Abstract
The goal of this experiment was to investigate the protective effect and the molecular mechanism of Panax Notoginseng Saponins (PNS) on cisplatin-induced nephrotoxicity through mitochondrial pathway of apoptosis. The rats underwent intraperitoneal injection with a single dose of cisplatin, a subset of rats were also intraperitoneally injected with 31.35 mg/kg PNS once a day for 8 days. At day 1, 4 and 8 after exposure to cisplatin, the concentrations of blood urea nitrogen (BUN), serum creatinine (Scr) and urinary N-acetyl-β-D-Glucosaminidase (NAG) were determined using commercial kits. The pathological change of renal tissue were examined using H & E staining and transmission electron microscopy. The rate of apoptosis and the expression of Bcl-2 in rat renal tissue were detected by using TUNEL staining and Western bloting, respectively. And the expressions of Bax and caspases 9 were detected by immunnohistochemistry. The results showed that PNS significantly protected against cisplatin-induced nephrotoxicity, as evidenced by the decrease in concentration of blood BUN, Scr and urinary NAG, as well as the attenuation of renal histopathological damage. Furthermore, PNS reduced the rate of apoptosis, and the mechanism studies showed that PNS inhibited the expression of Bax and caspase 9, while increased the expression of Bcl-2. This study first demonstrated that PNS can protect against cisplatin-induced nephrotoxicity and reduce renal tissue apoptosis via inhibiting the mitochondrial pathway.
Keywords: Panax notoginseng saponins (pns), cisplatin, apoptosis, mitochondrial pathway
Introduction
Cisplatin is one of the most commonly used chemotherapy drugs, but its renal toxicity limits cisplatin clinical use. The molecular mechanism of cisplatin-induced renal toxicity has not been fully elucidated. Studies had reported that apoptosis of renal tubular epithelial cells induced by cisplatin is one of the main mechanism in cisplatin-induced nephrotoxicity [1]. Increasing apoptosis of tubular epithelial cells may contribute to the increased nephrotoxicity induced by cisplatin [2]. Mitochondrial damage is an important factor involved in the renal tissue apoptosis induced by cisplatin [3]. Bax protein was translocated to mitochondrial [4], and then caspase 9 was activated after exposure of renal cells to cisplatin, finally the intrinsic mitochondrial pathway of apoptosis in renal cells was activated [5]. That is to say, cisplatin induces the apoptosis of renal cells via the mitochondrial pathway [6]. Thus, inhibition of renal tissue apoptosis via mitochondrial pathway can protect against cisplatin-induced nephrotoxicity [7].
It is lack of effective drugs to remedy cisplatin-induced nephrotoxicity in clinic at present. Panax Notoginseng Saponins (PNS) is the main active ingredients of Panax notoginseng. Clinical studies showed that PNS could reverse the multi-drug resistance of cancer, and improve the patients’ immune function [8]. Previous researches reported that PNS could protect ischemic myocardial cells and diabetic nephropathy via inhibiting apoptosis [9,10]. Recent study found that PNS had protection against cisplatin-induced renal injury [11], but the mechanism is still unclear. It has not been reported that whether or not PNS protected against cisplatin-induced renal damage through inhibiting apoptosis. In this study, we hypothesized that PNS would attenuate cisplatin-induced nephrotoxicity by inhibiting the mitochondrial pathway of renal cells apoptosis in rats.
Materials and methods
Chemicals
Cisplatin powder for injection (Batch number 0070152DB) was obtained from Qilu Pharmaceutical Co., Ltd. (Jinan, China). Panax notoginseng saponins (PNS) are a marketed drug. In this study, PNS (Batch number 20120305) was purchased from Guangxi Wuzhou Pharmaceutical Co., Ltd. (Wuzhou, China). BUN (urinary enzyme method), Scr, and N-acetyl-β-D-Glucosaminidase (NAG) kits were purchased from Nanjing Jiancheng Bioengineering Research Institute (Nanjing, China).
Animals
Male Sprague-Dawley (SD) rats (200 g ± 20 g) were provided by the Experimental Animal Center of Guangxi Medical University (Guangxi, China Approval No.: GX MU2010032418) and were housed four per cage. The research was conducted according to protocols approved by the institutional ethics committee of Guangxi Medical University.
Experimental design of drug treatment
Animals were divided into the following three groups (n = 36):
(I) The control group: rats received the same volume of saline from day 1 to 8.
(II) The cisplatin-only group: rats received a single dose of cisplatin (5 mg/kg) at day 1, and the same volume of saline once daily from day 1 to 8.
(III) The cisplatin + PNS group: rats received a single dose of cisplatin (5 mg/kg) at day 1, and PNS (31.35 mg/kg) once daily from day 1 to 8.
