Abstract
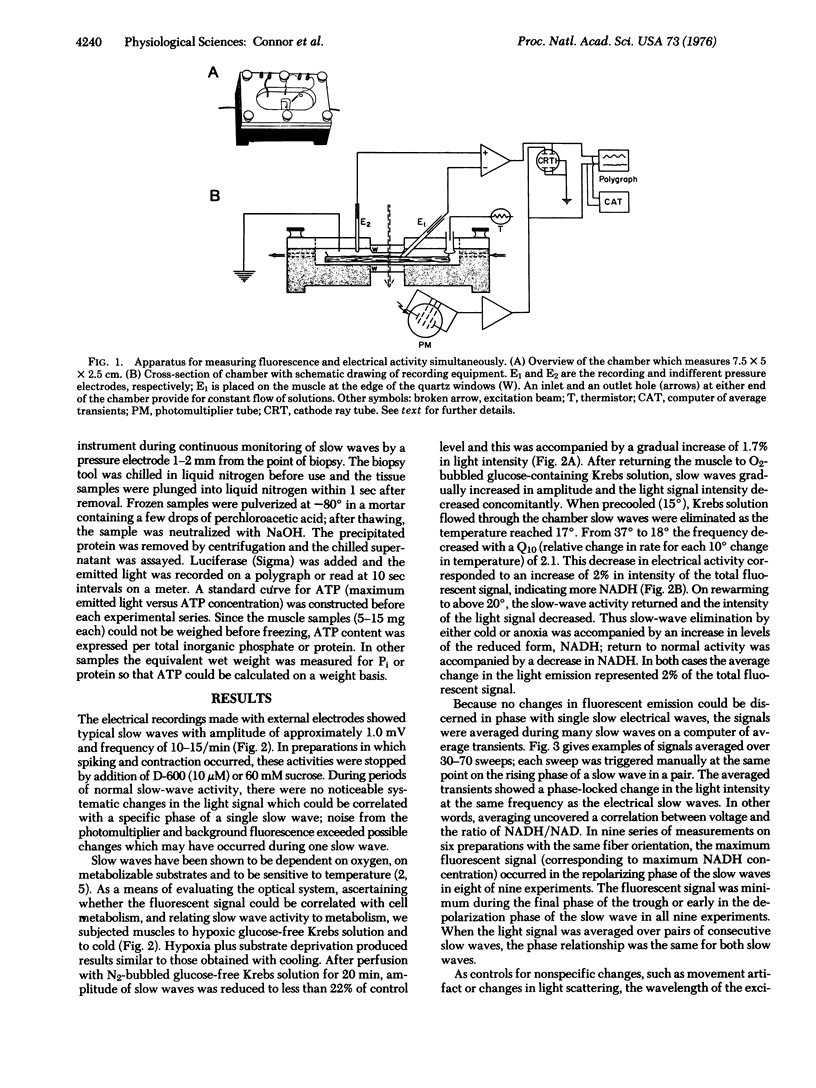
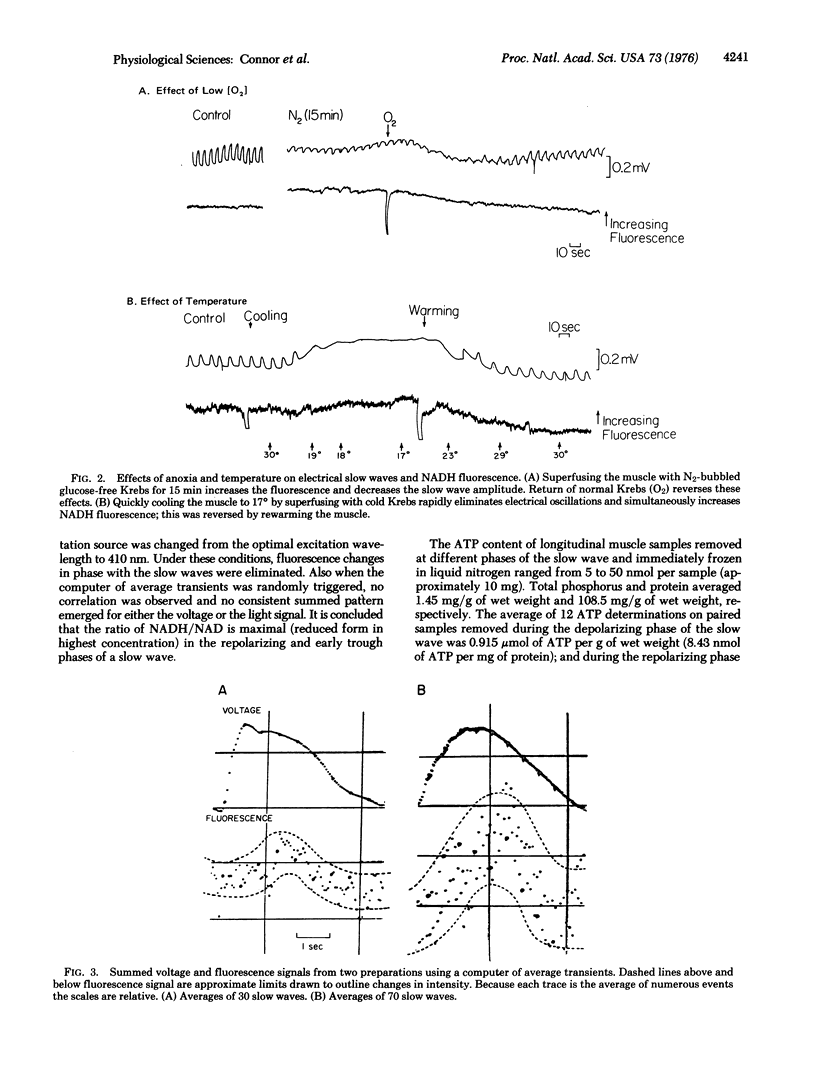
Intact muscle layers separated from the small intestine of the cat were mounted in a specially designed chamber to measure electrical slow waves and NADH fluorescence simultaneously. Cooling the muscle to 17 degrees eliminated slow waves and simultaneously increased the level of fluorescence. Likewise, superfusing the muscle with a N2-bubbled glucose-free Krebs solution decreased the amplitude of slow waves and concomitantly increased fluorescence emission. In both cases, return to normal conditions reversed the effects on both slow waves and fluorescence. When signals were averaged over 30-70 slow waves, a pattern emerged with the fluorescence oscillations in phase with the electric oscillations. The NADH:NAD+ ratio reached a maximum at the most depolarized point of the slow waves and a minimum at the most polarized point between slow waves. This indicates maximum ATP utilization during the repolarization process. The correlation between redox oscillations and electrical slow wave generation is associated with cell metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BETZ A., CHANCE B. INFLUENCE OF INHIBITORS AND TEMPERATURE ON THE OSCILLATION OF REDUCED PYRIDINE NUCLEOTIDES IN YEAST CELLS. Arch Biochem Biophys. 1965 Mar;109:579–584. doi: 10.1016/0003-9861(65)90403-0. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Widdicombe J. H. An estimate of sodium-potassium pump activity and the number of pump sites in the smooth muscle of the guinea-pig taenia coli, using (3H)ouabain. J Physiol. 1974 Apr;238(2):235–249. doi: 10.1113/jphysiol.1974.sp010521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueding E., Bülbring E., Gercken G., Hawkins J. T., Kuriyama H. The effect of adrenaline on the adenosine otriphosphate and creatine phosphate content of intestinal smooth muscle. J Physiol. 