Abstract
Neuroendocrine (NE) differentiation in prostate carcinomas can be seen in two settings: as a focal finding in conventional acinar adenocarcinoma, identifiable by immunohistochemical staining, or as a primary NE tumor of the prostate gland, such as carcinoid, small cell carcinoma, or large cell NE carcinoma. Of particular interest is the large cell NE carcinoma, which had been previously reported in isolated cases or in limited case series. In this report, we describe a case of a large cell NE carcinoma diagnosed in a 48-year-old man who presented with difficulty in voiding and urine retention. A cystoscopy revealed an enlarged, elongated prostate with an intra-urethral obstructing mass in the prostatic urethra. Subsequently, a transurethral resection of prostate (TURP) was performed at an outside hospital under the clinical diagnosis of benign prostatic hyperplasia (BPH). Microscopic examination of the TURP specimen revealed several foci of low-grade transitional-zone-type adenocarcinoma corresponding to Gleason score 5 (3 + 2), and a focus of high-grade large cell NE carcinoma. Concurrent x-ray computed tomography scans of the chest, abdomen, and pelvis demonstrated an enlarged left pelvic lymph node, which was biopsied and the patient was diagnosed with metastatic large cell NE carcinoma. He subsequently underwent 8 cycles of neoadjuvant chemotherapy with Lupron, a laparoscopic robotic-assisted radical retropubic prostatectomy, and pelvic lymphadenectomy. He died of widely metastatic prostatic carcinoma with leptomeningeal metastases 13 months after radical prostatectomy. Here, we present a rare case of large cell NE carcinoma with a review of the published literature.
Keywords: Neuroendocrine carcinoma, large cell, prostate, adenocarcinoma
Introduction
Prostatic adenocarcinomas may often exhibit focal neuroendocrine (NE) differentiation when stained with NE markers [1,2]. However, tumors with both morphologic NE features and immunohistochemical evidence of NE differentiation, also called NE carcinomas, are rare [3]. Of these NE tumors of the prostate, most are classified as small cell carcinomas or carcinoid tumors. Importantly, foci of NE differentiation present in classic prostatic adenocarcinomas are associated with worsened clinical prognosis, with studies indicating that the NE tumor cells influence angiogenesis and lead to a poor response to early treatment [4].
Although well described in the lung, the large cell NE carcinoma of the prostate is rare and has been described only in a handful of case reports. Moreover, these reports mostly outline instances of recurrent, previously-treated prostatic adenocarcinoma, while reports of de novo large cell NE carcinoma have been limited. For example, Evans et al. reported seven cases of large cell NE carcinoma of the prostate, of which six patients had a history of adenocarcinoma that was previously treated with hormone therapy, and only one patient was diagnosed with a de novo tumor [5].
In the present report, we describe a case of a de novo large cell NE carcinoma in a patient who was initially diagnosed with a benign prostatic hyperplasia (BPH) after presenting with symptoms of urinary retention. Following transurethral resection of prostate (TURP), microscopic analysis of the mass demonstrated several foci of transition-zone-type adenocarcinoma and a focus of large cell NE carcinoma. Moreover, a subsequent staging work up detected an enlarged left pelvic lymph node that was biopsied to confirm the diagnosis of metastatic large cell NE carcinoma.
Case presentation
A 48-year-old man presented with difficulty in voiding and severe urinary retention for a period of two weeks, which required catheterization prior to admission to the hospital. His medical history included hypertension, bilateral orchidopexy for torsion 26 years prior to presentation, and prostate cancer diagnoses in his father and grandfather, both of whom were diagnosed at the age of 72. A cystoscopy was performed and revealed an enlarged, elongated prostate with an intra-urethral obstructing mass in the prostatic urethra. The clinical impression was BPH, and a TURP procedure was subsequently performed at an outside hospital. Microscopic examination of the TURP specimen showed several foci of transition-zone-type adenocarcinoma of the prostate with Gleason score 5 (3 + 2), and a separate small focus of large cell NE carcinoma.