The rats in Group II and III were administered cisplatin to induce kidney damage. The doses of cisplatin and PNS were adopted according to a previous study [12]. All drugs were administered by intraperitoneal injection.
Urine samples, blood samples and renal tissues were collected after exposure to cisplatin 1 day, 4 days and 8 days. Rats were anesthetized with pentobarbital sodium (30 mg/kg b.w., i.v.). Kidneys were lavaged in situ with ice-cold saline through the abdominal aorta. Next, the kidneys of rat were dissected and washed immediately with ice-cold saline. A portion of the renal samples was fixed for 2 hours in 3% glutaraldehyde for histopathological analysis. The other portion of the renal samples was embedded in paraffin and cut into slices for immunohistochemistry analysis.
Determinate the concentration of Serum BUN, Scr and urinary NAG
The concentration of serum BUN, Scr was determined respectively using a rate assay, oxidase method. The concentration of urinary NAG was detected using the nitrophenol colorimetric method. All performed procedures were strictly in accordance with the kit requirements.
H & E staining
Renal tissues were fixed with 10% formalin. Then, the renal tissues were cut into sections. Finally, the sections were stained with hematoxylin and eosin (H & E) reagents for pathological histological examination.
Transmission electron microscopy (TEM) examination
Renal tissues were fixed for 2 hours with 3% glutaraldehyde. Next, the renal tissues were embedded in paraffin and cut into slices. Finally, the slices were processed in standard fashion for pathological examination using transmission electron microscopy (Hitachi H7650, Japan). All histological examinations were performed by an experienced pathologist who was blinded to the experimental groups.
Terminal deoxynucleotidyl transferase-mediatednick-end labeling (TUNEL) assays
The apoptosis of renal tissue was detected using the TUNEL assay accordance with the instruction provided by the kit (Roche Diagnostics, Penzberg, Germany). In brief, the paraffin-embedded renal tissues were cut into 4-μm sections. The sections were deparaffinized and incubated with 10 g/ml proteinase K for 15 minutes at room temperature. Then, the sections were exposed to terminal deoxynucleotidyl transferase labeling reaction mixture for 90 minutes, and incubated with antiFITC-horseradish peroxidase conjugate for 30 minutes in a 37°C humidified chamber. Finally, the sections were reacted with a kit containing 3, ’-diaminobenzidine (DAB) (Boster, Wuhan, China). The TUNEL-positive cell was count using microscope and photos were taken with the imaging analysis system (Olympus Soft Imaging Solutions, Germany).
Immunohistochemical detection of Bax and caspase9 protein expression
The expression of Bax and caspase9 was detected using immunohistochemical method. Sections were cut from paraffin-embedded rat renal tissues, 0.3% H2O2 was used to block the endogenous peroxidase activity. The sections were incubated with the Bax antibodies (1:500 dilution in PBS, Cell Signaling technology Co, Danvers, MA) or Caspase9 antibodies (1:300 dilution in PBS, Epitomicsan Abcam Co. Cambridge, England) overnight at 4°C in a moist chamber. Next, biotinylated secondary antibodies and ABC reagent were added according to the kit’s instructions (Boster, Wuhan, China). Color development was induced by incubation with DAB kit (Boster, Wuhan, China) for 3 to 5 minutes. Finally, the sections were dehydrated in ethanol, transparent in xylene and sealed. The specific staining was visualized and images were acquired using Upright microscope (Olympus Soft Imaging Solutions, Germany). The morphometric examination was performed in a blinded manner by two independent investigators.
Western blotting detection of Bcl-2 protein expression
The protein Bcl-2 expression of rat renal tissues was detected by Western blotting. In brief, proteins in renal specimen were separated using 12% Tris-glycine gel electrophoresis, and then transferred to polyvinylidene fluoride (PVDF) membrane (Milli-pore, USA). The transfer films were incubated with Bcl-2 rabbit monoclonal antibody (1:1,000 dilution in tPBS; Cell Signaling, Danvers, MA), or internal loading control protein β-actin (1:1,000 in tPBS; Santa Cruz Biotechnology, Santa Cruz, CA) overnight on a 4°C shaker. Finally, the PVDF membranes were incubated using a secondary fluorescent antibody (Anti-Rabbit IgG, LICOR, USA) and detected using Odyssey infrared fluorescence scanning imaging system (Odyssey, LICOR, USA).
Statistical analysis
Statistical analysis was performed using SPSS 16.0 for Windows. Differences between the groups were assessed using a one-way analysis of variance (ANOVA). The quantitative data were presented as the means ± SE. A P-value < 0.05 was considered to be statistically significant.
