Abstract
Background: Restenosis is a common adverse event of endovascular procedures and troubles cardiologists. However, the mechanism underlying restenosis is still not fully understood. To evaluate whether disheveled-1 (Dvl-1) is involved in the Wnt4/β-catenin signaling pathway to participate in the mechanisms of vascular restenosis. Methodology: Rat model of balloon-injured carotid artery was established and atorvastatin was used to treat artery injury. Vascular smooth muscle cells (VSMC) were isolated from rats and cultured in DMEM exposed to AngII. Down-regulation and overexpression of Dvl-1 were conducted in cells to explore the role underlying its effects on VSMC proliferation and collagen expression. Adenovirus with overexpressing Dvl-1 was injected into rats to evaluate the role of Dvl-1 in artery injury rats. Results: The results in vivo found that Wnt4, Dvl-1 and β-catenin expression as well as collagen volume fraction (CVF) in injured artery were significantly increased. The results in vitro showed that Dvl-1 overexpression reversed the treatment effects of atorvastatin on VSMCs proliferation and collagen expression. It was also canceled by overexpressing Dvl-1 that the decrease of β-catenin protein treated with atorvastatin in cells exposed to AngII. In addition, treated artery injury rats with atorvastatin, the group with injection of Ad-Dvl-1 had higher levels of intima thickness, intimal/medial area ratio and CVF. Conclusion: Dvl-1 was probably a key regulator in the pathway of wnt4/β-catenin to take part in the vascular restenosis partly, and Dvl-1 is a potential gene to anti- restenosis.
Keywords: Restenosis, wnt, disheveled-1, β-catenin, statin, vascular smooth muscle cells, rat
Introduction
Although the restenosis rate has been reduced dramatically in the drug-eluting stent (DES) era [1-3], it is still a major limitation for the long-term prognosis after percutaneous coronary intervention (PCI). Intensive study in the genetic mechanisms of vascular restenosis after injury may be a key to resolve the problem ultimately.
Base on the fact that mass SMC can be seen proliferating in artery intima after PTCA; most scholars believe that vascular restenosis after PTCA is reduced by neointimal thickening, which smooth muscle proliferation; migration and formation of large extracellular matrix secretion contribute to. Previous experiments conformed some drugs can ease vascular restenosis after PCI by inhibiting SMC proliferation.
It is well known that Wnt signal pathway is involved in the pathophysiology of many tumors [4,5]. The role of Wnt signal pathway in vascular restenosis has recently been recognized [6,7]. Wnt signal pathway are a group of proteins that perform signal transduction in and out of cells through cell surface receptors. β-catenin acts as a downstream signaling molecule of Wnt pathway to enters into the nucleus and activate the Wnt signaling pathway [8]. Embryonic and developmental study showed Wnt signal pathway activated in the early stage of VSMCs formation [9]. The inhibition of Wnt canonical pathway lead to reduced VSMC proliferation [10]. It was also reported that the β-catenin/TCF signal in a rat carotid artery injury model was activated [11].
The family of disheveled (dvl) are cytoplasmic scaffold protein, contains three members in human encoded genes, which including dvl-1, dvl-2 and dvl-3. Dvl is a key component in Wnt signaling to both the β-catenin-dependent and β-catenin-independent pathways [12], playing important role in cell proliferation. However, if Wnt/β-catenin mediated by dvl-1 is involved in VSMC is still unknown.
Atorvastatin effectively alleviates the vascular smooth muscle cell proliferation and reduces the incidence of cardiovascular disease [13,14], which has been widely reported. In this study, treatment of atorvastatin was used to be positive control to evaluate the dvl-1 effect on artery restenosis and explore the mechanisms of vascular restenosis. The rat model of balloon-injured artery restenosis and cultured VSMC from rat aorta exposed to AngII were employed to determine whether dvl-1 is involved in the Wnt4/β-catenin signaling pathway to participate in the mechanisms of vascular restenosis.
Materials and methods
Animal models of intimal thickening
32 SD rats (weighted 350~400 g, purchased from the Animal Experiment Center of Zhejiang Chinese Medicine University) were randomized into 3 groups: ① operation group (n = 6), anesthesia was induced by intraperitoneal injection of 30 mg/kg pentobarbital, the proximal ends of the right common carotid arteries were occluded by vascular clamps temporarily. A 1.5×12 mm PTCA balloon catheter was inserted gently from the incision on external carotid arteries to common carotid arteries of about 2.0 cm, then the balloon was inflated at 5 atm, drew back and pushed forward for 5 times. Then withdrew the catheter and ligated the external carotid arteries. ② sham operation group (n = 8), only received the common carotid arteries clamp and external carotid arteries ligation. ③ atorvastatin group (n = 7): 20 mg/kg/d atorvastatin was given intragastricly 1 week before balloon injury and 6 weeks later. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication No. 93-23, revised 1985) and were approved by the Institutional Care and Use Committee.
Histochemistry and immunohistochemistry
Ligated carotid arteries were removed at 2 or 6 weeks after balloon injury and embedded longitudinally in paraffin wax to assess intimal lesion size by HE staining. Image analysis was performed by Image Pro Plus 6.0 software, and the intimal/medial ratio was calculated: Intimal thickness = (internal plastic layer surrounding area/π)1/2-(luminal area/π)1/2, medial thickness = (external plastic layer surrounding area/π)1/2-(internal plastic layer surrounding area/π)1/2, neointimal area = internal plastic layer surrounding area-luminal area. Each measurement was taken 3 times to obtain the mean.
