Abstract
The transcriptional factor AP-2α is a tumor suppressor gene and is downregulated in various neoplasms including glioma. Although the level of AP-2α is negatively associated with the grade of human glioma, the specific functions of AP-2α in glioma are still unknown. In this study, we experimentally showed that artificial overexpression of AP-2α in glioma T98G and U251 cells significantly downregulated the mRNA levels of Bcl-xl, Bcl-2, c-IAP2 and survivin, together with upregulation of the Hrk mRNA levels. Reintroduction of AP-2α also induced downregulation of the protein levels of survivin and VEGF in glioma cells. In biological assays with T98G and U251 cells, AP-2α reduced tumor cell growth, increased cell death, attenuated cell migration and endothelial tube formation. The AP-2α transcription factor may play an important role in suppressing glioma progression.
Keywords: AP-2 alpha, glioma, cell growth, apoptosis, invasion
Introduction
AP-2α, which is located in the short arm of chromosome 6 adjoining to the HLA gene, is a member of the transcription factor activator protein-2 (AP-2) family {Britto, 2007 #42} {Britto, 2007 #42}. AP-2α binds to CG-rich sequences such as 5’-GCCN3GGC, 5’-GCCN4GGC and 5’-GCCN3/4GGG via a DNA-binding domain within the COOH-terminal half of the protein and plays key roles in transcriptional regulation [1].
In addition to its important function in physiological processes such as embryo development, AP-2α acts as a tumor suppressor gene, inhibiting the initiation and progression of a variety of malignancies. AP-2α expression was decreased in many neoplasms such as glioma [2-4], prostate cancer [5], breast cancer [6-9], colon cancer [10], melanoma [11,12], ovarian cancer [13] and renal carcinoma [14].
AP-2α has a pivotal role in regulating the expression of key genes, the products of which are involved in tumor initiation and progression. For example, AP-2α regulates genes that are involved in proliferation (c-MYC), cell cycle regulation (HER-2 and p21WAF1), apoptosis (c-KIT, Bcl-2, and FAS/APO-1), cell adhesion(MCAM/MUC18 and E-cadherin), and tumor invasion/angiogenesis (MMP-9, PAI-I, and PAR-1) [15-19].
Decreased AP-2α level was associated with grade of glioma [20]. However, the specific effects of AP-2α on glioma cells were not clearly understand. In the present study, we artificially overexpressed AP-2α in glioma cells, and investigated its effects on glioma cell growth, apoptosis, migration and endothelial tube formation.
Materials and methods
Cell lines, tissue samples and general reagents
Human glioma cells T98G, U251, adenovirus-immortalized human embryonic kidney epithelial cell HEK-293, and human umbilical vein endothelial cells (HUVEC) were all from the American Type Culture Collection and were cultured in DMEM with 10% FCS (Life Technologies, TakaRa, Japan). Archived formalin-fixed, paraffin-embedded samples of glioma (n = 2) and normal brain (n = 2), were also used. All tissue samples were from West China Hospital and were collected and used according to the ethical guidelines and procedures approved by the institutional supervisory committee. Tris base, EDTA, Tween 20 and dithiothreitol were from Amresco (Solon, OH). PMSF, aprotinin and pepstatin were from Roche Diagnostics (Mannheim, Germany).
RT-PCR and real-time qPCR
Total RNA was isolated using the TRIzol reagent (Invitrogen/Life Technologies, TakaRa, Japan). cDNA was synthesized from the isolated RNA by RT and amplified by PCR. PCR primer sequences and product lengths were as follows. AP-2α: 5’-ACT CCT TAC CTC ACG CCA TC-3’, 5’-ATA GGG ATG GCG GAG ACG-3’, 136 bp; actin: 5’-TGG AGA AAT CTG GCA CCA C-3’, 5’-GAG GCG TAC AGG GAT AGC AC-3’, 190 bp; Bcl-xl: 5’-ctg tgc gtg gaa agc gta g-3’, 5’-CTCGGCTGCTGCATTGTTC-3’, 159 bp; Bcl-2: 5’-GTC ATG TGT GTG GAG AGC GTC-3’, 5’-GAG TCT TCA GAC AGC CAG G-3’, 193 bp; Hrk: 5’-CTA GGC GAC GAG CTG CAC CAG-3’, 5’-GCA CAG CCA AGG CCA GTA GGT G-3’, 102 bp; c-IAP2: 5’-AGG GAA GAG GAG AGA GAA AGA GC-3, 5’-CGG CAG TTA GTA GAC TAT CCA GG-3’, 133 bp; Survivin: 5’-GCA GTT TGA AGA ATT AAC CCT TG-3’, 5’-CAC TTT CTC CG CAG TTT CCT C-3’, 121 bp. PCR products were separated by agarose gel electrophoresis and visualized by the fluorescent dye GoldView (Beijing SBS Genetech, Beijing, China) under UV light. Real time PCR was carried out as described [21].