Results
Effects of PNS on levels of serum Cr, BUN and urine NAG
The levels of serum Cr, BUN and urine NAG in the control group has no obvious change at day 1, 4 and 8. The levels of serum Cr and BUN in the cisplatin-only group significantly increased at day 1, 4 and 8, and the level of urine NAG significantly increased at day 4 and 8 when compared with the control group (Figure 1). Compared with the cisplatin-only group, both serum Cr and BUN levels in the cisplatin + PNS group significantly decreased at day 1, 4 and 8, and the level of urine NAG significantly decreased at day 4 and 8 (Figure 1).
Figure 1.
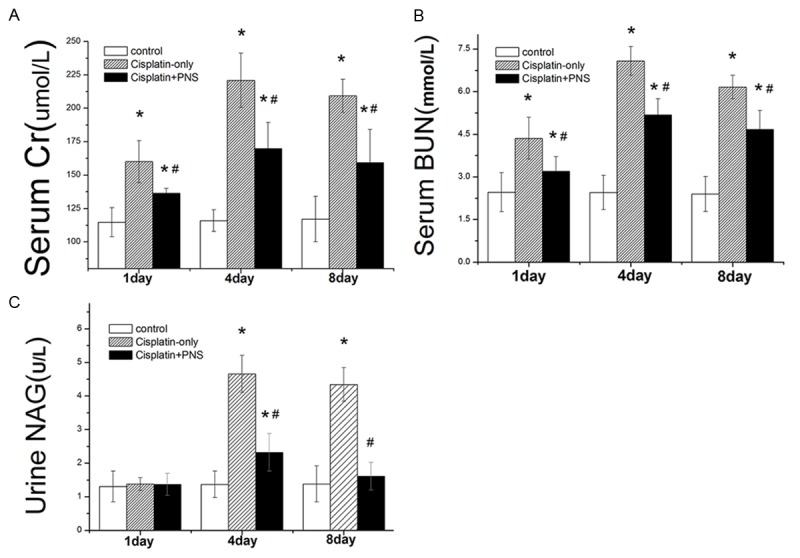
The effects of PNS on the levels of Serum Cr, BUN and urine NAG. The results are presented as the means ± SE. *P < 0.01 when compared with the control group, #P < 0.01 when compared with the cisplatin-only group.
Pathological examination of renal tissue using H & E-staining
The structure of rat renal tissues in the control group is normal (Figure 2A, 2D, 2G). In the cisplatin-only group, the renal tubular epithelial cells were edema, and renal tubular lumens were local stenosis at day 1 (Figure 2B). The edema of cells were aggravated, a lot shedding of epithelial cells and a large number of protein cast or red blood cell casts were found in the cisplatin-only group (Figure 2E, 2H) at day 4 and day 8. The pathological damages of renal tissues in the cisplatin + PNS group were obviously improved when compared with the cisplatin-only group (Figure 2C, 2F, 2I).
Figure 2.
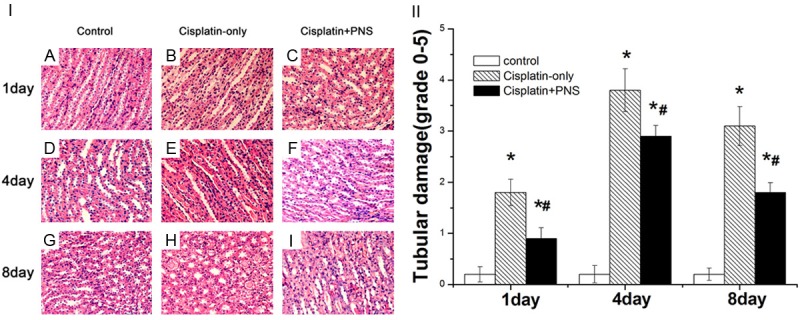
Histomorphological examination of rat renal tissue (H & E staining, 400×). A, D, G: The control group at day 1, 4 and 8; B, E, H: The cisplatin-only group at day 1, 4 and 8; C, F, I: The cisplatin-PNS group at day 1, 4 and 8.
Transmission electron microscopy examination of renal tubular epithelial cell
The structure and shape of renal tubular epithelial cells in the control group were normal (Figure 3A, 3D, 3G) at day 1, 4 and 8. The lysosome number in renal tubular epithelial cells was increased, the mitochondrial were swelling and mitochondrial cristae were vague in the cisplatin-only group (Figure 3B) at day 1. The mitochondrial swelling of renal tubular epithelial cells was aggravated, mitochondrial cristae were ruptured or melted in the cisplatin-only group (Figure 3E, 3H) at day 4 and 8. PNS could improve the mitochondrial injury of renal tubular epithelial cells when compared with the cisplatin-only group (Figure 3C, 3F, 3I) at day 1, 4 and 8.
