Abstract
The aim of this study was to evaluate the clinical response to chemotherapy and treatment outcome of breast cancers patients in the presence of the GSTM1 null/present, GSTT1 null/present, and GSTP1 IIe105Val polymorphisms. Genotyping of GSTP1 rs1695, GSTT1 deletion and GSTM1 deletion was carried out on a 384-well plate format on the Sequenom MassARRAY platform. Of 382 patients, 202 patients showed good response to chemotherapy, 51 died, and 155 showed progression at the end of the study. Patients carrying GG genotype and G allele of GSTP1 rs1695 were associated with poor response to chemotherapy. In the Cox proportional hazards model, after adjusting for potential confounding factors, patients carrying GG genotype and G allele of GSTP1 rs1695 were correlated with a shorter overall survival (OS). Variants of GSTP1 rs1695 are associated with response to chemotherapy and PFS and OS of breast cancer patients, and this gene polymorphism could help in the design of individualized therapy.
Keywords: Glutathione S-transferases, polymorphism, breast cancer
Introduction
Breast cancer is the leading cause of cancer mortality in women worldwide and the incidence is still increasing in China [1,2]. Breast cancer is by far the most frequent cancer among women and the leading cause of malignancy-related death in many countries [1]. Chemotherapy for breast cancer is used as an adjuvant systemic therapy following primary surgery or as neoadjuvant chemotherapy before surgery in patients with locally advanced breast cancers (LABC) [2]. Although many clinicopathologic characteristics (such as patient age, clinical disease stage, and lymph node status) have been identified as being imprecise in predicting the efficacy of chemotherapy, increasing evidence has suggested an important role for drug-metabolizing enzyme (DME) in determining inter-individual variations in therapeutic response [3,4].
The human glutathione S-transferases (GSTs), a superfamily of dimeric phase II metabolic enzymes, play an important role in the cellular defense system [5]. The GSTs enzymes detoxify chemotherapeutic drugs or their metabolites by catalyzing the reduction of these compounds through its conjugation with glutathione. And it has been suggested that genetic polymorphisms in GSTs genes could reduce the effectiveness of detoxifying cytotoxins generated by chemotherapeutic agents in the treatment of breast carcinoma [6].
GSTP1 (encoding GST π) also is subject to polymorphic variations; the single-nucleotide substitution A313G (rs1695 or rs947894) results in an amino acid change at codon 105 (Ile/Val) that is associated with lower substrate specific catalytic activity. These polymorphisms may alter the metabolism of chemotherapeutic drugs and modify the effectiveness of therapy, as suggested by reports that GSTs polymorphisms predict differences in outcomes of treatment for breast cancer [7,8].
Up to now, many studies have assessed the role of GSTs gene polymorphisms on the response to chemotherapy of breast cancer, but the results are inconsistent. Therefore, the aim of this study was to evaluate the clinical response to chemotherapy and treatment outcome of breast cancers patients in the presence of the GSTM1 null/present, GSTT1 null/present, and GSTP1 rs1695 polymorphisms.
Materials and methods
Patients, treatments and clinical variables
A total of 382 consecutive patients diagnosed with breast cancer were selected from Department of Oncology Radiotherapy of Henan Provincial People’s Hospital between May 2009 and May 2010. Clinical data were recorded when patients were enrolled into study, including age, year of birth, and family history of cancer. Blood samples were obtained from all patients. Written informed consent was provided by all patients. Our study was approved by the ethics committee of the Henan Provincial People’s Hospital.
All the patients received chemotherapy after surgery. The demographic and clinical information of all patients were collected from medical record. All the patients were followed up every month by telephone until death or the end of the study. Overall survival (OS) was calculated from the date of chemotherapy to the date of death or last clinical follow-up.