Immunohistochemistry was performed on rat carotid artery paraffin-wax-embedded sections using primary antibodies against β-catenin (Rabbit mAb, Epitomics, USA), DVL-1 (Rabbit pAb, Bioss USA), Wnt4 (Rabbit pAb, Bioss USA). Nonimmune IgG was used as a negative control. Biotin labeled goat-anti-rabbit IgG served as second antibody. Wnt4 was located on cellular membrane as well as in cytoplasm, either dvl-1 or β-catenin in cytoplasm. Picric acid-sirius red staining was also performed on rat carotid artery paraffin-wax-embedded sections to stain collagen specifically.
VSMC culture
Aortas were obtained from SD rats, and VSMCs were grown from aortic explants and used at passages 3 to 6. Cells were treated with different manners when cells grew to 75%-85% confluence: ① DMEM ② Angiotensin (Ang) II (Sigma, USA) at 1×10-6 mol/L (determined by pre-study) for 24 h, 48 h or 72 h ③ SiRNA-dvl-1 + lipofectamine2000 (5’-CACGCCUACAAAUUCUU-CUTT-3’; GenePharma, China) for 6 h, then AngII (1×10-6 mol/L) for 24 h, 48 h, 72 h ④ atorvastatin (Pfizer, USA) 0.1 µmol/L, 1 µmol/L, 10 µmol/L for 24 h, 48 h, 72 h ⑤ atorvastatin for 24 h and then 1×10-6 mol/L AngII for another 24 h, 48 h, 72 h ⑥ SiRNA-dvl-1 + lipofectamine2000 and atorvastatin pre-cultured for 24 h, then AngII (1×10-6 mol/L) for another 24 h, 48 h, 72 h. Each group was performed for at least 3 times.
SiRNA to silence dvl-1 gene transcription
Firstly, fluorescently labeled siRNA (FAM-siRNA, negative control) blended with lipofectamine2000 was adopted to determine cell transfection efficiency. Secondly, optimize the siRNA-Dvl-1 oligo among the three siRNA-Dvl-1 oligo provided by Shanghai GenePharma Company. Freeze drying powder of siRNA dissolved in RNase-free water (DEPC) to prepare 20 nM storage solutions. Liposome transfection reagent (lipofectamine2000) of concentration 1 μmol/L was diluted with 50 μl Opti-MEM without serum (This liposome concentration was recommended by the company and showed minimal cytotoxity in the pre experiment), gently mix after incubation for 5 min at room temperature. Different volume of FAM-SiRNA was diluted with 50 μl Opti-MEM without serum to achieve different final concentration in the medium. Gently blended the diluted FAM-siRNA with lipofectamine2000 and standed at room temperature in 20 min to form FAM-siRNA- lipofectamine2000 mixture. The mixture was added into the culture system then incubated on the condition of 37°C, 5% CO2 for 6 h. Cells were washed 2 times with sterile PBS and observed with inverted fluorescence microscopy (excited by blue light, excitation wavelength 480 nm, emission wavelength 520 nm). Transfection efficiency was defined as percentage of bright green fluorescence of the transfected cells to total cells. Optimized the most efficient concentration of FAM-SiRNA. Total mRNA and protein were extracted from cells after transfected 24 h and dvl-1 was assayed by real-time PCR and western blot. Most efficient siRNA-Dvl-1 oligo was selected for later used.
MTT proliferation assay
The SMCs proliferation was determined by MTT assay. After being incubated as above described, EPC were digested with 0.25% trypsin and 0.02% EDTA. After centrifugation and resuspension in DMEM, 10% FBS, 104 cells were cultured in serum-free medium in 96-well culture plate (200 ml per well), while the serum-free medium served as a control. After being cultured for 72 h, SMCs were supplemented with 20 ml MTT (5 g/L) and incubated for another 4 h. Then the supernatant was discarded by aspiration and the SMCs preparation was shaken with 150 ml MDSO for 10 min, before the OD value was measured at 490 nm.
Real-time quantitative PCR
After reverse transcription, cDNA was subjected to quantitative-PCR for several Wnt genes using quantitect primers (β-actin: 5’-TCATCACTATTGGCAACGAGC, 5’-AACAGTCCGCCTAGAAGCAC; Wnt4: 5’-TCAGGTTGGCCACGCACTAAAGGAGAA, 5’-AGTCTGGACTTGGCTCCAGGTACACC; β-catenin: 5’-GCTGACCTGACGGAGTTGGA, 5’-GCTACTTGCTCTTGCGTGAA; Dvl-1: 5’-CCTTCCATCCAAATGTTGC, 5’-GTGACTGACCATAGACTCTGTGC; col I: 5’-TACAGCACGCTTGTGGATG, 5’-TTGAGTTTGGGTTGTTGGTC; col III: 5’-TGATGGGATCCAATGAGGGAGA, 5’-GAGTCTCATGGCCTTGCGTGTTT; Sangon, China) and the real-time PCR Kit (TaKaRa, JAPAN), as described in the instructions of the manufacturer. Quantification was achieved after normalization using 18S ribosomal RNA values. The following primers for 18S ribosomal RNA were used: 5’-CTCGATGCTCTTAGCTGAGT, 5’-CTTCAAACCTCCGACTTTCG (Sigma, USA).
