Abstract
Cyclin kinase subunit 2 (CKS2) protein is a small cyclin-dependent kinase-interacting protein, which is essential for the first metaphase/anaphase transition of mammalian meiosis. CKS2 is up-regulated in various malignancies, suggesting that CKS2 maybe an oncogene. However, data on its expression pattern and clinical relevance in breast cancer are unknown. The aim of this study is to investigate CKS2 expression and its prognostic significance in breast cancer. The CKS2 expression was examined at mRNA and protein levels by real-time quantitative polymerase chain reaction (RT-PCR) and Western blotting analysis in paired breast cancer tissues and the adjacent normal tissues. The expression of CKS2 protein in 126 specimens of breast cancer was determined by immunohistochemistry assay. The relations between CKS2 expression and clinicopathological features were analyzed. The result show the expression of CKS2 mRNA and protein was higher in breast cancer than the adjacent normal tissues. Compared with adjacent normal breast tissues, Overexpression of CKS2 was detected in 56.3% (71/126) patients. Overexpression of CKS2 was significantly associated with large tumor size (P = 0.035), poor cellular differentiation (P = 0.016), lack expression of progesterone receptor (P = 0.006), and decreased overall survival (P = 0.001). In multivariate analysis, CKS2 expression was an independent prognostic factor for overall survival (Hazard ratio [HR] = 3.404, 95% confidence interval [CI] 1.482-7.818; P = 0.004). CKS2 is up-regulated in breast cancer and associated with large tumor size, lack expression of progesterone receptor, poor tumor differentiation and survival. CKS2 may serve as a good prognostic indicator for patients with breast cancer.
Keywords: Breast cancer, cyclin kinase subunit 2, prognosis
Introduction
Cyclin kinase subunit (CKS) proteins are small cyclin-dependent kinase-interacting proteins, which are essential components for cell cycle control [1]. There are two CKS proteins in mammals, CKS1 and CKS2. CKS1 expression has been shown to be up-regulated in various tumors, including breast, lung, colorectal, prostate and renal cancer [2-7]. Furthermore, overexpression of CKS1 is associated with tumor development and poor prognosis in breast cancer [2,3]. There is accumulating evidence that SKS2 is also up-regulated in a variety of cancers, including colorectal, prostate, bladder and liver cancer [8-12]. However, the expression of CKS2 and its significance in breast cancer is unclear.
In this study, we sought to investigate the expression of CKS2 and its prognostic significance in breast cancer. We found that CKS2 was significantly up-regulated in breast cancer tissues compared with adjacent non-tumor tissues both at mRNA and protein levels.CKS2 overexpression was connected with large tumor size, lack expression of progesterone receptor, poor tumor differentiation, and decreased overall survival. The results suggest that CKS2 may have a diagnostic and prognostic value for patients with breast cancer.
Materials and methods
Patients and specimens
The ethics committee at the Third Affiliated Hospital of Sun Yat-Sen University approved this study, and all the patients gave written informed consent on the use of clinical specimens for medical research. For real-time PCR and Western blotting analysis, paired primary breast cancer and adjacent normal tissues were collected. A total of 126 patients with primary breast cancer undergoing curative resection at our institution between March 2001 and December 2003 were included in this study. No patients received any type of neoadjuvant therapy, and all underwent curative surgery.
Postoperative specimens were examined by at least two pathologists specialized in breast cancer. Tumor size, retrieve and involved lymph nodes were ascertained based on final pathologic assessment. Tumors were staged according to the American Joint Committee on Cancer (seventh Edition) staging system. Human epidermal growth factor receptor-2 (HER2), estrogen receptor (ER), and progesterone receptor (PR) were also routinely reported in the final pathologic report. ER and PR status was assessed by immunohistochemistry (IHC) technique and at least 1% of nuclear staining was defined as positive. HER2 status was assessed by IHC or fluorescence in situ hybridization (FISH). The criteria of determining HER-2 positive were in accordance with a previous study [13].
Patients who underwent breast-conserving surgery or had 4 or more than 4 involved lymph nodes were suggested to receive adjuvant radiotherapy. Adjuvant chemotherapy therapy was delivered to patients with tumor size ≥ 2 cm or lymph node involvement. Patients with positive ER or/and PR received at least 5-year adjuvant endocrine therapy. Anti-HER2 therapy was administered to patients with positive HER-2 status.
