Abstract
This study is to test the efficacy of stromal cell-derived factor-1α (SDF-1α)-coated coils together with endothelial progenitor cells (EPCs) transplantation in occluding aneurysms. Bone marrow-derived EPC surface markers were analyzed using flow cytometry. The migratory function of EPCs in response to SDF-1α was evaluated using a modified Boyden chamber assay. Capillary-like tube formation was assessed using Matrigel gel. Coil morphologies before and after coating with SDF-1α were observed under a scanning electron microscope. The level of SDF-1α in supernatants was measured by ELISA. Sprague-Dawley rats were randomly allocated into five groups. Histological analysis was performed on days 14 and 28 after coil implantation. The bone marrow-EPCs could express CD133, CD34, and VEGFR-2 and form tubule-like structures in vitro. Migratory ability of EPCs in the presence of SDF-1α-coated coils was similar to that in the presence of 5 ng/ml SDF-1α gradient. Sustained release of SDF-1α was achieved using silk fibroin as a carrier. In SDF-1α-coated coils + EPCs transplantation group, a well-organized fibrous tissue bridging the orifice of aneurysms was shown on days 14 and 28. On day 28, tissue organization was greater in the SDF-1α-coated coils group than in the unmodified coils group. Immunofluorescence showed α-smooth muscle actin-positive cells in organized tissue in sacs. Combined treatment with SDF-1α-coated coils and EPCs transplantation is a safe and effective treatment for rat aneurysms. This may provide a new strategy for endovascular therapy following aneurysmal subarachnoid hemorrhage.
Keywords: Stromal cell-derived factor-1α, endothelial progenitor cells, silk fibroin, cerebral aneurysm, recanalization, endothelialization
Introduction
Coil embolization of intracranial aneurysms has become an effective technique alternative to standard surgical clipping [1,2]. In many hospitals, aneurysms are treated by coiling [3]. However, the long-term stability of aneurysms treated with coils is still controversial [1,4]. Furthermore, recurrence of aneurysms after embolization is a major obstacle in the field of neurosurgery. Previous studies on large heterogeneous groups of patients with intracranial aneurysms treated by coiling reported an overall recurrence rate of 34% [5]. In addition, about 50% of unruptured, untreated aneurysms increase in size within 3 years [6]. Factors related to recanalization include packing density, coil compaction, and rare endothelialization at aneurysm orifice [7,8]. Numerous studies suggested that endothelial layer formation of the aneurysm neck after embolization was important in preventing the recurrence of aneurysm, as well as the potential risks of late rebleeding [8,9].
Different surface modification technologies have been developed to reduce the aneurysm recanalization rate after coil embolization, including polymers, collagen, fibroblasts and growth factors [10-14]. The objectives of these modifications were to increase the inflammatory response at the neck of the aneurysm, to accelerate thrombus organization in the aneurysm cavity that aids “healing” at the neck, and to improve packing [15]. Their recanalization rates are similar to or higher than that of bare platinum coils [16].
To overcome the above limitation, we developed a novel coated coil that stimulates endothelial healing at the neck of the aneurysm. Endothelial progenitor cells (EPCs) are receiving much attention due to their contribution to neoangiogenesis in vascular injuries, such as wound healing, limb ischemia, myocardial infarction, atherosclerosis and stroke [17]. EPCs are circulating pluripotent stem cells that have been used to restore endothelial function in denuded vessels, to facilitate the vascular repairing process and to promote neovascularization. When used for vascular repair and angiogenesis or in case of vascular stress, EPCs enter the peripheral blood and migrate to areas with endothelial damage to begin the reparative process [18]. Chemokine, stromal cell-derived factor-1α (SDF-1α), and its receptor, C-X-C motif receptor 4 (CXCR4), play a crucial role in the recruitment and retention of EPCs in the reparative process. Based on previous reports, we hypothesize that local SDF-1α recruit EPCs to participate in endothelial cell repairing and reconstruction process in the neck of embolized aneurysms. In this study, SDF-1α is coated onto platinum coils with silk fibroin. Accordingly, the efficacy of silk protein formulation with SDF-1α is tested in vitro, and the extent of embolization is evaluated in rats. We hypothesize that such coating would increase the degree of luminal occlusion and decrease the rate of recanalization compared with standard unmodified coils.
Materials and methods
Animals
Sprague-Dawley rats (weighing 250-300 g) were obtained from the Animal Center of the Southern Medical University. A total of 60 male rats were randomly divided into five groups: sham-operated group (group A), unmodified coils group (group B), unmodified coils + transplantation of EPCs group (group C), SDF-1α-coated coils group (group D), and SDF-1α-coated coils + transplantation of EPCs group (group E). The aneurysms were constructed as previously described [10-13,19]. Briefly, rats were intraperitoneally injected with 60 mg/kg sodium pentobarbital and the right common carotid artery (CCA) was carefully dissected. Each of the coils was inserted into the right CCA (Figure 1). Vasodilation and pulsation in the CCA were confirmed on the removal of the temporary clips, and the wound was closed by using 4-0 nylon sutures. EPCs (3 × 106) were infused into tail vein immediately after coil implantation. The rats were returned to their cages where they continued to have access to food and water ad arbitrium. On day 14 and 28 after coil implantation, 6 rats from each group were sacrificed by intraperitoneal injection of 300 mg/kg sodium pentobarbital. CCA segments containing the coils were collected and placed into 4% paraformaldehyde. The coil segments were then carefully removed from the vessel segment. After rinsing in PBS for three times, the segments were preserved in 1% osmium tetroxide at 4°C. All procedures involving the use of rats were approved by the Animal Research Committee of Southern Medical University and were in accordance with the Guide for the Care and Use of Laboratory Animals (Washington, DC: National Academy Press, 1996).
Figure 1.
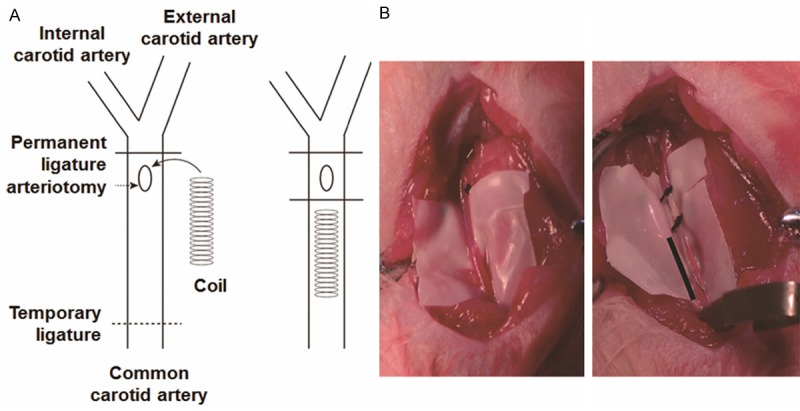
Experimental aneurysm creation and coil insertion. The insertion of coils was performed in the right common carotid artery. A. Diagram of coil insertion. B. Common carotid artery before (left) and after (right) coil insertion.