Genotyping
All the patients were asked to provide 5 ml venous blood, and the genomic DNA was isolated using a Qiagen Blood Kit (Qiagen, Chastworth, CA) according to the manufacturer’s instructions. Genotyping of GSTP1 rs1695, GSTT1 deletion and GSTM1 deletion was carried out on a 384-well plate format on the Sequenom MassARRAY platform (Sequenom, San Diego, USA). Sequenom Assay Design 3.1 software (Sequenom®) was conducted to design primers for polymerase chain reaction amplification and single base extension assays. PCR reaction was performed in a total volume of 25 μL, containing 50 ng of genomic DNA, 0.1 μl dNTP, 1.25 U Taq DNA polymerase (Promega Corporation, Madison, WI, USA) and 21 μl forward and reverse primers. The cycling programme involved preliminary denaturation at 95°C for 2 min, followed by 45 step cycles of denaturation at 95°C for 30 s, annealing at 56°C for 30 s, 72°C for 60 s, and a final extension at 72°C for 5 min. The PCR products were analyzed by 1.0% agarose gel electrophoresis. For quality control, 10% of subjects were randomly selected, and the results of repeated samples showed 100% concordance.
Statistical analysis
Continuous variables were shown by mean ± standard deviation (SD), while categorical variables were expressed as frequencies and percentages (%). The odds ratios (OR) and corresponding 95% confidence intervals (CIs) were calculated by unconditional logistic regression analysis and utilized to assess the potential association between GSTP1 rs1695, GSTT1 deletion and GSTM1 deletion and response to chemotherapy. The homozygote for the most frequent allele was regarded as reference group. The Cox proportional hazards models were used to evaluate the effect of GSTP1 rs1695, GSTT1 deletion and GSTM1 deletion and OS and PFS of breast cancer. Kaplan-Meier method was used to plot the OS curves. SPSS® statistical package, version 11.0 (SPSS Inc., Chicago, IL, USA) for Windows® was used for statistical analyses. All P values were two-tailed, and a difference was considered statistically significant when P < 0.05.
Results
Of 382 patients, 202 (52.88%) presented good response to chemotherapy and 51 (13.35%) died followed up until May 2014. The follow-up period of all patients was ranged from 1 month to 60 months. The median age of breast cancer patients was 51.4 ± 14.6 years (range, 22-78 years). 10 (2.62%) of the patients had family history of breast cancer, 156 (40.84%) had clinical tumor size > 5, 16 (4.19%) had clinical lymph node > 10, and 21 (5.50%) had Metastasis of M1 (Table 1).
Table 1.
Characteristics of the study population
| Characteristics | Breast cases N = 382 | % |
|---|---|---|
| Mean age (mean ± SD) | 51.4 ± 14.6 | |
| < 50 | 47.38 | 181 |
| ≥ 50 | 52.62 | 201 |
| Family history of breast cancer | ||
| No | 97.38 | 372 |
| Yes | 2.62 | 10 |
| Clinical tumor size (cm) | ||
| < 2 | 12.04 | 46 |
| 2-5 | 47.12 | 180 |
| > 5 | 40.84 | 156 |
| Clinical lymph node | ||
| < 3 | 77.49 | 296 |
| 3-10 | 18.32 | 70 |
| > 10 | 4.19 | 16 |
| Metastasis | ||
| M0 | 94.50 | 361 |
| M1 | 5.50 | 21 |
| Death | ||
| Yes | 13.35 | 51 |
| No | 86.65 | 331 |
| Response to chemotherapy | ||
| Yes | 52.88 | 202 |
| No | 47.12 | 180 |
Patients were classified into good and poor responders, and significantly different genetic distribution of GSTP1 rs1695 were observed between groups. Patients carrying GG genotype and G allele of GSTP1 rs1695 were more likely to have a poorer response to chemotherapy when compared with AA genotype, with ORs (95% CI) of 0.46 (0.26-0.84) and 0.65 (0.42-0.99), respectively. However, we did not find any association between GSTT1 or GSTM1 and response to chemotherapy in breast cancer patients (Table 2).
Table 2.
