Abstract
Activation of the hedgehog (Hh) signaling pathway has been implicated in the development of many human malignancies. Hh signaling target genes, such as patched (PTCH), smoothened (SMO) and sonic hedgehog (SHH), are markers of Hh signaling activation in most Hh-associated tumors. The protein kinase LKB1 has been shown to slow proliferation and induce cell-cycle arrest in many cell lines. However, the function of LKB1 in prostate cancer development remains largely unclear. In this study, the expression of LKB1 in human prostate cancer tissue samples and prostate cancer cell lines was detected, and the effects of LKB1 on prostate cancer cell proliferation and invasion were evaluated. Moreover, the influence of LKB1 on target genes of the Hh signaling pathway was analyzed. The results indicated that knockdown of LKB1 expression by RNA interference promoted cell proliferation, colony formation and invasion. Meanwhile, we observed that LKB1 siRNA increased the expression of factors related to Hh signaling reporter activity in prostate cancer cells, including PTCH, SMO and SHH. These findings suggest that LKB1 is a putative tumor suppressor gene in prostate cancer, and that LKB1 is negatively correlated with the expression of Hh signaling related transcription factors. Our results suggest that LKB1 may inhibit tumorigenesis by regulating the Hh signaling pathway in certain cancers.
Keywords: Prostate cancer, LKB1, proliferation, invasion, hedgehog (Hh) signaling pathway
Introduction
Prostate cancer is one of the most common malignancies and the leading cause of cancer-related death in men worldwide [1-4]. Surgery and radiation therapy are effective for localized disease [5], but there is no effective treatment strategy for recurrent or metastatic prostate cancer that has failed to respond to surgery, radiation or hormonal therapy. Therefore, it is important to investigate the molecular mechanisms underlying the progression and metastasis of prostate cancer to provide better strategies for the prevention and therapy of prostate cancer. With the recent advances in the understanding of molecular pathways involved in prostate cancer progression, targeted therapies that are designed to interfere with the way in which cancer cells grow and survive offer new hope in prostate cancer therapeutics.
Liver kinase B1 (LKB1), a serine/threonine kinase, functions as a tumor suppressor. The gene is located on chromosome 19p13.3, encodes a ~48 kDa multitasking kinase protein-LKB1 [6] and plays a crucial role in the control of cell structure, apoptosis, and cellular metabolism [7]. It is mutated in Peutz-Jeghers syndrome (PJS), a disease characterized by a predisposition to gastrointestinal polyposis, an increased risk of benign and malignant tumors in multiple tissues and mucocutaneous pigmentation [8] and in epithelial cancers, including hormone-sensitive organs such as the breast, ovaries, testes, and prostate [9]. LKB1 is emerging as a multifunctional protein, acting as a key metabolic enzyme, regulator of cell polarity, and transcription factor [10]. Recent studies have demonstrated that LKB1 regulates cell growth, cell proliferation and cell survival in response to different stresses. Altered LKB1 expression has been linked with the development and growth of various cancers [11,12]. It is well known that the Hedgehog (Hh) signaling pathway controls a variety of developmental processes, including cell proliferation, differentiation and survival [13]. Dysregulation of the Hh signaling pathway results in cellular proliferation and contributes to the formation and progression of human cancers, including lung, breast, pancreatic and prostate cancers [14-17]. Recently, the functional roles of the Hh signaling pathway in prostate cancer have been widely studied [18,19]. Increasing evidence suggests an active role for Hh signaling in the development and progression of prostate cancer. However, the role and mechanism of LKB1 in prostate cancer is still unclear. In this study, we investigated the potential role of LKB1 in the development and progression of prostate cancer. Further studies designed to determine the regulatory mechanism of LKB1 on the Hh signaling pathway are currently underway.
The results showed that the knockdown of LKB1 promoted the proliferation and invasion of prostate cancer cells. Moreover, LKB1 is negatively correlated with the expression of Hh signaling molecules in prostate cancer cells and has a negative effect on Hh signaling pathway activity in human prostate cancer cells.
Materials and methods
Patients and specimens
The study included 42 patients with prostate cancer. In addition, 16 normal prostate tissues, which served as controls, were obtained from patients who underwent total cystectomy at the Department of Urology, the First Affiliated Hospital of Zhengzhou University, from July 2012 to October 2013. None of the patients in our study received neoadjuvant chemotherapy. Informed consent was obtained from all patients prior to entering this study, and the study protocols were approved by the Research Ethics Committee for Clinical Research of the First Affiliated Hospital of Zhengzhou University, China.