1967 Nov;193(1):187–212. doi: 10.1113/jphysiol.1967.sp008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor C., Prosser C. L. Comparison of ionic effects on longitudinal and circular muscle of cat jejunum. Am J Physiol. 1974 May;226(5):1212–1218. doi: 10.1152/ajplegacy.1974.226.5.1212. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Prosser C. L., Weems W. A. A study of pace-maker activity in intestinal smooth muscle. J Physiol. 1974 Aug;240(3):671–701. doi: 10.1113/jphysiol.1974.sp010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradmann D., Slayman C. L. Oscillations of an electrogenic pump in the plasma membrane of Neurospora. J Membr Biol. 1975 Aug 29;23(2):181–212. doi: 10.1007/BF01870250. [DOI] [PubMed] [Google Scholar]
- Job D. D. Effect of antibiotics and selective inhibitors of ATP on intestinal slow waves. Am J Physiol. 1971 Feb;220(2):299–306. doi: 10.1152/ajplegacy.1971.220.2.299. [DOI] [PubMed] [Google Scholar]
- Job D. D. Ionic basis of intestinal electrical activity. Am J Physiol. 1969 Nov;217(5):1534–1541. doi: 10.1152/ajplegacy.1969.217.5.1534. [DOI] [PubMed] [Google Scholar]
- Liu J., Prosser C. L., Job D. D. Ionic dependence of slow waves and spikes in intestinal muscle. Am J Physiol. 1969 Nov;217(5):1542–1547. doi: 10.1152/ajplegacy.1969.217.5.1542. [DOI] [PubMed] [Google Scholar]
- NAGAI T., PROSSER C. L. Electrical parameters of smooth muscle cells. Am J Physiol. 1963 May;204:915–924. doi: 10.1152/ajplegacy.1963.204.5.915. [DOI] [PubMed] [Google Scholar]
- Pye K., Chance B. Sustained sinusoidal oscillations of reduced pyridine nucleotide in a cell-free extract of Saccharomyces carlsbergensis. Proc Natl Acad Sci U S A. 1966 Apr;55(4):888–894. doi: 10.1073/pnas.55.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehler B. L. Bioluminescence assay: principles and practice. Methods Biochem Anal. 1968;16:99–181. doi: 10.1002/9780470110348.ch2. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]