Western blot assay
A total of 20 µg protein was separated on NuPAGE Novex 4-12% Bis-Tris Gel (Invitrogen, Carlsbad, CA, USA) and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% nonfat dry milk in PBS containing 0.05% Tween (PBST) for 1 h. After being washed with PBST, the membrane was separately incubated with primary antibody overnight at 4°C and washed four times and incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz, CA, USA) for 1 h. Film was developed by chemiluminescence (Perkin Elmer, Boston, MA, USA). Primary antibodies against rat Dvl-1 (Santa Cruz, USA), Wnt4 (Proteintech, USA), COL1A1 (C-18; Santa Cruz, USA), COL3A1 (C-15; Santa Cruz, USA) and cyclinD1 (C-20; Santa Cruz, USA) were employed to detect the expression of protein in SMCs. Anti-β-actin (Abcam) was used to normalize loading variability.
Statistical analysis
All data were expressed as mean ± standard deviation (SD). Continuous variable were tested for normal distribution with the Kolmogorov-Smirnov test. Differences between groups were assessed by using one-way analysis of variance (ANOVA), followed by LSD test as post hoc comparison; P < 0.05 was considered significant. All statistical analysis were performed using SPSS 16.0 for Windows.
Results
Establishment of an intimal thickening rat model
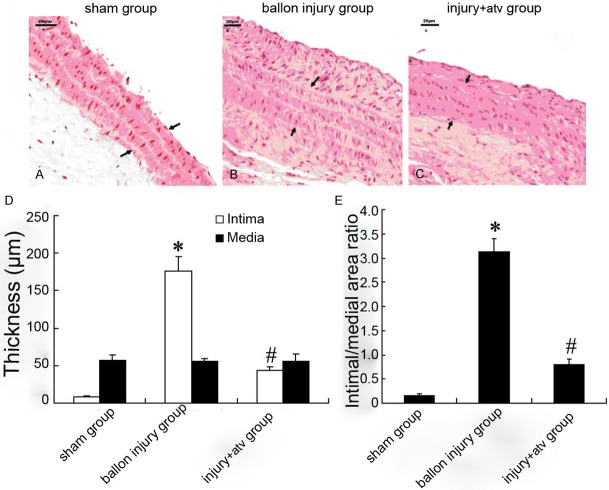
Six weeks after carotid artery balloon injury, HE staining showed that carotid artery intima of rats was markedly thickened, neointima protruding into the lumen, cells and fibers were irregular, and part of the internal elastic plate was damaged seriously with extracellular matrix (ECM) deposition (Figure 1). Compared with the injury group, the atorvastatin significantly decreased the intimal hyperplasia (46.19 ± 4.19 μm vs. 178.56 ± 12.22 μm, P < 0.05), while the external elastic plate was reserved more completely and the neointimal area as well as intima/media area ratio were decreased significantly (0.755 ± 0.045 μm vs. 1.592 ± 0.206 μm, P < 0.05).
Figure 1.
Representative histological of carotid arteries after balloon injury with or without atorvastatin treatment in rat. HE-staining was performed to observe the intima and media of carotid arteries in sham-operation group (A), balloon injury group (B) and atorvastatin group (C). Intimal and medial layer thickness of carotid artery after balloon injury with or without atorvastatin treatment was showed in (D). Intimal/medial area ratio after balloon injury with or without atorvastatin treatment was showed in (E). Bars represent means ± s.d. *: P < 0.05 versus control, #: P < 0.05 versus balloon injury group.
Collagen volume in carotid artery of rats
Picric Sirius red specific staining was used to determine the collagen volume in carotid artery. As showed in Figure 2A, the regular collagen and elastic fibers was visible in carotid artery intima-media organization in sham-operation group, while obvious intimal hyperplasia could be observed in injury model group (Figure 2B). Atorvastatin significantly decreased the collagen volume in intima (Figure 2C). The results of collagen volume integral (CVF) also presented collagen content in model group were increased compared with sham operation group (20.99 ± 3.52% vs. 25.23 ± 4.02%, P < 0.05), and atorvastatin significantly decreased the CVF (18.26 ± 3.21% vs. 25.23 ± 4.02%, P < 0.05) value in vascular intimal after balloon injury (Figure 2D).
Figure 2.
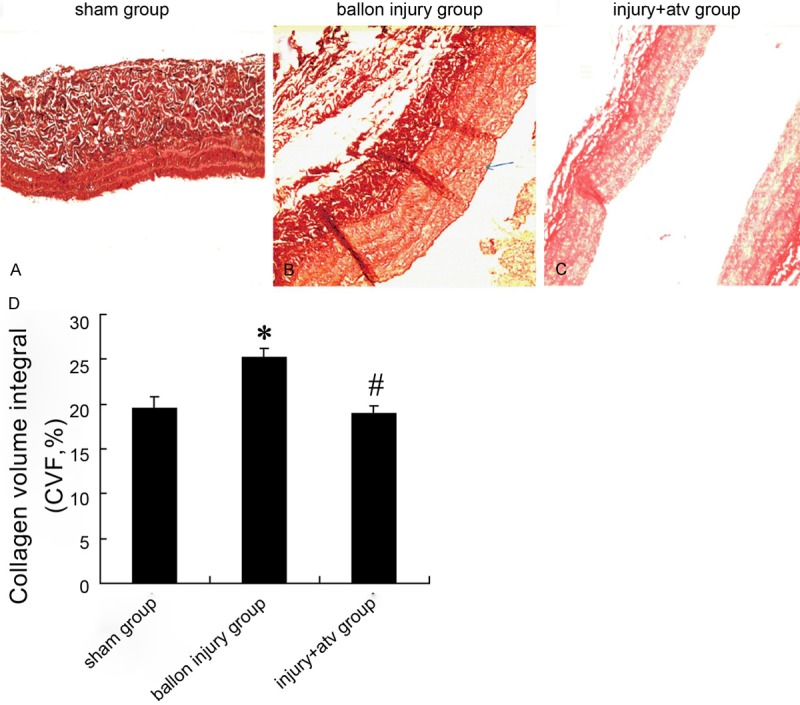
Collagen volume in carotid artery after balloon injury with or without atorvastatin treatment in rat. Picric Sirius red specific staining were used to observe the collagen volume of carotid arteries in sham-operation group (A), balloon injury group (B) and atorvastatin group (C). Collagen volume integral (CVF) of carotid artery after balloon injury with or without atorvastatin treatment were showed in (D). Bars represent means ± s.d. *: P < 0.05 versus control, #: P < 0.05 versus balloon injury group.
