Abstract
We investigated the anti-inflammatory properties of chlorogenic acid (CGA) in interleukin-1β-induced chondrocytes. The nitric oxide (NO) and prostaglandin E2 (PGE2) were detected by Griess and Enzyme-linked immunosorbent assay (ELISA) respectively. Quantitative real-time PCR and western blot were performed to measure the expression of inducible NO synthase (iNOS) and cyclooxygenase (COX)-2. Our results indicate that CGA inhibited the production of NO and PGE2 as well as the expression of iNOS and COX-2 in chondrocytes. Our data suggest that CGA possess potential value in the treatment of OA.
Keywords: Chlorogenic acid, inducible NO synthase, cyclooxygenase, chondrocyte
Introduction
Osteoarthritis (OA) is the most disorder of the joint which is characterized by cartilage damage progressively. It is well known that matrix metalloproteinases (MMPs) is the main mediator in cartilage degradation in OA [1]. However, the importance of inflammation in OA is also recognized [2]. The pro-inflammatory cytokine interleukin-1β (IL-1β) plays a critical role in OA progression. Previous studies show that IL-1β can trigger a cascade of inflammation events in OA [3], it can also induce the expression of MMPs, leading to cartilage matrix degradation [4]. In addition, IL-1β can induce the production of nitric oxide (NO) and prostaglandin E2 (PGE2), two inflammatory mediators which are associated with cartilage degradation in OA [5].
Nonsteroidal anti-inflammatory drugs (NSAIDs) were wide-spread used in treatment of OA to reduce the pain of joint. These drugs can exert side effect for long-term use, thus, it is necessary to find new agent for alternative treatment in OA.
Chlorogenic acid (CGA) is a type of polyphenol which is rich in many plants. CGA been reported to possess antioxidative, anti-cancer and anti-inflammatory properties in vitro and in vivo [6,7]. In previous study, we found that CGA reduced the MMPs expression in chondrocytes [8]. However, the effect of CGA on IL-1β-induced inflammatory responses of chondrocytes is still unclear. In the present study, we investigated the effect of CGA on the expression of inflammatory mediators in IL-1β-induced chondrocytes.
Materials and methods
Reagents and antibodies
Recombinant human IL-1β and CGA was purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium DMEM penicillin, streptomycin, fetal bovine serum (FBS), 0.25% trypsin, and collagenase II were purchased from Gibco BRL (Grand Island, NY, USA). Monoclonal antibodies for Inducible NO synthase (iNOS), Cyclooxygenase-2 (COX-2), β-actin and peroxidase-conjugated secondary antibody were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Preparation of chondrocytes
This study was approved by the local Ethics Committee. Cartilage was obtained from OA patients undergoing total knee arthroplasty after obtaining informed consents from patients. Chondrocytes were isolated and cultured as previously described [9]. In brief, Cartilage received enzymatic digestion with 0.25% trypsin for 30 min and 2 mg/ml collagenase II for 6 hours. Cells were cultured in DMEM with 10% FBS and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) at 37°C with 5% CO2 and 95% air. Cells from passages 3-4 were used in the experiment.
Measurements of PGE2 and NO production
Chondrocytes were cultured in 6-well plate, cells were serum starved, pre-treated with different concentrations of CGA for 1 h followed by stimulation with IL-1β (10 ng/ml) for 24 h. culture supernatants were collected. For PGE2 production, ELISA kit was used and Griess reaction was performed for NO production, Cells were collected for real-time polymerase chain reaction (PCR) and western blotting.
Quantitative real-time polymerase chain reaction (PCR)
Quantitative PCR was performed as previously reported [9]. In brief, total RNA was isolated by TRIzol reagents (Invitrogen, Carlsbad, CA, USA). Reverse transcription was performed to obtain cDNA. Then, quantitative real-time PCR were performed using an iCycler apparatus system (Bio-Rad). The primer sequences were as follows: PCR as described above. The primers used were as follows: for iNOS: 5’-CTCAGCAGCATCCACGCCAAGAA-3’ and 5’-CCTGAGAAGGACGAGTGGACAT-3’; for COX-2: 5’-CAGCAAAGGCTACTTGGATCAGG-3’ and 5’-CCTGAGAAGGACGAGTGGACAT-3’; for GAPDH: 5’-CAACGGCACAGTCAAGGCTGAGA-3’ and 5’-CTCAGCACCAGCATCACCCCAT-3’; GAPDH was used as an internal control. The real-time PCR data were quantified using the ΔCT method with the formula: n = 100 × 2-(ΔCT targeted gene-ΔCT GAPDH).
Western blotting
Chondrocytes were washed with PBS, collected by scraper and lysed using the cell lysis buffer. Then, the lysates were resolved in SDS-PAGE, transferred to PVDF membranes, probed with primary antibodies for iNOS and COX-2 overnight at 4°C. After washing, membranes were incubated with HRP-linked secondary antibodies for 1 h at room temperature. Membranes were visualized by Enhanced Chemiluminescence (ECL) kit and exposed to X-ray films (Kodak, Hangzhou, China).