Figure 3.
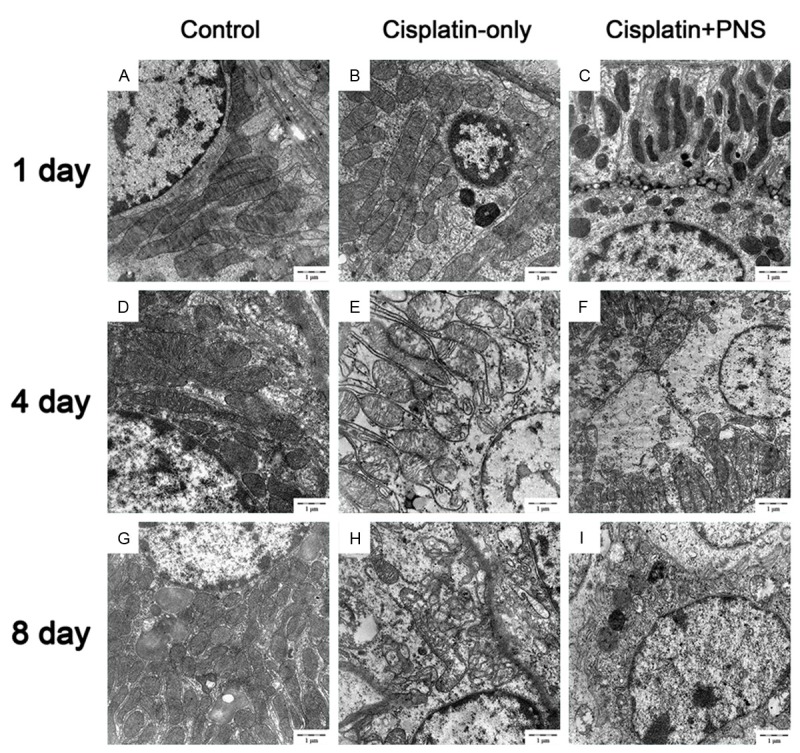
The effects of PNS on the mitochondrial of renal tubular epithelial cells. A, D, G: The control group at day 1, 4 and 8, the structure and shape of renal tubular epithelial cells were normal; B, E, H: The cisplatin-only group at day 1, 4 and 8, the mitochondrial were swelling, mitochondrial cristae were vague, rupture and melted; C, F, I: The cisplatin-PNS group at day 1, 4 and 8, the mitochondrial injury was improved.
Effects of PNS on the apoptosis of renal tissue
The apoptosis rate of renal tissue was detected using TUNEL staining (Figure 4). As shown in Figure 4, the apoptosis rate of renal tissue in the control group had no changes at day 1, 4 and 8. The apoptosis rate was significantly higher in the cisplatin-only group when compared with the control group at day 1, 4 and 8. Compared with the cisplatin-only group, the apoptosis rate of renal tissue in the cisplatin + PNS group was significantly lower at day 4 and 8 (Figure 4).
Figure 4.
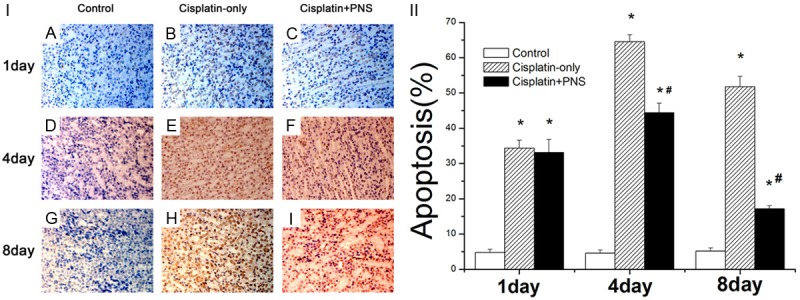
The effect of PNS on the apoptosis rate of rat renal tissue detected using TUNEL staining. A, D, G: The control group at day 1, 4 and 8; B, E, H: The cisplatin-only group at day 1, 4 and 8; C, F, I: The cisplatin-PNS group at day 1, 4 and 8. *P < 0.01 when compared with the control group, #P < 0.01 when compared with the cisplatin-only group.
Effects of PNS on the expression of caspase9 in renal tissue of rat
The caspase9 protein was mainly expressed in renal tubular epithelial cells (Figure 5). The level of caspase9 protein expression in the control group had no changes at day 1, 4 and 8 (Figure 5). Compared with the control group, the expression of caspase9 protein in the cisplatin-only group was significantly increased, it increased respectively by 1.13, 2.23, 1.75 times at day 1, 4 and 8. PNS significantly reduced the expression of caspase9 when compared with the cisplatin-only group, it respectively decreased by 16.9%, 19.7%, 30.2% at day 1, 4 and 8 (Figure 5).