After hospital discharge, patients were suggested to visit the doctors every six month within first five years and yearly thereafter. During each follow-up, patients received a series of evaluations, including physical examination, complete blood count, and liver function test. Breast, abdominal and pelvic ultrasound, and chest X-ray were conducted every 6 months after surgery. Mammography was performed per year after surgery.
RT-PCR analysis
Total RNA from human breast cancer tissues and their adjacent normal breast tissues was extracted using Trizol reagent (Invitrogen) according to the manufacturer’s instructions, cDNA was synthesized from 1 ug of total RNA using the First-Strand Synthesis System (Thermo), then Real-time PCR procedure was carried out using an CFX96 Real-Time System (BIO-RAD). The primer sequences were as follows: CKS2 sense 5’-CGCTCTCGTTTCATTTTCTGC-3’, antisense 5’-TGGAAAGTTCTCTGGGTAACATAACA-3’. GAPDH (sense 5’-TGTTGCCATCAATGACCCC-3’, antisense 5’-CTCCACGACGTACTCAGC-3’) was used as an internal control. All reactions were run in triplicate in three independent experiments.
Western blotting analysis
Total 8 paired tissue samples were collected and solubilized in SDS lysis buffer, and then the protein concentrations were detected by the BCA protein assay kit (PIERCE, Rockford, IL). Equal amounts of protein samples (30 μg) were separated by electrophoresis through resolving SDS-polyacrylamide gel, the protein was transferred to PVDF membranes (Amersham Pharmacia Biotech Inc in Piscataway, NJ). The membranes were incubated with a primary polyclonal antibody to CKS2 for 2 hr at room temperature. After washing 3 times with TBST, the membranes were incubated with secondary antibody (1:2000) for 40 min at room temperature. Then, the membranes were washed 3 times with TBST and proteins were detected by enhanced chemiluminescence system (Amersham Pharmacia Biotech). GAPDH was used as loading control (1:2000; Santa Cruz Biotechnology). Image J software version 1.38 (National Institutes of Health, Bethesda, Md) was used to analyze the amount of CKS2 expression.
Immunohistochemistry
Immunohistochemical (IHC) was performed according to the manufacturer’s protocol (Zymed®, Life Technologies, Carlsbad, CA, USA). The section was baked 1 h at 63°C and deparaffined with xylenes and rehydrated through graded ethanol series to distilled water. Then, the section was submerged in sodium citrate buffer and heated for antigenic retrieval. The section was blocked by the endogenous peroxidase with 0.3% H2O2 for 15 min at room temperature, and then incubated with normal goat serum for 30 min at room temperature to reduce the nonspecific binding. Next, the section was incubated with rabbit polyclonal anti-CKS2 antibody (1:100; Abcam) overnight at 4°C. After 3 washings in sterile phosphate-buffered saline, the section was incubated with a biotinylated anti-rabbit secondary antibody (Zymed) followed by further incubation with streptavidin-horseradish peroxidase (Zymed) at 37°C for 30 min. Diaminobenzidine (DAB) was used for color reaction, and the antibody was replaced by normal goat serum for negative controls.
Evaluation of immunostaining
Tissue section immunoreactivities were viewed and scored separately by two independent pathologists, who were blind to the histopathological features and patient information of the samples. Scores given by the two pathologists were averaged for further comparative evaluation of CKS2 expression. Immunoreactivities were scored by the intensity of staining (0, no staining; 1, weak = light yellow; 2, moderate = yellow brown; 3, strong = brown) and the percentage of stained cells (0, no staining; 1, 1-25%; 2, 26-50%; 3, 51-75%; 4, > 75%). By multiplication of both values, a final score ranging between 0 and 12 was obtained. The expression level of CKS2 was defined as follows: “-” (negative, score 0), “+” (weakly positive, score 1-4), “++” (positive, score 5-8), “+++” (strongly positive, score9-12). CKS2 overexpression was defined as final score more than zero.