Cell culture
The human prostate cancer cells line DU-145 (ATCC, Rockville, MD) and PC-3 (ATCC, Rockville, MD) were cultured in RPMI-1640 medium (Gibco, USA) supplemented with 10% fetal calf serum (Hyclone, USA), penicillin and streptomycin at 37°C in 5% CO2. Cells were regularly passaged to maintain exponential growth.
Cell transfection
The prostate cancer cells DU-145 and PC-3 were seeded into 6-well plates and incubated overnight, and were then transfected using Lipofectamine®2000 (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions, with slight modifications. The experiments were divided into three groups respectively: the control group (treated with Lipofectamine®2000 only), the vector group (treated with Lipofectamine®2000 and control siRNA), and the LKB1 siRNA (Santa Cruz, CA) group (treated with Lipofectamine®2000 and LKB1 siRNA). For every 105 cells, 0.5 μg LKB1 siRNA and control siRNA were diluted and mixed with 3 µl transfection reagents. After mixing and incubating 30 min, the transfection mixture was added to the cells. After 6 hours, the medium was changed to growth medium.
Gene expression analysis by Reverse Transcription and Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from prostate cancer cells with TRIzol Reagent (Invitrogen, Carlsbad, CA USA) according to the manufacturer’s protocol. Then, 2 µg of total RNA was subjected to reverse-transcription using a High-Capacity RNA-to-cDNA Kit (Applied Biosystems) according to the manufacturer’s instructions. RT-PCR was performed in a BioRad MyIQ single-color real-time PCR detection system using SYBR Green Supermix (BioRad, Carlsbad, CA). Relative quantification of gene expression was performed using the 2-ΔΔCt method and with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA as an internal control. The specific primers and their product size, cycles and renaturation temperature are listed in Table 1.
Table 1.
Prime sequence, product size, cycles and Renaturation temperature of different gene
| Gene | Prime sequence(5’-3’) | Product size | Cycles | Renaturation temperature (°C) |
|---|---|---|---|---|
| SHH | F: GACTCAGAGGTGTAAGGACAAGTT | 85 bp | 30 | 59 |
| R: CTCGGTCACCCGCAGTTT | ||||
| SMO | F: CAACCTCTTTGCGTTTCCTT | 154 bp | 30 | 59 |
| R: ACTCACTGCTCCTATCCCACTC | ||||
| PTCH | F: CAGGCAGCGGTAGTAGTGG | 193 bp | 25 | 59 |
| R: TGTAGCGGGTATTGTCGTGT | ||||
| LKB1 | F: CGGCAAGGTGAAGGAA | 141 bp | 25 | 59 |
| R :ACGCCCAGGTCGGAGAT | ||||
| GAPDH | F: GGGAAACTGTGGCGTGAT | 299 bp | 25 | 56 |
| R: GAGTGGGTGTCGCTGTTGA |
Western blot analysis
The proteins were extracted from tissue and cells using RIPA lysis buffer (Beyotime, Nantong, China). The cell lysates were collected after centrifugation and the protein concentration was quantified by the bovine serum albumin (BSA) method. Western blotting was performed according to standard methods. Equal amounts of proteins (40 μg) were subjected to SDS-PAGE electrophoresis on 12% polyacrylamide gels and transferred onto PVDF membranes (Millipore, Bedford, MA), which were blocked in non-fat dry milk and incubated in 1:500 diluted primary antibodies at 4°C overnight. After three washes with TBST, the membranes were incubated with HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, USA) for 1 h at room temperature. The membranes were developed using the ECL kit (Santa Cruz Biotechnology, USA) and exposed to X-ray film. β-actin was used as a loading control. The density of the bands on the membrane was scanned and analyzed with an image analyzer (LabWorks Software, UVP Upland, CA, USA).
Cell proliferation assays
To measure the effects of LKB1 on cell proliferation of prostate cancer cells, a colorimetric 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay was used. DU145 and PC3 cells were seeded in a 96-well culture plate and transiently transfected with LKB1 siRNA or control vectors. After 1-5 days of transfection, cells were washed twice with PBS and 10 µl MTT (5 mg/mL) was added to each well. Then, cells were incubated at 37°C for 4 h and 100 µl DMSO was added to dissolve the formazan crystals. The optical density [20] value was measured at a wavelength of 490 nm using a spectrophotometer (Multiskan MK3, Thermo, Waltham, MA). Experiments were carried out in triplicate.