Correlation between eight polymorphisms and tumor response
| Genotype | Patients | % | Tumor response | OR (95% CI)1 | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Good | % | Poor | % | ||||||
| GSTP1 rs1695 | AA | 150 | 39.27 | 88 | 43.56 | 62 | 34.44 | 1.0 (Ref.) | - |
| AG | 154 | 40.31 | 83 | 41.09 | 71 | 39.44 | 0.82 (0.51-1.33) | 0.41 | |
| GG | 78 | 20.42 | 31 | 15.35 | 47 | 26.11 | 0.46 (0.26-0.84) | 0.007 | |
| Allele | A | 227 | 59.42 | 130 | 64.36 | 97 | 53.89 | 1.0 (Ref.) | - |
| G | 155 | 40.58 | 72 | 35.64 | 83 | 46.11 | 0.65 (0.42-0.99) | 0.04 | |
| GSTT1 | Present | 167 | 43.72 | 93 | 46.04 | 74 | 41.11 | 1.0 (Ref.) | - |
| Null | 215 | 56.28 | 109 | 53.96 | 106 | 58.89 | 0.82 (0.53-1.25) | 0.33 | |
| GSTM1 | Present | 228 | 59.69 | 124 | 61.39 | 104 | 57.78 | 1.0 (Ref.) | - |
| Null | 154 | 40.31 | 78 | 38.61 | 76 | 42.22 | 0.86 (0.56-1.32) | 0.47 | |
Adjusted for age, family history of breast cancer, clinical tumor size, clinical lymph node and metastasis.
During the follow up period, 51 patients (13.35%) died at the end of the study. The median overall survival time was 47.1 ± 12.5 months (ranged from 1 to 60 months). In the Cox proportional hazards model, after adjusting for potential confounding factors, patients carrying GG genotype and G allele of GSTP1 rs1695 were correlated with higher risk of breast cancer when compared with homozygote of the most frequent genotype, and the adjusted HRs were 3.21 (1.34-7.81) and 2.23 (1.37-3.62), respectively (Table 3; Figure 1). However, we did not find significant association between polymorphisms in GSTT1 and GSTM1 and risk of death in breast cancer patients.
Table 3.
Cox regression analysis of genes and polymorphisms with OS of breast cancer patients
| Genotype | Patients | Events | Alive | OS1 | ||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| N=51 | % | N=331 | % | HR (95% CI)1 | P value | |||
| GSTP1 rs1695 | AA | 150 | 12 | 23.53 | 138 | 41.69 | 1.0 (Ref.) | - |
| AG | 154 | 22 | 43.14 | 132 | 39.88 | 1.92 (0.87-4.42) | 0.08 | |
| GG | 78 | 17 | 33.33 | 61 | 18.43 | 3.21 (1.34-7.81) | 0.003 | |
| Allele | A | 227 | 46 | 45.10 | 181 | 54.68 | 1.0 (Ref.) | - |
| G | 155 | 56 | 54.90 | 99 | 29.91 | 2.23 (1.37-3.62) | 0.001 | |
| GSTT1 | Present | 167 | 19 | 37.25 | 148 | 44.71 | 1.0 (Ref.) | - |
| Null | 215 | 32 | 62.75 | 183 | 55.29 | 1.36 (0.71-2.65) | 0.32 | |
| GSTM1 | Present | 228 | 30 | 58.82 | 198 | 59.82 | 1.0 (Ref.) | - |
| Null | 154 | 21 | 41.18 | 133 | 40.18 | 1.04 (0.54-1.97) | 0.89 | |
Adjusted for age, family history of breast cancer, clinical tumor size, clinical lymph node and metastasis.
Figure 1.
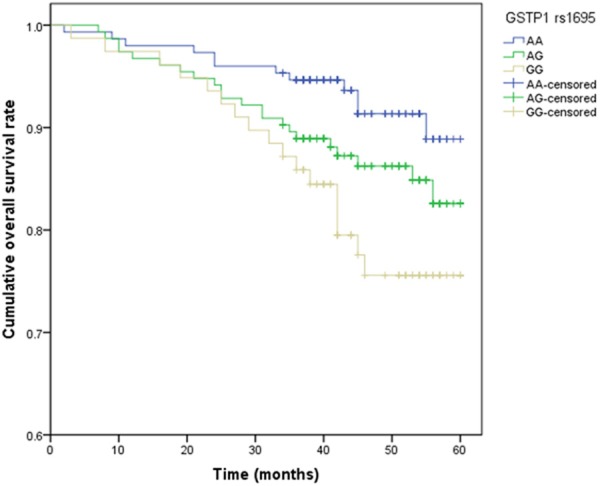
Kaplan-Meier analysis for OS curve of GSTP1 rs1695.