Statistical analysis
Continuous variables are expressed as median and range, and were analyzed with the Student’s t-test, while categorical ones are expressed as numbers with percentages, and were analyzed by chi-square test or Fisher’s exact test when appropriate. Overall survival (OS) was defined from the date of operation to the date of death. Kaplan-Meier method was used to analyze overall survival (OS) of patients, and comparisons were analyzed by log-rank test. Cox’s proportional hazards model was used for multivariate analysis, adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated. All the statistical analyses were evaluated using the Statistical Package for the Social Science (SPSS) 18.0 for Windows (SPSS Inc. Chicago, IL, USA). All statistical tests were two-sided and a P value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
The demographic features and clinicopathologic data were detailed in Table 1. A total of 126 patients with primary invasive ductal carcinoma were included in this study. All patients were female and the median age was 50 years (range 29-83 years). Pathologic tumor staging was stage I in 10 (7.9%) patients, stage II in 76 (60.3%) patients, stage III in 40 (31.7%) patients. 81 (64.3%) patients were found to be ER positive, and PR was positive in 72 (57.1%) patients. 36 (28.6%) patients had tumors with positive HER-2.
Table 1.
Correlation of CKS2 expression with clinicopathologic features
| Characteristics | Total (n = 126) | CKS2 | P value | |
|---|---|---|---|---|
|
| ||||
| Negative (n = 55) | Positive (n = 71) | |||
| Age (years) | 0.214 | |||
| ≥ 60 | 37 (29.4%) | 13 (23.6%) | 24 (33.8%) | |
| < 60 | 89 (70.6%) | 42 (76.4%) | 47 (66.2%) | |
| T stage | 0.035 | |||
| 1 | 26 (20.6%) | 17 (30.9%) | 9 (12.7%) | |
| 2 | 87 (69.0%) | 32 (58.2%) | 55 (77.5%) | |
| 3 | 13 (10.3%) | 6 (10.9%) | 7 (9.9%) | |
| N stage | 0.334 | |||
| 0 | 49 (38.9%) | 24 (43.6%) | 25 (35.2%) | |
| 1 | 39 (31.0%) | 19 (34.5%) | 20 (28.2%) | |
| 2 | 30 (23.8%) | 10 (18.2%) | 20 (28.2%) | |
| 3 | 8 (6.3%) | 2 (3.6%) | 6 (8.5%) | |
| TNM stage | 0.156 | |||
| I | 10 (7.9%) | 6 (10.9%) | 4 (5.6%) | |
| II | 76 (60.3) | 36 (65.5%) | 40 (56.3%) | |
| III | 40 (31.7) | 13 (23.6%) | 27 (38.0%) | |
| Tumor differentiation | 0.016 | |||
| Well | 13 (10.3%) | 10 (18.2%) | 3 (4.2%) | |
| Moderate | 94 (74.6%) | 40 (72.7%) | 54 (76.1%) | |
| Poor | 19 (15.1%) | 5 (9.1%) | 14 (19.7%) | |
| ER | 0.322 | |||
| Positive | 81 (64.3%) | 38 (69.1%) | 43 (60.6%) | |
| Negative | 45 (35.7%) | 17 (30.9%) | 28 (39.4%) | |
| PR | 0.006 | |||
| Positive | 72 (57.1%) | 39 (70.9%) | 33 (46.5%) | |
| Negative | 54 (42.9%) | 16 (29.1%) | 38 (53.5%) | |
| HER2 | 0.140 | |||
| Positive | 36 (28.6%) | 12 (21.8%) | 24 (33.8%) | |
| Negative | 90 (71.4%) | 43 (78.2%) | 47 (66.2%) | |
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor-2.
CKS2 is up-regulated in human breast cancer
To determine whether the CKS2 expression levels were differentially between breast cancer and normal breast samples, we first queried the Oncomine database, in the meta-analysis, CKS2 expression significantly higher than the corresponding normal tissues in breast cancer with a median rank of 143 and a P-value of 7.06E-6 (Figure 1). To confirm this result, we obtained paired breast cancer tissues and adjacent normal breast tissues for real-time RT-PCR and Western blot analysis. The result as shown in Figure 2, the expression level of CKS2 mRNA is significantly higher in 10 breast cancer tissues compared to the adjacent non-tumor tissues with a P-value of 0.008. Western blotting analysis revealed that CKS2 protein was differentially increased in 8 breast cancer tissues compared to the adjacent non-tumor tissues, indicating that CKS2 is up-regulated in breast cancer (Figure 3). For immunostaining result, overexpression of CKS2 was observed in 56.3% (71/126) patients. CKS2 protein staining was weak in the adjacent non-tumor tissues.CKS2 was predominantly present in the cytoplasm of tumor cells (Figure 4). These data showed that CKS2 was significantly increased in breast cancer tissues.