Soft agar colony formation assay
Assays of colony formation in soft agar were performed according to a previous report [21]. The control group, the control siRNA-transfected group, and the LKB1 siRNA-transfected group cells were evenly seeded into 100 mm petri dishes with soft agar. The cells were allowed to grow for 3 weeks until the colonies were formed. Colonies were then stained with crystal violet and counted under a microscope and Open CFU 3.8-BETA software [22]. All experiments were conducted in triplicate.
Cell invasion assays
The invasion ability of DU-145 and PC-3 cells was evaluated in the transwell chamber assay. According to the manufacturer’s protocol, chambers were coated with matrigel (50 µl per filter) (BD, USA) and incubated at 37°C for 30 min to create the matrigel membrane and divide the chamber into upper and lower compartments. The control, the vector-transfected, and the LKB1-transfected cells were cultured for 48 h and added to the upper chambers with serum-free DMEM (1 × 105 cells per transwell). DMEM containing 10% fetal bovine serum (FBS) was added to the lower chamber. After 20 h incubation at 37°C in a 5% CO2 incubator, cells on the upper surface of the filters were removed by wiping with a cotton swab. The filters were fixed in 4% paraformaldehyde and stained with 0.2% crystal violet. The stained cells were counted under a microscope in five randomly selected fields. At least three chambers from three different experiments were analyzed.
Statistical analysis
All statistical analyses were performed using Microsoft Excel and SPSS v12 software. Data are presented as the mean ± SEM for the indicated number of separate experiments. Statistical analysis of data for multiple groups was performed using one-way analysis of variance (ANOVA). Student’s t test is applied for comparison of the two groups. P values less than 0.05 were considered statistically significant.
Results
Expression of LKB1 in prostate cancer tissue
To analyze LKB1 expression in human prostate tissues, we analyzed the protein level of LKB1 in normal and different prostate cancer patient tissues by Western blotting. The results showed that the relative expression levels of LKB1 were much lower in prostate cancer than that in normal tissues (Figure 1). These results indicate that LKB1 might be critical in prostate cancer.
Figure 1.
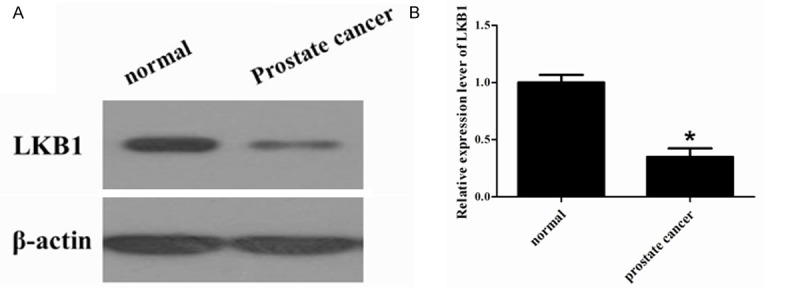
LKB1 protein expression level in prostate cancer tissue. LKB1 expression in the normal tissues and prostate cancer tissue was detected by Western blot assay. β-actin served as a loading control. *P < 0.05 prostate cancer tissue compared with normal tissue.
Expression of LKB1 mRNA and protein in prostate cancer cell lines
To further verify the expression of LKB1 in normal and prostate cancer cells, we used RT-PCR to detect LKB1 mRNA levels and Western blotting to analyze the protein levels in RWPE-1, DU-145 and PC-3 cell lines. RT-PCR analysis showed that the mRNA levels of LKB1 in two prostate cancer cell lines were down-regulated compared to levels seen in normal cells (Figure 2A). Western blotting results were consistent with RT-PCR (Figure 2B). Therefore, the low expression of LKB1 was confirmed to exist in prostate tissues as well as the prostate cell lines. It is noteworthy that both the mRNA and protein levels of LKB1 in DU-145 cells were higher than those in PC-3 cells.
Figure 2.