Expression of Wnt4/dvl-1/β-catenin and collagen in carotid artery of rats
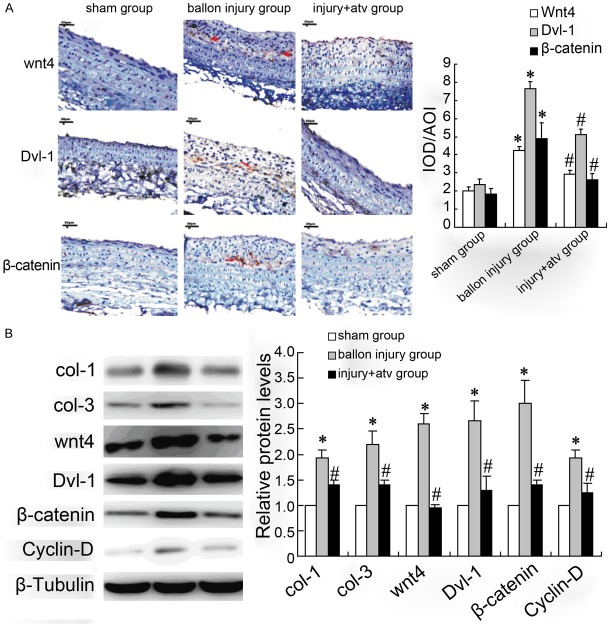
Semi-quantitative immunohistochemistry analysis and western blotting were performed to detect the expression of Wnt4/dvl-1/β-catenin and collagen in carotid artery. The results in Figure 3A, 3B showed that Wnt4/dvl-1/β-catenin in balloon injury group had significant increase than that in sham-operation group, and the injection of atorvastatin significantly down-regulated the Wnt4/Dvl-1/β-catenin proteins expression in the injury site of the rat carotid arteries. In addition, expression of col-1 and col-3 in balloon injury group were up-regulated and atorvastatin reversed this effect (Figure 3B).
Figure 3.
The expression of Wnt4/dvl-1/β-catenin and collagen in carotid artery after balloon injury with or without atorvastatin treatment in rat. Semi-quantitative immunohistochemistry (A) and Western blotting (B) were used to determine the Wnt4/dvl-1/β-catenin and col-1/col-3 expression in sham-operation group, balloon injury group and atorvastatin group. Bars represent means ± s.d. *: P < 0.05 versus control, #: P < 0.05 versus balloon injury group.
Dvl-1 involves in the atorvastatin regulation of VSMCs proliferation exposed to AngII
To investigate the role of Dvl-1 and Wnt signaling in restenosis, cell experiments were employed. We started with evaluating the effect of atorvastatin on growth of vascular smooth muscle cells (VSMCs) exposed to AngII. As showed in Figure 4A, AngII (10-6 mol/L) significantly induced the growth of VSMCs, and atorvastatin reversed this effect. Overexpressing Dvl-1 plasmid was transfected into VSMCs, the viability was significantly elevated in atorvastatin treated cells exposed to AngII. Transfected VSMCs with pcDNA-Dvl-1 in the absence of AngII and atorvastatin, the cell viability was slightly higher than control pcDNA group. In addition, the down-regulation of Dvl-1 by siRNA was conducted in VSMCs, the results showed that si-Dvl-1 significantly reduced the cell viability induced by AngII. Si-Dvl-1 also markedly decreased VSMCs viability alone.
Figure 4.
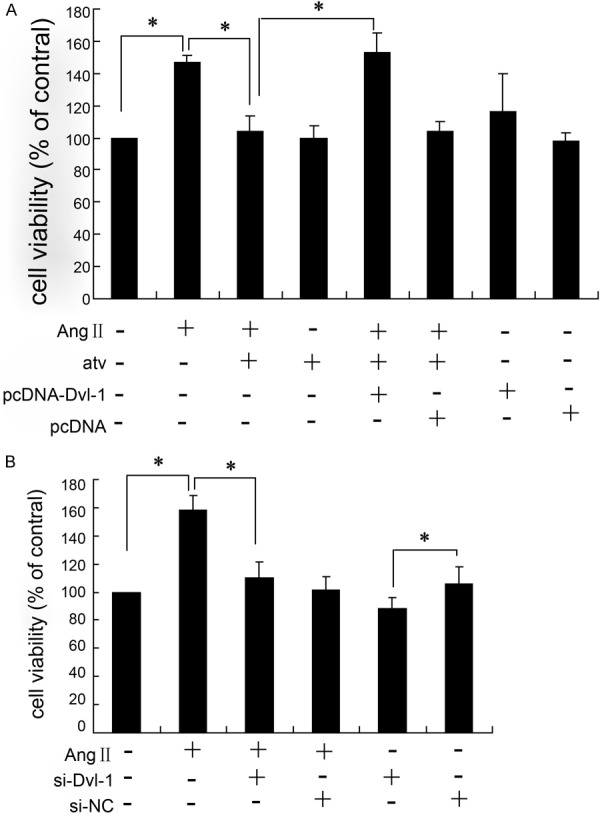
Dvl-1 involves in the atorvastatin regulation of VSMCs proliferation exposed to AngII. MTT assay was used to determine the viability of VSMCs treated with different interventions for 24 h; all results were repeated for three times; *: P < 0.05.