A computed tomography scan (CT scan) of the chest, abdomen, and pelvis was performed and revealed an enlarged left pelvic lymph node. The lymph node was biopsied and microscopic evaluation confirmed the presence of metastatic large cell NE carcinoma. The patient underwent 8 cycles of neoadjuvant chemotherapy (Taxol, VP-16, and cisplatin) in combination with Lupron, followed by a laparoscopic robotic-assisted radical retropubic prostatectomy and pelvic lymphadenectomy. His prostate-specific antigen (PSA) levels were less than 0.1 ng/mL when measured at three different times post-surgery. The patient was last examined in the clinic one year after the surgery and was discharged to hospice care due to widely metastatic prostatic carcinoma with leptomeningeal metastases. He passed away 13 months after the radical retropubic prostatectomy.
Pathologic findings
The high-grade carcinoma cells were arranged in solid nests and trabeculae, relatively large, and characterized by moderate amounts of eosinophilic cytoplasm and oval or round nuclei with stippled chromatin, a hallmark of NE features.
Histologic examination of the biopsied left pelvic lymph node revealed a metastatic NE carcinoma with solid and trabecular nests of tumor cells without gland formation, replacing the normal lymph node architecture. The tumor cells possessed nuclei with “salt and pepper”-appearing chromatin, occasional prominent nucleoli, and moderate amounts of eosinophilic cytoplasm that was morphologically consistent with large cell NE carcinoma (Figure 1A). Further immunohistochemical analysis demonstrated that the tumor cells expressed CD56 (Figure 1B), chromogranin, and synaptophysin, but were negative for the expression of PSA and prostatic acid phosphatase (PAP), which are both prostatic markers. Thus, the morphologic and immunohistochemical analyses were consistent with the diagnosis of metastatic large cell NE carcinoma of the prostate. The patient received 8 cycles of neoadjuvant chemotherapy with Lupron, followed by a radical prostatectomy with pelvic lymphadenectomy.
Figure 1.
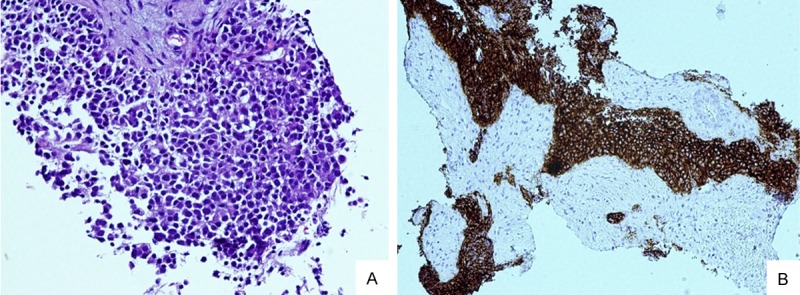
(A) Lymph node biopsy exhibited a poorly differentiated cohesive epithelial tumor consistent with metastatic carcinoma (100 ×). (B) Strong and diffuse positivity for CD56 demonstrates neuroendocrine differentiation in the metastatic carcinoma presentedin (A) (100 ×).
The prostatectomy specimen consisted of a prostate with attached bilateral seminal vesicles, which weighed 40 g in toto, while the prostate itself weighed 36 g. Whole-mount histological examination of the prostate revealed a tumor measuring 3.8 cm in its largest dimension, which showed extensive treatment-related changes. The viable areas comprised approximately 30% of the total tumor volume. The tumor was composed of an infiltrating crushed NE carcinoma arranged in solid nests, trabeculae, and single infiltrating cells (Figure 2A), which involved both the right and left posterior halves of the peripheral zone with multiple tumor foci in the apical portion of the prostate gland. A desmoplastic reaction (Figure 2B) and multifocal perineural and lymphovascular invasion were noted. The tumor cells were large, with a visible amount of cytoplasm and occasionally prominent nucleoli. Atypical mitoses were numerous and accounted for more than 20/10 high power fields (HPFs). Small cell carcinoma or microacinar adenocarcinoma components were not observed. The left seminal vesicle showed NE carcinoma infiltration in the muscular wall, but the right seminal vesicle was free of tumor, although NE carcinoma infiltration into the perivesicular fibroadipose tissue and associated lymphovascular invasion were noted.
Figure 2.
