Abstract
Objective: This study was designed to investigate the protective effects of salidroside (SDS) via suppressing the expression of transforming growth factor-β1 (TGF-β1) in rat acute lung injury (ALI) induced by paraquat (PQ) and to explore the potential molecular mechanisms. Methods: A total of 90 male rats (190-210 g) were randomly and evenly divided into 9 groups: control group, PQ groups (4 groups), and PQ + SDS groups (4 groups). The rats in control group were treated with equal volume of saline intraperitoneally. The rats in PQ groups were exposed to PQ solution (20 mg/kg) by gastric gavage for 1, 6, 24, and 72 hours, respectively. The rats in PQ + SDS groups were intraperitoneally injected once with SDS (10 mg/kg) every 12 hours after PQ perfusion. Pulmonary pathological changes were observed by hematoxylin and eosin (HE) staining. The expression of TGF-β1 and the mRNA were evaluated by immunohistochemical (IHC) scoring and real time quantitative reverse transcription polymerase chain reaction (real-time qRT-PCR), respectively. Results: SDS alleviated the symptoms of PQ induced ALI. Moreover, SDS reduced the expression of the inflammatory cytokine TGF-β1 including TGF-β1 IHC scores (at each time point from 6 to 72 hours after PQ perfusion) and mRNA level (at each time point from 1 to 72 hours after PQ perfusion) compared with PQ groups (P < 0.05). Conclusion: SDS alleviated the pulmonary symptoms of PQ-induced ALI, at least partially, by repressing inflammatory cell infiltration and the expression of TGF-β1 resulting in delayed lung fibrosis.
Keywords: Salidroside, paraquat, acute lung injury, transforming growth factor-β1
Introduction
Paraquat (PQ) is a highly effective and none-selective quaternary ammonium herbicide, which is widely used in the world, controlling weeds in a huge variety of crops [1]. However, it could cause acute lung injuries (ALI) and damages to other organ systems once being ingested by animals [2,3]. The signs of ALI, usually observed in severe PQ-poisoned cases, were Clara and alveolar epithelial cell disruption, damage of surfactant generation, oedema, hypoxaemia, haemorrhage and infiltration of inflammatory cells into the interstitial and alveoli spaces [4-6]. Moreover, there have been frequent PQ poisoning incidents which have become a severe public health issue all over the world, especially in Asian region. The statistical data show that organophosphorus pesticide poisoning is the major toxic diseases, and among which PQ poisoning is the one with highest mortality (> 50%) [1,7]. Currently, the mechanism of PQ-induced lung toxicity has not been fully understood and there are no widely accepted guidelines on treatment of PQ poisoning. So developing an effective therapeutic method for PQ poisoning is badly needed.
Since no antidote is useful for PQ poisoning so far. Preventing the leukocyte infiltration and alveolar-capillary barrier dysfunction may be helpful to prevent PQ-induced ALI regardless of etiology. Qian et al. showed FTY720 (fingolimod) could alleviate PQ-induced lung injury [8]. Furthermore, methylprednisolone and cyclophosphamide have been reported to be valid in PQ poisoning [9]. Some studies also demonstrated that Rhodiola rosea exerts anti-inflammatory [10] and relieves pulmonary edema in rats [11].
Salidroside (SDS), a glucoside of tyrosol isolated from the plant Rhodiola rosea, is thought to be responsible for the antidepressant and anxiolytic actions [12]. SDS also has anti-inflammatory, anti-hypoxia, anti-tumor, anti-aging, and anti-liver fibrosis effects [13,14]. Reports have shown that SDS has obvious therapeutic effects to rat’s secondary lung injury and pulmonary dysfunction induced by burning [14-17]. SDS could not only alleviate bleomycin-induced rat pulmonary fibrosis by regulating the balance of MMP-2 (matrix metalloproteinase 2) and TIMP-1 (tissue inhibitor of metalloproteinases 1) to some extent [18], but also ease lipopolysaccharide and oleic acid induced ALI by repressing inflammatory mediators and anti-oxygenation [19]. Transforming growth factor-β1 (TGF-β1) is a polypeptide cytokine that promotes inflammatory responses by releasing related inflammatory cells [20]. TGF-β1 plays a critical role in tissue injury development of multiple organs, including lung [21]. Expression level of TGF-β1 is dramatically increased as early as 2 days after the lung injury, which is a critical mediator of ALI [20]. A previous study has demonstrated that TGF-β1 not only participates in the early phase of ALI (exudative) contributing to pulmonary edema, but also is related with the late phase of ALI (fibrotic) leading to pulmonary fibrosis [4]. Additionally, TGF-β1 has been reported to play an important role in ALI induced with PQ and hyperoxia [22]. Therefore, in this study, we investigated the protective role of SDS to PQ-induced ALI in rats, and explored the underlying mechanism by focusing on the change of TGF-β1 level.