Dvl-1 involves in the atorvastatin regulation of collagen expression inVSMCs exposed to AngII
Collagen expression was detected by western blotting as shown in Figure 5. The VSMCs exposed to AngII exhibited a higher expression of col-1, col-3, β-catenin and Cyclin-D compared with the level in control, and atorvastatin significantly decreased the protein expression of them in cells with AngII treatment. However, the effect of atorvastatin on collagen expression was reversed by overexpressing Dvl-1 (Figure 5A). The down-regulation of Dvl-1 significantly reduced the col-1, col-3, β-catenin and Cyclin-D expression induced by AngII in VSMCs (Figure 5B).
Figure 5.
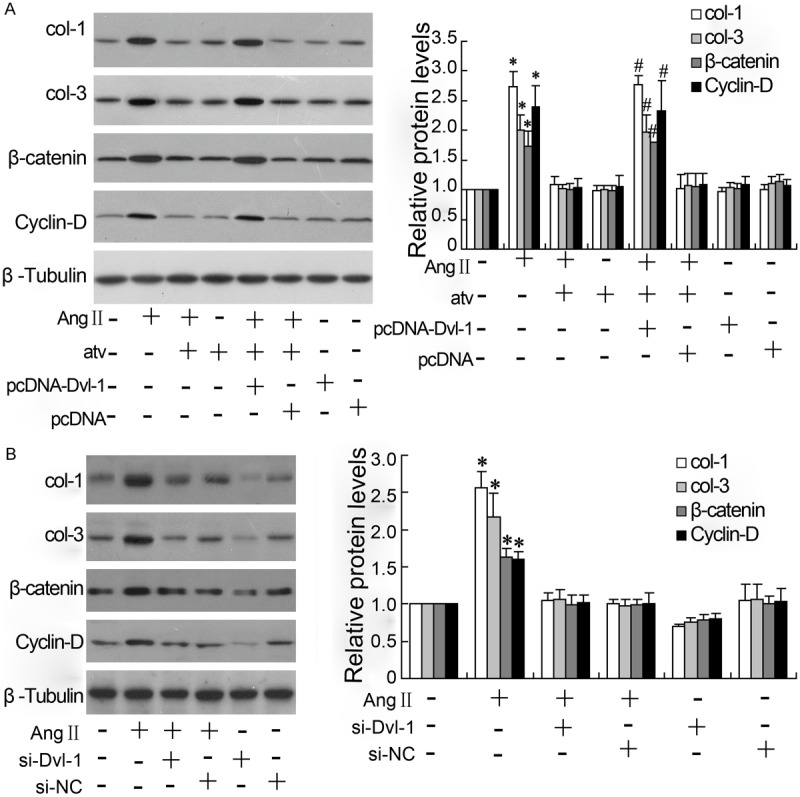
Dvl-1 involves in the atorvastatin regulation of collagen and β-catenin expression in VSMCs exposed to AngII. The col-1, col-3, β-catenin and Cyclin-D were detected by western blotting, and with β-Tubulin as control protein; all results were repeated for three times; *: P < 0.05 vs control without treatment; #: P < 0.05 vs group treated with AngII and atorvastatin.
Effect of si-dvl-1 on the Wnt/dvl-1/β-catenin expression of VSMCs exposed to AngII
As showed in Figure 6A, 6B, siRNA-dvl-1 effectively silenced the expression of dvl-1 both in mRNA and protein levels. Treatment with atorvastatin (1 µmol/L and 10 µmol/L) down-regulated the expression of Wnt/dvl-1/β-catenin in VSMCs exposed to AngII in a dose-dependent manner. siRNA-dvl-1 also decreased the mRNA and protein expression of dvl-1 and β-catenin, which was more effective than 10 µmol/L atorvastatin treatment.
Figure 6.