Cells
Sprague-Dawley rat was anesthetized with intraperitoneal injection of 60 mg/kg sodium pentobarbital. Bone marrow mononuclear cells were isolated from the long bones of rats by density gradient centrifugation with Ficoll-PaqueTM (Tianjin Haoyang Bio Co. Ltd, China) and cultured in endothelial basal medium-2 (EBM-2) supplemented with microvascular endothelial growth medium (EGM-2MV) SingleQuots (Lonza, USA) containing 5% fetal bovine serum (Hyclone, USA), hydrocortisone, vascular endothelial growth factor, basic human fibroblast growth factor, insulin-like growth factor-1, ascorbic acid, human epidermal growth factor, and CA-1000. After 3 days of culture, nonadherent cells were removed. Thereafter, the media was changed every 3 days. Phenotypic characterization of early EPCs was analyzed by flow cytometry after staining with anti-CD34, anti-VEGFR-2 (KDR), and anti-CD133.
Migration assay
The migratory function of EPCs in response to SDF-1α was evaluated using a modified Boyden chamber assay. In brief, EPCs on day 21 were detached from the wells, and seeded in the upper chamber of 24-well Transwell plate with polycarbonate membrane (8 μm pores; BD Biosciences, USA) in serum-free EBM-2. The lower chamber was filled with EBM-2 containing 10 ng/ml SDF-1α gradient, SDF-1α-coated coils, silk fibroin (SF)-coated coils, unmodified coils or nothing. After 12 h of incubation at 37°C, all cellular nuclei were stained with 4’,6-diamidino-2-phenylindole and counted in 3 random fields (200×) in each well.
Tube formation assay
Capillary-like tube formation was assessed using Matrigel gel (BD Biosciences, USA). Briefly, Matrigel gel was thawed at 4°C overnight and 300 μL/well gel was placed in a 24-well culture plate before incubation for 1 h at 37°C to allow solidification. EPCs (1 × 105) were placed onto Matrigel gel and incubated in EGM-2 for 12 h. Tubular structures were photographed by phase-contrast microscopy (40×).
Preparation of coils
Silk fibroin aqueous solution was prepared as previously described [20]. Briefly, cocoons of Bombyx mori were boiled in ultra-purified water containing 0.02 M Na2CO3, thoroughly rinsed with distilled water, and then dissolved in 9 M LiBr at 55oC to obtain a 3% aqueous SF (w/v) solution. SDF-1α (PeproTech, USA) was embedded by adding 10 μg SDF-1α to 500 μl of SF solution. The SDF-1α-SF solution was subsequently sterilized using a 0.22 μm filter. Platinum coils of 300 μm diameter were purchased from Boston Scientific Corporation (USA) and cut into 5-mm length segments. The coil segments were sterilized using 75% alcohol for 30 min, and then rinsed with sterile phosphate-buffered saline (PBS) for three times. Under sterile conditions, the coil segments were immersed in SDF-1α-SF solution at 4°C for 4 h under continuous gentle agitation. Then, the coil segments were rinsed with sterile PBS for 10 min and subsequently treated with 90% methanol for 15 min to induce SF crystalline β-sheet structure formation. The procedure was repeated for three times to obtain SDF-1α-coated coils. The coil segments were maintained at -80°C and rinsed with sterile PBS for three times before use.
Scanning electron microscopy (SEM)
Coil morphologies before and after coating with SDF-1α were observed under a scanning electron microscope (JSM-6380LA model, JEOL Ltd., Japan) at 15 kV. Prior to analysis, the specimens were carefully sputter-coated with gold. Measurements were performed in both coronal and axial directions.
Enzyme-linked immunosorbent assay (ELISA)
Coil segments were used for the detection of the kinetics of SDF-1α immobilization on platinum coils. Each segment was incubated in 50 µl PBS (0.1 M, pH = 7.4) at 37°C with continuous gentle agitation. At various time points, the supernatants were collected and replaced with fresh buffer. The level of SDF-1α in supernatants was measured by ELISA (R&D systems, MN, USA). The absorbance was measured at 450 nm using a plate reader (Bio-Rad, CA, USA).
Histopathological stainings
Paraformaldehyde-fixed CCA segments were embedded in paraffin and sectioned at a thickness of 3 μm. Each specimen was stained with hematoxylin and eosin, Masson trichrome, and Elastica van Gieson according to standard protocols. Coil segments were sputter-coated with gold and visualized by SEM at 15 kV.
Morphometric analysis was performed on cross-sections of each artery stained with hematoxylin and eosin. The inside area of the arterial media encircled by the internal elastic lamina was determined as the vascular lumen, and cellular and fibrotic compartments in the lumen were defined as organized areas. Lumen area, diameter and wall thickness were calculated by using image analysis software (Scion Corp, Frederick, MD, USA). In addition, the neointimal area and media area were measured and the intima/media ratio was calculated.
Immunofluorescence
Immunofluorescence was performed to identify smooth muscle cells. Sections were incubated overnight at 4°C with the primary antibodies rabbit anti-α-smooth muscle actin (1:200; Abcam, USA). The sections were then washed with PBS and incubated with secondary antibody (AlexaFluor488-conjugated goat anti-rabbit, 1:300). The nuclei were stained with 4’,6-diamidino-2-phenylindole. Glass slides were photographed under a fluorescence microscope (Leica, Germany).
Statistical analysis
Statistical analysis was performed using SPSS 17.0 (USA). Two-tailed t-test was used to determine the statistical difference between groups. One-way ANOVA and post hoc Scheffe’s method were performed to analyze the differences among groups. All data were presented as means ± SD. P < 0.05 was considered statistically significant.
Results
Rat bone marrow-derived EPCs develop endothelial-like shapes and express endothelial stem cell markers
To examine morphologic changes of rat bone marrow-derived EPCs, a phase contrast microscope (Leica, Germany) was used. EPCs showed spindle-like phenotype after being isolated (Figure 2A). After continued culturing of EPCs for 1 week, cord-like structures were observed (Figure 2B). At the same time, alignment structure and network formation were observed (Figure 2C). After 3 weeks of culture, EPCs exhibited cobblestone appearance that was distinct from the spindle-shaped morphology of EPCs two weeks ago, suggesting that the cells differentiated into endothelial-like cells (Figure 2D). Furthermore, tubular-like structures were detected on Matrigel (Figure 2E). Flow cytometry analysis of the surface markers on EPCs at passage three demonstrated that the cells expressed endothelial stem cell markers such as CD133, CD34, and VEGFR-2(KDR) (Figure 2F-H). These data suggested that EPCs developed endothelial-like shapes and expressed endothelial stem cell markers as they grew.
Figure 2.