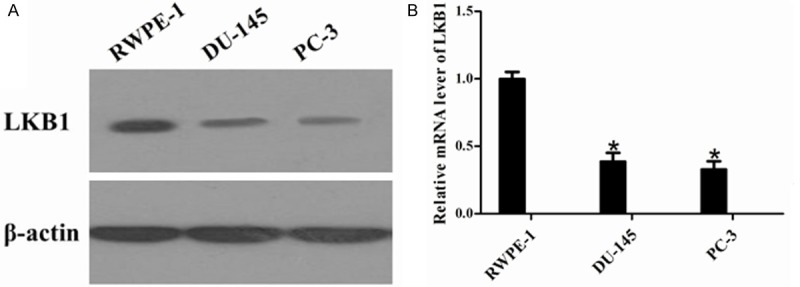
LKB1 mRNA and protein levels in prostate cancer cell lines. A. The protein expression profiles of the LKB1 in normal control cell lines (RWPE-1) and prostate cancer cell lines (DU-145 and PC-3) were determined by Western blot analysis. β-actin served as a loading control. B. The mRNA levels of LKB1 in normal control cell lines (RWPE-1) and prostate cancer cell lines (DU-145 and PC-3) were compared by RT-PCR. *P < 0.05 compared with RWPE-1.
Knockdown of LKB1 in prostate cancer cells
To evaluate the functional significance of LKB1 in prostate cancer, we silenced LKB1 expression using siRNA in DU-145 and PC-3 cells and then examined the expression of LKB1 in two human prostate cancer cell lines by RT-PCR and western blot analysis. As shown in the Figure 3A and 3B, RT-PCR and Western blot analysis showed that the mRNA and protein expression levels of LKB1 were significantly decreased by LKB1 siRNA when compared with their expression in control siRNA.
Figure 3.
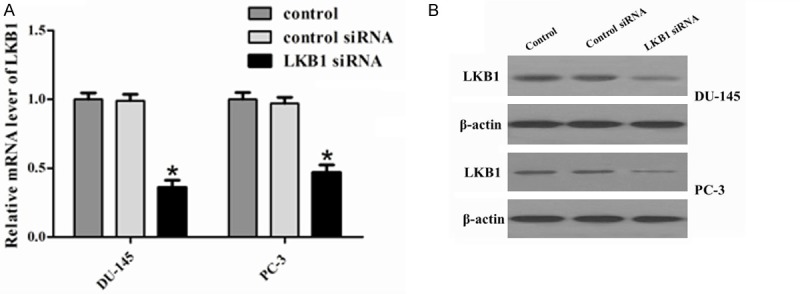
Expression of LKB1 in the prostate cancer cell lines DU-145 and PC-3. A. mRNA expression of LKB1 was detected by RT-PCR. GAPDH served as an internal control. Protein expression of LKB1 in (B) DU-145 and (C) PC-3 cells was detected by western blot analysis. β-actin served as a loading control. *P < 0.05 compared to control and siRNA control. The mRNA and protein expression of LKB1 was significantly downregulated in LKB1 siRNA cells compared to control and control siRNA.
Knockdown of LKB1 promotes cell proliferation in prostate cancer cells
To evaluate the effect of LKB1 on cell proliferation, we performed an MTT assay. The results indicated that knockdown of LKB1 promoted the proliferation of DU-145 and PC-3 cells (Figure 4A and 4B). Moreover, to confirm the inhibitory effect of LKB1 on the growth of prostate cancer cell lines, we used a colony formation assay using DU-145 and PC-3 cells. Knockdown of LKB1 significantly increased colony formation compared with that of cells transfected with control siRNA (Figure 4C). Knockdown of LKB1 in DU-145 and PC-3 cells showed a significant growth, which was increased by the promotion of cell proliferation and colony formation. These results further indicated that LKB1 acts as a potential tumor suppressor gene in prostate cancer.
Figure 4.
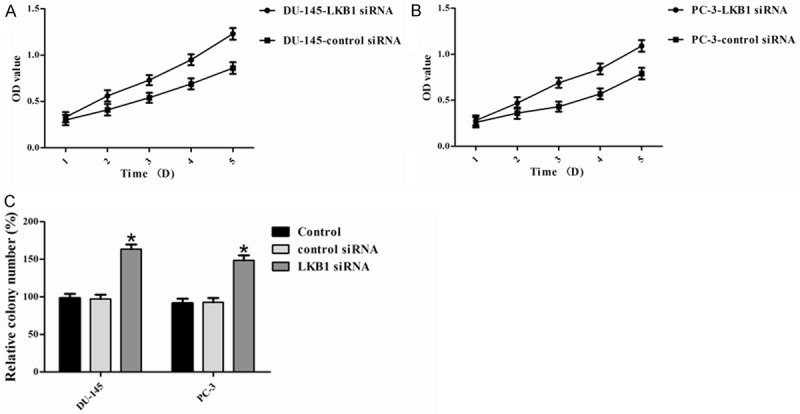
LKB1 inhibits cell growth. LKB1 significantly inhibited the proliferation of (A) DU-145 and (B) PC-3 cells, as shown by the MTT assay. These data are shown as the mean ± S.D. of three independent experiments. Cell proliferation was significantly inhibited by the knockdown of LKB1. C. Colony formation assays were performed to confirm the effect of LKB1 on cell growth. Compared with control and control siRNA, colony numbers were significantly increased by knockdown of LKB1. *P < 0.05.