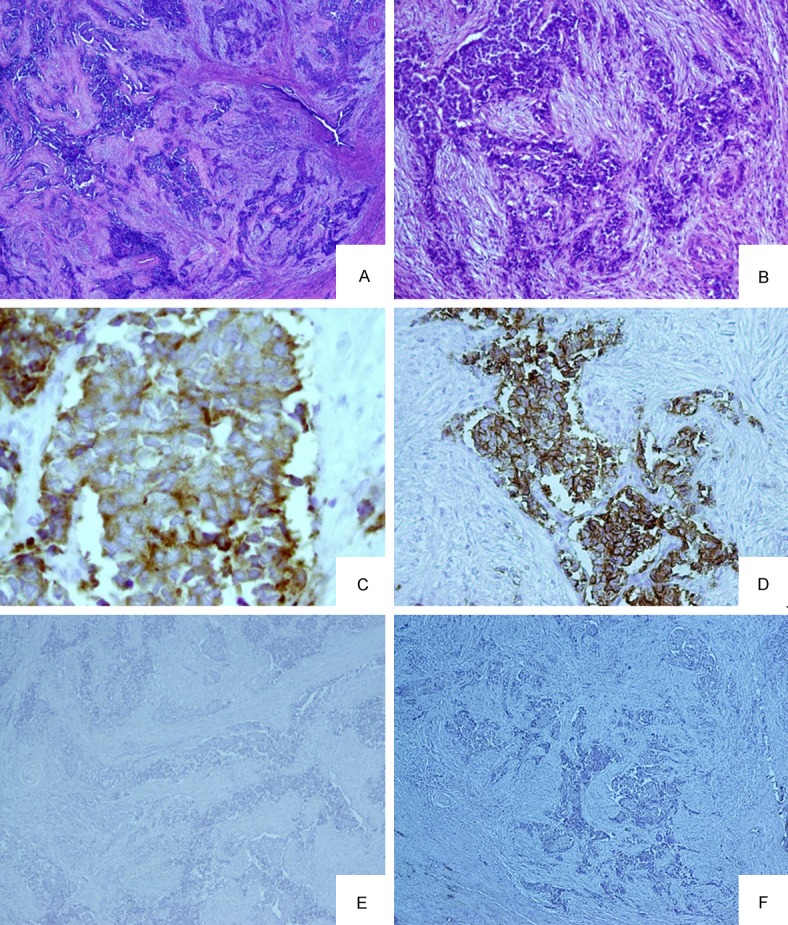
(A) A low power view of the prostatectomy specimen demonstrates an infiltrative tumor composed of solid nests and trabeculae (40 ×). (B) NE tumor comprised of irregular infiltrative trabeculae with surrounding desmoplasia (100 ×). (C) Tumor cells showing strong membranous staining for neuroendocrine marker synaptophysin (200 ×). (D) Tumor cells demonstrate membranous positivity for CD 56. (E and F) Tumor cells are negative for the expression of prostatic lineage markers PSA (E) and PAP (F).
Seven lymph nodes were identified in the right and left pelvic lymph node dissection specimens, and two were positive for metastatic NE carcinoma. The tumor morphology was similar to that seen in the prostate gland, and the largest metastatic tumor deposit measured 2.0 cm and exhibited focal extranodal extension. Other findings noted in the prostate included high-grade prostatic intraepithelial neoplasia (HGPIN), atrophy, urothelial and squamous metaplasia, and incomplete basal cell hyperplasia associated with neoadjuvant treatment.
Immunohistochemical analysis of the tumor revealed strong diffuse membranous expression of CD56, and cytoplasmic expression of chromogranin and synaptophysin (Figure 2C and 2D). Moreover, the tumor cells lacked the expression of PSA and PAP (Figure 2E and 2F). The final diagnosis was large cell NE carcinoma of the prostate architecturally consistent with Gleason score 10 (5 + 5), with pelvic lymph node metastases.
Discussion
Large cell NE carcinoma of the prostate is rare and has been previously described only in isolated case reports, with the largest case series presented by Evans et al. [5]. Although not included in the 2004 World Health Organization (WHO) classification, large cell NE carcinoma has been considered a variant of prostatic carcinoma [6], and was shown to have features similar to pulmonary large cell NE carcinoma, which was first presented by Travis et al. in 1991 [7]. Per the WHO criteria, pulmonary large cell NE carcinoma is defined by three features, which have also been adapted for the characterization of large cell NE carcinomas of the prostate [5]. First, these tumors must contain a high-grade lesion with a mitotic rate greater than 10 mitoses per 10 HPFs and accompanying large necrotic zones [8]. Secondly, the morphologic classification should identify NE architecture at a low-power magnification, including organoid nests, palisading, rosettes, trabeculae, and large tumor cells that exhibit prominent nucleoli, vesicular clumped chromatin, and abundant cytoplasm. Lastly, the tumor cells should express at least one NE marker other than neuron-specific enolase (NSE) [8].