Figure 1.

CKS2 expression is upregulated in human breast carcinoma in Oncomine datebase. Oncomine heat map of CKS2 gene expression in clinical breast carcinoma samples compared with the normal breast tissues.
Figure 2.
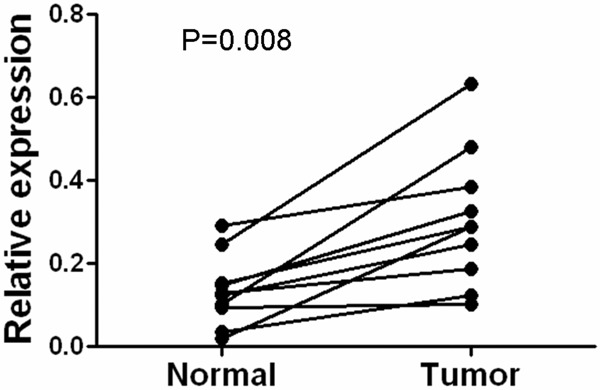
Expression levels of CKS2 mRNA in 10 paired breast cancer tissues. Expression levels of CKS2 mRNA in 10 paired breast cancer tissues by real-time PCR. Normal, para carcinoma (normal) breast tissues. Tumor, breast cancer tissues.
Figure 3.
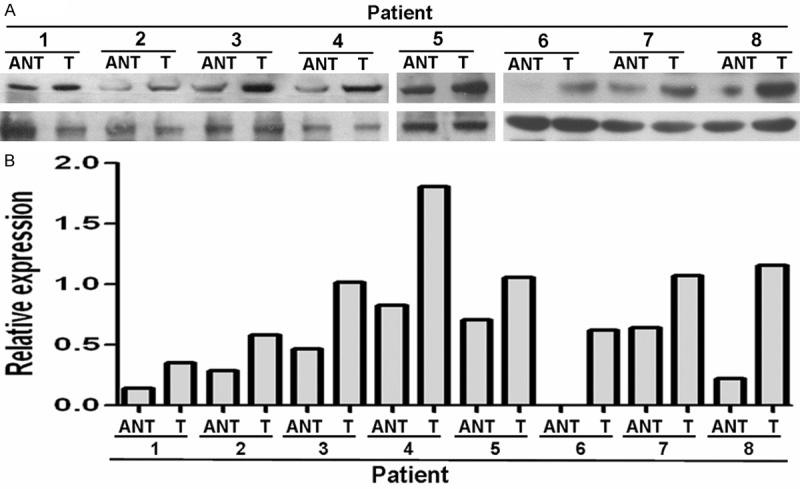
Expression levels of CKS2 protein in 8 paired breast cancer tissues. Expression levels (A) and quantitative analysis (B) of CKS2 protein in 8 paired breast cancer tissues by Western blotting. T, breast cancer tissues, ANT, matched adjacent non-tumor breast tissues.
Figure 4.