Statistical analysis
Data are expressed as the mean ± standard deviation (SD) and were analyzed statistically using one-way ANOVA. Differences were considered significant when P values less than 0.05.
Results
Effects of CGA on NO and PGE2 production in chondrocytes
We investigated whether CGA had inhibitory effect on IL-1β-induced NO and PGE2 production. Our results showed that CGA suppressed IL-1β-induced NO and PGE2 production (Figure 1).
Figure 1.
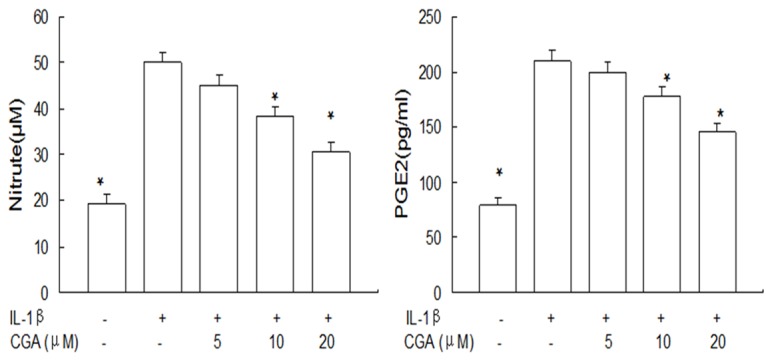
Effects of CGA on NO and PGE2 production in IL-1β-induced chondrocytes. Cells were pre-treated with 5-20 μM of CGA for 1 h, followed with or without IL-1β (10 ng/ml) for 24 h. NO and PGE2 production were measured by Griess reaction and ELISA respectively. Data are expressed as mean ± standard deviation (SD). *P < 0.05 compared with cells stimulated with IL-1β. The experiment is representative of three experiments performed.
Effects of CGA on iNOS and COX-2 expression in chondrocytes
We next investigate the effect of CGA on iNOS and COX-2 gene expression and protein levels in IL-1β-induced chondrocytes. Our results showed that CGA suppressed the iNOS and COX-2 mRNA expression (Figure 2) as well as the protein levels of iNOS and COX-2 (Figure 3).
Figure 2.
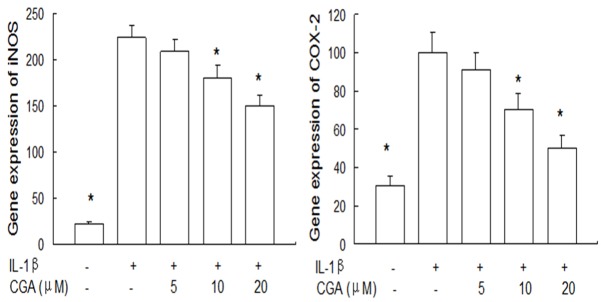
Effects of CGA on iNOS and COX-2 gene expression in IL-1β-induced chondrocytes. Cells were pre-treated with different concentrations of CGA for 1 h prior to IL-1β (10 ng/ml) for 24 h. Gene expression of iNOS and COX-2 was detected by quantitative real-time PCR. Data are expressed as mean ± standard deviation (SD). *P < 0.05 compared with cells stimulated with IL-1β. The experiment is representative of three experiments performed.
Figure 3.
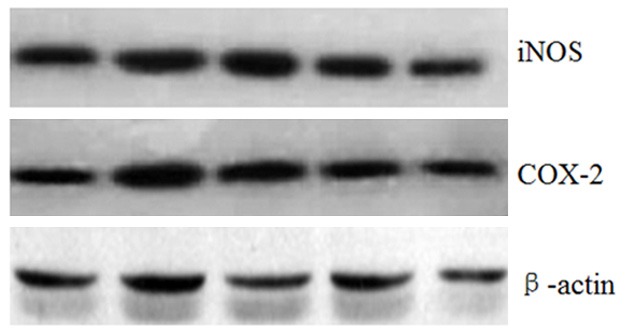
Effects of CGA on IL-1β-induced iNOS and COX-2 protein levels in IL-1β-induced chondrocytes. Cells were pre-treated with different concentrations of CGA for 1 h prior to IL-1β (10 ng/ml) for 24 h. The protein levels of iNOS and COX-2 in chondrocytes were assessed by western blot analysis.
Discussion
Until now, disease modifying anti-OA drugs (DMOADs) are lacking, so there is necessary to find new agent to modify OA. In this field, natural products are considered as a source of new agents. Previous studies have shown that CGA has anti-inflammatory activities and inhibitory effects on MMPs. However, the anti-inflammatory effects of CGA in chondrocytes are still unclear. Therefore, we investigated the anti-inflammatory effect of CGA in IL-1β-stimulated chondrocytes. We demonstrated that CGA not only suppressed the production of NO and PGE2, but also inhibited iNOS and COX-2 expression in IL-1β-induced chondrocytes.