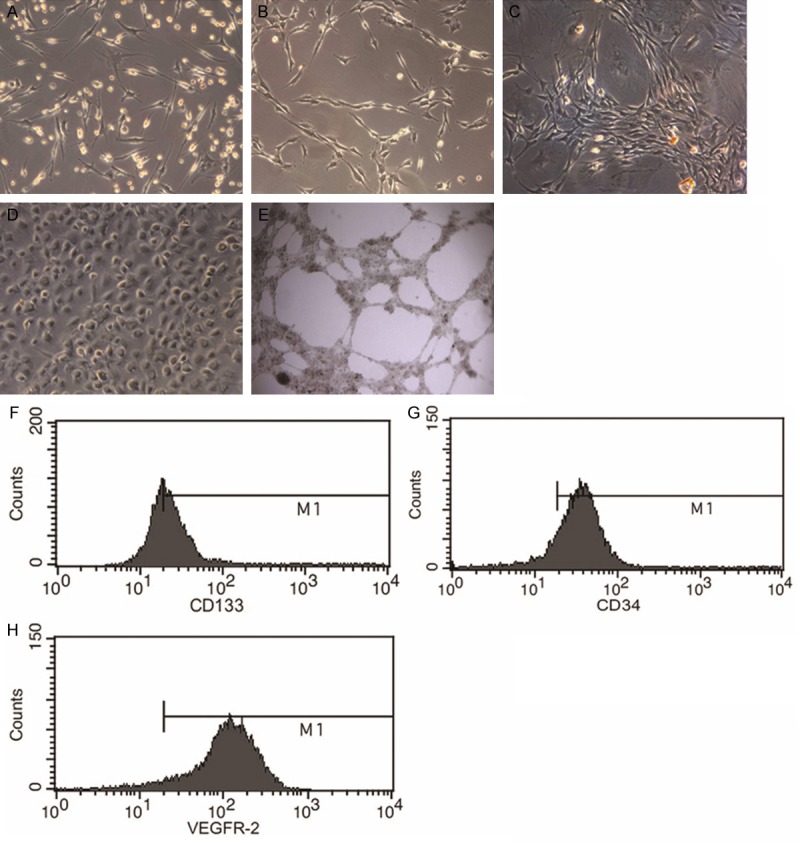
Characteristics of rat bone marrow-derived EPCs. A. EPCs showing typical spindle-like morphology (100×). B. Cord-like structures observed after 7 days of culture (100×). C. Network formation observed after 7 days of culture (100×). D. EPCs showing cobblestone-like morphology after 3 weeks of culture (100×). E. EPCs take part in network formation tubular-like structures on Matrigel (40×). F-H. Flow cytometric analysis of surface markers (CD133, CD34, and VEGFR-2) on EPCs.
SDF-1α promotes the migration of EPCs
To study the effect of SDF-1α on the migration of EPCs, transwell migration assay was performed. The results showed that EPCs had remarkable migration in response to SDF-1α, especially SDF-1α-coated coils, in the lower chamber compared with that in the control group (P < 0.01; Figure 3). However, no significant difference was observed between SDF-1α (5 ng/ml) group and SDF-1α-coated coils group (P > 0.05). These data indicated that SDF-1α promoted the migration of EPCs.
Figure 3.
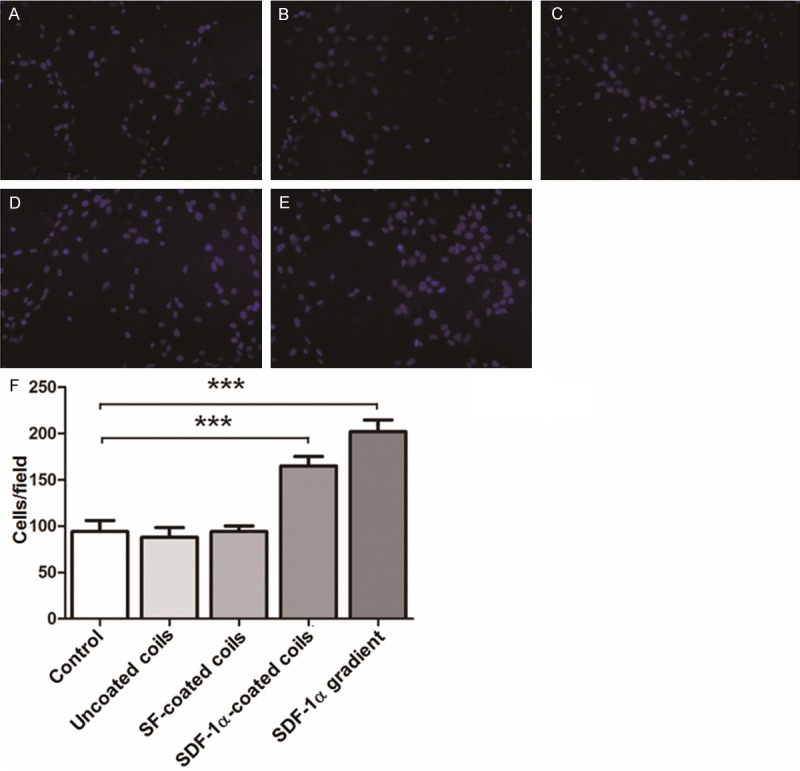
EPC migration. Migration of EPCs in medium containing (A) nothing (control), (B) unmodified coils, (C) SF-coated coils, (D) SDF-1α-coated coils, and (E) SDF-1α gradient (10 ng/ml) was measured using modified Boyden chamber migration assay (200×). (F) Quantification of EPC migration. Values are means ± SD (n = 3). **P < 0.01 compared with control; ***P < 0.001 compared with control.
SDF-1α coating generates an integral thin layer on coil surface, with stable in vitro release of SDF-1α
To examine coil characteristics and in vitro release of SDF-1α, SEM was used to observe the coil surface and ELISA was used to detect the concentration of released SDF-1α after SDF-1α/SF solution was embedded and mixed into surface. SEM showed that SDF-1α-coated coils had complete surface integrity with a thin film component in contrast to uncoated coils (Figure 4A-D). In vitro release of SDF-1α fromSDF-1α-coated coils showed exponential release profile on day 1. After 3 days, the release speed was decreased with a linear growth up to day 21, after which a continuous release thereafter could be extrapolated (Figure 4E). These data demonstrated that SDF-1α coating generated an integral thin layer on coil surface, with stable in vitro release of SDF-1α after day 3.
Figure 4.