Discussion
It is generally known that traditional selection of chemotherapy regimen could improve response and survival of cancer patients. Personalized chemotherapy according to molecular biomarkers could further augment response rates and improve survival of breast cancer. Our study assessed the association between GSTP1 rs1695, GSTT1 deletion and GSTM1 deletion and response to chemotherapy and OS of breast cancer patients. Our study suggests that variant of GSTP1 rs1695 can influence the response to chemotherapy, and are associated with OS of breast cancer patients. This gene variant could be useful as prognostic markers in breast cancer patients, and help in the design of individualized therapy.
The GST super-family of enzymes belongs to the phase II group of enzymes, which are involved in the metabolism of a wide range of xenobiotics and drugs including a variety of cytotoxic cancer chemotherapeutic agents [9]. Since the first evidence that glutathione S-transferases may play an important role in chemotherapy efficacy [10], the results of various subsequent studies have shown the inconsistent nature of this relationship. Our analysis showed that GG genotype and G allele of GSTP1 rs1695 were associated with better chemotherapy response in breast cancer patients. This association was independent of age and other traditional predictors of prognosis.
Recent studies have investigated that genetic polymorphisms involving GSTM1, GSTP1, and GSTT1 have attracted much interest in relation to cancer susceptibility. People with the GSTs variant genotypes are less able to detoxify the metabolites of drugs and carcinogens. As a result, they are more susceptible to the development of certain cancers, including breast cancer. On the other hand, because of the same property of these metabolizing enzymes, these people are likely to respond better to chemotherapeutic drugs, resulting in a survival advantage. Tulsyan et al. observed that patients with present genotype of GSTM1/GSTT1 had a poor response as compared with absent genotype [11]. On the contrary, another study in Indian, Mishra et al. did not find a significant association between glutatinione S-transferases and responses to chemotherapy [12]. One other study of 159 women from Beijing, China, found a significant association of GSTM1 null genotype and GSTP1 Val/Val genotypes with breast cancer survival and response to chemotherapy [13]. In two reports based on the same sample, breast cancer patients with null mutations for GSTM1 and GSTT1 had reduced risk of death compared to women with alleles present and a reduction in mortality risk for women homozygous for the variant GSTP1 Val-allele compared to those homozygous for the Ile allele [14-16]. These inconsistency results might be due to differences in ethnicities, source of patients, disease stages, sample size, and by chance.
Several limitations should be considered in our study. Firstly, all the breast cancer patients were selected from one hospital, and these cases may not better represent the patients from other populations. There may be a selection bias and information bias. However, potential bias may be minimized by further adjustment for age, family history of breast cancer, clinical tumor size, clinical lymph node and metastasis in final analysis, which could minimize the selection bias. Secondly, the sample size of our study is relative small, which would reduce the statistical power to find the difference between groups. Therefore, further large sample size and well designed studies are greatly needed to confirm the association between GSTs gene polymorphisms and prognosis of breast cancer patients.
In summary, the finding of our study provided statistical evidence that variant of GSTP1 rs1695 can influence the prognosis of breast cancer patients treated with chemotherapy. Therefore, we suggested that GSTP1 rs1695 polymorphism could be used as surrogate markers toward individualizing breast cancer treatment strategies.