Influences of silencing of LKB1 on invasion of prostate cancer cells
To further investigate the role of LKB1 in the invasion of prostate cancer cells, we employed the loss of function approach to knockdown LKB1 expression in DU-145 and PC-3 cells. We performed a transwell invasion assay and the results demonstrated that the invaded cell numbers in control, control siRNA and LKB1 siRNA were 37.4 ± 3.3, 38.5 ± 3.4, 97.6 ± 3.0, respectively, in DU-145 and 29.3 ± 2.9, 31.3 ± 3.2, 90.2 ± 3.3, respectively, in PC-3 (Figure 5). Compared with control and control siRNA, the invaded cell number in LKB1 siRNA significantly increased; the difference was significant (P < 0.05). The results suggest that silencing of LKB1 promotes the invasion of prostate cancer cells, indicating that LKB1 plays an important role in invasive capability.
Figure 5.
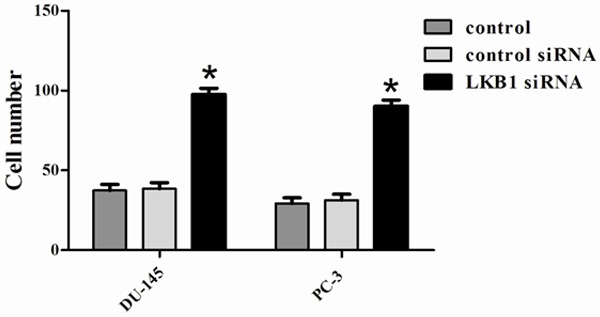
Invasion was measured by determining the percentage of cells that penetrated through Matrigel-coated Transwell chambers. These experiments were repeated three times. Invasion through Matrigel is significantly increased in siRNA LKB1 cells compared with control and siRNA control cells. *P < 0.05 compared to control and siRNA control.
Effects of knockdown of LKB1 on hedgehog signaling molecules in prostate cancer cells
To further investigate the molecular mechanism of LKB1 in prostate cancer, we examined the expression of Hh signaling molecules, including SHH, SMO, and PTCH by RT-PCR and Western blot analysis. As shown in Figure 6, the mRNA levels of SHH, SMO, and PTCH (P < 0.05) were significantly increased in siRNA LKB1-transfected (A) compared with control and control siRNA, which was reflected in the protein levels of SHH, SMO, and PTCH (B and C).
Figure 6.
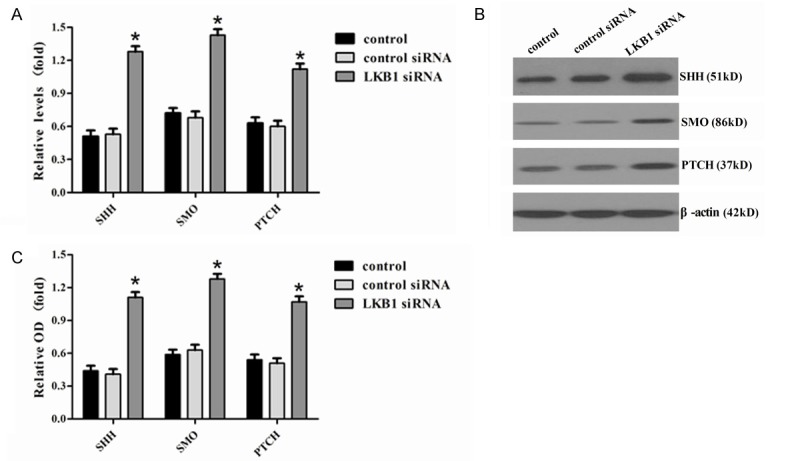
Analysis of effector molecules in Hh. A. The relative mRNA levels for SHH, SMO, and PTCH, which were detected by RT-PCR in DU-145 cells. B, C. The relative protein levels of SHH, SMO, and PTCH, which were detected by Western blotting in control, control siRNA, and siRNA LKB1. Representative quantitation from three independent experiments. The values of DU-145 are expressed relative to the respective controls (β-actin), which were given an arbitrary value of 1. Bars, SE. *P < 0.05.
