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds JE, Yang T, Qian L, Jenkinson JD, Zhou P, Eastman A, Craig RW. Mcl-1, a member of the Bcl-2 family, delays apoptosis induced by c-Myc overexpression in Chinese hamster ovary cells. Cancer Res. 1994;54:6348–6352. [PubMed] [Google Scholar]
- 23.Perciavalle RM, Stewart DP, Koss B, Lynch J, Milasta S, Bathina M, Temirov J, Cleland MM, Pelletier S, Schuetz JD, Youle RJ, Green DR, Opferman JT. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat Cell Biol. 2012;14:575–583. doi: 10.1038/ncb2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 26.Dalal SR, Kwon JH. The Role of MicroRNA in Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y) 2010;6:714–722. [PMC free article] [PubMed] [Google Scholar]
- 27.Koukos G, Polytarchou C, Kaplan JL, Morley-Fletcher A, Gras-Miralles B, Kokkotou E, Baril-Dore M, Pothoulakis C, Winter HS, Iliopoulos D. MicroRNA-124 regulates STAT3 expression and is down-regulated in colon tissues of pediatric patients with ulcerative colitis. Gastroenterology. 2013;145:842–852. doi: 10.1053/j.gastro.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan YG, Zhang YF, Guo CJ, Yang M, Chen MY. Screening of differentially expressed microRNA in ulcerative colitis related colorectal cancer. Asian Pac J Trop Med. 2013;6:972–976. doi: 10.1016/S1995-7645(13)60174-1. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt MJ, Margue C, Behrmann I, Kreis S. MiRNA-29: a microRNA family with tumor-suppressing and immune-modulating properties. Curr Mol Med. 2013;13:572–585. doi: 10.2174/1566524011313040009. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Zhang X, Li H, Yu J, Ren X. The role of miRNA-29 family in cancer. Eur J Cell Biol. 2013;92:123–128. doi: 10.1016/j.ejcb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lu J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei W, He HB, Zhang WY, Zhang HX, Bai JB, Liu HZ, Cao JH, Chang KC, Li XY, Zhao SH. miR-29 targets Akt3 to reduce proliferation and facilitate differentiation of myoblasts in skeletal muscle development. Cell Death Dis. 2013;4:e668. doi: 10.1038/cddis.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010;59:775–784. doi: 10.1136/gut.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu W, Wu B, Wang X, Buchanan ME, Regueiro MD, Hartman DJ, Schoen RE, Yu J, Zhang L. PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J Clin Invest. 2011;121:1722–1732. doi: 10.1172/JCI42917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bamias G, Kaltsa G, Ladas SD. Cytokines in the pathogenesis of ulcerative colitis. Discov Med. 2011;11:459–467. [PubMed] [Google Scholar]
- 36.Sands BE, Kaplan GG. The role of TNFalpha in ulcerative colitis. J Clin Pharmacol. 2007;47:930–941. doi: 10.1177/0091270007301623. [DOI] [PubMed] [Google Scholar]
- 37.Bernardo D, Vallejo-Diez S, Mann ER, Al-Hassi HO, Martinez-Abad B, Montalvillo E, Tee CT, Murugananthan AU, Nunez H, Peake ST, Hart AL, Fernandez-Salazar L, Garrote JA, Arranz E, Knight SC. IL-6 promotes immune responses in human ulcerative colitis and induces a skin-homing phenotype in the dendritic cells and Tcells they stimulate. Eur J Immunol. 2012;42:1337–1353. doi: 10.1002/eji.201142327. [DOI] [PubMed] [Google Scholar]
- 38.Ranjha R, Paul J. Micro-RNAs in inflammatory diseases and as a link between inflammation and cancer. Inflamm Res. 2013;62:343–355. doi: 10.1007/s00011-013-0600-9. [DOI] [PubMed] [Google Scholar]
- 39.O’Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol. 2012;30:295–312. doi: 10.1146/annurev-immunol-020711-075013. [DOI] [PubMed] [Google Scholar]
- 40.Chen T, Li Z, Tu J, Zhu W, Ge J, Zheng X, Yang L, Pan X, Yan H, Zhu J. MicroRNA-29a regulates pro-inflammatory cytokine secretion and scavenger receptor expression by targeting LPL in oxLDL-stimulated dendritic cells. FEBS Lett. 2011;585:657–663. doi: 10.1016/j.febslet.2011.01.027. [DOI] [PubMed] [Google Scholar]


