Abstract
This study will provide guide for the terminal ileitis in clinical diagnosis and treatment. The animals were been done terminal ileum-cecum side to side anastomosis, terminal ileum operation line and only anesthesia treatment, respectively. The model group presented acute inflammation after surgery for 2 weeks and the inflammation was limited to the mucosal layer. Animals presented chronic inflammation to 8 weeks, mucosal membrane was given priority to with lymphocytic infiltrates. In 2 weeks and 4 weeks, the number of Peyer’s patches (PP knot) and PP knot lymphocytes increased significantly in the model group (P < 0.05, P < 0.01). At 8 weeks, the suture group and the model group presented a large number of lymphocytic apoptosis (P < 0.01). Rat ileal PP knot lymphocyte small molecule DNA showed typical “trapezoid” bands. We observed apparent morphology of apoptosis and crescent-shaped nucleus. Continuous immune response in terminal ileitis plays a considerable role in the process of the disease.
Keywords: Cell apoptosis, lymphocytes, PP knot, terminal ileitis
Introduction
After more than 60 years, the etiology of pig proliferative ileitis has been clear joint efforts of many scientists [1]. Both acute and chronic pig ileitis is infectious intestinal disease caused by intracellular Lawson bacteria infection. The etiology and pathogenesis of human acute and chronic terminal ileitis have researched many years. Although there are a lot of progresses, in general, its etiology and pathogenesis is more complex. At present most of scholars believe that chronic terminal ileitis has relationship with infection, immune factors, colon and ileal reverse flow, allergic factors, drug factors and so on [2-4]. These researchers thought infection was initiator and immune response was the key factor. The allergic factors in the final analysis were the body’s immune response and drug factors may also be related to immune response.
In recent years, the pathogenic role of the colon and ileal reverse flow has particular concern. Whatever the reason resulting in ileum-cecum function imbalance, all can cause colon and ileal reverse flow. Then this reverse flow caused the transplantation or translocation of bacteria, the formation of endogenous infection. A series of changes mentioned above stimulated terminal ileum mucosal immune reaction. This mucosa has abundant lymphoid tissue. After mucosal immune reaction local mucosa was damaged and this harm caused terminal ileitis [5]. So that the main mechanism of chronic terminal ileitis happening may be: colon intestinal dysmotility disorders, ileocecal dysfunction, colon-ileum reflux, bacterial translocation, bacterial infection, the end of ileum mucosal inflammatory lesions. They had effect on next factor one by one.
Peyer’s knot (Peyer’s patches, PP) is small intestinal tissue of mucosa follicular. PP knot found by Swiss physician Johann Peyer and exactly was defined it as part of the lymphatic system. They located mainly in the distal small intestine mucosa lamina propria, especially in terminal ileum. PP knot is a kind of white elliptic uplift structure, slightly protruding in the small mesenteric. PP knot is aggregated by lymphoid follicles. Each PP knot contains about 5~900 lymph follicles and this number will change with age. PP knot of the experimental animals was found in the middle of pregnancy. Proliferation of lymphoid tissue happened in the embryo stage and lymphoid cell density reached to peak at birth [6]. After the birth, size and the number of PP knot increased expeditiously which showed the mucosal immune system response to antigen in the environment. This immune response occurred especially after microbial invasion happening. PP knot of germ-free animals is generally small, but it will increase contacted with microbes. If adult intestinal immune barrier is damage, bacterial translocation and intestinal infection can cause PP knot and immune cells (T lymphocytes, B lymphocytes and macrophages) apoptosis [7,8]. We could understand terminal ileum immune response through studying PP knot and PP knot lymphocytes.
In this study, we made SD rat model for terminal ileitis to discuss the mechanism of it. We observed ileal histopathology. At the same time, we researched the number, apoptosis and proliferation of PP knot and lymphocytes in these animals. We wanted to know the changes of PP knot and lymphocytes in terminal ileitis model animals.
Materials and methods
Rats
Selected male SD rats were provided by the Ministry of Hua University and kept in a controlled environment with free access to normal food and sufficient water. These 90 rats weighed 250~300 g. The experimental animals were randomly divided into three groups: control, the suture group and modeling group, 30 rats in each group. All experiments were approved by the Animal Experimental Committee of The Chinese people’s liberation army 169 hospital.