Methods
Laboratory animals
Ninety healthy male Sprague-Dawley rats (SPF grade, Rattus rattus, 190-210 g) were provided by The Laboratory Animal Center, Zhejiang Chinese Medicine University [Animal production license No.: SCXK (z) 2008-0022; animal use permit No.: SYXK (z) 2008-0115]. The rats were kept under a 12-h light/12-h dark cycle with free access to food and water. The animal experiments were approved by local ethics committee.
Animal grouping and model establishment
PQ and SDS were purchased from Shandong Sanyuan Industry and Trading, Co., Ltd. and National Institutes for Food and Drug Control, respectively. PQ and SDS were dissolved in 0.9% saline solution. The molecule structure of SDS was shown in Figure 2. A total of 90 male rats were randomly and evenly divided into 9 groups: control group, PQ groups (4 groups) and PQ + SDS groups (4 groups). The rats in control group were treated with equal volume of physiological saline intraperitoneally. The rats in PQ groups were exposed to PQ solution (20 mg/kg) by gastric gavage at 1, 6, 24, and 72 hours, respectively. While the rats in PQ + SDS groups were intraperitoneally injected once with 1% SDS (10 mg/kg) every 12 hours after PQ perfusion. The injection style of SDS was done as previously reported [19].
Figure 2.
Molecular structure of SDS.
Pathological observation of lung tissue
Five mice were randomly chosen from each group and sacrificed for further study at 1, 6, 24, 72 hours after PQ perfusion respectively. The animals were anesthetized with intraperitoneal injections of 10% chloral hydrate and rapidly perfusion fixed with saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Lung tissue was obtained and stored at -70°C until use. The upper right lung tissue was used to prepare paraffin sections as follows: fixation in neutral formaldehyde overnight at 4°C; decalcifiation by immersion in 0.12 M EDTA, pH 7.0 for 1 week at room temperature (RT); dehydration by gradient concentration of ethanol; vitrification by dimethylbenzene; embedding in 52-54°C paraffin. Finally, sections were cut in a microtome. Then, all sections were collected for hematoxylin and eosin (HE) and immunohistological (IHC) staining. The pathological changes were observed under optical microscope.
HE staining
To observe general histological pathology of lung tissue, HE staining was used. Digital images were collected by a conventional light microscope in five visual fields/per section with 100× magnification under bright-field viewing.
IHC observation and scoring
The TGF-β1 kit (Invitrogen, San Diego, CA, USA) and IHC kit (Boster Company, Wuhan, China) were used in this experiment. The expression of TGF-β1 was detected using an IHC staining kit. The lung slides were dewaxed and hydrated. Endogenous peroxidase was inactivated by H2O2. The antigens were repaired in sodium citrate at pH 6.0 by microwave. Goat serum was used to block non-specific antigen sites. Then, the slides were incubated successionally with 50 μl diluted primary antibody (TGF-β1) overnight at 4°C. The sections were then washed with PBS and incubated with biotin-conjugated secondary antibody for 1 hour at RT, then, streptavidin/HRP was added and 50 μl diaminobenzidine was added until reaction terminated by the tap water, with dyeing time controlled under microscope. Finally, the slides were re-dyed with hematoxylin, dehydrated, vitrified and mounted before observed under light microscope. Two slides were chosen randomly for negative control, which were incubated with antibody dilution instead of primary antibody. Under microscope observation, the positive cells for TGF-β1 were counted in 5 randomly selected fields with Image-Pro Plus 6.0 software.