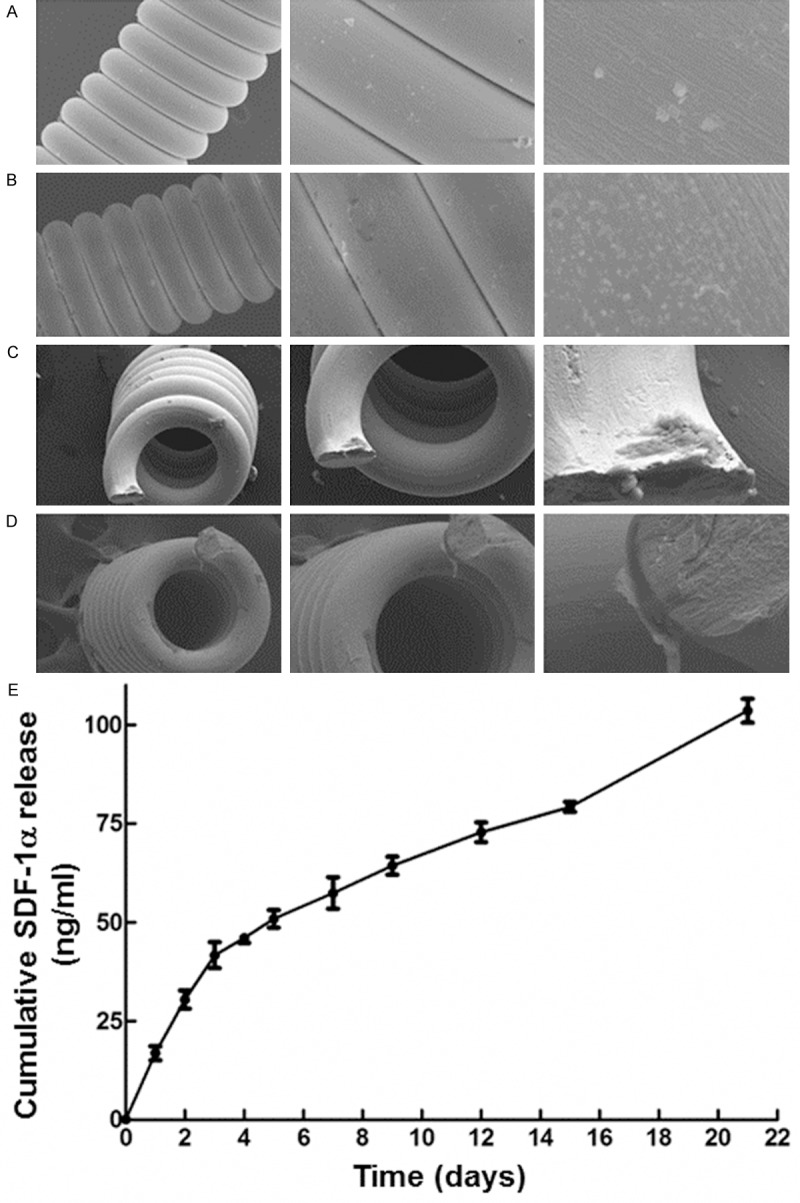
Characteristics of coils and in vitro release of SDF-1α. Scanning electron micrographs of (A) unmodified coils (Left: 15 kV, 300×, scale bar = 50 μm; Middle: 15 kV, 1000×, scale bar = 10 μm; Right: 15 kV, 5000×, scale bar = 5 μm); (B) Coils coated with SDF-1α/SF (Left: 15 kV, 300×, scale bar = 50 μm; Middle: 15 kV, 1000×, scale bar = 10 μm; Right: 15 kV, 5000×, scale bar = 5 μm); (C) Cross section of coils before immobilization of SDF-1α/SF (Left: 15 kV, 300×, scale bar = 50 μm; Middle: 15 kV, 500×, scale bar = 50 μm; Right: 15 kV, 2000×, scale bar = 10 μm); (D) Cross section of coils after immobilization of SDF-1α/SF (Left: 15 kV, 300×, scale bar = 50 μm; Middle: 15 kV, 500×, scale bar = 50 μm; Right: 15 kV, 2000×, scale bar = 10 μm). (E) Cumulative time-course release of SDF-1α from SDF-1α/SF-coated coils. Data are means ± SD (n = 3).
SDF-1α-coated coils had the best organized fibrous tissue response and neck coverage ratio, as well as the most fibrotic accumulation on the surface
To observe embolized aneurysms with coils, light microscopy and SEM were used. Low magnification light microscopy showed that fibrous scarring was covered with neoendothelium arising from the edges of the neck of the aneurysm on day 14 (Figure 5A). In addition, well-organized fibrous tissue response was observed at the neck of aneurysms embolized with SDF-1α-coated coils on day 28 (Figure 5B). A thin layer of fibrin-like material and partial endothelialization were seen in aneurysms embolized with unmodified coils (Figure 5A and 5B). Furthermore, the proportion of neck coverage between different groups was compared on day 14 and 28. On day 14, the proportion of neck coverage in group D and group E was significantly higher than that in the unmodified coil group (group B) (P < 0.01). On day 28, only the proportion of neck coverage in group E was higher than the unmodified coil group (group B) (P < 0.05). However, there was no significant difference between group D and group E on day 14 or day 28 (P > 0.05) (Figure 5E and 5F).
Figure 5.
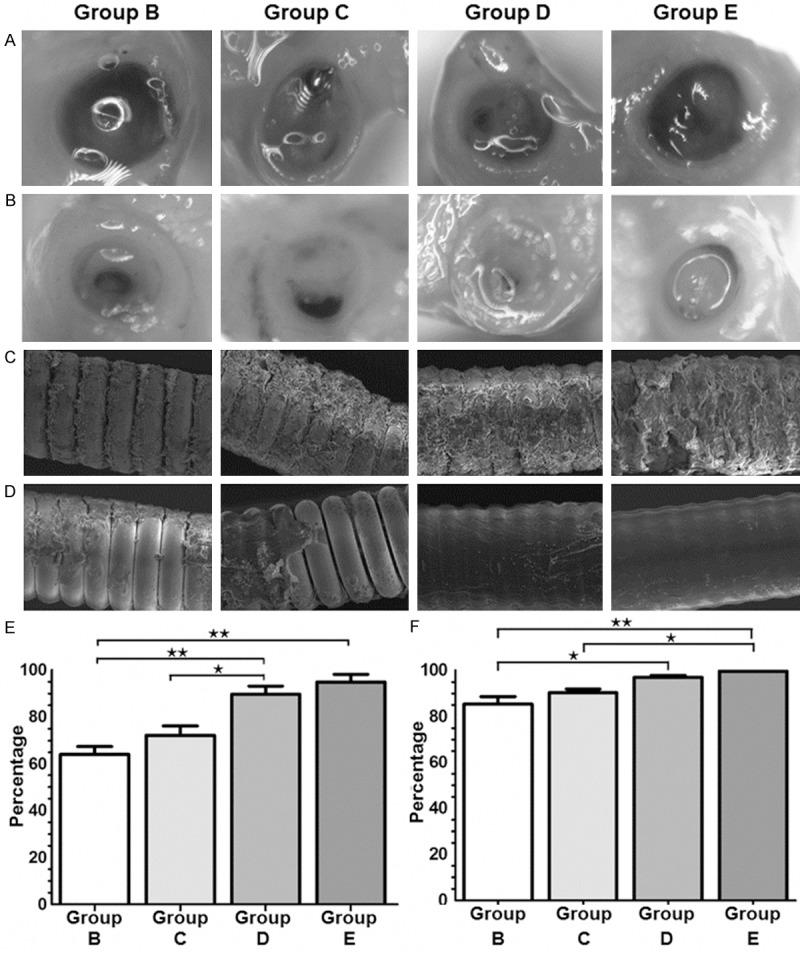
Light microscopic and SEM observations of embolized aneurysms. (A, B) Representative microscopic photographs of an aneurysmal orifice on (A) day 14 after treatment and (B) day 28 after treatment. (C, D) SEM images of the surfaces of coil segments on (C) day 14 after treatment (15 kV, 300×; Scale bar, 50 μm) and (D) day 28 after treatment (15 kV, 300×; Scale bar, 50 μm). (E, F) Neck coverage ratio (fibrous membrane/orifice) for treated aneurysms on (E) day 14 and (F) day 28 after treatment. Data are means ± SD. *P < 0.05; **P < 0.01.