Animal models
Preoperative fasting for 12 hours, rats were intraperitoneal anesthesia with 3% sodium pentobarbital 0.3-0.4 ml. In sterile conditions we did abdominal surgery to look for intestinal ileocecal. We separated distant ileum and colon from the peritoneal cavity. At ileocecal valve distant from ileum 3 cm and the end of colon we did a 1cm incision respectively. We performed the terminal ileum and cecum side to side anastomosis (dysfunction of the ileocecal valve to cause colon-ileum reflux). And then we had a postoperative abdominal closure, stitched incision and povidone-iodine disinfection. Animals had fasting 1d after surgery and resumed amount of about 1/3 normal diet 3 d after surgery. They could have 3/4 normal diet 7 d after surgery. Suture group: We preformed operation like model group before surgery. At the end of ileum distant from intestinal ileocecal 3 cm we did a 1 cm incision and surgical sutured. Then animals had the same diet as model group.
Specimen collection
An ileal pathology specimen collection and handling: The animals were sacrificed immediately after removal of cervical cesarean section exposed to the whole abdomen. Under lower temperatures we took the terminal ileum tissue (0.3 × 1.5 cm) away quickly. Tissues were rinsed with ice-cold saline to remove blood and feces. Then these tissues were fixed by 10% formalin and performed paraffin-embedded.
PP knot and lymphocytes specimen collection and handling: After taking the terminal ileum tissue finishing, we cut PP knot with curved scissors from the ileocecal valve up to the end of ileum for 20 cm. PP knot were rinsed with ice-cold saline to remove blood and feces. Then we set these PP knot to a pre-cooled RPMI1640 medium (Gibco) containing 10% fetal bovine serum (Sijiqing, Hangzhou).
Histology
We made the end of ileum for paraffin wax blocks. The sections (5 μm) were stained with hematoxylin and eosin (HE) [9]. We observed inflammatory cell infiltration, changes in blood vessels, lymphatic vessels, thoracic duct and intestinal villus.
Counting PP knot lymphocytes
First counting the number of PP knot from the ileocecal valve up to the end of ileum for 20 cm. PP knot cultured in RPMI1640 medium then we made mechanical compression on a 100 mesh stainless steel sieve. The sieve was rinsed with RPMI1640 medium. The filtrate filtrated with 400 mesh stainless steel sieve. Then the filtrate was washed once by centrifugation, 500 rpm for 10 min. We got PP knot single cell suspension. SD rat lymphocyte separation medium (The Chinese academy of medical sciences institute of biological engineering) was added to the cell suspension, 2000 rpm centrifuged for 20 min. Cells were carefully draw from the interface and then washed by culture medium. Lymphocytes were resuspended in an appropriate amount of the culture medium. Adjusting the concentration of the cell of 1 × 106/ml, we calculated each SD rat PP knot total lymphocyte count.
Detection PP knot lymphocyte apoptosis
According to the introduction of kit box, we made SD rats PP knot lymphocyte suspension first. Then we used fluorescein isothiocyanate (FITC) combined anexin-V and propidium iodide (PI) apoptosis detection kit box for cell staining. We obtained the total PP knot lymphocyte apoptosis by Flow cytometry detection.
PP knot lymphocyte DNA agarose gel electrophoresis
For typical apoptotic, 200 bp nucleotide segment size and a large fold is formed in the core body. Rather than the typical apoptotic cells, the degradation of DNA in the nucleus is not entirely and form only a large 300-50 kb of DNA. With this feature, we can determine the occurrence and development of apoptosis by DNA gel electrophoresis.
According to manual operation, we used apoptotic DNA extraction kit to extract apoptotic molecule DNA. 15 g/L agarose gel electrophoresis, SYBR Green-1 staining, the gel was observed by the visible light camera and stored the results pictures.
PP knot lymphocytes Hochst33258/PI nuclear staining
PP node cells were collected and adjusted the cell concentration about 1 × 106/ml. Lymphocytes were suspended in l mL RPMI 1640 medium (containing 10% Fetal bovine serum, FBS) then the suspension was added 10 μL Hoehst33258 (0.1 μg/ml). The mixture was incubated 37°C for 5-15 min. Then the stained cells were placed on ice and centrifuged at 4°C after cooling. Cells were resuspended by l mL PBS. After that we added 5 μL PI (l mg/ml) stock solution mix into cell suspension and 4°C dark stained for 15 min. We dropped cell suspension on a glass slide and observed under a fluorescence microscope photograph.
Statistical analysis
Experimental results data expressed as mean ± standard deviation (± s) and the groups were compared using ANOVA (one-wayANOVA). If the difference was statistically significant, homogeneity of variance was conducted by LSD test; if not, did Dunnet’s test. SPSS17.0 was used for statistical analysis and P < 0.05 was considered statistically significant.