There is cumulated evidences showed that iNOS-NO signaling pathway are implicated in the pathogenesis of OA [10]. Previous study has shown that IL-1β can induce NO production via iNOS in chondrocytes [11]. Inhibition of iNOS-NO signaling pathway is beneficial to OA [12]. In the present study, we found that CGA reduced IL-1β-induced iNOS-NO activation in a dose-dependent manner. Our results are partly supported by previous study which reported that CGA reduced NO and iNOS expression in vitro [13].
In the present study, we demonstrate that CGA suppressed the PGE2 via inhibiting COX-2 expression in IL-1β-induced chondrocytes. PGE2 is an inflammatory mediator involved in the pathogenesis of OA. Because the synthesis of PGE2 is dependent on COX-2, in the present study, we investigated whether CGA possessed inhibitory effect on PGE2 and COX-2, our results showed that CGA inhibited the elevated PGE2 and COX-2 expression in chondrocyte. Our results are consistent with previous studies showing that CGA reduced the COX-2-PGE2 signaling pathway in other cells [14].
Nuclear factor-kappaB (NF-κB) is a transcription factor that plays an important role in inflammation events including OA. It is known that the effects of IL-1β in OA are associated with NF-κB [15]. In our previous study, we demonstrated that CGA inhibited NF-κB activation in chondrocytes. Thus, we speculated that the anti-inflammatory effects of CGA in IL-1β-induced chondrocytes are partly associated with the inhibition of NF-κB.
In conclusion, our findings showed that CGA exerts an anti-inflammatory effect by the inhibition of COX-2/PGE2 and iNOS/NO expression and the anti-inflammatory effect may partly associate with the inhibition of NF-κB.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81201429).
Disclosure of conflict of interest
None.
References
- 1.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: Role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 2.Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aigner T, McKenna L, Zien A, Fan Z, Gebhard PM, Zimmer R. Gene expression profiling of serum- and interleukin-1 beta-stimulated primary human adult articular chondrocytes--a molecular analysis based on chondrocytes isolated from one donor. Cytokine. 2005;31:227–240. doi: 10.1016/j.cyto.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: Differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801–811. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 5.El MF, Chabane N, Zayed N, Kapoor M, Benderdour M, Martel-Pelletier J, Pelletier JP, Duval N, Fahmi H. Contribution of H3K4 methylation by SET-1A to interleukin-1-induced cyclooxygenase 2 and inducible nitric oxide synthase expression in human osteoarthritis chondrocytes. Arthritis Rheum. 2011;63:168–179. doi: 10.1002/art.27762. [DOI] [PubMed] [Google Scholar]
- 6.Dos SM, Almeida MC, Lopes NP, de Souza GE. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol Pharm Bull. 2006;29:2236–2240. doi: 10.1248/bpb.29.2236. [DOI] [PubMed] [Google Scholar]
- 7.Jin UH, Lee JY, Kang SK, Kim JK, Park WH, Kim JG, Moon SK, Kim CH. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: Isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005;77:2760–2769. doi: 10.1016/j.lfs.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 8.Chen WP, Tang JL, Bao JP, Hu PF, Shi ZL, Wu LD. Anti-arthritic effects of chlorogenic acid in interleukin-1beta-induced rabbit chondrocytes and a rabbit osteoarthritis model. Int Immunopharmacol. 2011;11:23–28. doi: 10.1016/j.intimp.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Chen WP, Wang YL, Tang JL, Hu PF, Bao JP, Wu LD. Morin inhibits interleukin-1beta-induced nitric oxide and prostaglandin E2 production in human chondrocytes. Int Immunopharmacol. 2012;12:447–452. doi: 10.1016/j.intimp.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Studer R, Jaffurs D, Stefanovic-Racic M, Robbins PD, Evans CH. Nitric oxide in osteoarthritis. Osteoarthritis Cartilage. 1999;7:377–379. doi: 10.1053/joca.1998.0216. [DOI] [PubMed] [Google Scholar]
- 11.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;427(Suppl):S27–36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 12.Bentz M, Zaouter C, Shi Q, Fahmi H, Moldovan F, Fernandes JC, Benderdour M. Inhibition of inducible nitric oxide synthase prevents lipid peroxidation in osteoarthritic chondrocytes. J Cell Biochem. 2012;113:2256–2267. doi: 10.1002/jcb.24096. [DOI] [PubMed] [Google Scholar]
- 13.Hwang SJ, Kim YW, Park Y, Lee HJ, Kim KW. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm Res. 2014;63:81–90. doi: 10.1007/s00011-013-0674-4. [DOI] [PubMed] [Google Scholar]
- 14.Shan J, Fu J, Zhao Z, Kong X, Huang H, Luo L, Yin Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-kappaB and JNK/AP-1 activation. Int Immunopharmacol. 2009;9:1042–1048. doi: 10.1016/j.intimp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Tak PP, Firestein GS. NF-kappaB: A key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]