Figure 5.
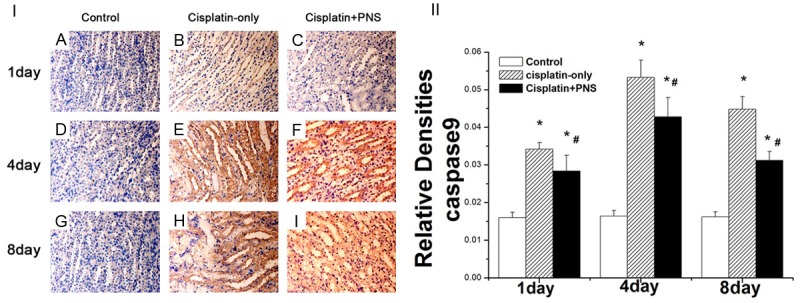
The effects of PNS on the expression of caspase9 detected using immunohistochemical method. A, D, G: Normal control group at 1, 4 and 8 d; B, E, H: Cisplatin-only group at day 1, 4 and 8; C, F, I: Cisplatin-PNS group at day 1, 4 and 8. The results are presented as the means ± SE. *P < 0.01 when compared with the control group, #P < 0.01 when compared with the cisplatin-only group.
Effects of PNS on the expression of Bax in renal tissue in rat
The Bax protein was expressed mainly in renal tubular epithelial cells (Figure 6). The level of Bax protein expression in the control group didn’t change at day 1, 4 and 8 (Figure 6). Compared with the control group, the expression of Bax protein in the cisplatin-only group was significantly increased at day 1, 4 and 8, it was increased by 2.34, 4.51, 5.38 times, respectively. The expression level of Bax protein in the cisplatin + PNS group was significantly decreased when compared with the cisplatin-only group at day 1, 4 and 8, it decreased respectively by 40.7%, 22.2%, 50.9% (Figure 6).
Figure 6.
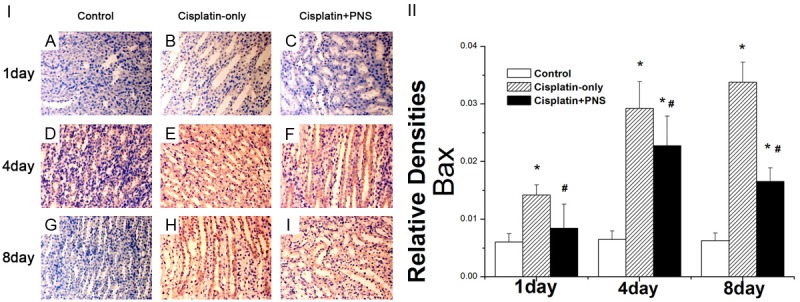
The effects of PNS on the expression of Bax and caspase9 in rat renal tissue detected using immunohistochemical method. A, D, G: The control group at day 1, 4 and 8; B, E, H: The cisplatin-only group at day 1, 4 and 8; C, F, I: The cisplatin-PNS group at day 1, 4 and 8. The results are presented as the means ± SE. *P < 0.01 when compared with the control group, #P < 0.01 when compared with the cisplatin-only group.
Effects of PNS on the expression of Bcl-2 in renal tissue of rat
The expression of Bcl-2 protein in the control group had no obvious changes at day 1, 4 and 8 (Figure 7). The expression of Bcl-2 protein in the cisplatin-only group was significantly increased when compared with the control group, it increased respectively by 0.79, 1.39, 3.47 times at day 1, 4 and 8 (Figure 7). PNS significantly increased the expression of Bcl-2 protein when compared with the cisplatin-only group, it increased by 0.66, 0.70, 1.00 times respectively at day 1, 4 and 8 (Figure 7).
Figure 7.