Results
Ileal histopathology
In control group, all tissues were normal (Figure 1A-C). In suture group, we observed capillaries, lymphatic vessels and thoracic duct dilatation after two weeks of surgery. In the mean time, tissue showed inflammatory cell infiltration, intestinal villus became thicker and shorter (Figure 1D). From Figure 1E we observed inflammation reduced significantly, epithelium and intestinal villus had integrity, inflammation cells reduced. In suture group 8 weeks after surgry, tissue had epithelial integrity and proliferation of fibroblasts, inflammatory cells reduced (Figure 1F). Model group presented acute inflammation after two weeks of the surgery performance. The inflammation was limited to the mucosa and local tissue showed mucosal neutrophil infiltration (Figure 1G). Then the inflammation gradually deepened after surgery for four weeks. Inflammatory cells infiltration became lymphocytes gradually. And congestion bleeding acute inflammation was gradually moderated, while lesions invaded the submucosa accompanied by a small amount of fibroblasts hyperplasia (Figure 1H). To eight weeks, tissue presented a typical chronic inflammation and showed mucosal lymphocyte infiltration (Figure 1I).
Figure 1.
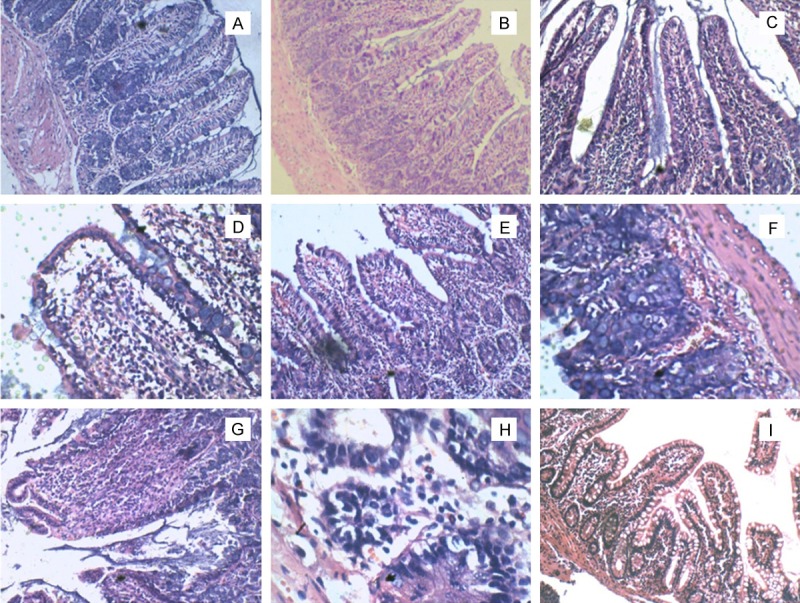
The HE staining of rats terminal ileum (H × 100, others × 40). A-C. Control group at 2 weeks, 4 weeks and 8 weeks. In these pictures we observed mucosal epithelium and intestinal villus had integrity, meantime had less inflammatory cells. D. Suture group 2 weeks after surgry: the capillaries, lymphatics and lacteal had expansion, inflammatory cells infiltration, dilatation of the villi. E. Suture group 4 weeks after surgry: inflammation reduced significantly, mucosal epithelium and intestinal villus had integrity, inflammation cells reduced. F. Suture group 8 weeks after surgry: epithelial integrity, inflammatory cells reduced, proliferation of fibroblasts. G. Model group 2 weeks after surgry: the capillaries, lymphatics and lacteal had expansion, inflammatory cells infiltration, dilatation of the villi. H. Model group 4 weeks after surgry: capillary slightly atrophy compared with earlier, mucosal epithelial cell falls off, inflammatory cells infiltration. I. Model group 4 weeks after surgry: submucosal structure damaged, inflammatory cells invasion submucosa, capillary hyperplasia, erosion formation.
Changes of SD rat PP knot number and lymphocytes
Compared with the control group, the suture group at 2 weeks and 4 weeks, PP knot had a small increasing in the number of nodes (P 2 week > 0.05, P 4 week < 0.05). The number of PP knot was similar with the control at 8 weeks (P 8 week > 0.05) (Table 1). In model group, the number of PP knot was increased significantly at first 2 weeks and 4 weeks (P 2 week < 0.05, P 4 week < 0.01); at 8 weeks, PP knot had a small increase (P 8 week > 0.05) (Table 1). The changes of the lymphocytes total number in PP knot were similar with PP knot (Table 1).
Table 1.