Seven cases of large cell NE carcinomas of the prostate have been previously reported in the largest case series, with six of these recorded in patients who had a history of adenocarcinoma treated with hormone therapy [5]. In this series, the mean age was 67 years, with a range of 43 to 81 years [5]. The patient we describe herein was 48 years old and had a previous diagnosis of transition-zone-type conventional adenocarcinoma (Gleason score 5) with a large cell NE carcinoma component (as per the pathology report from a referring institution). He had not been previously treated with hormonal therapy or chemotherapy, but subsequently received neoadjuvant and hormonal therapies followed by a definitive radical prostatectomy.
Although the exact etiology of NE differentiation arising in patients with conventional adenocarcinoma who were treated with hormonal therapy has not been well defined, it has been hypothesized that androgen deprivation can cause transdifferentiation of the carcinoma cells into androgen receptor-negative, B cell lymphoma-2 (bcl-2)-negative NE cells [5,9,10]. Moreover, the NE cells are thought to lack androgen receptor expression and play a regulator role in proliferative and secretory activity of prostatic glandular epithelium [5]. As previously stated, the largest case series describing large cell NE carcinoma of the prostate identified that six of the seven patients were previously treated with androgen deprivation therapy. In the present case, the patient had not received prior hormonal therapy. However, it is of note that this patient had undergone a bilateral orchidopexy 26 years prior to presentation, as a result of testicular torsion. Therefore, it effectively placed him into an androgen-deprived state and may have played a role in the development of the NE prostatic tumor.
Another interesting aspect of our case is the fact that the patient was relatively young at the time of diagnosis, compared to the patients described in all other reported cases. The advanced disease stage and earlier presentation may be accredited to the prolonged functional androgen deprivation, although the patient also had a strong family history of prostate cancer. The initial microscopic examination of the TURP specimen revealed a relatively common microacinar adenocarcinoma that corresponded to Gleason score 5, along with a single focus of NE carcinoma. However, subsequent examination of the radical prostatectomy specimen demonstrated only the NE carcinoma in the absence of a glandular component. This phenomenon may be explained by its total eradication during TURP or by the administration of neoadjuvant hormonal therapy subsequent to the metastatic carcinoma diagnosis.
Although strongly associated with androgen deprivation therapy, studies show that it may be possible for NE carcinoma to arise de novo, as in our case. For example, animal studies of prostate cancer have shown a direct malignant transformation of resident prostatic NE cells [10]. Based on our experience, we concur with this theory, but emphasize that our patient underwent a bilateral orchidopexy, which culminated in a functional state of decreased androgens.
In spite of the fact that this particular case had an initial TURP that showed an adenocarcinoma corresponding to Gleason score 5, presence of a large pelvic node infiltrated with metastatic NE carcinoma presents an interesting potential differential diagnosis. Had the enlarged lymph node been noted in isolation, or prior to the discovery of prostatic carcinoma, the differential diagnosis would also have included metastasis from another primary site, such as the lung. Moreover, it is important to note that, as in this case, large cell NE carcinoma of the prostate can be entirely negative or display only focal positivity for prostatic origin markers, such as PAP and PSA [6]. Therefore, immunohistochemical staining for these markers, in the event that large cell NE carcinoma of prostatic origin is suspected, should not necessarily rule out a primary prostatic tumor.
There have been very few reports of large cell NE carcinoma of the lung metastasizing to the prostate [11,12]. In both cases, the tumor cells comprising the primary pulmonary lesion were positive for thyroid transcription factor-1 (TTF-1), which is helpful in confirming the pulmonary origin of the primary tumor. In our case, the prostatic tumor was negative for TTF-1, and the bulk of the tumor was located in the prostate gland, occupying approximately 40% of the gland volume. Furthermore, no concomitant lung lesion was identified on CT scan of the chest, while CT of the pelvis revealed an enlarged lymph node that was ultimately determined to be a regional lymph node metastasis. Together, these findings effectively ruled out a pulmonary large cell NE carcinoma that metastasized to the prostate gland.