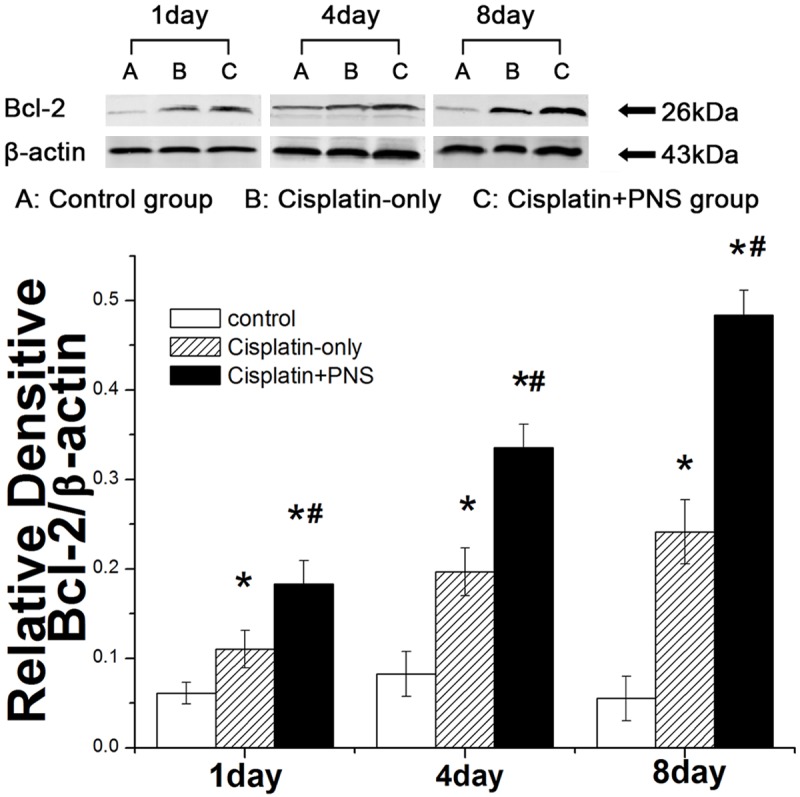
The effect of PNS on the expression of Bcl-2 in rat renal tissue detected by WB. A: The control group; B: The cisplatin-only group; C: The cisplatin + PNS group. The results are presented as the means ± SE. *P< 0.01 when compared with the control group, #P < 0.01 when compared with the cisplatin-only group.
Discussion
The main pathological feature of cisplatin-induced nephrotoxicity is renal tubular epithelial cell injury, and cisplatin can reduce the rate of glomerular filtration, increase the levels of serum Cr, BUN [13-15] and urinary NAG [16]. This study found that the levels of serum Cr, BUN and urinary NAG in the cisplaitn-only group were significantly elevated when compared with the control group.
Apoptosis is also called programmed cell death. It is a form of cell death. Many studies have demonstrated that the apoptosis of renal tissue induced by cisplatin plays an important role in cisplatin-induced nephropathy [17,18]. Apoptosis of tubular epithelial cells induced by cisplatin may increase nephrotoxicity [2]. In this experiment, the apoptosis rate of rat renal cells in the cisplaitn-only group was significantly higher when compared with the control group at day 1, 4 and 8. The apoptosis rate at day 4 was the highest among these three time points. The renal damage and the mitochondria injury of renal tubular epithelial cells at day 4 were the most serious among these three time points too. That is to say, the apoptosis rate was consistent with the damages of renal tissue shown in pathological examination using H & E staining and TEM. These results suggest that cisplatin can induce apoptosis of renal tissue and cause kidney damage. It is consistent with literature reports [19,20].
However, the molecular mechanisms of cisplatin-induced apoptosis of renal tubular epithelial cells have not been fully elucidated. According to the reports, the apoptosis of renal tubular epithelial cells induced by cisplatin was mainly through the mitochondrial pathway [21]. Caspase 9 plays an important role in the mitochondrial pathway of apoptosis. Cytochrome C is released from mitochondria to the cytosol when mitochondrial pathway is activated by cisplatin, leading to the conversation of procaspase9 to caspase9 [5] and the formation of apoptosis bodies. Only the combination of caspase9 and apoptosis body can effectively activate downstream of caspase, including caspase 3/6/7, and eventually leads to cell apoptosis [22]. Thus, caspase9 is a key protein and promoter in the mitochondrial pathway of apoptosis [23]. It has not been reported that whether or not caspase9 participates in the apoptosis of renal cells induced by cisplatin. In this study, the caspase9 expressions of rat renal tissue in the cisplatin-only group were significantly increased when compared with the control group at day 1, 4 and 8, the expression of caspase9 at day 4 was the highest among these three time points. It was at the same time that the apoptosis rate of renal cells was the highest, and the mitochondrial damage of renal cells was the most serious among these three time points in the cisplatin group. These results first suggest that caspase9 may be involved in the apoptosis of renal cells induced by cisplatin.
Mitochondrial pathway of apoptosis induced by cisplatin can be regulated by the pro-apoptotic protein Bax and the anti-apoptosis protein Bcl-2 [7]. Studies found that Bax genes were activated to increase the expression of Bax protein when cells were stimulated by cisplatin [24]. Bax protein binds to mitochondria membrane after conformational change, and then causes the release of cytochrome C from mitochondria [25], eventually promotes the apoptosis. In contrast, the anti-apoptosis protein Bcl-2 can stabilize the mitochondrial membrane potential through a variety of ways to inhibit the release of cytochrome C [26,27], thereby inhibits the mitochondrial pathway of cell apoptosis. Thus, the expressions of protein Bax and Bcl-2 are very important in the regulatory of apoptosis [28], the increasing of Bax expression promotes apoptosis while the decreasing of Bcl-2 expression inhibits apoptosis. In this experiment, the Bax expression in the cisplatin-only group was significantly higher when compared with the control group. This result shows that cisplatin can increase the Bax expression of renal tissues to promote apoptosis.