Discussion
As the second most common cause of cancer-related death, prostate cancer is a global public health problem. Unfortunately, to date, very limited information is available to discern which cases of prostate cancer are likely to remain latent, versus those that are likely to metastasize and warrant more aggressive management [23]. Therefore, there is an urgent need to identify novel biomarkers of prostate cancer that could distinguish benign versus malignant or even metastatic prostate cancer.
LKB1 is emerging as a multifunctional protein, acting as a key metabolic enzyme, regulator of cell polarity, and transcription factor. Altered LKB1 expression has been linked with various cancers and may be a potential prognostic marker. In the current investigation, LKB1 protein expression was decreased in primary breast tumors compared to normal breast epithelium [24]. In our study, loss of LKB1 expression in a significant number of prostate cancer tissues is consistent with the notion that LKB1 is a tumor suppressor. Several recent studies have implicated the involvement of LKB1 gene inactivation in differentiation, invasion and metastasis of lung cancer cells [25,26]. It also has been reported that that low expression of the LKB1 protein promotes breast cancer cells proliferation, migration and invasion [27,28], and these results clearly suggested that LKB1 might exert tumor inhibitory effects on human breast cancer. Moreover, it also has been reported that overexpression of LKB1 in NSCLC cells inhibited cell migration and invasion. In this study, we knocked-down LKB1 expression in prostate cancer cells and found that the depletion of LKB1 led to increased cell proliferation and colony formation. Furthermore, knockdown of LKB1 was shown to increase the invasion of prostate cancer cells. The present results are consistent with those previous findings, demonstrating that LKB1 can inhibit the growth and invasion of prostate cancer and further supporting the involvement of LKB1 inactivation in the malignant progression of prostate cancer. Therefore, the above results provided evidence that LKB1 may play a potential tumor suppressor role in prostate cancer progression.
Aberrant activation of the Hh signaling pathway correlates with a variety of human tumors where the pathway is implicated in tumorigenesis, malignancy, metastasis and cancer stem cell development [29,30]. The development of advanced disease is a complex process involving up-regulation of anti-apoptotic genes and activated growth factor and signaling pathways [31]. Deregulation of Hh signaling molecules has also been demonstrated in the pathogenesis of carcinoma [32-34]. The Hh signaling pathway is integral in human organ embryogenesis. Recent evidence suggests that the Hh signaling pathway is activated in human prostate cancers [17,35,36]. Expression of SHH, SMO and PTCH, all of which are known markers of the Hh signaling pathway activation, are increased in cancer compared with normal prostate epithelium [35]. Accordingly, activation of this pathway has been observed in prostate cancer metastases. Thus, the Hh signaling pathway seems to be associated with advanced prostate cancer and tumor progression [37]. Moreover, several studies have found that PKA plays an important role in the Hh signaling pathway, which in turn determines the activity of cAMP. As LKB1 can also affect the state of cAMP [24], this raises the question of the relationship between LKB1 and Hh in human prostate cancer patients. In our study, we knocked-down LKB1, the results indicated the significant up-regulation of expression of Hh signaling target genes SHH, SMO and PTCH at both the mRNA and protein levels in siRNA LKB1-transfected DU-145 cells. Based on the above results suggesting that LKB1 is negatively correlated with Hh signaling molecule expression and may be an important negative regulator of the Hh signaling pathway, we speculated that LKB1 can affect the expression of Hh signaling molecules, which are in turn involved in many biological processes including embryogenesis and carcinogenesis of prostate cancer growth. The results suggest that LKB1 contributes to the suppression of tumorigenesis by decreasing cell proliferation and invasion by regulating the Hh signaling pathway.