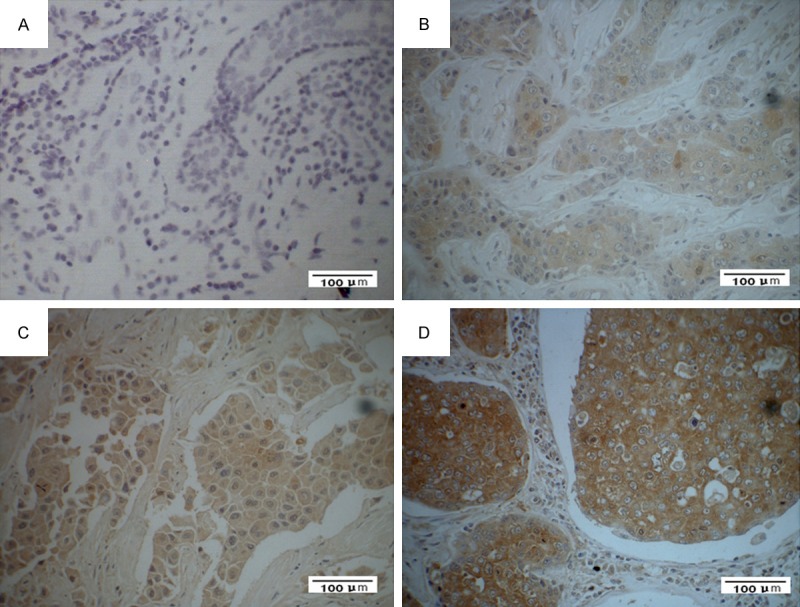
Expression analysis of CKS2 protein by immunohistochemistry. CKS2 expression was mainly localized in the cytoplasm of tumor cells. A. Negative CKS2 staining in cancer cells (×400). B. Weak CKS2 staining in cancer cells (×400). C. Median CKS2 staining in cancer cells (×400). D. Strong CKS2 staining in cancer cells (×400).
CKS2 expression correlates with clinicopathological features of breast cancer
For better understanding of the potential roles of CKS2 in breast cancer development and progression, patients were grouped according to CKS2 expression (Table 1). CKS2 expression was significantly associated with large tumor size (P = 0.035), poor cellular differentiation (P = 0.016), and lack expression of PR (P = 0.006). However, there was no correlation of CKS2 expression with other clinicopathologic features, such as age, ER and HER2 status (Table 1).
Expression of CKS2 in breast cancer patients correlates with worse overall survival
After a median follow-up of 111 months (range 2-131 months), 35 (27.8%) patients died. The estimated 5-year overall survival (OS) was 81.7% (95% CI 75.0-88.4). In univariate analysis, tumor stage, ER status, PR status, and CKS2 expression was significantly associated with OS (Table 2). Patients with negative CKS2 expression had a 5-year OS rate of 90.9% (95% CI 83.3-98.5), whereas those with positive CKS2 expression had a 5-year OS rate of 74.6% (95% CI 64.4-84.8; P = 0.001; Figure 5). In multivariate analysis (Table 3), factors associated with OS were CKS2 overexpression (HR =3.404, 95% CI 1.482-7.818; P = 0.004), tumor stage (HR = 1.843; 95% CI 1.010-3.365; P = 0.046), and ER expression (HR = 0.420; 95% CI 0.215-0.818; P = 0.011). These results indicated that CKS2 expression maybe an independent prognostic marker for OS in breast cancer patients.
Table 2.
Univariate analysis of the effect of covariates on overall survival
| Characteristics | Overall survival | |
|---|---|---|
|
| ||
| 5-year (%) | P value | |
| Age (years) | 0.517 | |
| ≥ 60 | 81.1 | |
| < 60 | 82.0 | |
| T stage | 0.281 | |
| 1 | 88.5 | |
| 2 | 82.8 | |
| 3 | 61.5 | |
| N stage | 0.103 | |
| 0 | 87.8 | |
| 1 | 84.6 | |
| 2 | 70.0 | |
| 3 | 75.0 | |
| TNM stage | 0.034 | |
| I | 100.0 | |
| II | 85.5 | |
| III | 70.0 | |
| Tumor differentiation | 0.144 | |
| Well | 92.3 | |
| Moderate | 80.9 | |
| Poor | 78.9 | |
| ER | 0.004 | |
| Positive | 88.9 | |
| Negative | 68.9 | |
| PR | 0.001 | |
| Positive | 87.5 | |
| Negative | 74.1 | |
| HER2 | 0.987 | |
| Positive | 83.3 | |
| Negative | 81.1 | |
| CKS2 | 0.001 | |
| Positive | 90.9 | |
| Negative | 74.6 | |
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor-2; CKS2, cyclin kinase subunit 2.
Figure 5.