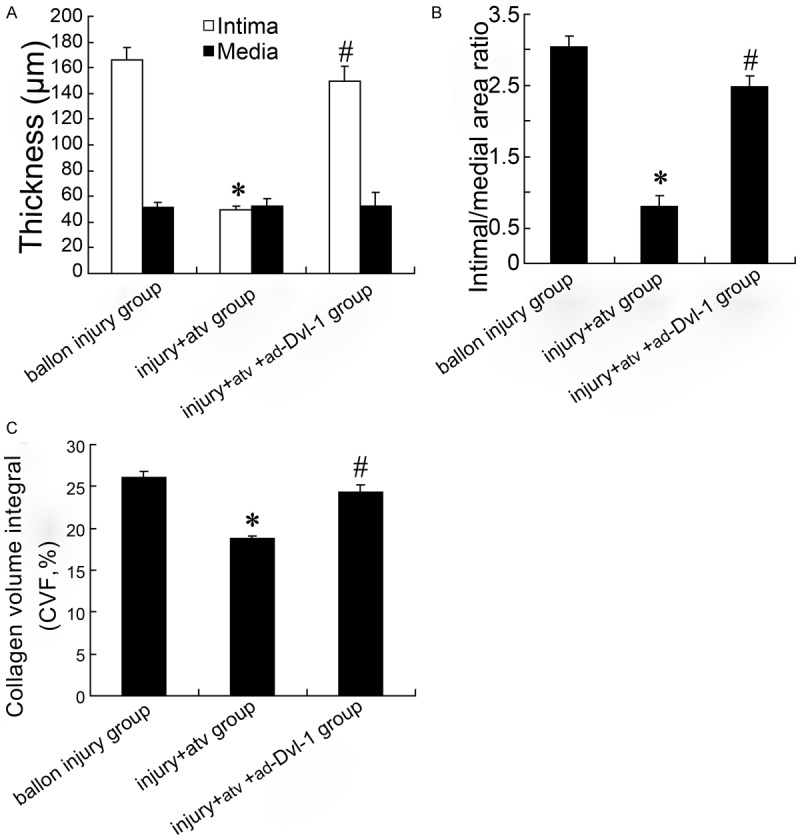
Overexpressing Dvl-1 involves in restenosis of balloon injury rat. Intima and media thickness of rat carotid artery were showed in (A); Intimal/medial area ratio of rat carotid artery was showed in (B); Collagen volume integral (CVF) of carotid artery was showed in (C). *: P < 0.05 versus balloon injury group; #: P < 0.05 versus balloon injury + atorvastatin group.
Discussion
Most scholars believe abnormal proliferation and migration of VSMCs in media result in the balloon-injured artery restenosis. When intima was injured by balloon, VSMCs in media may switch from contractile to synthetic phenotype following proliferation, migration and collagen secretion under effects of many cytokines or growth factors. In this complicated biological processes, changes in gene expression of VSMC were inevitable.
At present, the studies on the fields of tumor and embryonic development suggest that Wnt signaling pathway is involved in cellular proliferation, differentiation and apoptosis [15-18]. Recent studies showed that Wnt signaling pathway may also play a role in vascular restenosis [10,11,19,20]. Tsaousi et al. [7] referred that the mRNA expression of Wnt2 and Wnt4 were increased in process of VSMCs proliferation, but only up-regulation of Wnt4 protein expression played a key role in this process. Therefore, the Wnt4 was determined to be discussed in this study. Canonical and non-canonical Wnt pathways were contained in Wnt signaling. B-catenin is regarded as a key gene of canonical Wnt signaling pathway and the increased intracellular β-catenin has an effect on its downstream target gene cyclin D1, regulating the proliferation of SMC [10]. Dvl is a key component in canonical and non-canonical Wnt pathways, transmitting signals to downstream effectors from receptors, which affecting cell proliferation and migration. It is reported that dvl is involved in the formation of retinal neovascularization in retinopathy [21]. However, few reports have been published regarding the effect of dvl on VSMCs.
In vivo experiments, the pathological intimal hyperplasia appeared at 2nd week after balloon injured (not shown in the article) and was aggravated significantly at 6th weeks, some VSMCs enlarged and disarranged with massive extracellular matrix deposition among them, which indicated rats model of balloon-injured carotid artery restenosis had been made successfully. Immunohistochemistry showed that expression of dvl-1 as well as Wnt4 and β-catenin in hyperplasia intima and media were increased remarkably, especially near the internal elastic lamina where is the junction of intima and media. The pathological intimal hyperplasia was significantly reduced in the carotid artery balloon injury site by atorvastatin treatment, and the expression of dvl-1, Wnt4, β-catenin and collagen by immunohistochemistry were also down-regulated in atorvastatin group. The results above indicated that up-regulation of Wnt4 signaling pathway modulates balloon-injured artery restenosis via promoting VSMCs proliferation and collagen secretion. This pathological changes can be relieved by down-regulating Wnt signaling pathway.
AngII was used to induce the VSMCs proliferation in vitro experiments. It was proved that the expression of wnt4/Dvl-1/β-catenin in mRNA and protein levels were significantly increased in VSMCs exposed to AngII, which was consistent with the results in vivo experiments. To investigate whether dvl-1 is involved in the Wnt4/β-catenin signaling pathway to participate in the mechanisms of VSMCs proliferation, siRNA-dvl-1 was employed to silence the expression of dvl-1. As expected, the expression of Wnt4 and β-catenin were decreased by down-regulation of dvl-1. In addition, siRNA-dvl-1 also inhibited the expression of col-1 and col-3 in VSMCs, implying dvl-1 is involved in the production of collagen in VSMCs. In the other hand, atorvastatin had an inhibitory effect on cell proliferation, Wnt4/dvl-1/β-catenin and collagen expression in cultured VSMCs exposed to AngII in dose-dependent manner. Compared with atorvastatin treatment, siRNA-dvl-1 was more effective to inhibit the expression of Wnt4//β-catenin and collagen in cells. Accordingly, we thought Wnt signaling pathway mediated by dvl-1 is involved in the AngII-induced proliferation of VSMC. Additionally, VSMCs proliferation could be further inhibited by siRNA-dvl-1 combined with atorvastatin, which suggesting besides canonical Wnt pathway, noncanonical Wnt signaling pathway and any other signaling pathways [22,23] as well were part of AngII-induced SMC proliferation.