SEM images showed that coils coated with SDF-1α/SF (group D) had more accumulation of cellular debris on the surface than unmodified coils (group B) on day 14. There was evidence of slightly more fibrosis on those coils of group E than those of group D (Figure 5C). However, unmodified coils (group B) showed minimal fibrotic accumulation on the surface, and the fibrosis could not completely cover the surface of coils on day 28 (Figure 5D). These data demonstrated that SDF-1α-coated coils had the best organized fibrous tissue response and neck coverage ratio, as well as the most fibrotic accumulation on the surface.
SDF-1α-coated coils and transplanted EPCs have better effect on intracranial aneurysm
To investigate the histology of aneurysm tissue, representative tissue sections from each group were stained with hematoxylin & eosin, Masson trichrome, and Elastica van Gieson. In the unmodified coils group (group B), blood clots and cellular tissues were detected by hematoxylin & eosin staining in the arterial lumen of aneurysms on days 14 and 28. On the contrary, sacs in group D were almost entirely filled with newly formed fibrotic components such as intimal hyperplasia. The intimal hyperplasia of group E was much thicker than that of group B or group D on both days 14 and 28 (Figure 6A). Moreover, proliferated tissue in the aneurysm sacs was mainly stained blue by Masson trichrome, suggesting that the tissue was composed of fibrosis (Figure 6B). After staining with Elastica van Gieson, the internal elastic lamina was distinct and intact in all specimens of the sham group and most specimens of the other four embolized groups. Minimal damages in some specimens of embolized group were observed (Figure 6C).
Figure 6.
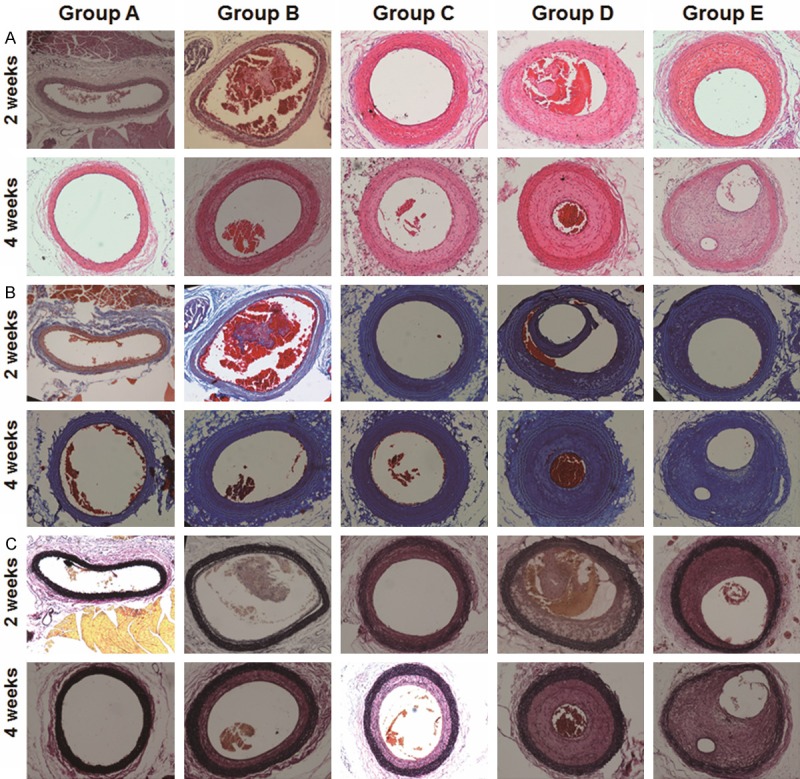
Histopathological investigation of CCA segments on days 14 and 28. Representative microscopic images of tissue sections from CCA segments in rat models on days 14 and 28 after treatment. Blood clots and their organization in these tissue sections were identified using hematoxylin & eosin, Masson trichrome, and Elastica van Gieson (magnification: 100×).
Using immunofluorescence analysis, we detected α-SMA-positive cells in organized tissues and vascular walls of different groups. Within the group D and group E, the entire vascular wall consisted of smooth muscle cells and was significantly increased compared with the unmodified (group B) or sham-operative group (group A). Within the group E, α-SMA positive cells were present in organized tissue in the sac (Figure 7).
Figure 7.
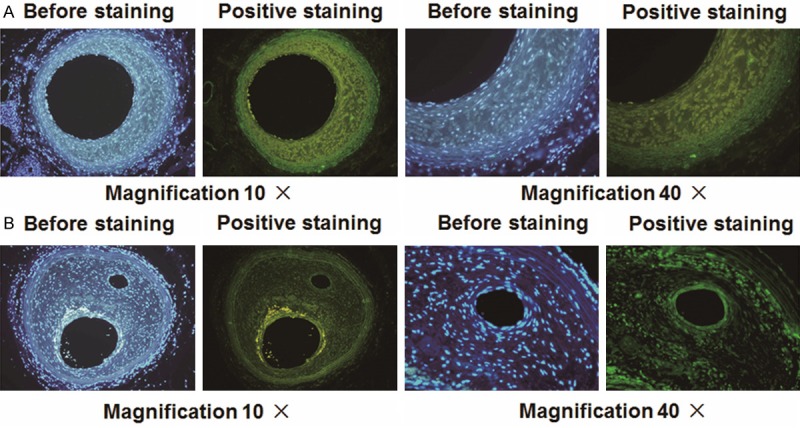
Immunofluorescence analysis for α-SMA. Photomicrographs showing α-SMA cells in (A) organized tissue and (B) vascular walls with different magnification. Positive staining was indicated by green color.
Quantification of the organized area after coil implantation showed that, after 14 days of implantation, group E demonstrated significant increases of intimal area and intima/media ratio compared with group B and C (P < 0.001) (Figure 8A). However, there was no significant difference between the group D and group E on day 14 (P > 0.05) (Figure 8A). On day 28, the percentage of organized area in the group D and group E was significantly higher than the unmodified coil group (group B) (Figure 8B). In addition, there was significant difference between the group D and group E. These data indicated that SDF-1α-coated coils and transplanted EPCs had better effect on intracranial aneurysm.
Figure 8.