Description of the level of TGF-β1 is on the basis of the ratio of positive cells and intensity of the reaction. The parameter is classified and a combined score is used to decide positive or negative result according to criteria defined previously [23-25]. For the score of TGF-β1 positive cell ratio, 0-1%, 1-10%, 10-50%, 50-80% and 80-100% were scored as 0, 1, 2, 3 and 4, respectively. For intensity score, negative, weakly positive, positive and strongly positive were scored as 0, 1, 2, and 3, respectively. IHC score value = positive cell ration score × intensity score.
Quantitative polymerase chain reaction (QPCR)
The total RNA of lung tissue was extracted with Trizol method (Invitrogen, San Diego, CA, USA). The reverse transcription (RT) reaction was performed in 20 μl system using RT kit from TaKaRa. TGF-β1 and GAPDH mRNA sequences were obtained from PUBMED database. All the primers were designed with Primer Premier 5.0. qPCR assay was used to detect the mRNA expression level of TGF-β1. qPCR reaction was performed in 20 μl system containing 10 μl SYBR® Premix Ex TaqTM, 0.4 μl forward primer (10 μM), 0.4 μl reverse primer (10 μM), 2 μl cDNA template, and 7.8 μl distilled H2O (dH2O). PCR program: 95°C pre-denaturation 2 min, 95°C denaturation 15 s, 60°C annealing 30 s, 40 cycles. The Primers used were listed in Table 1.
Table 1.
Real-time PCR reactions for each gene-specific primer
| Gene | Primer | Sequence | Product Length (bp) |
|---|---|---|---|
| TGF-β1 | TGF-F-uc | 5’-GAGATGCAATGCTATTCCT-3’ | 101 |
| TGF41TC | 5’-CGACGTCACAGCGCACTT-3’ | ||
| GAPDH | GAPDH-F | 5’-CCTCAAGATTGTCAGCAAT-3’ | 141 |
| GAPDH-R | 5’-CCATCCACAGTCTTCTGAGT-3’ |
Statistical analysis
All the data were presented as X̅ (mean) ± s (standard deviation). SPSS 17.0 was used for statistical analysis. Multiple comparisons among groups were performed by one-way ANOVA. Paired comparisons were carried out using least significant difference method. P < 0.05 was designated as significant difference.
Results
PQ poisoning symptoms
In the PQ groups, the rats displayed series of symptoms including listlessness, decreased appetite, squinting, back arching, sluggish, and an increased respiratory rate half an hour after PQ perfusion. Six hours after PQ perfusion, rats showed lip cyanosis, buccal respiration or respiratory distress. The poisoning symptoms became progressively severe from 24 to 72 hours after PQ perfusion, the damage of respiratory system is the most obvious along with weight loss, diarrhoea, and hematuresis compared to the control group. Compared with PQ groups, rats in PQ + SDS groups displayed milder symptoms. Twelve rats died in PQ groups (2 at 1 hour, 2 at 6 hours, 4 at 24 hours, 4 at 72 hours) and 5 rats died in PQ + SDS groups (1 at 1 hour, 1 at 6 hours, 2 at 24 hours, 1 at 72 hours). The mortality between PQ and PQ + SDS groups were significantly different (P < 0.05).
Pathological changes of lung tissue
The HE staining results of control group showed clear alveolar structure, thin alveolar wall, no alveolar edema, and no inflammatory cell infiltration in pulmonary interstitial. While in PQ groups, the rats showed alveolar inflammation features 1 hour after PQ perfusion, such as obvious telangiectasia and hyperemia. The rats of 6 hours after PQ perfusion displayed widened alveolar septum, edema fluid in alveolar cavity, and partial hyaline membrane formation. More serious symptoms, such as obvious lung tissue inflammation and exudation, hemorrhage, necrotic and inflammatory cell infiltration, partial bronchiolar epithelium exuviation, alveolar structure collapse, atelectasis, and pneumonectasis, were observed in rats at 24-72 hours post PQ perfusion. Compared with PQ groups, the rats in PQ + SDS groups showed similar but slighter symptoms at each time point from 1 to 72 hours after PQ perfusion (Figure 1).
Figure 1.