PNS is the effective components extracted from traditional Chinese medicine herb Panax Notoginseng. It contains a variety of active ingredients including notoginseng saponin R1 and ginseng saponin Rg1, Rb1, Re, Rd, etc. Therefore, PNS has a variety of activities such as antioxidant [29], anti-proliferative and anti-apoptotic. According to the previous study, PNS can reduce the renal damage induced by cisplatin [11]. Other reports are that PNS plays a role of protection on ischemic myocardial cells and diabetic nephropathy by inhibiting apoptosis [3,9]. In this experiment, the levels of Cr, BUN and urinary NAG in the cisplatin + PNS group were decreased significantly when compared with the cisplatin-only group, the renal tubular necrosis and the mitochondrial injury of renal tubular epithelial cells in the cisplatin + PNS group were obviously improved too. Moreover, the apoptosis rate of rat renal in the cisplatin + PNS group was significantly decreased when compared with the cisplatin-only group at day 4 and 8. These results first prompt that PNS may be protect against cisplatin-induced nephrotoxicity by inhibiting the apoptosis of renal cells. In addition, PNS significantly reduced the expression of caspase9 and Bax, while it significantly increased the Bcl-2 expression when compared with the cisplatin-only group. These results show that PNS inhibits the apoptosis of renal cells via mitochondrial pathway.
From what has been discussed above, PNS possible plays a role of protection against cisplatin-induced nephrotoxicity by inhibiting the apoptosis of renal cells induced by cisplatin via the mitochondrial pathway dependent caspase 9.
Acknowledgements
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (No. 81260598).
Disclosure of conflict of interest
None.
References
- 1.Schwerdt G, Freudinger R, Schuster C, Weber F, Thews O, Gekle M. Cisplatin-induced apoptosis is enhanced by hypoxia and by inhibition of mitochondria in renal collecting duct cells. Toxicol Sci. 2005;85:735–42. doi: 10.1093/toxsci/kfi117. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Grunz-Borgmann EA, Parrish AR. Loss of alpha(E)-catenin potentiates cisplatin-induced nephrotoxicity via increasing apoptosis in renal tubular epithelial cells. Toxicol Sci. 2014;141:254–262. doi: 10.1093/toxsci/kfu130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 4.Lee YM, Bae SY, Won NH, Pyo HJ, Kwon YJ. Alpha-lipoic acid attenuates cisplatin-induced tubulointerstitial injuries through inhibition of mitochondrial bax translocation in rats. Nephron Exp Nephrol. 2009;113:e104–112. doi: 10.1159/000235754. [DOI] [PubMed] [Google Scholar]
- 5.Park MS, De Leon M, Devarajan P. Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J Am Soc Nephrol. 2002;13:858–865. doi: 10.1681/ASN.V134858. [DOI] [PubMed] [Google Scholar]
- 6.dos Santos NA, Carvalho Rodrigues MA, Martins NM, dos Santos AC. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol. 2012;86:1233–1250. doi: 10.1007/s00204-012-0821-7. [DOI] [PubMed] [Google Scholar]
- 7.Jiang M, Wang CY, Huang S, Yang T, Dong Z. Cisplatin-induced apoptosis in p53-deficient renal cells via the intrinsic mitochondrial pathway. Am J Physiol Renal Physiol. 2009;296:F983–993. doi: 10.1152/ajprenal.90579.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang JH, Wang JP, Wang HJ. Clinical study on effect of total panax notoginseng saponins on immune related inner environment imbalance in rheumatoid arthritis patients. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27:589–92. [PubMed] [Google Scholar]
- 9.Chen S, Liu J, Liu X, Fu Y, Zhang M, Lin Q, Zhu J, Mai L, Shan Z, Yu X, Yang M, Lin S. Panax notoginseng saponins inhibit ischemia-induced apoptosis by activating PI3K/Akt pathway in cardiomyocytes. J Ethnopharmacol. 2011;137:263–270. doi: 10.1016/j.jep.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Uzayisenga R, Ayeka PA, Wang Y. Anti-diabetic potential of Panax notoginseng saponins (PNS): a review. Phytother Res. 2014;28:510–516. doi: 10.1002/ptr.5026. [DOI] [PubMed] [Google Scholar]