Changes of SD rat PP knot number and lymphocytes in three groups (n=10, x̅±s)
| Group | Rat No. | PP knot number | PP knot lymphocytes (× 106) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 2 W | 4 W | 8 W | 2 W | 4 W | 8 W | ||
| Control | 10 | 4.8±1.1 | 5.2±1.3 | 5.0±0.6 | 2.54±0.51 | 2.69±0.76 | 2.63±0.64 |
| Suture roup | 10 | 5.5±0.9 | 7.0±1.2 | 5.1±1.5 | 3.17±0.35 | 3.92±1.07 | 2.66±0.79 |
| Model group | 10 | 7.1±1.4 | 9.4±2.6 | 6.3±0.7 | 4.01±1.12 | 6.45±1.36 | 3.11±0.93 |
Rate of SD rat PP knot lymphocyte apoptosis and proliferation
Compared with the control, there was a small number of PP knot lymphocyte apoptosis in the suture group and model group at first 2 weeks and 4 weeks. And these two groups had no significant difference (P 2, 4 weeks > 0.05). At 8 weeks, the suture group and the model group had a large number of apoptotic lymphocytes (P 8 week < 0.01) (Table 2 and Figure 2). At the mean time, between the suture group and the model group had a significant difference (P < 0.01) (Table 2). Proliferation of lymphocytes in PP knot had a small increase at 4 weeks compared with the control (P 4 week < 0.05). There were on significant changes at 2 weeks and 8 weeks in these two groups (Table 1 and Figure 3).
Table 2.
Rate of SD rat PP knot lymphocyte apoptosis and proliferation in three groups (n=10, X̅±s)
| Group | Rat No. | Rate of lymphocyte apoptosis (%) | Rate of lymphocyte proliferation (%) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| 2W | 4W | 8W | 2W | 4W | 8W | ||
| Control | 10 | 1.85±0.21 | 1.91±0.28 | 1.79±0.23 | 25.1±3.3 | 23.9±4.6 | 22.3±2.7 |
| Suture group | 10 | 2.07±0.34 | 2.32±0.45 | 34.8±5.45 | 28.7±6.1 | 41.6±7.3 | 24.8±5.9 |
| Model group | 10 | 2.26±0.29 | 2.47±0.52 | 81.1±9.68 | 32.8±7.0 | 55.0±8.7 | 27.4±4.8 |
Figure 2.
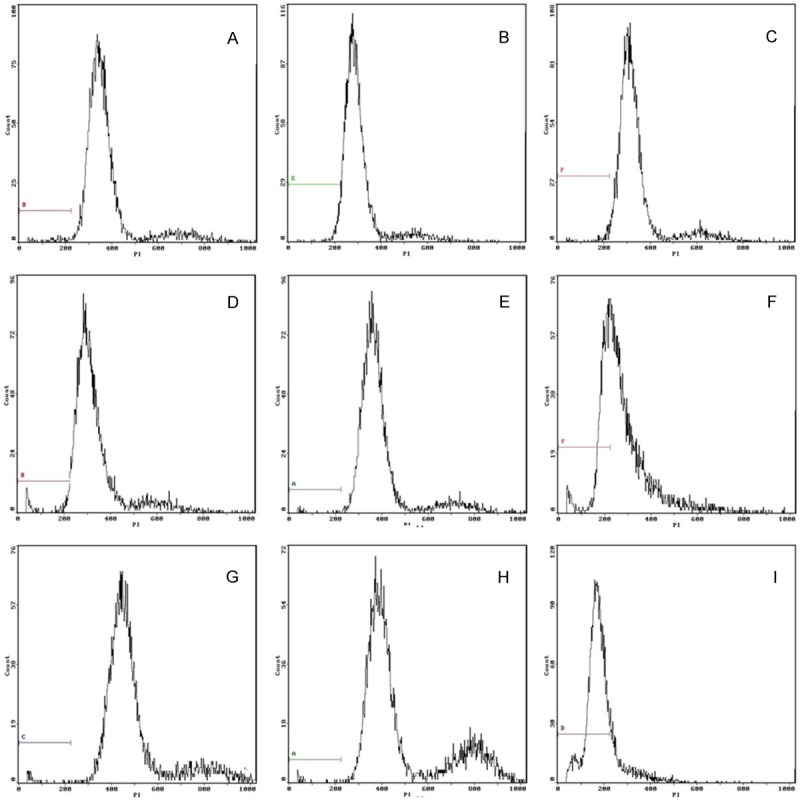
Flow cytometry to detect cell apoptosis in three groups. A-C. Control group at 2 weeks, 4 weeks and 8 weeks. The rate of apoptosis cell was 1.85%, 1.91% and 1.79% respectively. D-F. Suture group at 2 weeks, 4 weeks and 8 weeks after surgry. The rate of apoptosis cell was 2.07%, 2.32% and 34.8% respectively. G-I. Model group at 2 weeks, 4 weeks and 8 weeks after surgry. The rate of apoptosis cell was 2.26%, 2.47% and 81.10% respectively.