Western blot
The primary antibodies used were as follows: AP-2α (rabbit monoclonal, 1:500, Santa Cruz Biotechnology, USA), VEGF (mouse Polyclonal, 1:500, Boster, China), Survivin (rabbit polyclonal, 1:500, R& USA), GAPDH (mouse monoclonal, 1:10000, Kangcheng, China). Horseradish peroxidase-labeled secondary antibodies were from Zymed Laboratories (South San Francisco, CA). Western blotting was carried out as previously described [22].
Recombinant adenoviral vectors for overexpression of AP-2α
The coding sequence of AP-2α (transcript variant 3, 1321 bp) was cloned from HEK-293 cells by RT-PCR with the following primers: 5’-CTC GAG CCG CGA TGT CCA TAC TTG C-3’, 5’-AAG CTT GCC TCA CTT TCT GTG CTT CTC-3’. The adenovirus vector for AP-2α was constructed as described [21] and named AD-AP2α. The pAdTrack-CMV vector was used as control (AD-control). The titers and multiplicity of infection (MOI) were determined according to the manufacturer’s protocols.
MTT assays
T98G and U251 cells transfected with AD-AP2α or AD-control were incubated in 96-well tissue culture plates for the indicated times. MTT (Sigma Aldrich, USA) was then added to each well and incubated for 4 hours. Then the supernatant was removed, replaced with 100 μl dimethylsulfoxide and incubated for 10 min in dark. The absorbance at 570 nm (A570) was measured by spectrophotometer (Bio-Tek, USA).
Migration assays
Transwell migration assays were used to examine the effect of AP-2α on glioma cell migration. T98G or U251 cell were transfected with AD-AP2α (AD-control as negative control). 24 h later, cells were isolated and 1 × 105 cells suspended in serum-free media were placed in the upper compartment of 5 mm-pore transwells (Corning Costar, Lowell, MA). Cells were allowed to migrate for 24 h and the transwell slides were finally fixed with paraformaldehyde and stained with crystal violet.
Tube formation assay
Tube formation assay was performed as previously described [23]. Conditioned media were obtained by incubating the cells transfected with AD-AP2α or AD-control in DMEM without serum for 24 h. The 96-well plate was coated with 50 µl growth factor-reduced matrigel (BD Biosciences). A total of 4 × 105HUVEC cells was resuspended in 100 µl of conditioned media with 1% FCS and seeded on matrigel-coated wells. HUVEC cells were incubated for 18 h to allow formation of tube-like structures. Total tube lengths formed were measured and compared from three different viewing fields at 10 × magnification using an inverted microscope (Nikon TMS). Tube formation assays were performed in triplicate.
Terminal deoxynucleotidyl transferase-mediated biotinylated dUTP nick end-labeling
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) was performed by using in situ cell death detection kit (Roche, USA) as previously described [22].
Statistical analysis
All experiments were repeated at least thrice. PASW Statistics 18.0 software package was used for statistical analysis. P value less than 0.05 was considered significant statistically.
Results
AP-2α expression was significantly decreased in human glioma cells
RT-PCR and western blot confirmed significant reduction of AP-2α level in human glioma cells (T98G, U251) and glioma tissues (G1, G2) as compared with normal human brain (N1, N2) at both the mRNA and protein levels (Figure 1).
Figure 1.
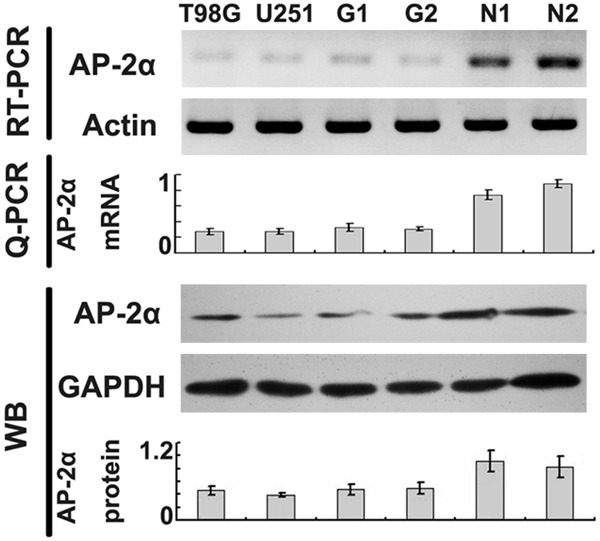
AP-2α expression in glioma. AP-2α expression in glioma cell lines (U251 and T98G), glioma tissues (G1 and G2) and normal brain tissues (N1 and N2). RT-PCR (upper), Q-PCR (middle) and western blot analysis (lower, with semiquantitative histograms) showed decreased AP-2α levels (relative to actin or GAPDH, mean ± SD of three independent experiments, P < 0.05) in glioma cell lines and tissues compared with that in normal brain tissues.