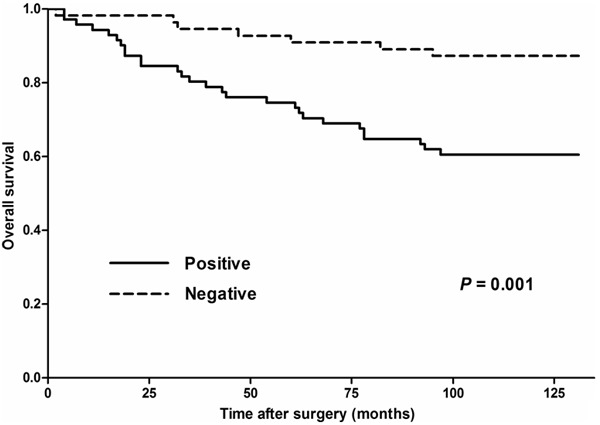
Overall survival according to CKS2 expression.
Table 3.
Multivariate analysis of the effect of covariates on overall survival
| Characteristics | Overall survival | ||
|---|---|---|---|
|
| |||
| HR | 95% CI | P value | |
| CKS2 expression | 3.404 | 1.482-7.818 | 0.004 |
| Tumor stage | 1.843 | 1.010-3.365 | 0.046 |
| ER expression | 0.420 | 0.215-0.818 | 0.011 |
CKS2, cyclin kinase subunit 2; ER, estrogen receptor.
Discussion
Breast cancer is the most common malignancy diagnosed in women worldwide and the second leading cause of cancer-related death among women [14]. It has been clearly demonstrated that breast cancer is a heterogeneous disease including various pathological entities that are showed to have distinct clinical features and outcomes. Patients with similar clinical characters may vary in their response to therapy and show distinct prognoses. Approximately one-third of patients with early stage breast cancer will eventually develop recurrence, while about one-third of patients with lymph node involvement remain free of metastases [15,16]. The development of gene expression profiling has led to a novel way of classifying tumor, breast cancer can be divided at least into 5 types with distinct clinical features: luminal type A and B, HER2/neu type, normal breast-like, and basal-like types [17,18]. Although gene expression profiling classification shows strong overall association with patients’ features and outcomes, it has its own limitation and has not been widely used in clinical practice [18,19]. Currently, tumor staging system and molecular biomarkers instead of genotyping are routinely used, including ER, PR, and HER-2 [20]. However, it has been estimated that all these traditional clinicopathological and molecular factors divide breast cancer patients into different risk groups at an approximate absolute specificity level of only 10% to achieve an acceptable degree of sensitivity [21]. Therefore, new biomarkers are in great need to improve patients risk stratification and guide treatment.
The CKS family, including CKS1 and CKS2, are essential components of cyclin/cyclin-dependent kinase (CDK) complexes contributing to the cell cycle control. The structures of CKS proteins are highly conserved and have 81% identical amino acid sequence [22]. CKS has been shown to play a direct role in cell-cycle regulation [23]. Complete silencing of CKS leads to cell-cycle arrest in G2 in somatic cells [24]. A variety of tumors have showed the differential dysregulation of CKS. CKS1 has been revealed to be up-regulated in various tumors, including breast, lung, colorectal, prostate and renal cancer [2-7]. Wang et al. [3] found that overexpression of CKS1 was strongly associated with lymph node metastasis and poor prognosis in patients with breast cancer. Similarly, Slotky et al. [2] reported that overexpression of CKS1 in breast cancer was associated with younger age, loss of tumor differentiation, lack of expression of ER and PR, and decreased survival. There is also accumulating evidence that CKS2 is also elevated in a variety of cancers, including colorectal, prostate, bladder and liver cancer. However, the expression of CKS2 and its significance in breast cancer is unclear.
Based on previous studies, we investigated the expression of CKS2 in breast cancer and its prognostic values in predicting survival following curative resection in the present study. Our results clearly showed that CKS2 was up-regulated in breast cancer tissues compared with matched non-tumor breast tissues. In accordance with our results, Chen et al. [12] demonstrated that CKS2 gene expression significantly increased after superficial bladder cancer progressing to muscle-invasive cancer. The difference in CKS2 expression among different tissues may indicate that CKS2 is a key regulator in tumorigenesis.