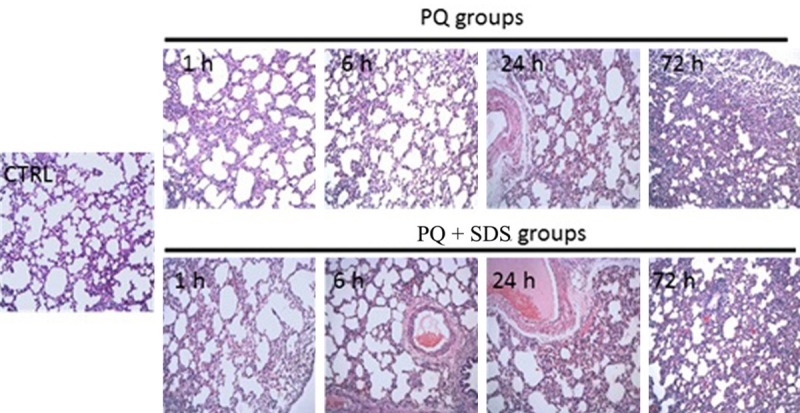
Pathological changes of lung tissue from different groups of rats determined by HE staining (×100) after PQ poisoning. PQ groups: rats were treated by paraquat solution; PQ + SDS groups: rats were treated by PQ plus SDS at each time point from the 1 hour after PQ poisoning; CTRL (control group): rats were only treated by saline intraperitoneally.
IHC detection of TGF-β1 in lung tissue
Compared with control group, TGF-β1 positive cells and TGF-β1 IHC scores in PQ groups were significantly increased at each time point (P < 0.01) from 6 hours after PQ perfusion and peaked at the 72 hours after PQ perfusion. While in PQ + SDS groups, TGF-β1 positive cells and TGF-β1 IHC scores (P < 0.05) were decreased significantly at each time point from 6 hours after PQ perfusion compared with PQ groups (Table 2).
Table 2.
TGF-β1-positive cell number (X̅ ± s) and TGF-β1 IHC scoring for all the groups
| Group | TGF-β1 positive cell number | TGF-β1 IHC scores | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 1 h | 6 h | 24 h | 72 h | 1 h | 6 h | 24 h | 72 h | |||||
| Control | 16±1.31 | 2.11±0.20 | ||||||||||
| PQ groups | 17±1.34 | 21.33±2.591 | 26.67±3.121 | 32.39±4.361 | 2.35±0.19 | 3.54±0.541 | 5.63±0.981 | 6.46±1.411 | ||||
| PQ + SDS groups | 16±1.54 | 18.73±2.711,2 | 22.67±3.031,2 | 28.31±4.411,2 | 2.22±0.18 | 2.85±0.461,2 | 4.15±1.091,2 | 5.15±1.321,2 | ||||
P < 0.01 vs. control group;
P < 0.05 vs. PQ groups.
Detection of TGF-β1 mRNA level via qPCR
Gene expression level for TGF-β1 was examined by qPCR in the lung tissue of rats in 9 groups. The gene expression of TGF-β1 in PQ groups was significantly increased at each time point from 1 to 72 hours after PQ perfusion compared with that of control group (Table 3, P < 0.01), while TGF-β1 mRNA level in PQ + SDS groups was significantly decreased at each time point from 1 to 72 hours after PQ perfusion relative to PQ groups (P < 0.05) (Table 3).
Table 3.
TGF-β1 mRNA levels detection via qPCR for all the groups
| Group | Fold changes | |||
|---|---|---|---|---|
|
| ||||
| 1 h | 6 h | 24 h | 72 h | |
| Control | 1 | |||
| PQ groups | 2.15±0.241 | 3.31±0.761 | 4.47±1.021 | 5.11±1.121 |
| PQ + SDS groups | 1.55±0.221 | 2.45±0.66 1,2 | 3.52±1.031,2 | 4.12±1.05 1,2 |
P < 0.01 vs. control group;
P < 0.05 vs. PQ groups.
Discussion
In the present study, we demonstrated that SDS ameliorated the symptoms of PQ-induced ALI as measured by HE staining, IHC and qPCR for inflammatory cytokine level of TGF-β1. Our observations implied that SDS exhibited protective effects though repressing inflammatory cell infiltration and the expression of TGF-β1 at least partially in ALI induced by PQ as potentially pathophysiological mechanisms explaining the functional impairment.
