Abstract
Descending control of nociceptive processing in the rostral ventromedial medulla (RVM) has been implicated in the inhibition and facilitation of spinal nociceptive transmission. Here we investigated the contribution of serotonergic (5-HT) pathway at the RVM to pruritic behavior. Selective lesion of the descending serotonergic pathway by intra-RVM injection of focal neurotoxin 5,7-dihydroxytryptamine (2 μg/0.5 μl) attenuated pruritic behavior at the 30-min observation period following an intradermal microinjection of compound 48/80 (100 μg/100 μl) in the nape of the neck. Intradermal microinjection of compound 48/80 resulted in a dramatic increase in itch behavior between naive group and saline group. 5,7-DHT-treated mice showed profound scratching deficits after intradermal injection of compound 48/80. 5,7-DHT treatment resulted in a significant decrease in the number of 5-HT positive neurons in the RVM by using intracisternal injection of the serotonin neurotoxin 5,7-DHT. These findings demonstrate that pruritic behavior is dependent in part on descending facilitation via the RVM, and identify a modulatory role of serotonergic pathway at the RVM for pruritic behavior.
Keywords: Itch, serotonergic signals, the rostral ventromedial medulla
Introduction
Itch has been defined as an unpleasant skin-specific sensation with negative affective component, and produces a characteristic desire or reflex of scratching behavior [1-3]. The mechanisms that generate itch are poorly understood and effective treatment for chronic itch is lacking despite its clinical importance. It’s widely acknowledged that both pain and itch belong to nociceptive sensation [4], and many C-fibers respond both to algesic (pain-inducing) and pruritic (itch-inducing) compounds [5,6]. The rostral ventromedial medulla (RVM) of the brainstem is a prominent component of the descending modulatory system for nociception at the spinal cord level, and neurons in the RVM have been implicated in the inhibition and facilitation of spinal nociceptive transmission [7-10]. Recent studies have established that the RVM facilitation of nociceptive transmission in the spinal cord contributes to neuropathic pain [8,11-13]. Thereby, it has been hypothesized that RVM facilitation of nociceptive transmission might involve in chronic pathological itch.
Behavioral data suggest that scratching behavior may be used as an index for the study of itch in experimental rodents [2,14]. Several lines of evidence suggested that 5, 7-dihydroxytriptamine (5,7-DHT), a serotonergic neurotoxin, selectively diminished tissue 5-HT content [15-17]. To date, the role of descending facilitation in pruritic behavior has not been investigated. Here, it was reported that the changes of scratching behaviors induced by histamine-dependent pruritogen compound 48/80 after selectively ablated serotonergic signals at the RVM in mice.
Methods and materials
Animal care
Male C57BL/6J mice (8-10 weeks old) at the time of surgery were maintained in a climate-controlled room on a 12-hlight/dark cycle (light on at 07:00 h). Mice were housed (5/cage), but they were individually caged during each experiment. Food and water were available ad libitum. All testing and surgeries were performed in accordance with the policies and recommendations of the National Guides for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Experimental design
Mice were randomly assigned to one of three groups for these experiments: (1) naive group (naive group, n = 6); (2) saline group (saline group, n = 6); (3) 5,7-DHT group (DHT group, n = 6). 0.5 µl saline was microinjected into the RVM in saline group whereas 0.5 µl 5,7-DHT (2 µg/0.5 µl) into the RVM in DHT group. 10 d after surgical procedures, pruritic behavior was measured. All procedures and behavioral assay were conducted in an isolated quiet room to reduce variance.
Intra-RVM microinjections
As previously described [8], mice were anesthetized with 2% Sodium pentobarbital (50 mg/kg) and placed in a stereotaxic instrument. The coordinates for the RVM were as follows: 5.8-6.0 mm caudal to bregma, midline, and 5.5-5.7 mm ventral to the surface of the cerebellum (Franklin and Paxinos, 2007) [18]. A midline incision was made to expose the skull with a dental drill to insert a microinjection needle into the RVM. Microinjections were performed by delivering 5,7-DHT (2 µg/0.5 µl) or saline (0.5 µl) slowly over a 10 min period using a 0.5 µl Hamilton syringe with a 32 gauge needle. To prevent damage of noradrenergic neurons in the RVM, desipramine (20 mg/kg, i.p.), an inhibitor of neuronal noradrenergic reuptake, was pretreated animals 30 min before 5,7-DHT (or saline) administration [7]. The wound was closed and animals returned to their cages after they recovered from anesthesia.