CKS2 over-expression in breast cancer was associated with large tumor size, lack expression of progesterone receptor, poor tumor differentiation and survival. Similar to our results, Kang et al. [10] found CKS2 was significantly up-regulated in gastric cancers and overexpression of CKS2 was correlated with tumor differentiation and tumor size, lymph node, and metastasis. Wang et al. [25] also showed a high level of CKS2 in esophageal carcinoma was closely associated with poor pathological features, including higher histological grade of tumor, regional lymph nodes invasion, and neoplastic embolus. In present study, we found that CKS2 over-expression was associated with worse outcome, and it was an independent prognosis factor for breast cancer patients after curative resection (HR = 3.404, 95% CI 1.482-7.818; P = 0.004). These results indicate that CKS2 could be a new biomarker for breast cancer and a potential therapeutic target. However, the molecular mechanism connecting worse outcome and CKS2 overexpression is unclear and remains to be elucidated.
In conclusion, CKS2 is up-regulated in breast cancer and correlates with tumor size, cellular differentiation, and PR expression. Most importantly, CKS2 overexpression is associated with worse outcome in breast cancer patients, and CKS2 may serve as a potential prognostic biomarker for breast cancer. To the best of our knowledge, this is the first report to indentify CKS2 as an independent prognostic factor for breast cancer patients. Further studies are warranted to reveal the molecular mechanism of CKS2 in the development and progression of breast cancer.
Acknowledgements
This study was supported by Key Clinical Disciplines of Guangdong Province (20111219) and the PhD Start-up Fund of Natural Science Foundation of Guangzhou Medical University (2013C28).
Disclosure of conflict of interest
None.
References
- 1.Pines J. Cell cycle: reaching for a role for the Cks proteins. Curr Biol. 1996;6:1399–402. doi: 10.1016/s0960-9822(96)00741-5. [DOI] [PubMed] [Google Scholar]
- 2.Slotky M, Shapira M, Ben-Izhak O, Linn S, Futerman B, Tsalic M, Hershko DD. The expression of the ubiquitin ligase subunit Cks1 in human breast cancer. Breast Cancer Res. 2005;7:R737–44. doi: 10.1186/bcr1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang XC, Tian J, Tian LL, Wu HL, Meng AM, Ma TH, Xiao J, Xiao XL, Li CH. Role of Cks1 amplification and overexpression in breast cancer. Biochem Biophys Res Commun. 2009;379:1107–13. doi: 10.1016/j.bbrc.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 4.Shapira M, Ben-Izhak O, Linn S, Futerman B, Minkov I, Hershko DD. The prognostic impact of the ubiquitin ligase subunits Skp2 and Cks1 in colorectal carcinoma. Cancer. 2005;103:1336–46. doi: 10.1002/cncr.20917. [DOI] [PubMed] [Google Scholar]
- 5.Lan Y, Zhang Y, Wang J, Lin C, Ittmann MM, Wang F. Aberrant expression of Cks1 and Cks2 contributes to prostate tumorigenesis by promoting proliferation and inhibiting programmed cell death. Int J Cancer. 2008;123:543–51. doi: 10.1002/ijc.23548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z, Fu Q, Lv J, Wang F, Ding K. Prognostic implication of p27Kip1, Skp2 and Cks1 expression in renal cell carcinoma: a tissue microarray study. J Exp Clin Cancer Res. 2008;27:51. doi: 10.1186/1756-9966-27-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inui N, Kitagawa K, Miwa S, Hattori T, Chida K, Nakamura H, Kitagawa M. High expression of Cks1 in human non-small cell lung carcinomas. Biochem Biophys Res Commun. 2003;303:978–84. doi: 10.1016/s0006-291x(03)00469-8. [DOI] [PubMed] [Google Scholar]
- 8.Yu M, Zhong M, Qiao Z. Expression and clinical significance of cyclin kinase subunit 2 in colorectal cancer. Oncol Lett. 2013;6:777–80. doi: 10.3892/ol.2013.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawakami K, Enokida H, Tachiwada T, Gotanda T, Tsuneyoshi K, Kubo H, Nishiyama K, Takiguchi M, Nakagawa M, Seki N. Identification of differentially expressed genes in human bladder cancer through genome-wide gene expression profiling. Oncol Rep. 2006;16:521–31. [PubMed] [Google Scholar]