- 11.Liu SJ, Zhou SW. Panax notoginseng saponins attenuated cisplatin-induced nephrotoxicity. Acta Pharmacol Sin. 2000;21:257–260. [PubMed] [Google Scholar]
- 12.Xi JX, Liu XX, Yang YF, Zhang CH, Liu HG. Effect of Xueshuantong on Renal Function and Oxidation Indexes in Cisplatin-induced Nephroxicity Rats. Chin J Exp Tradit Med Form. 2012;18:263–266. [Google Scholar]
- 13.Chen Y, Brott D, Luo W, Gangl E, Kamendi H, Barthlow H, Lengel D, Fikes J, Kinter L, Valentin JP, Bialecki R. Assessment of cisplatin-induced kidney injury using an integrated rodent platform. Toxicol Appl Pharmacol. 2013;268:352–361. doi: 10.1016/j.taap.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 14.Moslemi F, Nematbakhsh M, Eshraghi-Jazi F, Talebi A, Nasri H, Ashrafi F, Moeini M, Mansouri A, Pezeshki Z. Inhibition of Nitric Oxide Synthase by L-NAME Promotes Cisplatin-Induced Nephrotoxicity in Male Rats. ISRN Toxicol. 2013;2013:242345. doi: 10.1155/2013/242345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazaheri S, Nematbakhsh M, Bahadorani M, Pezeshki Z, Talebi A, Ghannadi AR, Ashrafi F. Effects of Fennel Essential Oil on Cisplatin-induced Nephrotoxicity in Ovariectomized Rats. Toxicol Int. 2013;20:138–145. doi: 10.4103/0971-6580.117256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdelrahman AM, Al Salam S, AlMahruqi AS, Al husseni IS, Mansour MA, Ali BH. N-acetylcysteine improves renal hemodynamics in rats with cisplatin-induced nephrotoxicity. J Appl Toxicol. 2010;30:15–21. doi: 10.1002/jat.1465. [DOI] [PubMed] [Google Scholar]
- 17.Tsuruya K, Ninomiya T, Tokumoto M, Hirakawa M, Masutani K, Taniguchi M, Fukuda K, Kanai H, Kishihara K, Hirakata H, Iida M. Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int. 2003;63:72–82. doi: 10.1046/j.1523-1755.2003.00709.x. [DOI] [PubMed] [Google Scholar]
- 18.Jeong JJ, Park N, Kwon YJ, Ye DJ, Moon A, Chun YJ. Role of annexin A5 in cisplatin-induced toxicity in renal cells: molecular mechanism of apoptosis. J Biol Chem. 2014;289:2469–2481. doi: 10.1074/jbc.M113.450163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baek SM, Kwon CH, Kim JH, Woo JS, Jung JS, Kim YK. Differential roles of hydrogen peroxide and hydroxyl radical in cisplatin-induced cell death in renal proximal tubular epithelial cells. J Lab Clin Med. 2003;142:178–186. doi: 10.1016/S0022-2143(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 21.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 22.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 23.Cain K, Bratton SB, Langlais C, Walker G, Brown DG, Sun XM, Cohen GM. Apaf-1 oligomerizes into biologically active approximately 700-kDa and inactive approximately 1.4-MDa apoptosome complexes. J Biol Chem. 2000;275:6067–6070. doi: 10.1074/jbc.275.9.6067. [DOI] [PubMed] [Google Scholar]
- 24.Wei Q, Dong G, Franklin J, Dong Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int. 2007;72:53–62. doi: 10.1038/sj.ki.5002256. [DOI] [PubMed] [Google Scholar]
- 25.Tayem Y, Johnson TR, Mann BE, Green CJ, Motterlini R. Protection against cisplatin-induced nephrotoxicity by a carbon monoxide-releasing molecule. Am J Physiol Renal Physiol. 2006;290:F789–794. doi: 10.1152/ajprenal.00363.2005. [DOI] [PubMed] [Google Scholar]
- 26.Cummings BS, Schnellmann RG. Cisplatin-induced renal cell apoptosis: caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther. 2002;302:8–17. doi: 10.1124/jpet.302.1.8. [DOI] [PubMed] [Google Scholar]
- 27.Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608–8618. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- 28.Yuan G, Dai S, Yin Z, Lu H, Jia R, Xu J, Song X, Li L, Shu Y, Zhao X, Chen Z, Fan Q, Liang X, He C, Yin L, Lv C, Lei Q, Wang L, Mi Y, Yu X, Zhang M. Sub-chronic lead and cadmium co-induce apoptosis protein expression in liver and kidney of rats. Int J Clin Exp Pathol. 2014;7:2905–2914. [PMC free article] [PubMed] [Google Scholar]
- 29.Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng) J Pharm Pharmacol. 2006;58:1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]