Behavioral assay
Ten d after Intra-RVM microinjections, pruritic behavior was evaluated. The mice were adapted to the testing situation for at least 15 min. They were anesthetized with isoflurane and received an intradermal microinjection of 100 µL compound 48/80 (100 µg/100 µl) or saline in the nape of the neck. As previously described [16,17], immediately after the injection, the mouse was placed into the arena and videotaped from above for 30 min. Videotapes were reviewed by the observers of the pruritic behaviors. All investigators were blind to the treatment of the mice, and the number of scratch bouts was recorded at 5 min intervals. All pruritic behavior was evaluated as previously described [10,17,18] with minor modifications.
Histology
After performing the behavioral assay, the animals were deeply anesthetized with ketamine and xylazine. Then, they were transcardially perfused with 0.9% saline and 4% PFA solution as described [2,19-21]. The brains were dissected and immersed in the same fixative for 2h at 4°C. Subsequently, the brains were immersed in 30% sucrose overnight at 4°C. Free-floating sections (30 μm) were made at the levels of the target site and stored at -80°C until staining. The neuroanatomical locations of the target site were confirmed using mouse brain atlas. Only the animals with correct target site in the RVM were included in the data analysis.
For the 5-HT staining, sections were washed with 0.01 M PBS (3 × 10 min) before being incubated primary with rabbit anti-5-HT (1:2000) and secondary with donkey anti-rabbit 5-HT (1:1000). Finally, sections were collected onto glass microscope slides, covered with a coverslip, and examined under the fluorescence microscopy by an investigator blinded as to treatment.
Statistical analysis
All the data were presented as x̅ ± s or x̅ ± s x. Two-way repeated-measures ANOVA was used to analyze the data with multiple time points (time course of itch behaviors). A P value of < 0.05 was considered statistically significant.
Results
Grouping and evaluation
In the RVM area by saline or focal neurotoxin 5,7-DHT (2 µg/0.5 µl) injection, we evaluated scratching behaviors of mice response to intradermal injection of compound 48/80 (100 µg/100 µl), a histamine-dependent pruritogenic agent. Unlike the saline-treated and naïve mice, which exhibited vigorous scratching response after intradermal injection of compound 48/80, 5,7-DHT-treated mice showed profound scratching deficits (reduced by 77%) (Figure 1A and 1B).
Figure 1.
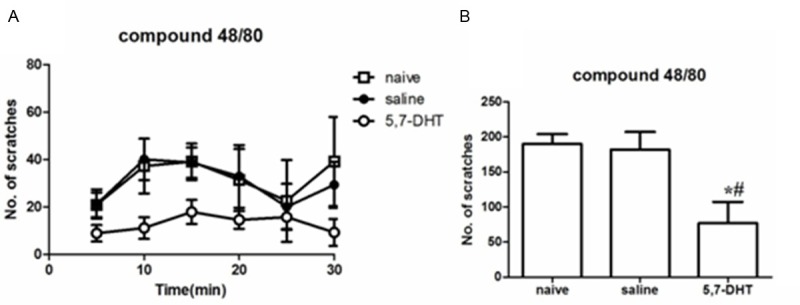
Pruritogen-evoked scratching behavior in DHT-treated, saline-treated and naïve mice. (A) Bar graph plots mean number of scratch bouts recorded at 5 min intervals over the 30-min observation period by an intradermal microinjection of compound 48/80 (100 μg/100 μl). (B) Graph by recording the total numbers of scratching bouts at 0-30 min as in (A). Square bars: naïve (untreated) group; Black bars: control saline treatment; open bars: 5,7-DHT treatment group. *DHT group significantly different compared naïve group (P < 0.05; repeated-measures ANOVA). #DHT group significantly different compared saline group (P < 0.05; repeated-measures ANOVA). There was no difference between saline and naïve group for pruritogens-evoked scratching behavior (n = 5/group). All data are presented as means ± s.e.m. Error bars represent s.e.m.
5-HT immunoreactivity in the RVM 10 d after intra-RVM microinjections
We found that 5,7-DHT treatment resulted in a significant decrease in the number of 5-HT positive neurons in the RVM by using intracisternal injection of the serotonin neurotoxin 5,7-DHT. 5-HT positive neurons in the RVM (including nucleus raphe magnus (NRM) and the adjacent gigantocellularreticular nucleus α part (NGCα) were widely expressed between naive group and saline group. 5-HT expression between the NRM and NGCα was significantly decreased in DHT group (n = 6) (Figure 2A) compared to saline group (n = 6) (Figure 2B).
Figure 2.

5-HT immunoreactivity in the RVM 10 d after intra-RVM microinjections. A. DHT group; B. saline group. 5-HT positive neurons in the RVM (NRM and NGCα) were widely expressed in saline group. 5-HT expression between the NRM and NGCα was significantly decreased in DHT group compared to saline group. NRM, nucleus raphe magnus; NGCα, gigantocellular reticular nucleus α part; Py, Pyramidal tract. Scale bar: 50 μm.