Due to the presence of polyamines uptake system in lung tissue, PQ could accumulate in lung against a concentration gradient, reaching to 6-10 times of PQ concentration in serum [13,14]. Compared with other tissues, the PQ clearance rate in lung tissue is much slower. After PQ poisoning, the early pulmonary symptoms are alveolar epithelial injury, hemorrhagic edema, and inflammatory cell infiltration, while the latter symptom is the alveolar and interstitial fibrosis [3]. There is considerable evidence to suggest that the inflammatory features, histological pulmonary impairment and metabolic processes are similar in human and rats in acute PQ intoxication [26]. In our study the rats in PQ groups showed the similar pulmonary symptoms at both early and latter stages post PQ perfusion, which indicates our rat model is very resemble to the symptoms of human PQ poisoning. The rats in PQ + SDS groups showed much slighter symptoms, indicating SDS has therapeutic effects to PQ poisoning.
Growing evidences showed that several cytokines are involved in the initiation and development of lung injuries and fibrosis [27,28]. TGF-β1 is a polypeptide cytokine that not only controls proliferation, differentiation, and apoptosis [29], but also promotes inflammatory responses by releasing related inflammatory cells during the development of ALI and lung fibrosis [20]. The down-regulation of the inflammatory processes is an important mechanism for decreasing PQ-induced ALI. TGF-β1 could also be involved in ALI and lung fibrosis by enhancing the pulmonary extracellular matrix deposition and inducing the synthesis of collagen and fibronectin [30]. TGF-β1 is now an acknowledged starting hub in the development of lung injury and fibrosis. Previous researches had exhibited that inhibition of TGF-β1 expression or its signaling pathways can abrogate pulmonary fibrosis induced by bleomycin [31]. In the current study, the number and scores of TGF-β1 positive cells measured by IHC was up-regulated in lung tissue following PQ administration, this result is in line with previous research results [32]. While that of TGF-β1 positive cells decreased after SDS intervention. Additionally, quantitative analysis of qPCR demonstrated that level of TGF-β1 was accordance with the outcomes of the IHC. Obviously, our results showed that SDS treatment repressed the expression of TGF-β1 along with inflammatory indicating that the anti-inflammatory effect of SDS may be partly contributed to the inhibition of TGF-β1, which further improved the symptom of PQ induced ALI.
Compared to western medicine, there is more interest in natural plant products which may be used in the development of new drugs and applications in lung diseases induced by toxic agents [33,34]. SDS is herbal extract from Rhodiola rosea L. Compared with synthetic drugs, natural compounds has less side effects to human body. Guan et al. also exhibited that SDS treatment caused the reduction of inflammatory cells in the bronchoalveolar lavage fluid in LPS-induced ALI in mice [19]. But necessary evaluations are needed for the usage of SDS in PQ poisoning treatment.
Although we observed that SDS plays an important role of alleviating the symptoms of PQ induced ALI, there remained some shortcomings in our study. We examine the pathological changes only under optical microscope, but transmission electron microscopy (TEM) was not used which might have shown the structure changes of lung tissue more clearly. Additionally, the level of TGF-β1 of lung tissue was only observed under microscope, but electrophoregram was not observed which might be more convincing. Moreover, the mechanisms of PQ poisoning are so complicated and unclear. Hence, further experiments are necessarily to solve these problems above in our future study.
To conclude, our study suggested that SDS alleviated the symptoms of PQ induced ALI in rats via down-regulation of the expression of TGF-β1. To a certain degree, our results could provide informative clues for pathogenesis of PQ induced ALI and shed lights for developing clinical treatment for PQ poisoning.
Acknowledgements
We wish to express our warm thanks to all the authors who contributed to the research. The research was supported by the Scientific Research Fund of Zhejiang Province Chinese Medicine (2012ZB039, 2013ZA056) and Zhejiang Medicines and Health Sciences Research Foundation (2013KYA141).
Disclosure of conflict of interest
None.