Figure 3.
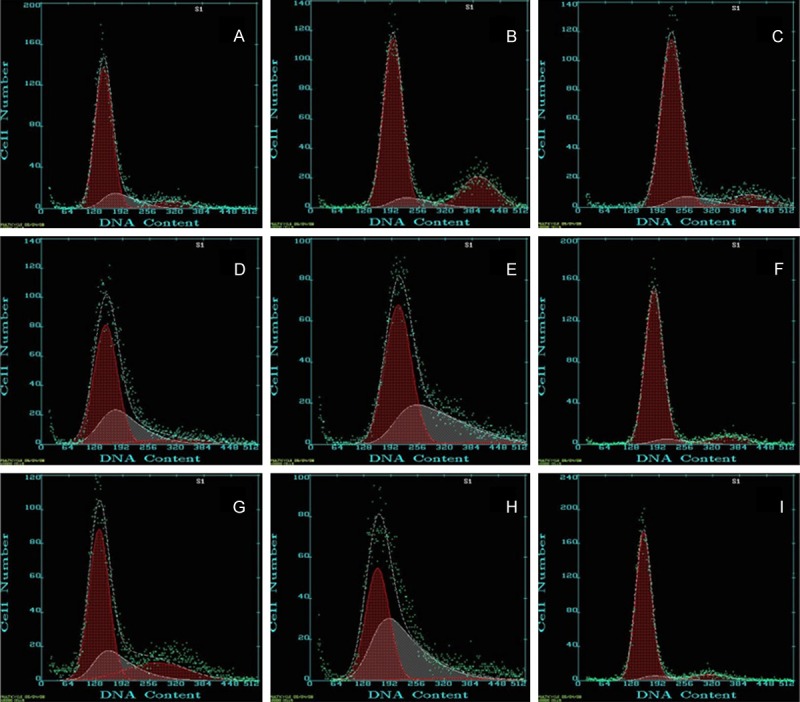
Flow cytometry to detect the cell cycle in three groups. A-C. Control group at 2 weeks, 4 weeks and 8 weeks. The rate of G1 stage cell was 74.9%, 76.1% and 77.7% respectively. D-F. Suture group at 2 weeks, 4 weeks and 8 weeks after surgry. The rate of G1 stage cell was 71.3%, 58.4% and 75.2% respectively. G-I. Model group at 2 weeks, 4 weeks and 8 weeks after surgry. The rate of G1 stage cell was 67.2%, 45.0% and 72.6% respectively.
SD lymphocyte apoptosis in rat PP knot characteristic DNA ladder bands
There were no small molecule 200 bp DNA bands in ileum PP knot lymphocyte DNA agarose gel electrophoresis of control group (Figure 4, 5, 6 and 7 lanes), suture group 2, 4 weeks (Figure 4, 3 and 4 lanes) and model group 2, 4 weeks (Figure 4, 1 and 2 lanes). In the suture group 8 weeks and the model group 8 weeks rat ileal PP knot lymphocyte small molecule DNA showed typical “trapezoid” (200 bp and 400 bp) bands (Figure 4, 8 and 9). The 200 bp band in model group was more evident than suture group at 8 weeks.
Figure 4.
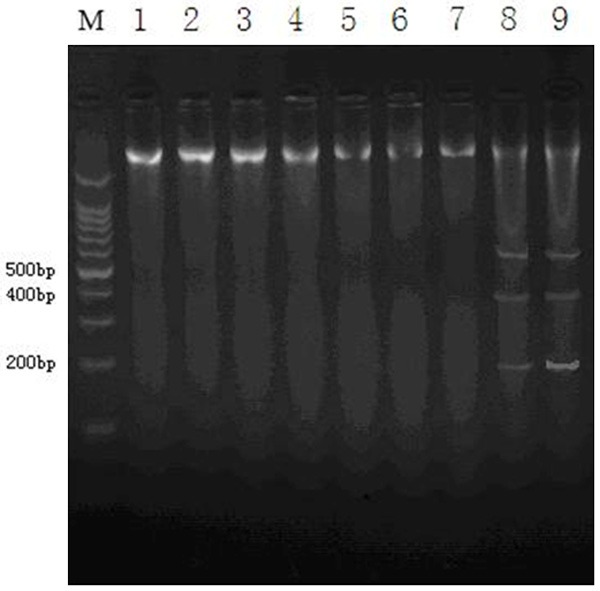
SD lymphocyte apoptosis in rat PP knot characteristic DNA ladder bands. 5-7 lanes repressed the control group DNA ladder bands at 2 weeks, 4 weeks and 8 weeks. 3, 4 and 8 lanes repressed the suture group DNA ladder bands at 2 weeks, 4 weeks and 8 weeks. 1, 2 and 9 lanes repressed the model group DNA ladder bands at 2 weeks, 4 weeks and 8 weeks. In the suture group 8 weeks and the model group 8 weeks rat ileal PP knot lymphocyte small molecule DNA showed typical “trapezoid” (200 bp and 400 bp) bands.