Discussion
In the present study, we used intra-RVM microinjections and fluorescence immunohistochemistry, and assessed the contribution of the RVM 5-HT system to descending itch modulation by examining pruritic behavior in the histamine-dependent pruritogenic model. The principal findings we made were as follow: (1) Intradermal microinjection of compound 48/80 resulted in a dramatic increase in itch behavior between naive group and saline group; (2) 5,7-DHT-treated mice showed profound scratching deficits after intradermal injection of compound 48/80; (3) 5,7-DHT treatment resulted in a significant decrease in the number of 5-HT positive neurons in the RVM by using intracisternal injection of the serotonin neurotoxin 5,7-DHT.
Anatomical and physiological studies have shown that serotonergic (5-HT) system comprises one of the major components of descending pain control pathways [22]. In addition to this finding, serotonergic projection has been shown to inhibit nociceptive afferents at the level of the spinal dorsal horn neurons [23,24]. There is growing recognition that serotonergic neurons play a complex and crucial role as an underlying neurobiological mechanism to modulate acute and chronic pain [25-27]. Braz et al [28] used a transgenic line of mice in which Cre recombinase is selectively expressed in 5-HT neurons (ePet-Cre mice), and had shown an anatomical substrate that a noxious stimulus can activate 5-HT neurons of the NRM and in turn could trigger descending serotonergic antinociceptive controls. Thereby, it is speculated that serotonergic signaling in RVM may modulate itch-related responses. Our results may confirm this hypothesis, and provide a novel exploration of mechanism for pruritic signal pathway.
The present data provide the experimental evidence for behavioral reduction of itch-related scratching after selectively ablated serotonergic signals at the RVM, potentially mediated via sensitization of central pruritogen-sensitive signals. This method will be proved for future studies of transmission mechanisms of itch signal and potential treatment of chronic itch.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (No. 81071307, No. 81271766) and Special Fund of Fundamental Scientific Research Business Expense for Higher School of Central Government (2012TS060) and Clinical Key Disciplines Construction Grant from the Ministry of Health of P.R. China. Experiments were approved by the Institutional Animal Care and Use Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology University, Wuhan, China.
Disclosure of conflict of interest
None.
References
- 1.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song Y, Pan X, Liu C, Xiang H. Role of nociceptive arcuate nucleus neurons in chloroquine-induced pruritic behaviors in mice. J Huazhong Univ Sci Technolog Med Sci. 2012;32:919–922. doi: 10.1007/s11596-012-1058-7. [DOI] [PubMed] [Google Scholar]
- 3.Liu T, Berta T, Xu ZZ, Park CK, Zhang L, Lu N, Liu Q, Liu Y, Gao YJ, Liu YC, Ma Q, Dong X, Ji RR. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J Clin Invest. 2012;122:2195–2207. doi: 10.1172/JCI45414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, Tolias C, Corfas R, Chen S, Inquimbert P, Xu Y, McInnes RR, Rice FL, Corfas G, Ma Q, Woolf CJ, Greenberg ME. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paus R, Schmelz M, Biro T, Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest. 2006;116:1174–1186. doi: 10.1172/JCI28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leong ML, Gu M, Speltz-Paiz R, Stahura EI, Mottey N, Steer CJ, Wessendorf M. Neuronal loss in the rostral ventromedial medulla in a rat model of neuropathic pain. J Neurosci. 2011;31:17028–17039. doi: 10.1523/JNEUROSCI.1268-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei F, Dubner R, Zou S, Ren K, Bai G, Wei D, Guo W. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J Neurosci. 2010;30:8624–8636. doi: 10.1523/JNEUROSCI.5389-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan XC, Song YT, Liu C, Xiang HB, Lu CJ. Melanocortin-4 receptor expression in the rostral ventromedial medulla involved in modulation of nociception in transgenic mice. J Huazhong Univ Sci Technolog Med Sci. 2013;33:195–198. doi: 10.1007/s11596-013-1096-9. [DOI] [PubMed] [Google Scholar]