Disclosure of conf锘匡豢锘匡豢lict of interest
None.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J. Clin. Oncol. 2001;19:4224–37. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 3.Arun BK, Granville LA, Yin G, Middleton LP, Dawood S, Kau SW, Kamal A, Hsu L, Hortobagyi GN, Sahin AA. Glutathione-s-transferase-pi expression in early breast cancer: association with outcome and response to chemotherapy. Cancer Invest. 2010;28:554–9. doi: 10.3109/07357900903286925. [DOI] [PubMed] [Google Scholar]
- 4.Franco RL, Schenka NG, Schenka AA, Rezende LF, Gurgel MS. Glutathione S-transferase Pi expression in invasive breast cancer and its relation with the clinical outcome. J BUON. 2012;17:259–64. [PubMed] [Google Scholar]
- 5.Strange RC, Fryer AA. The glutathione S-transferases: influence of polymorphism on cancer susceptibility. IARC Sci Publ. 1999:231–49. [PubMed] [Google Scholar]
- 6.Townsend DM, Tew KD. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22:7369–75. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz AJ, Hoehn JL, Lees AW, Dimitrov NV, Bear HD. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J. Clin. Oncol. 1998;16:2672–85. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 8.Pierga JY, Mouret E, Laurence V, Dieras V, Savigioni A, Beuzeboc P, Dorval T, Palangie T, Jouve M, Pouillart P. Prognostic factors for survival after neoadjuvant chemotherapy in operable breast cancer. the role of clinical response. Eur J Cancer. 2003;39:1089–96. doi: 10.1016/s0959-8049(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 9.Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–20. [PubMed] [Google Scholar]
- 10.Anderer G, Schrappe M, Brechlin AM, Lauten M, Muti P, Welte K, Stanulla M. Polymorphisms within glutathione S-transferase genes and initial response to glucocorticoids in childhood acute lymphoblastic leukemia. Pharmacogenetics. 2000;10:715–26. doi: 10.1097/00008571-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Tulsyan S, Chaturvedi P, Agarwal G, Lal P, Agrawal S, Mittal RD, Mittal B. Pharmacogenetic influence of GST polymorphisms on anthracycline-based chemotherapy responses and toxicity in breast cancer patients: a multi-analytical approach. Mol Diagn Ther. 2013;17:371–9. doi: 10.1007/s40291-013-0045-4. [DOI] [PubMed] [Google Scholar]
- 12.Mishra A, Chandra R, Mehrotra PK, Bajpai P, Agrawal D. Glutathione S-transferase M1 and T1 polymorphism and response to neoadjuvant chemotherapy (CAF) in breast cancer patients. Surg Today. 2011;41:471–6. doi: 10.1007/s00595-009-4310-4. [DOI] [PubMed] [Google Scholar]
- 13.Bai YL, Zhou B, Jing XY, Zhang B, Huo XQ, Ma C, He JM. Predictive role of GSTs on the prognosis of breast cancer patients with neoadjuvant chemotherapy. Asian Pac J Cancer Prev. 2012;13:5019–22. doi: 10.7314/apjcp.2012.13.10.5019. [DOI] [PubMed] [Google Scholar]
- 14.Sweeney C, McClure GY, Fares MY, Stone A, Coles BF, Thompson PA, Korourian S, Hutchins LF, Kadlubar FF, Ambrosone CB. Association between survival after treatment for breast cancer and glutathione S-transferase P1 Ile105Val polymorphism. Cancer Res. 2000;60:5621–4. [PubMed] [Google Scholar]
- 15.Yang G, Shu XO, Ruan ZX, Cai QY, Jin F, Gao YT, Zheng W. Genetic polymorphisms in glutathione-S-transferase genes (GSTM1, GSTT1, GSTP1) and survival after chemotherapy for invasive breast carcinoma. Cancer. 2005;103:52–8. doi: 10.1002/cncr.20729. [DOI] [PubMed] [Google Scholar]
- 16.Ambrosone CB, Sweeney C, Coles BF, Thompson PA, McClure GY, Korourian S, Fares MY, Stone A, Kadlubar FF, Hutchins LF. Polymorphisms in glutathione S-transferases (GSTM1 and GSTT1) and survival after treatment for breast cancer. Cancer Res. 2001;61:7130–5. [PubMed] [Google Scholar]