References
- 1.Sittipunt C. Paraquat poisoning. Respir Care. 2005;50:383–5. [PubMed] [Google Scholar]
- 2.Dinis-Oliveira RJ, Duarte JA, Sanchez-Navarro A, Remiao F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38:13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita M, Ando Y. A long-term follow-up of lung function in survivors of paraquat poisoning. Hum Exp Toxicol. 2000;19:99–103. doi: 10.1191/096032700678815729. [DOI] [PubMed] [Google Scholar]
- 4.Dhainaut JF, Charpentier J, Chiche JD. Transforming growth factor-beta: a mediator of cell regulation in acute respiratory distress syndrome. Crit Care Med. 2003;31:S258–64. doi: 10.1097/01.CCM.0000057901.92381.75. [DOI] [PubMed] [Google Scholar]
- 5.Rocco PR, Souza AB, Faffe DS, Passaro CP, Santos FB, Negri EM, Lima JG, Contador RS, Capelozzi VL, Zin WA. Effect of corticosteroid on lung parenchyma remodeling at an early phase of acute lung injury. Am J Respir Crit Care Med. 2003;168:677–84. doi: 10.1164/rccm.200302-256OC. [DOI] [PubMed] [Google Scholar]
- 6.Park HK, Kim SJ, Kwon do Y, Park JH, Kim YC. Protective effect of quercetin against paraquat-induced lung injury in rats. Life Sci. 2010;87:181–6. doi: 10.1016/j.lfs.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Bertolote JM, Fleischmann A, Eddleston M, Gunnell D. Deaths from pesticide poisoning: a global response. Br J Psychiatry. 2006;189:201–3. doi: 10.1192/bjp.bp.105.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian J, Ye Y, Lv L, Zhu C, Ye S. FTY720 attenuates paraquat-induced lung injury in mice. Int Immunopharmacol. 2014;21:426–31. doi: 10.1016/j.intimp.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Eddleston M, Wilks MF, Buckley NA. Prospects for treatment of paraquat-induced lung fibrosis with immunosuppressive drugs and the need for better prediction of outcome: a systematic review. QJM. 2003;96:809–24. doi: 10.1093/qjmed/hcg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bawa AS, Khanum F. Anti-inflammatory activity of Rhodiola rosea--“a second-generation adaptogen”. Phytother Res. 2009;23:1099–102. doi: 10.1002/ptr.2749. [DOI] [PubMed] [Google Scholar]
- 11.Lee SY, Li MH, Shi LS, Chu H, Ho CW, Chang TC. Rhodiola crenulata Extract Alleviates Hypoxic Pulmonary Edema in Rats. Evid Based Complement Alternat Med. 2013;2013:718739. doi: 10.1155/2013/718739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhingra D, Sharma A. A review on antidepressant plants. Natural product radiance. 2006;5:144–52. [Google Scholar]
- 13.Hoet PH, Lewis CP, Demedts M, Nemery B. Putrescine and paraquat uptake in human lung slices and isolated type II pneumocytes. Biochem Pharmacol. 1994;48:517–24. doi: 10.1016/0006-2952(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Li X, Simoneau AR, Jafari M, Zi X. Rhodiola rosea extracts and salidroside decrease the growth of bladder cancer cell lines via inhibition of the mTOR pathway and induction of autophagy. Mol Carcinog. 2012;51:257–67. doi: 10.1002/mc.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi C, Zhang J, Chen X, Zhang J, Yang P, Jiao Q, Zhang P, Lu H, Liu Y. Salidroside protects cultured rat subventricular zone neural stem cells against hypoxia injury by inhibiting Bax, Bcl-2 and caspase-3 expressions. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:962. [PubMed] [Google Scholar]
- 16.Mao GX, Wang Y, Qiu Q, Deng HB, Yuan LG, Li RG, Song DQ, Li YY, Li DD, Wang Z. Salidroside protects human fibroblast cells from premature senescence induced by H2O2 partly through modulating oxidative status. Mech Ageing Dev. 2010;131:723–31. doi: 10.1016/j.mad.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang J, Gao Z, Ren Z, Hong D, Qiao H, Chen Y. Synergistic effects of rMSCs and salidroside on the experimental hepatic fibrosis. Pharmazie. 2010;65:607–13. [PubMed] [Google Scholar]
- 18.Yuan-yuan W. Effect of integr ipetal rhodiola herb on the expression of MMP-2 and TIMP-1 in rats with pulmonary fibrosis. Journal of Chongqing Medical University. 2010;5:017. [Google Scholar]