Finally, it is important to consider the possibility of large cell prostatic NE carcinoma because its prognosis is worse and treatment options differ from other prostate tumor types [4,5]. Specifically, it is possible that some large cell NE carcinoma cases may have been misdiagnosed as poorly-differentiated adenocarcinomas. However, large cell NE carcinomas and poorly-differentiated adenocarcinomas differ in both prognosis and treatment. Therefore, when a high-grade prostate carcinoma with organoid appearance is identified on biopsy or resection specimens, we recommend that it be subjected to further immunohistochemical analyses for prostate-specific (PSA and PAP) and NE markers. This strategy will also help to create a database of similar cases, which along with patient outcomes, will enable a better understanding of their biologic behavior. In summary, further studies using standardized criteria and terminology are needed to correctly document this group of tumors [13].
Disclosure of conflict of interest
None.
References
- 1.di Sant’ Agnese PA. Neuroendocrine differentiation in carcinoma of the prostate: diagnostic, prognostic and therapeutic implication. Hum Pathol. 1992;23:287–96. doi: 10.1002/1097-0142(19920701)70:1+<254::aid-cncr2820701312>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Wynn SS, Nagabundi S, Koo J, Chin NW. Recurrent prostate carcinoma presenting as omental large cell carcinoma with neuroendocrine differentiation and resulting in bowel obstruction. Arch Pathol Lab Med. 2000;124:1074–6. doi: 10.5858/2000-124-1074-RPCPAO. [DOI] [PubMed] [Google Scholar]
- 3.Freschi M, Colombo R, Naspro R, Rigatti P. Primary and pure neuroendocrine tumor of the Prostate. Eur Urol. 2004;45:166–70. doi: 10.1016/j.eururo.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Heinrich E, Trojan L, Friedrich D, Voss M, Weiss C, Michel MS, Grobholz R. Neuroendocrine tumor cells in prostate cancer: evaluation of the neurosecretory products serotonin, bombesin, and gastrin-mpact on angiogenesis and clinical follow-up. Prostate. 2011;71:1752–8. doi: 10.1002/pros.21392. [DOI] [PubMed] [Google Scholar]
- 5.Evans AJ, Humphrey PA, Belani J, van der Kwast TH, Srigley JR. Large cell neuroendocrine carcinoma of prostate: a clinicopathologic summary of 7 cases of a rare manifestation of advanced prostate cancer. Am J Surg Pathol. 2006;30:684–93. doi: 10.1097/00000478-200606000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Humphrey PA. Histological variants of prostatic carcinoma and their significance. Histopathology. 2012;60:59–74. doi: 10.1111/j.1365-2559.2011.04039.x. [DOI] [PubMed] [Google Scholar]
- 7.Travis WD, Linnoila RI, Tsokos MG, Hitchcock CL, Cutler GB Jr, Nieman L, Chrousos G, Pass H, Doppman J. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma: an ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15:529–53. doi: 10.1097/00000478-199106000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Travis WD, Brambilia E, Muller-Hermelink HK, Harris CC. World Health Organization Classification of Tumors. Vol. 10. Lyon, France: IARC Press; 2004. Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart. [Google Scholar]
- 9.Furtado P, Lima MV, Nogueira C, Franco M, Tavora F. Review of small cell carcinomas of the prostate. Prostate Cancer. 2011;2011:543272. doi: 10.1155/2011/543272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garabedin EM, Humphery PA, Gordon JI. A transgenic mouse model of metastatic prostate cancer originating from neuroendocrine cells. Proc Natl Acad Sci U S A. 1998;95:15382–7. doi: 10.1073/pnas.95.26.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo JH, Lee JH, Kim EK, Hong YK, Lee Y, Jeong HC. Prostatic metastasis of large cell neuroendocrine carcinoma of the lung. Respirology. 2009;14:772–5. doi: 10.1111/j.1440-1843.2009.01545.x. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu K, Goto T, Maeshima A, Oyamada Y, Kato R. Prostatic metastasis of pulmonary large cell neuroendocrine carcinoma. J Cancer. 2012;3:96–9. doi: 10.7150/jca.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein JI, Amin MB, Beltran H, Lotan TL, Mosquera JM, Reuter VE, Robinson BD, Troncoso P, Rubin MA. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol. 2014;38:756–67. doi: 10.1097/PAS.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]