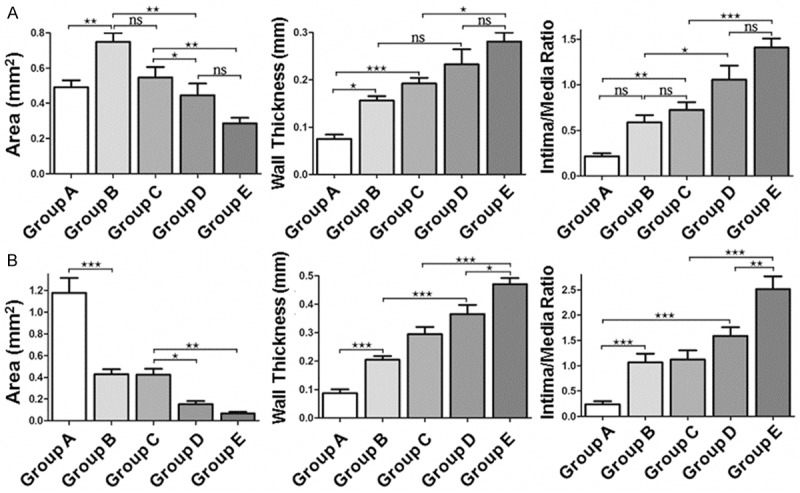
Analysis of organized area in rat aneurysm models. The area, wall thickness, and intima/media ratio of the five groups were quantified on (A) day 14 and (B) day 28 after implantation. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Current endovascular coil embolization of intracranial aneurysms is a safe and effective method [1,3,5]. The purpose of implanting platinum-based coils into the fundus is to exclude aneurysms from the circulation and hence, preventing the risk of subarachnoid hemorrhage. However, not all coiled aneurysms develop tissue reactions required for permanent cure. Postoperative coil compaction and recanalization are persistent problems, particularly for patients with large or giant aneurysms [14]. This is because the platinum coil is biologically inert and can only induce incomplete thrombus organization, which is a major obstacle in the reconstruction of endothelial cell layer in the aneurysm neck orifice. Moreover, some studies demonstrated that lack of complete endothelialization at the aneurysm neck is a key histological mechanism for coil compaction and aneurysm recurrence [21]. For complete fibrovascular tissue formation within the aneurismal sac and endothelial formation of the aneurysm orifice, coils that effectively promote clot organization and endothelial cell proliferation are necessary.
Many growth factors have been used to modify coil surface to increase its biological activity [10,11,19,22]. Evaluation of the histological response of these modified coils to enhanced thrombus organization showed some good effects but with some limitations. Alternative growth factors that allow coiling and endothelialization are worth exploring in order to decrease recanalization rate. The CXC chemokine SDF-1α, known as CXCL12, is a key regulator of B cell lymphopoiesis, hematopoietic stem cell mobilization, and leukocyte migration [23]. SDF-1α binds and activates two G-protein coupled receptors, CXCR4 and CXCR7. Migration and proliferation of stem cells and progenitor cells are regulated via the activation of CXCR4. Involvement of SDF-1α and CXCR4 in brain pathologies has also been described. Stroke and traumatic injuries to the central nervous system lead to an inflammatory response that includes local secretion of several chemokines that attract neural stem cell-derived neuroblasts from the subventricular zone [23]. Increase in SDF-1α expression by astrocytes after brain ischemia attracts transplanted human umbilical cord cells expressing CXCR4 to the injury site [24]. SDF-1α-CXCR4 axis plays important roles in the homing of CXCR4-expressing cells. Previous studies showed that SDF-1α was primarily expressed in bone marrow and a high level of CXCR4 was expressed in EPCs. SDF-1α could induce EPC migration and protect EPCs from apoptosis. In addition, SDF-1α/CXCR4 axis has been well confirmed to play a key role in EPC mobilization in response to hypoxia or injury [25]. EPCs that mainly exist in postnatal bone marrow can differentiate into mature endothelial cells in vitro [26,27]. Growing evidence indicated that EPCs could migrate to peripheral blood and proliferate at the site of endothelial disruption and hence, incorporating into nascent endothelium [28]. Moreover, EPCs secrete a variety of proangiogenic, prosurvival and anti-inflammatory cytokines, contributing to vascular repair, neovascularization and cardiac function [29]. A recent study proved that endovascular injection of EPC-seeded fibrin glue resulted in neointima formation at the aneurysm neck in rabbits. Another study demonstrated that bone marrow-derived EPCs were involved in the aneurysm repairing process [30]. However, the precise mechanism of action of the injected EPCs in vivo was not shown in the study by Aronson et al. [8]. It is also unknown whether increased number of circulating EPCs can accelerate the repairing process of the aneurysm neck endothelial lineage [31].
In the present study, SF, a natural protein that has excellent biocompatibility but leads to minimal inflammatory reaction, was used as a growth factor-releasing carrier for endovascular treatment of aneurysms. After being processed, SF can be used as particles, fibers, films and gels in a lot of applications [32]. Growth factors can be incorporated into silk matrix due to the aqueous and mild processing conditions [33]. To develop bioactive coils, the coils were coated with SF that was able to become water-insoluble due to β-sheet formation. SF and SDF-1α coating was confirmed by SEM. In contrast to unmodified coils, a thin layer could be seen on coils coated with SDF-1α/SF. Because of the beneficial mechanical properties shown in pervious studies, we speculate that the embedding of SDF-1α into a SF matrix can produce coils with good handing properties. The release profile of SDF-1α from SDF-1α/SF-coated coils was determined by ELISA. Though the release rate of SDF-1α from SF was fast at early stage, but no burst release was observed in this study. In the late stage of incubation, diffusion of SDF-1α from SF was steady. In general, the degradation rate decreases with the increase of overall β-sheet content. In the present study, the coils were coated with SDF-1/SF via β-sheet formation. Therefore, we speculate that SF may have a slow degradation rate and excellent biological activity for the controlled release of SDF-1.
In a previous study, we knew that growth factor release kinetics from SF was likely influenced by both ionic charge and molecular weight of the protein [20]. Interestingly, due to the clusters of positively charged residues of SDF-1α and negatively charged SF at isohydric pH, we speculate that the interaction between SDF-1α and SF governed by ionic attraction is strong. Moreover, SDF-1α with a low molecular weight (8 kDa) was immobilized on the coils more quickly and efficiently. Binding led to the accumulation of SDF-1α on the bioactive coils and localized SDF-1α gradients capable of attracting EPCs over time. However, SDF-1α release was prolonged to more than 3 weeks in vitro. The length of the sustained release of SDF-1α from SDF-1α/SF-coated coils was not determined in the present study.
EPCs, bone marrow stem cells widely used in proangiogenic applications [34], are thought to play an important role in endogenous vascular repair after vascular injury and in the maintenance of endothelial integrity. EPCs are originated from bone marrow after vascular injury, followed by homing to the site of neovascularization [35], where they differentiate into endothelial cells. However, EPCs are very rare in the peripheral blood, and SDF-1α released from the aneurysm wall is not enough for the chemotaxis of EPCs. Therefore, the increase in the number of EPCs and the implantation of SDF-1α/SF-coated coils in the embolized aneurysms are strategies for accelerating the repairing process of aneurysm neck endothelial lineage. Safe and controlled delivery of SDF-1α for the effective recruitment of EPCs is achieved by SF. Chemotactic properties of SDF-1/SF-coated coils were investigated in the modified Boyden chamber assay in vitro. Microscopic images of the stained EPCs clearly showed increased chemotactic response of the cells with increasing amount of SDF-1 immobilized within the coils. EPCs contributed to neck neointima formation after the implantation of SDF-1-coated coils.