In conclusion, the expression of LKB1 is negatively correlated with factors in the Hh signaling pathways. The LKB1 gene inhibits cell growth by suppressing Hh signaling pathway in prostate cancer cells. The crosstalk between these pathways may play an important role in prostate cancer growth, suggesting that LKB1 could be a potential molecular target for prostate cancer gene therapy.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, Bray F. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 3.Flourakis M, Prevarskaya N. Insights into Ca2+ homeostasis of advanced prostate cancer cells. Biochim Biophys Acta. 2009;1793:1105–1109. doi: 10.1016/j.bbamcr.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Wissenbach U, Niemeyer B, Himmerkus N, Fixemer T, Bonkhoff H, Flockerzi V. TRPV6 and prostate cancer: cancer growth beyond the prostate correlates with increased TRPV6 Ca2+ channel expression. Biochem Biophys Res Commun. 2004;322:1359–1363. doi: 10.1016/j.bbrc.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 5.Klein EA, Kupelian PA. Localized prostate cancer: radiation or surgery? Urol Clin N Am. 2003;30:315–330. doi: 10.1016/s0094-0143(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Yu C, Ren J, Ye S, Ou W, Wang Y, Yang W, Zhong G, Chen X, Shi H. Synergistic effects of eukaryotic coexpression plasmid carrying LKB1 and FUS1 genes on lung cancer in vitro and in vivo. J Cancer Res Clin Oncol. 2014;140:895–907. doi: 10.1007/s00432-014-1607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu JL, Liu SP, Lee CC, Hsu LC, Ho YF, Huang HS, Guh JH. A unique amidoanthraquinone derivative displays antiproliferative activity against human hormone-refractory metastatic prostate cancers through activation of LKB1-AMPK-mTOR signaling pathway. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:979–90. doi: 10.1007/s00210-014-0998-9. [DOI] [PubMed] [Google Scholar]
- 8.Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Höglund P. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 9.Nath-Sain S, Marignani PA. LKB1 catalytic activity contributes to estrogen receptor α signaling. Mol Biol Cell. 2009;20:2785–2795. doi: 10.1091/mbc.E08-11-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linher-Melville K, Zantinge S, Singh G. Liver kinase B1 expression (LKB1) is repressed by estrogen receptor alpha (ERα) in MCF-7 human breast cancer cells. Biochem Biophys Res Commun. 2012;417:1063–1068. doi: 10.1016/j.bbrc.2011.12.096. [DOI] [PubMed] [Google Scholar]
- 11.Esteller M, Avizienyte E, Corn PG, Lothe RA, Baylin SB, Aaltonen LA, Herman JG. Epigenetic inactivation of LKB1 in primary tumors associated with the Peutz-Jeghers syndrome. Oncogene. 2000;19:164–168. doi: 10.1038/sj.onc.1203227. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang Z, Wang K, Cheng X, Qu X, Jiang B, Li Z, Luo J, Shao Z, Duan T. LKB1 inhibits breast cancer partially through repressing the Hedgehog signaling pathway. PLoS One. 2013;8:e67431. doi: 10.1371/journal.pone.0067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur J Cancer. 2006;42:437–445. doi: 10.1016/j.ejca.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 14.Huang S, He J, Zhang X, Bian Y, Yang L, Xie G, Zhang K, Tang W, Stelter AA, Wang Q. Activation of the hedgehog pathway in human hepatocellular carcinomas. Carcinogenesis. 2006;27:1334–1340. doi: 10.1093/carcin/bgi378. [DOI] [PubMed] [Google Scholar]
- 15.Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 16.Kubo M, Kuroki S, Tanaka M. [New therapeutic target of breast cancer] . Nihon Rinsho. 2007;65(Suppl 6):142–147. [PubMed] [Google Scholar]
- 17.Sheng T, Li C, Zhang X, Chi S, He N, Chen K, McCormick F, Gatalica Z, Xie J. Activation of the hedgehog pathway in advanced prostate cancer. Mol Cancer. 2004;3:29. doi: 10.1186/1476-4598-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonnissen A, Isebaert S, Haustermans K. Hedgehog signaling in prostate cancer and its therapeutic implication. Int J Mol Sci. 2013;14:13979–14007. doi: 10.3390/ijms140713979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson SE, Furic L, Buchanan G, Larsson O, Pedersen J, Frydenberg M, Risbridger GP, Taylor RA. Hedgehog signaling is active in human prostate cancer stroma and regulates proliferation and differentiation of adjacent epithelium. Prostate. 2013;73:1810–1823. doi: 10.1002/pros.22720. [DOI] [PubMed] [Google Scholar]