The staining characteristics of SD rat PP knot lymphocytes apoptosis nucleus
Compared with the control, PP knot stained lymphocytes increased significantly in the suture group and the model group at 2 weeks and 4 weeks. This result was consistent with increasing the total number of PP knot lymphocytes (Table 1 and Figure 2). At 8 weeks, compared with the control, the suture group and the model group showed a lot of lymphocyte apoptosis (Figure 5F and 5I). In the model group, we observed apparent morphology of apoptosis, nuclear condensation state and crescent-shaped nucleus (Figure 5I arrow showed).
Figure 5.
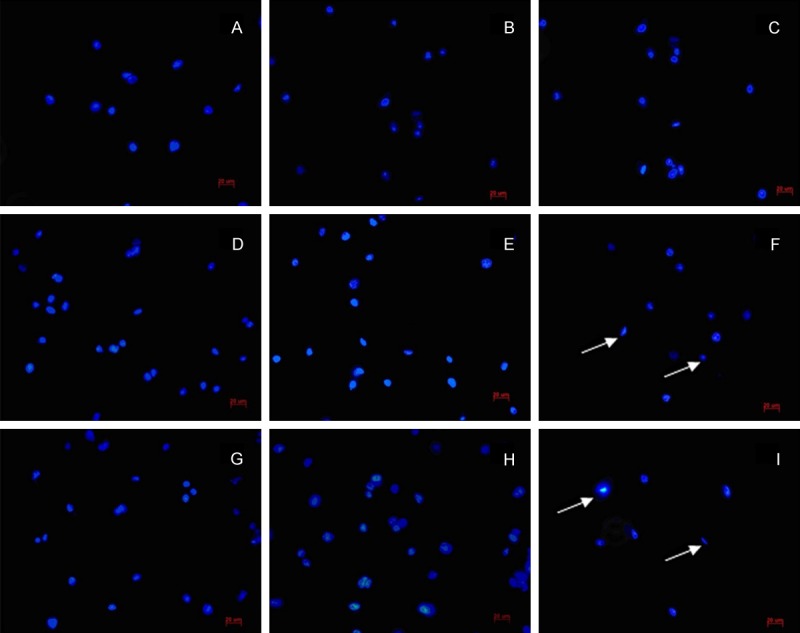
The staining characteristics of SD rat PP knot lymphocytes apoptosis nucleus (Bars =20 μm). A-C. Control group at 2 weeks, 4 weeks and 8 weeks. D-F. Suture group at 2 weeks, 4 weeks and 8 weeks after surgry. G-I. Model group at 2 weeks, 4 weeks and 8 weeks after surgry. At 8 weeks, compared with the control, the suture group and the model group showed a lot of lymphocyte apoptosis (F and I). In the model group, we observed apparent morphology of apoptosis, nuclear condensation state and crescent-shaped nucleus (arrow showed).
Discussion
Distant ileitis is mainly non-specific inflammation. Other previous studies suggested that this disease main pathogenesis: colon intestinal dysmotility disorders, ileocecal dysfunction, colon-ileum reflux, bacterial translocation, bacterial infection, the end of ileum mucosal inflammatory lesions. Typical pathological changes are: ileal mucosa and submucosa lymphoid tissue proliferation significant, lymph follicles expand and the biochemical centers significant [10,11]. In this study, we observed that surgery induced inflammation. The suture group and the model group had acute inflammation after surgery at 2 weeks. After performing terminal ileum and cecum side to side anastomosis cecum in model group, colon bacteria continued coming into the end of the ileum. So inflammation of the model group was significantly more serious than the suture group. At 8 weeks, the suture group surgical influence disappeared, inflammation outcome, approaching epithelial integrity, normal tissue, while making module colon-ileum reflux, colon bacterial translocation infection, the end of the ileum environment changes, continue to stimulate the lymphatic system of local immune response, resulting in inflammatory damage. Experimental pathology also confirmed two weeks ileal mucosa neutrophil infiltration, four weeks of inflammatory cells into neutrophil lymphocyte infiltration gradually, eight weeks mucosal lymphocyte infiltration.