In this study, histological healing was improved by SDF-1α-coated coils compared with unmodified coils. At 2 and 4 weeks after coil implantation, sham operation group displayed unorganized thrombus, and unmodified coils developed loose connective tissue, whereas SDF-1α-coated coils showed significant intimal proliferation response. As shown in our study, α-SMA-positive cells were present in the sacs of organized tissues, and the proliferated tissues were stained blue with Masson trichrome. The proliferated tissues were composed of cellular components that were probably fibroblasts, smooth muscle cells and collagen fibers. A report revealed that SDF-1α/CXCR4 axis played a crucial role in the recruitment of bone marrow-derived smooth muscle cell progenitor in response to arterial injury and the mediation of neointimal hyperplasia [36]. We speculate that SDF-1α released from SDF-1α/SF coils influenced fibroblasts or smooth muscle cells in the vascular lumen and hence, inducing fibrosis in the arterial lumen. A recent study described the arterial wall changes after HydroCoil implantation into rat aneurysm model. Because of the fully expandable property of hydrogel, disappearance of intima and damage of elastic lamina were found in the sacs [37]. Although blood clots and organized tissues appeared within the CCA sacs following the placement of SDF-1α-coated coils and the transplantation of EPCs, the vascular wall in this group remained similar to those of other groups. SEM showed more fibrin deposition on the surface of SDF-1α-coated coils and light microscopy showed wound healing at the necks of the embolized aneurysms. These results suggested that SDF-1α/SF-coated coils were bioactive and could functionally enhance clot organization and endothelial cell proliferation. The use of SDF-1-coated coils and EPC transplantation may improve long-term anatomical outcomes by decreasing aneurysm recanalization due to stronger in situ anchoring of coils by organized fibrous tissue.
The aim of this study was to explore the suitability of SDF-1α-loaded SF for the surface modification of platinum coils and its efficacy in promoting aneurysm healing at the neck of rat aneurysm model. However, the present study still has several limitations: We didn’t construct SF-coated coil group (SF alone) because it has been proven that SF-coated coils had no capability of recruiting EPCs in vitro. In addition, endovascular approach for the implantation of coils in small animal model used in this study was difficult. Moreover, the transplantation of autologous EPCs on SDF-1α-coated coils in embolized aneurysms was limited to unruptured aneurysms because of the time required for harvesting EPCs. For SDF-1α-coated coils alone, the coil could induce neointima formation at the aneurysm neck and decrease recanalization of ruptured aneurysm that needed urgent treatment. However, further experiments are required to determine the behavior of the injected EPCs in vivo and to test the effects in larger animals.
In the present study, we developed a bioactive platinum coil modified with SDF-1α/SF. Sustained release of SDF-1α from coils could be achieved through the degradation of SF. Implantation of coils coated with SDF-1α/SF combined with autogoulous transplantation of EPCs could accelerate clot organization and endothelial cell proliferation. In the experimental rat aneurysm, EPCs played a crucial role in the repairing and reconstruction of the aneurysm neck orifice after being embolized with SDF-1α/SF-coated coils, which could prevent the recurrence of coiled aneurysm. Our results suggested that SF might be a promising alternative carrier of growth factors for the preparation of bioactive platinum coils for endovascular treatment of aneurysms.
Acknowledgements
The authors wish to acknowledge Professors Daping Quan and Chi Zeng (School of Chemistry and Chemical Engineering, Sun Yat-Sen University, Guangzhou, China) for their valuable help in preparing SDF-1α-coated coils. This research was supported by the Special Program of Guangdong Province, China (No. 00136530154773042), and the Science and Technology Program of Haizhu District, Guangzhou, Guangdong Province, China (No. 2013-cg-28).
Disclosure of conflict of interest
None.
References
- 1.Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, Sandercock P. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–17. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 2.Bakker NA, Metzemaekers JD, Groen RJ, Mooij JJ, Van Dijk JM. International subarachnoid aneurysm trial 2009: endovascular coiling of ruptured intracranial aneurysms has no significant advantage over neurosurgical clipping. Neurosurgery. 2010;66:961–62. doi: 10.1227/01.NEU.0000368152.67151.73. [DOI] [PubMed] [Google Scholar]
- 3.Alshekhlee A, Mehta S, Edgell RC, Vora N, Feen E, Mohammadi A, Kale SP, Cruz-Flores S. Hospital mortality and complications of electively clipped or coiled unruptured intracranial aneurysm. Stroke. 2010;41:1471–6. doi: 10.1161/STROKEAHA.110.580647. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Pan R, Wang H, Rong X, Yin Z, Milgrom DP, Shi X, Tang Y, Peng Y. Clipping versus coiling for ruptured intracranial aneurysms: a systematic review and meta-analysis. Stroke. 2013;44:29–37. doi: 10.1161/STROKEAHA.112.663559. [DOI] [PubMed] [Google Scholar]
- 5.Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, Lamoureux J, Chagnon M, Roy D. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–403. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]
- 6.So TY, Dowling R, Mitchell PJ, Laidlaw J, Yan B. Risk of growth in unruptured intracranial aneurysms: a retrospective analysis. J Clin Neurosci. 2010;17:29–33. doi: 10.1016/j.jocn.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Kodama T, Iwata H. Comparison of bare metal and statin-coated coils on rates of intra-aneurysmal tissue organization in a rat model of aneurysm. J Biomed Mater Res B Appl Biomater. 2013;101:656–62. doi: 10.1002/jbm.b.32869. [DOI] [PubMed] [Google Scholar]
- 8.Aronson JP, Mitha AP, Hoh BL, Auluck PK, Pomerantseva I, Vacanti JP, Ogilvy CS. A novel tissue engineering approach using an endothelial progenitor cell-seeded biopolymer to treat intracranial saccular aneurysms. J Neurosurg. 2012;117:546–54. doi: 10.3171/2012.5.JNS091308. [DOI] [PubMed] [Google Scholar]
- 9.Reul J, Weis J, Spetzger U, Konert T, Fricke C, Thron A. Long-term angiographic and histopathologic findings in experimental aneurysms of the carotid bifurcation embolized with platinum and tungsten coils. AJNR Am J Neuroradiol. 1997;18:35–42. [PMC free article] [PubMed] [Google Scholar]
- 10.Agrawal SK. Aneurysm embolization with biologically active coils: an animal study. Neurol Res. 2013;35:37–43. doi: 10.1179/1743132812Y.0000000110. [DOI] [PubMed] [Google Scholar]