- 10.Kang MA, Kim JT, Kim JH, Kim SY, Kim YH, Yeom YI, Lee Y, Lee HG. Upregulation of the cycline kinase subunit CKS2 increases cell proliferation rate in gastric cancer. J Cancer Res Clin Oncol. 2009;135:761–9. doi: 10.1007/s00432-008-0510-3. [DOI] [PubMed] [Google Scholar]
- 11.Shen DY, Fang ZX, You P, Liu PG, Wang F, Huang CL, Yao XB, Chen ZX, Zhang ZY. Clinical significance and expression of cyclin kinase subunits 1 and 2 in hepatocellular carcinoma. Liver Int. 2010;30:119–25. doi: 10.1111/j.1478-3231.2009.02106.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen R, Feng C, Xu Y. Cyclin-dependent kinase-associated protein Cks2 is associated with bladder cancer progression. J Int Med Res. 2011;39:533–40. doi: 10.1177/147323001103900222. [DOI] [PubMed] [Google Scholar]
- 13.Hicks DG, Kulkarni S. HER2+ breast cancer: review of biologic relevance and optimal use of diagnostic tools. Am J Clin Pathol. 2008;129:263–73. doi: 10.1309/99AE032R9FM8WND1. [DOI] [PubMed] [Google Scholar]
- 14.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2013;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 15.Clahsen PC, van de Velde CJ, Goldhirsch A, Rossbach J, Sertoli MR, Bijnens L, Sylvester RJ. Overview of randomized perioperative polychemotherapy trials in women with early-stage breast cancer. J. Clin. Oncol. 1997;15:2526–35. doi: 10.1200/JCO.1997.15.7.2526. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Sun B, Li X, Zhang L, Niu Y, Xiao C Ning L, Fang Z, Wang Y, Zhang L, Cheng J, Zhang W, Hao X. Differentially expressed genes between primary cancer and paired lymph node metastases predict clinical outcome of node-positive breast cancer patients. Breast Cancer Res Treat. 2007;103:319–29. doi: 10.1007/s10549-006-9385-7. [DOI] [PubMed] [Google Scholar]
- 17.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 18.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 19.Pusztai L, Mazouni C, Anderson K, Wu Y, Symmans WF. Molecular classification of breast cancer: limitations and potential. Oncologist. 2006;11:868–77. doi: 10.1634/theoncologist.11-8-868. [DOI] [PubMed] [Google Scholar]
- 20.Rakha EA, Reis-Filho JS, Ellis IO. Combinatorial biomarker expression in breast cancer. Breast Cancer Res Treat. 2010;120:293–308. doi: 10.1007/s10549-010-0746-x. [DOI] [PubMed] [Google Scholar]
- 21.Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. Early Breast Cancer Trialists’ Collaborative Group. N Engl J Med. 1988;319:1681–92. doi: 10.1056/NEJM198812293192601. [DOI] [PubMed] [Google Scholar]
- 22.Richardson HE, Stueland CS, Thomas J, Russell P, Reed SI. Human cDNAs encoding homologs of the small p34Cdc28/Cdc2-associated protein of Saccharomyces cerevisiae and Schizosaccharomyces pombe. Genes Dev. 1990;4:1332–44. doi: 10.1101/gad.4.8.1332. [DOI] [PubMed] [Google Scholar]
- 23.Frontini M, Kukalev A, Leo E, Ng YM, Cervantes M, Cheng CW, Holic R, Dormann D, Tse E, Pommier Y, Yu V. The CDK subunit CKS2 counteracts CKS1 to control cyclin A/CDK2 activity in maintaining replicative fidelity and neurodevelopment. Dev Cell. 2012;23:356–70. doi: 10.1016/j.devcel.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinsson-Ahlzen HS, Liberal V, Grunenfelder B, Chaves SR, Spruck CH, Reed SI. Cyclin-dependent kinase-associated proteins Cks1 and Cks2 are essential during early embryogenesis and for cell cycle progression in somatic cells. Mol Cell Biol. 2008;28:5698–709. doi: 10.1128/MCB.01833-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JJ, Fang ZX, Ye HM, You P, Cai MJ, Duan HB, Wang F, Zhang ZY. Clinical significance of overexpressed cyclin-dependent kinase subunits 1 and 2 in esophageal carcinoma. Dis Esophagus. 2013;26:729–36. doi: 10.1111/dote.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]