Colonic bacterial translocation after infection terminal ileum, started the innate immune response quickly [12]. Then these responses caused acute inflammation and also induced the lymphocyte-mediated adaptive immune response. Adaptive immune response is usually divided into three stages. First, the activation of antigen recognition stage, lymphocytes are stimulated and began to proliferate. Second, cell proliferation and differentiation phase, lymphocytes were stimulated by antigen, then activated and started to proliferate. In the second stage, stimulated lymphocytes differentiated into immune effector cells, in the meanwhile the body initiated apoptosis control system. Third, immune effector phase, a large number of apoptotic lymphocytes were cleared up and had proliferation rarely. Intestinal immune system is composed of a large number of immune cells and immune molecules within the intestinal mucosa and submucosa as well as mucosa-associated lymphoid tissue composition such as dispersed PP knot [13]. PP knot is assembled by lymphoid follicles enriched T cells and B cells and has a large quantities distribution in the terminal ileum. It is the major site that participate the local adaptive immune response in the gut. PP knot plays a key role in inducting and disseminating immune effector cells to the site of mucosal and these cells play immune function [14].
The mucosal immune system comprises of three basic lymphocytes components [15]. One is intraepithelial lymphocytes (IEL) distributed into a variety of mucosal epithelial cell. Two is scattered into the mucosal lamina propria lymphocytes (LPL), which contain various phenotypes of T cells and B cells. Three is Peyer’s patches (PP knot) lymphocytes. They are different in composition and effectiveness. Current research on mucosal immunity has become the hot immunology research, but few domestic reports presented. Separation and purification process of the mucosal immune system cell is more complex, which affected the extensive mucosal immunity research. The characters and changes of intestinal mucosal lymphocytes are important mucosal immune studies. IEL and LPL separation process is relatively complicated in the three mucosal lymphocytes. IEL located in the epithelial cells and closed with them. LPL is located in the lamina propria of intestinal epithelial cells under the basement membrane. The dissociation degree of epithelial basement membrane and lamina propria will have a impact on IEL and LPL. On the one hand over dissociation of the epithelial layer may cause damage to the lamina propria, which resulted in mixing of IEL and IPL. On the other hand, epithelial cells and the basement membrane solution insufficient will induce lamina propria lymphocytes isolated by collagenase mixed with intraepithelial lymphocytes. Since PP knots were visible, they can completely ophthalmic cut with curved scissors. And these isolated PP knots were squeezed through the cell screening machinery. Then we got purified lymphocyte suspension. So, PP knot becomes a good choice of studying intestinal mucosal lymphocytes.
Apoptosis named program death is a life mechanism of multicellular organisms to regulate the body’s growth and maintain stability in the environment. This mechanism is controlled by genes. Program death is one of the main mode of cell aging natural death. Physiological significance of apoptosis is to ensure normal growth and development, maintain homeostasis, plays an active defense [16]. So apoptosis is a normal physiological process. In fact, too much or too little apoptosis can cause diseases. Similarly, we can also detect apoptosis to react the development of disease. Prominent feature of apoptotic cells is nuclear DNA fragmentation due to activation of the endogenous endonuclease. Under light microscope, apoptosis showed nuclear chromatin condensation, nucleolus fragmentation, a small bubble-like membrane formed inside the membrane. In fact, the membrane structure is still intact. The apoptotic cells eventually can be divided into several parcels of apoptotic bodies. The biochemical changes of cell apoptosis are: DNA fragment degraded regularly, DNA breakage happen between nucleosome. These degradation and breakage resulted fragment containing a different quantity nucleosome units. During agarose gel electrophoresis, small molecule DNA showed typical “trapezoid” (200 bp and 400 bp) bands. Its size is an integer multiple of 180-200 bp. If it is typical apoptosis, 200 bp nucleotide segment and a large number of size time’s segments are formed in the nucleus. With this feature, you can determine the occurrence and development of apoptosis by DNA gel electrophoresis.
In this experiment, 2, 4 and 8 weeks after surgery, suture group and model group were in the acute inflammatory phase (innate immune phase and antigen recognition activation stage), cell proliferation and differentiation phase, immune effector phase. Four weeks after surgery, in the suture group the number of PP knot and PP knot total lymphocyte count have increased compared with the control group; the rate of apoptosis increase was not obvious. In the model group, within the colon bacterial translocation and infection were observed; PP knot number and PP knot total lymphocyte count were significantly higher than the suture group and the control group; a slightly higher rate of lymphocyte apoptosis was showed. The activated lymphocytes played immune effector and then a large number of them had apoptosis after surgery 8 weeks. At this period, the number of PP knot and PP knot lymphocyte in experiment group (suture group and model group) decreased compared to 2 and 4 weeks surgery. Lymphocytes nuclear staining showed apoptotic nuclear morphology and typical “trapezoid” bands presented in DNA agarose gel electrophoresis. In the model group, colonic bacteria translocated to terminal ileum continuously. This translocation stimulated the immune response in intestinal lymphatic system. Lymphocyte apoptosis in the model group was significantly higher than the suture group, at the meantime PP knot and PP knot lymphocyte number slightly more than that in the suture group and the control group. In the pathogenesis of experimental terminal ileitis, SD rats experienced innate immunity and adaptive immunity stages. PP knot and PP knot lymphocyte presented significant proliferation first and apoptosis in the later time changes. Thus, we sustained that immune response played an essential role in its pathogenesis.