- 20.Grisaru-Granovsky S, Maoz M, Barzilay O, Yin YJ, Prus D, Bar-Shavit R. Protease activated receptor-1, PAR1, promotes placenta trophoblast invasion and β-catenin stabilization. J Cell Physiol. 2009;218:512–521. doi: 10.1002/jcp.21625. [DOI] [PubMed] [Google Scholar]
- 21.Zhang K, Tian F, Zhang Y, Zhu Q, Xue N, Zhu H, Wang H, Guo X. MACC1 is involved in the regulation of proliferation, colony formation, invasion ability, cell cycle distribution, apoptosis and tumorigenicity by altering Akt signaling pathway in human osteosarcoma. Tumour Biol. 2014;35:2537–2548. doi: 10.1007/s13277-013-1335-5. [DOI] [PubMed] [Google Scholar]
- 22.Geissmann Q. OpenCFU, a new free and open-source software to count cell colonies and other circular objects. PLoS One. 2013;8:e54072. doi: 10.1371/journal.pone.0054072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie C, Kim HJ, Haw JG, Kalbasi A, Gardner BK, Li G, Rao J, Chia D, Liong M, Punzalan RR. A novel multiplex assay combining autoantibodies plus PSA has potential implications for classification of prostate cancer from non-malignant cases. J Transl Med. 2011;9:43. doi: 10.1186/1479-5876-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fenton H, Carlile B, Montgomery EA, Carraway H, Herman J, Sahin F, Su GH, Argani P. LKB1 protein expression in human breast cancer. Appl Immunohistochem Mol Morphol. 2006;14:146–153. doi: 10.1097/01.pai.0000176157.07908.20. [DOI] [PubMed] [Google Scholar]
- 25.Upadhyay S, Liu C, Chatterjee A, Hoque MO, Kim MS, Engles J, Westra W, Trink B, Ratovitski E, Sidransky D. LKB1/STK11 suppresses cyclooxygenase-2 induction and cellular invasion through PEA3 in lung cancer. Cancer Res. 2006;66:7870–7879. doi: 10.1158/0008-5472.CAN-05-2902. [DOI] [PubMed] [Google Scholar]
- 26.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 27.Shen Z, Wen XF, Lan F, Shen ZZ, Shao ZM. The tumor suppressor gene LKB1 is associated with prognosis in human breast carcinoma. Clin Cancer Res. 2002;8:2085–2090. [PubMed] [Google Scholar]
- 28.Zhuang ZG, Di GH, Shen ZZ, Ding J, Shao ZM. Enhanced expression of LKB1 in breast cancer cells attenuates angiogenesis, invasion, and metastatic potential. Mol Cancer Res. 2006;4:843–849. doi: 10.1158/1541-7786.MCR-06-0118. [DOI] [PubMed] [Google Scholar]
- 29.Hao K, Tian XD, Qin CF, Xie XH, Yang YM. Hedgehog signaling pathway regulates human pancreatic cancer cell proliferation and metastasis. Oncol Rep. 2013;29:1124–1132. doi: 10.3892/or.2012.2210. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz i Altaba A. Therapeutic inhibition of Hedgehog-GLI signaling in cancer: epithelial, stromal, or stem cell targets? Cancer Cell. 2008;14:281–283. doi: 10.1016/j.ccr.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Narita S, So A, Ettinger S, Hayashi N, Muramaki M, Fazli L, Kim Y, Gleave ME. GLI2 knockdown using an antisense oligonucleotide induces apoptosis and chemosensitizes cells to paclitaxel in androgen-independent prostate cancer. Clin Cancer Res. 2008;14:5769–5777. doi: 10.1158/1078-0432.CCR-07-4282. [DOI] [PubMed] [Google Scholar]
- 32.Shaw A, Gipp J, Bushman W. The Sonic Hedgehog pathway stimulates prostate tumor growth by paracrine signaling and recapitulates embryonic gene expression in tumor myofibroblasts. Oncogene. 2009;28:4480–4490. doi: 10.1038/onc.2009.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 34.Ingham PW, Nakano Y, Seger C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet. 2011;12:393–406. doi: 10.1038/nrg2984. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez P, Hernández AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, Beyna M, Datta MW, Datta S, i Altaba AR. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. P Natl Acad Sci U S A. 2004;101:12561–12566. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 37.Datta S, Datta M. Sonic Hedgehog signaling in advanced prostate cancer. Cell Mol Life Sci. 2006;63:435–448. doi: 10.1007/s00018-005-5389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