- 11.Abrahams JM, Forman MS, Grady MS, Diamond SL. Delivery of human vascular endothelial growth factor with platinum coils enhances wall thickening and coil impregnation in a rat aneurysm model. AJNR Am J Neuroradiol. 2001;22:1410–7. [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto H, Terada T, Tsuura M, Itakura T, Ogawa A. Experimental polyvinyl alcohol core coil for a drug delivery system. Interv Neuroradiol. 2003;9:107–11. doi: 10.1177/15910199030090S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toma N, Imanaka-Yoshida K, Takeuchi T, Matsushima S, Iwata H, Yoshida T, Taki W. Tenascin-C-coated platinum coils for acceleration of organization of cavities and reduction of lumen size in a rat aneurysm model. J Neurosurg. 2005;103:681–6. doi: 10.3171/jns.2005.103.4.0681. [DOI] [PubMed] [Google Scholar]
- 14.Sano H, Toda M, Sugihara T, Uchiyama N, Hamada J, Iwata H. Coils coated with the cyclic peptide SEK-1005 accelerate intra-aneurysmal organization. Neurosurgery. 2010;67:984–91. doi: 10.1227/NEU.0b013e3181eb95da. discussion 992. [DOI] [PubMed] [Google Scholar]
- 15.Molyneux AJ, Clarke A, Sneade M, Mehta Z, Coley S, Roy D, Kallmes DF, Fox AJ. Cerecyte coil trial: angiographic outcomes of a prospective randomized trial comparing endovascular coiling of cerebral aneurysms with either cerecyte or bare platinum coils. Stroke. 2012;43:2544–50. doi: 10.1161/STROKEAHA.112.657254. [DOI] [PubMed] [Google Scholar]
- 16.Pierot L, Leclerc X, Bonafe A, Bracard S. Endovascular treatment of intracranial aneurysms with matrix detachable coils: midterm anatomic follow-up from a prospective multicenter registry. AJNR Am J Neuroradiol. 2008;29:57–61. doi: 10.3174/ajnr.A0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao EH, Fukuda N, Matsumoto T, Katakawa M, Yamamoto C, Han Y, Ueno T, Kobayashi N, Matsumoto K. Effects of the antioxidative beta-blocker celiprolol on endothelial progenitor cells in hypertensive rats. Am J Hypertens. 2008;21:1062–8. doi: 10.1038/ajh.2008.233. [DOI] [PubMed] [Google Scholar]
- 19.Pan H, Zimmerman T, Zakhaleva J, Abrahams JM, Jiang H, Chen W. Embolization of a common carotid aneurysm with rhVEGF coupled to a pH-responsive chitosan in a rat model. J Neurosurg. 2010;112:658–65. doi: 10.3171/2009.1.JNS08411. [DOI] [PubMed] [Google Scholar]
- 20.Uebersax L, Mattotti M, Papaloizos M, Merkle HP, Gander B, Meinel L. Silk fibroin matrices for the controlled release of nerve growth factor (NGF) Biomaterials. 2007;28:4449–60. doi: 10.1016/j.biomaterials.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 21.Murayama Y, Vinuela F, Suzuki Y, Akiba Y, Ulihoa A, Duckwiler GR, Gobin YP, Vinters HV, Iwaki M, Abe T. Development of the biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms. Part II: an experimental study in a swine aneurysm model. AJNR Am J Neuroradiol. 1999;20:1992–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumoto H, Terada T, Tsuura M, Itakura T, Ogawa A. Basic fibroblast growth factor released from a platinum coil with a polyvinyl alcohol core enhances cellular proliferation and vascular wall thickness: an in vitro and in vivo study. Neurosurgery. 2003;53:402–7. 407–8. doi: 10.1227/01.neu.0000073728.82721.8e. [DOI] [PubMed] [Google Scholar]
- 23.Filippo TR, Galindo LT, Barnabe GF, Ariza CB, Mello LE, Juliano MA, Juliano L, Porcionatto MA. CXCL12 N-terminal end is sufficient to induce chemotaxis and proliferation of neural stem/progenitor cells. Stem Cell Res. 2013;11:913–25. doi: 10.1016/j.scr.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Rosenkranz K, Kumbruch S, Lebermann K, Marschner K, Jensen A, Dermietzel R, Meier C. The chemokine SDF-1/CXCL12 contributes to the ‘homing’ of umbilical cord blood cells to a hypoxic-ischemic lesion in the rat brain. J Neurosci Res. 2010;88:1223–33. doi: 10.1002/jnr.22292. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Yu P, Wu R, Ge Y, Wu J, Zhu J, Jia R. Renal cell carcinoma-adjacent tissues enhance mobilization and recruitment of endothelial progenitor cells to promote the invasion of the neoplasm. Biomed Pharmacother. 2013;67:643–9. doi: 10.1016/j.biopha.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 27.Young PP, Vaughan DE, Hatzopoulos AK. Biologic properties of endothelial progenitor cells and their potential for cell therapy. Prog Cardiovasc Dis. 2007;49:421–9. doi: 10.1016/j.pcad.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Wang YC, Hu XB, Zhang BF, Dou GR, He F, Gao F, Feng F, Liang YM, Dou KF, Han H. Notch-RBP-J signaling regulates the mobilization and function of endothelial progenitor cells by dynamic modulation of CXCR4 expression in mice. PLoS One. 2009;4:e7572. doi: 10.1371/journal.pone.0007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villalvilla A, Moro M, Arruza L, Redondo S, Fernandez-Cruz A, Fernandez-Durango R. Circulating endothelial progenitor cells are reduced in rat oxygen-induced retinopathy despite a retinal SDF-1/CXCR4 and VEGF proangiogenic response. Life Sci. 2012;91:264–70. doi: 10.1016/j.lfs.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Fang X, Zhao R, Wang K, Li Z, Yang P, Huang Q, Xu Y, Hong B, Liu J. Bone marrow-derived endothelial progenitor cells are involved in aneurysm repair in rabbits. J Clin Neurosci. 2012;19:1283–6. doi: 10.1016/j.jocn.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 31.Wei H, Mao Q, Liu L, Xu Y, Chen J, Jiang R, Yin L, Fan Y, Chopp M, Dong J, Zhang J. Changes and function of circulating endothelial progenitor cells in patients with cerebral aneurysm. J Neurosci Res. 2011;89:1822–8. doi: 10.1002/jnr.22696. [DOI] [PubMed] [Google Scholar]
- 32.Rockwood DN, Preda RC, Yucel T, Wang X, Lovett ML, Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011;6:1612–31. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang S, Zhu J, Xu Y, Xiang AP, Jiang MH, Quan D. The effects of gradients of nerve growth factor immobilized PCLA scaffolds on neurite outgrowth in vitro and peripheral nerve regeneration in rats. Biomaterials. 2013;34:7086–96. doi: 10.1016/j.biomaterials.2013.05.080. [DOI] [PubMed] [Google Scholar]
- 34.Chang ZT, Hong L, Wang H, Lai HL, Li LF, Yin QL. Application of peripheral-blood-derived endothelial progenitor cell for treating ischemia-reperfusion injury and infarction: a preclinical study in rat models. J Cardiothorac Surg. 2013;8:33. doi: 10.1186/1749-8090-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsukada S, Kwon SM, Matsuda T, Jung SY, Lee JH, Lee SH, Masuda H, Asahara T. Identification of mouse colony forming endothelial progenitor cells for postnatal neovascularization: a novel insight highlighted by new mouse colony forming assay. Stem Cell Res Ther. 2013;4:20. doi: 10.1186/scrt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Mopps B, Mericskay M, Gierschik P, Biessen EA, Weber C. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–91. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 37.Zhang C, Chaudhary N, Gemmete JJ, Thompson BG, Xi G, Pandey AS. Reactive tissue proliferation and damage of elastic lamina caused by hydrogel coated coils in experimental rat aneurysms. J Neurointerv Surg. 2013;6:480–6. doi: 10.1136/neurintsurg-2013-010867. [DOI] [PubMed] [Google Scholar]