Chen et al [24] found dvl-1 was of importance in wound-healing response to myocardial infaction through effecting on proliferation and migration of fibroblast and vascular endothelial cell. Therefore, up-regulation of Wnt signals in injured vascular may have many other effects on various cells in vascular wall besides VSMC. Studies have shown that overexpression of MMP-2 in the injured vascular attributed to SMC migration and vascular remodeling [25]. Wu et al [26] reported Wnt/β-catenin/TCF/LEF pathway induced remarkable increasing of MMPs through acting directly on MMPs promoter, which favor T cells migration through basement membrane. The degradation of extracellular matrix is critical to VSMC migration in vascular wall. Accordingly, Wnt molecules expression nearby internal elastic lamina suggested Wnt pathway may have dual role on VSMCs, on the one hand, it promotes proliferation and collagen secretion of VSMC. On the other hand, it induces ECM degradation to facilitate VSMC migration toward intima. This seems to be interpreted why VSMCs inhabit in media can migrate toward intima and why not media but intima become thickening in process of restenosis.
At present, injured vascular restenosis is a pathological process with excessive inflammatory vascular responses [26-28]. Various Inflammatory factors and cytokines released during inflammation can further induce abnormal expression of certain genes of some histiocytes, which aggravate or bring about new pathological changes. Statins plays a role in anti-atherosclerosis and anti-restenosis because of its anti-inflammatory effect. The experiment showed that siRNA-dvl-1 and atorvastatin inhibit vascular restenosis partly through down-regulation Wnt signaling pathway. However, further studies are needed to make clear whether dvl is involved in the mechanism of restenosis and VSMC proliferation by anti-inflammation effect or direct effect.
Acknowledgements
This work was supported by Health Bureau of Zhejiang province and (No. 2010KWA155) and Administration of Traditional Chinese Medicine of Zhejiang province (No. 2100ZA027).
Disclosure of conflict of interest
None.
References
- 1.Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Popma JJ, Russell ME TAXUS-IV Investigators. A polymer-based, paclitaxel- eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–31. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 2.Ozer N, Tangurek B, Firat F, Ozer S, Tartan Z, Ozturk R, Ozay B, Ciloglu F, Yilmaz H, Cam N. Effects of drug-eluting stents on systemic inflammatory response in patients with unstable angina pectoris undergoing percutaneous coronary intervention. Heart Vessels. 2008;23:75–82. doi: 10.1007/s00380-007-1020-y. [DOI] [PubMed] [Google Scholar]
- 3.Patti G, Nusca A, Di Sciascio G. Meta-analysis comparison (nine trials) of outcomes with drug-eluting stents versus bare metal stents in patients with diabetes mellitus. Am J Cardiol. 2008;102:1328–34. doi: 10.1016/j.amjcard.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Sebio A, Kahn M, Lenz HJ. The potential of targeting wnt/beta-catenin in colon cancer. Expert Opin Ther Targets. 2014;18:611–5. doi: 10.1517/14728222.2014.906580. [DOI] [PubMed] [Google Scholar]
- 5.Ramachandran I, Thavathiru E, Ramalingam S, Natarajan G, Mills WK, Benbrook DM, Zuna R, Lightfoot S, Reis A, Anant S, Queimado L. Wnt inhibitory factor 1 induces apoptosis and inhibits cervical cancer growth, invasion and angiogenesis in vivo. Oncogene. 2012;31:2725–37. doi: 10.1038/onc.2011.455. [DOI] [PubMed] [Google Scholar]
- 6.Slater SC, Koutsouki E, Jackson CL, Bush RC, Angelini GD, Newby AC, George SJ. R-cadherin: beta-catenin complex and its association with vascular smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol. 2004;24:1204–10. doi: 10.1161/01.ATV.0000130464.24599.e0. [DOI] [PubMed] [Google Scholar]
- 7.Tsaousi A, Williams H, Lyon CA, Taylor V, Swain A, Johnson JL, George SJ. Wnt4/β-catenin signaling induces VSMC proliferation and is associated with intimal thickening. Circ Res. 2011;108:427–36. doi: 10.1161/CIRCRESAHA.110.233999. [DOI] [PubMed] [Google Scholar]
- 8.Kim W, Kim M, Jho EH. Wnt/Beta-catenin signalling: from plasma membrane to nucleus. Biochem J. 2013;450:9–21. doi: 10.1042/BJ20121284. [DOI] [PubMed] [Google Scholar]
- 9.Merki E, Zamora M, Raya A, Kawakami Y, Wang J, Zhang X, Burch J, Kubalak SW, Kaliman P, Izpisua Belmonte JC, Chien KR, Ruiz-Lozano P. Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci U S A. 2005;102:18455–60. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quasnichka H, Slater SC, Beeching CA, Boehm M, Sala-Newby GB, George SJ. Regulation of smooth muscle cell proliferation by beta-catenin/T-Cell factor signaling involves modulation of cyclin D1 and P21 expression. Circ Res. 2006;99:1329–37. doi: 10.1161/01.RES.0000253533.65446.33. [DOI] [PubMed] [Google Scholar]