- 10.Carr FB, Geranton SM, Hunt SP. Descending controls modulate inflammatory joint pain and regulate CXC chemokine and iNOS expression in the dorsal horn. Mol Pain. 2014;10:39. doi: 10.1186/1744-8069-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu N, Han M, Yang ZL, Wang YQ, Wu GC, Zhang YQ. Nociceptin/Orphanin FQ in PAG modulates the release of amino acids, serotonin and norepinephrine in the rostral ventromedial medulla and spinal cord in rats. Pain. 2010;148:414–425. doi: 10.1016/j.pain.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Vera-Portocarrero LP, Xie JY, Kowal J, Ossipov MH, King T, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains visceral pain in rats with experimental pancreatitis. Gastroenterology. 2006;130:2155–2164. doi: 10.1053/j.gastro.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Vera-Portocarrero LP, Zhang ET, Ossipov MH, Xie JY, King T, Lai J, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains nerve injury-induced central sensitization. Neuroscience. 2006;140:1311–1320. doi: 10.1016/j.neuroscience.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Horvath G, Joo G, Kekesi G, Farkas I, Tuboly G, Petrovszki Z, Benedek G. Inhibition of itch-related responses at spinal level in rats. Acta Physiol Hung. 2011;98:480–490. doi: 10.1556/APhysiol.98.2011.4.12. [DOI] [PubMed] [Google Scholar]
- 15.Godinez-Chaparro B, Lopez-Santillan FJ, Orduna P, Granados-Soto V. Secondary mechanical allodynia and hyperalgesia depend on descending facilitation mediated by spinal 5-HT(4), 5-HT(6) and 5-HT(7) receptors. Neuroscience. 2012;222:379–391. doi: 10.1016/j.neuroscience.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Choi S, Jonak E, Fernstrom JD. Serotonin reuptake inhibitors do not prevent 5,7-dihydroxytryptamine-induced depletion of serotonin in rat brain. Brain Res. 2004;1007:19–28. doi: 10.1016/j.brainres.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 17.Kondo Y, Ogawa N, Asanuma M, Hirata H, Tanaka K, Kawada Y, Mori A. Regional changes in neuropeptide levels after 5,7-dihydroxytryptamine-induced serotonin depletion in the rat brain. J Neural Transm Gen Sect. 1993;92:151–157. doi: 10.1007/BF01244874. [DOI] [PubMed] [Google Scholar]
- 18.Franklin KB, Paxinos G. The mouse Brain in Stereotaxic Coordinates. 3rd edition. San Diego, CA: Academic Press; 2007. [Google Scholar]
- 19.Hao Y, Tian XB, Liu C, Xiang HB. Retrograde tracing of medial vestibular nuclei connections to the kidney in mice. Int J Clin Exp Pathol. 2014;7:5348–5354. [PMC free article] [PubMed] [Google Scholar]
- 20.Xiang HB, Liu C, Liu TT, Xiong J. Central circuits regulating the sympathetic outflow to lumbar muscles in spinally transected mice by retrograde transsynaptic transport. Int J Clin Exp Pathol. 2014;7:2987–2997. [PMC free article] [PubMed] [Google Scholar]
- 21.Ye DW, Liu C, Tian XB, Xiang HB. Identification of neuroanatomic circuits from spinal cord to stomach in mouse: retrograde transneuronal viral tracing study. Int J Clin Exp Pathol. 2014;7:5343–5347. [PMC free article] [PubMed] [Google Scholar]
- 22.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 23.Braz JM, Basbaum AI. Genetically expressed transneuronal tracer reveals direct and indirect serotonergic descending control circuits. J Comp Neurol. 2008;507:1990–2003. doi: 10.1002/cne.21665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furst S. Transmitters involved in antinociception in the spinal cord. Brain Res Bull. 1999;48:129–141. doi: 10.1016/s0361-9230(98)00159-2. [DOI] [PubMed] [Google Scholar]
- 25.Eaton MJ, Widerstrom-Noga E, Wolfe SQ. Subarachnoid Transplant of the Human Neuronal hNT2.19 Serotonergic Cell Line Attenuates Behavioral Hypersensitivity without Affecting Motor Dysfunction after Severe Contusive Spinal Cord Injury. Neurol Res Int. 2011;2011:891605. doi: 10.1155/2011/891605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao ZQ, Chiechio S, Sun YG, Zhang KH, Zhao CS, Scott M, Johnson RL, Deneris ES, Renner KJ, Gereau RWt, Chen ZF. Mice lacking central serotonergic neurons show enhanced inflammatory pain and an impaired analgesic response to antidepressant drugs. J Neurosci. 2007;27:6045–6053. doi: 10.1523/JNEUROSCI.1623-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eaton MJ, Santiago DI, Dancausse HA, Whittemore SR. Lumbar transplants of immortalized serotonergic neurons alleviate chronic neuropathic pain. Pain. 1997;72:59–69. doi: 10.1016/s0304-3959(97)00015-8. [DOI] [PubMed] [Google Scholar]
- 28.Braz JM, Enquist LW, Basbaum AI. Inputs to serotonergic neurons revealed by conditional viral transneuronal tracing. J Comp Neurol. 2009;514:145–160. doi: 10.1002/cne.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]