Numerous studies demonstrated that lymphocytes, especially intestinal intraepithelial lymphocytes play an important role in the pathogenesis of terminal ileitis. Intestinal intraepithelial lymphocytes mainly transferred to the junction between the epithelium from the lamina propria lymphocytes or PP knot lymphocytes. The changes number and activity of intestinal intraepithelial lymphocytes and PP knot lymphocytes were still not very clear. Local immune response of the terminal ileum mainly cellular immunity or humoral immunity is also not understood. How the relationship between immune response, nervous and endocrine relationship? Since this experiment only studied the changes in PP knot and PP knot lymphocytes, those areas above mentioned were not involved. So we will study specific circumstances in further.
Disclosure of conflict of interest
None.
References
- 1.Wu TF, Carati CJ, Macnaughton WK, von der Weid PY. Contractile activity of lymphatic vessels is altered in the TNBS model of guinea pig ileitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G566–574. doi: 10.1152/ajpgi.00058.2006. [DOI] [PubMed] [Google Scholar]
- 2.Melo MM, Cury PM, Ronchi LS, Goncalves-Filho Fde A, Cunrath GS, Netinho JG. Terminal ileum of patients who underwent colonoscopy: endoscopic, histologic and clinical aspects. Arq Gastroenterol. 2009;46:102–106. doi: 10.1590/s0004-28032009000200005. [DOI] [PubMed] [Google Scholar]
- 3.Cuvelier C, Demetter P, Mielants H, Veys EM, De Vos M. Interpretation of ileal biopsies: morphological features in normal and diseased mucosa. Histopathology. 2001;38:1–12. doi: 10.1046/j.1365-2559.2001.01070.x. [DOI] [PubMed] [Google Scholar]
- 4.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merga Y, Campbell BJ, Rhodes JM. Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Dig Dis. 2014;32:475–483. doi: 10.1159/000358156. [DOI] [PubMed] [Google Scholar]
- 6.Reis BS, Mucida D. The role of the intestinal context in the generation of tolerance and inflammation. Clin Dev Immunol. 2012;2012:157948. doi: 10.1155/2012/157948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutella S, Locatelli F. Intestinal dendritic cells in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2011;17:3761–3775. doi: 10.3748/wjg.v17.i33.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koboziev I, Karlsson F, Grisham MB. Gut-associated lymphoid tissue, T cell trafficking, and chronic intestinal inflammation. Ann N Y Acad Sci. 2010;1207(Suppl 1):E86–93. doi: 10.1111/j.1749-6632.2010.05711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melton SD, Feagins LA, Saboorian MH, Genta RM. Ileal biopsy: Clinical indications, endoscopic and histopathologic findings in 10,000 patients. Dig Liver Dis. 2011;43:199–203. doi: 10.1016/j.dld.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 11.Cosnes J, Bourrier A, Nion-Larmurier I, Sokol H, Beaugerie L, Seksik P. Factors affecting outcomes in Crohn’s disease over 15 years. Gut. 2012;61:1140–1145. doi: 10.1136/gutjnl-2011-301971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai YN, Yeh SL, Lin MT, Shang HF, Yeh CL, Chen WJ. Glutamine supplementation enhances mucosal immunity in rats with Gut-Derived sepsis. Nutrition. 2004;20:286–291. doi: 10.1016/j.nut.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Gebert A, Steinmetz I, Fassbender S, Wendlandt KH. Antigen transport into Peyer’s patches: increased uptake by constant numbers of M cells. Am J Pathol. 2004;164:65–72. doi: 10.1016/S0002-9440(10)63097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cader MZ, Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut. 2013;62:1653–1664. doi: 10.1136/gutjnl-2012-303955. [DOI] [PubMed] [Google Scholar]
- 16.Tan C, Ramaswamy M, Shi G, Vistica BP, Siegel RM, Gery I. Inflammation-inducing Th1 and Th17 cells differ in their expression patterns of apoptosis-related molecules. Cell Immunol. 2011;271:210–213. doi: 10.1016/j.cellimm.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