- 19.Guan S, Xiong Y, Song B, Song Y, Wang D, Chu X, Chen N, Huo M, Deng X, Lu J. Protective effects of salidroside from Rhodiola rosea on LPS-induced acute lung injury in mice. Immunopharmacol Immunotoxicol. 2012;34:667–72. doi: 10.3109/08923973.2011.650175. [DOI] [PubMed] [Google Scholar]
- 20.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-beta is a critical mediator of acute lung injury. J Clin Invest. 2001;107:1537–44. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shull MM, Ormsby I, Kier AB, Pawlowskr S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvint D. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz V, Ordonez RM, Berumen J, Ramirez R, Uhal B, Becerril C, Pardo A, Selman M. Unbalanced collagenases/TIMP-1 expression and epithelial apoptosis in experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1026–36. doi: 10.1152/ajplung.00183.2003. [DOI] [PubMed] [Google Scholar]
- 23.Kaemmerer D, Peter L, Lupp A, Schulz S, Sanger J, Baum RP, Prasad V, Hommann M. Comparing of IRS and Her2 as immunohistochemical scoring schemes in gastroenteropancreatic neuroendocrine tumors. Int J Clin Exp Pathol. 2012;5:187–94. [PMC free article] [PubMed] [Google Scholar]
- 24.Kong CS, Balzer BL, Troxell ML, Patterson BK, Longacre TA. p16INK4A immunohistochemistry is superior to HPV in situ hybridization for the detection of high-risk HPV in atypical squamous metaplasia. Am J Surg Pathol. 2007;31:33–43. doi: 10.1097/01.pas.0000213347.65014.ee. [DOI] [PubMed] [Google Scholar]
- 25.Yildiz IZ, Usubutun A, Firat P, Ayhan A, Kucukali T. Efficiency of immunohistochemical p16 expression and HPV typing in cervical squamous intraepithelial lesion grading and review of the p16 literature. Pathol Res Pract. 2007;203:445–9. doi: 10.1016/j.prp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Dasta JF. Paraquat poisoning: a review. Am J Hosp Pharm. 1978;35:1368–72. [PubMed] [Google Scholar]
- 27.Gauldie J, Jordana M, Cox G. Cytokines and pulmonary fibrosis. Thorax. 1993;48:931–5. doi: 10.1136/thx.48.9.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rocco PR, Facchinetti LD, Ferreira HC, Negri EM, Capelozzi VL, Faffe DS, Zin WA. Time course of respiratory mechanics and pulmonary structural remodelling in acute lung injury. Respir Physiol Neurobiol. 2004;143:49–61. doi: 10.1016/j.resp.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005;23:1011–27. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Liu F, Peng Y, Yuan F, Liu Y. [Inhibition of TGFbeta1 expression in human peritoneal mesothelial cells by pcDU6 vector mediated TGFbeta1 shRNA] . Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2004;29:552–7. [PubMed] [Google Scholar]
- 31.Choe JY, Jung HJ, Park KY, Kum YS, Song GG, Hyun DS, Park SH, Kim SK. Anti-fibrotic effect of thalidomide through inhibiting TGF-beta-induced ERK1/2 pathways in bleomycin-induced lung fibrosis in mice. Inflamm Res. 2010;59:177–88. doi: 10.1007/s00011-009-0084-9. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Nie YC, Luo YL, Lin F, Zheng YF, Cheng GH, Wu H, Zhang KJ, Su WW, Shen JG, Li PB. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food Chem Toxicol. 2013;58:133–40. doi: 10.1016/j.fct.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Lo YC, Lin YL, Yu KL, Lai YH, Wu YC, Ann LM, Chen IJ. San-Huang-Xie-Xin-Tang attenuates inflammatory responses in lipopolysaccharide-exposed rat lungs. J Ethnopharmacol. 2005;101:68–74. doi: 10.1016/j.jep.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Novaes RD, Goncalves RV, Cupertino MC, Marques DC, Rosa DD, Peluzio Mdo C, Neves CA, Leite JP. Bark extract of Bathysa cuspidata attenuates extra-pulmonary acute lung injury induced by paraquat and reduces mortality in rats. Int J Exp Pathol. 2012;93:225–33. doi: 10.1111/j.1365-2613.2012.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