- 11.Corada M, Nyqvist D, Orsenigo F, Caprini A, Giampietro C, Taketo MM, Iruela-Arispe ML, Adams RH, Dejana E. The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev Cell. 2010;18:938–49. doi: 10.1016/j.devcel.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao C, Chen YG. Dishevelled: the hub of wnt signaling. Cell Signal. 2010;22:717–27. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Liu D, Cui W, Liu B, Hu H, Liu J, Xie R, Yang X, Gu G, Zhang J, Zheng H. Atorvastatin Protects Vascular Smooth Muscle Cells from Tgf-Beta1-Stimulated Calcification by Inducing Autophagy Via Suppression of the Beta-Catenin Pathway. Cell Physiol Biochem. 2014;33:129–41. doi: 10.1159/000356656. [DOI] [PubMed] [Google Scholar]
- 14.Shyu KG, Chen SC, Wang BW, Cheng WP, Hung HF. Mechanism of the inhibitory effect of atorvastatin on leptin expression induced by angiotensin ii in cultured human coronary artery smooth muscle cells. Clin Sci (Lond) 2012;122:33–42. doi: 10.1042/CS20110114. [DOI] [PubMed] [Google Scholar]
- 15.Kim YM, Kim IH, Nam TJ. Capsosiphon fulvescens glycoprotein inhibits ags gastric cancer cell proliferation by downregulating Wnt-1 signaling. Int J Oncol. 2013;43:1395–401. doi: 10.3892/ijo.2013.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terada K, Misao S, Katase N, Nishimatsu S, Nohno T. Interaction of wnt signaling with bmp/smad signaling during the transition from cell proliferation to myogenic differentiation in mouse myoblast-derived cells. Int J Cell Biol. 2013;2013:616294. doi: 10.1155/2013/616294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toivonen S, Lundin K, Balboa D, Ustinov J, Tamminen K, Palgi J, Trokovic R, Tuuri T, Otonkoski T. Activin A and Wnt-dependent specification of human definitive endoderm cells. Exp Cell Res. 2013;319:2535–44. doi: 10.1016/j.yexcr.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Coluccia AM, Vacca A, Mireia L, Dunach M, Mologni L, Redaelli S, Bustos VH, Benati D, Pinna LA, Gambacorti-Passerini C. Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J. 2007;26:1456–66. doi: 10.1038/sj.emboj.7601485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsaousi A, Williams H, Lyon CA, Taylor V, Swain A, Johnson JL, George SJ. Wnt4/β-catenin signaling induces VSMC proliferation and is associated with intimal thickening. Circ Res. 2011;108:427–36. doi: 10.1161/CIRCRESAHA.110.233999. [DOI] [PubMed] [Google Scholar]
- 20.Hilker M, Längin T, Hake U, Schmid FX, Kuroczynski W, Lehr HA, Oelert H, Buerke M. Gene expression profiling of human stenotic aorto-coronary bypass grafts by cdna array analysis. Eur J Cardiothorac Surg. 2003;23:620–5. doi: 10.1016/s1010-7940(03)00017-4. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Stahl A, Krah NM, Seaward MR, Dennison RJ, Sapieha P, Hua J, Hatton CJ, Juan AM, Aderman CM, Willett KL, Guerin KI, Mammoto A, Campbell M, Smith LE. Wnt signaling mediates pathological vascular growth in proliferative retinopathy. Circulation. 2011;124:1871–81. doi: 10.1161/CIRCULATIONAHA.111.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozasa Y, Akazawa H, Qin Y, Tateno K, Ito K, Kudo-Sakamoto Y, Yano M, Yabumoto C, Naito AT, Oka T, Lee JK, Minamino T, Nagai T, Kobayashi Y, Komuro I. Notch activation mediates angiotensin II-induced vascular remodeling by promoting the proliferation and migration of vascular smooth muscle cells. Hypertens Res. 2013;36:859–65. doi: 10.1038/hr.2013.52. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues Díez R, Rodrigues-Díez R, Lavoz C, Rayego-Mateos S, Civantos E, Rodríguez-Vita J, Mezzano S, Ortiz A, Egido J, Ruiz-Ortega M. Statins inhibit angiotensin II/smad pathway and related vascular fibrosis, by a tgf-beta-independent process. PLoS One. 2010;5:e14145. doi: 10.1371/journal.pone.0014145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, Wu Q, Guo F, Xia B, Zuo J. Expression of Dishevelled-1 in wound healing after acute myocardial infarction: possible involvement in myofibroblast proliferation and migration. J Cell Mol Med. 2004;8:257–64. doi: 10.1111/j.1582-4934.2004.tb00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park ES, Lee KP, Jung SH, Lee DY, Won KJ, Yun YP, Kim B. Compound K, an intestinal metabolite of ginsenosides, inhibits PDGF-BB-induced VSMC proliferation and migration through G1 arrest and attenuates neointimal hyperplasia afterarterial injury. Atherosclerosis. 2013;228:53–60. doi: 10.1016/j.atherosclerosis.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26:227–39. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molica F, Burger F, Thomas A, Staub C, Tailleux A, Staels B, Pelli G, Zimmer A, Cravatt B, Matter CM, Pacher P, Steffens S. Endogenous cannabinoid receptor CB1 activation promotes vascular smooth-muscle cell proliferation and neointima formation. J Lipid Res. 2013;54:1360–8. doi: 10.1194/jlr.M035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong J, Zhang J, Li L, Jiang G, Wang X, Liu X, Yu B. Urinary trypsin inhibitor reduced neointimal hyperplasia induced by systemic inflammation after balloon injury in rabbits. Inflamm Res. 2013;62:173–9. doi: 10.1007/s00011-012-0568-x. [DOI] [PubMed] [Google Scholar]