Artificial overexpression of AP-2α decreased mRNA levels of Bcl-xl, Bcl-2, c-IAP2 and survivin, increased mRNA levels of Hrk, and decreased protein levels of survivin and VEGF
To examine the effects of AP-2α on glioma cells, recombinant adenovirus for overexpression of AP-2α (AD-AP2α, MOI 50, 96 hours) was constructed (Figure 2B). RT-PCR, Q-PCR and western blot confirmed the efficient expression of AP-2α in AD-AP2α infected T98G and U251 cells (Figure 3).
Figure 2.
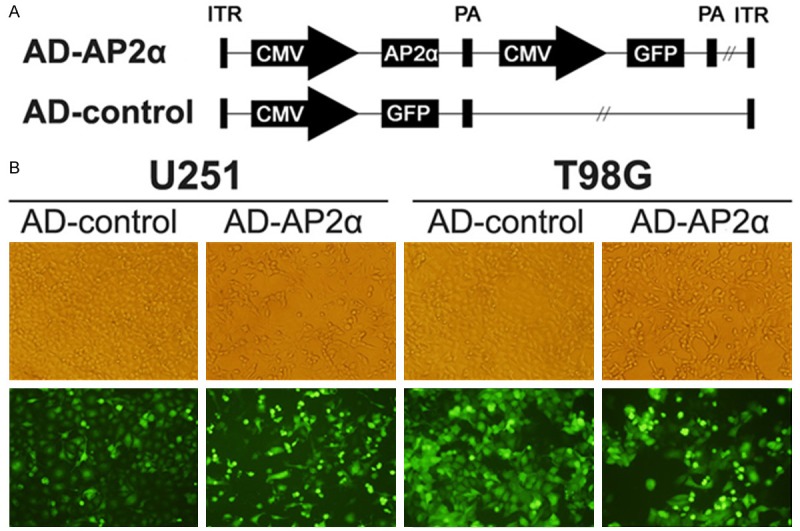
The AP-2α recombinant adenoviral vector was constructed. (A) Shows the recombinant adenoviral vector structure (ITR, inverted terminal repeat; CMV, cytomegalovirus; PA, poly A; GFP, green fluorescence protein.). The infection efficiency of AD-AP2α and AD-control of glioma cells was shown by homogenous green fluorescence protein expression of the infected cells (B).
Figure 3.
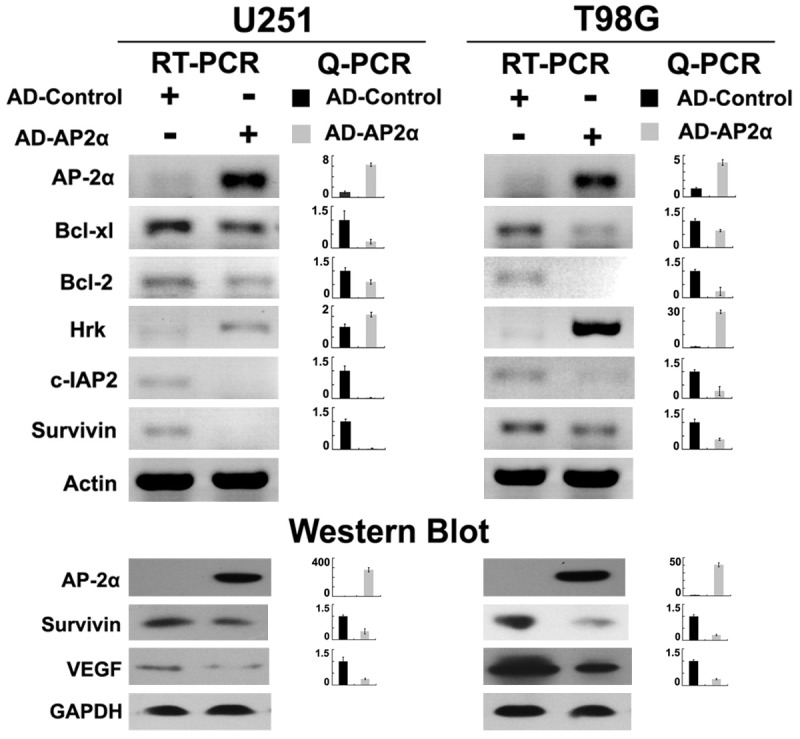
Overexpression of AP-2α was confirmed by PCR and WB. In glioma cells, ectopic expression of AD-AP2α resulted in significant downregulations of Bcl-2, Bcl-xl, c-IAP2 and Survivin mRNA levels, together with upregulation of Hrk mRNA level (upper) (relative to actin, means ± SD from three independent experiments, P < 0.05). Western blot showed decreased (relative to GAPDH, means ± SD from three independent experiments, P < 0.05) protein levels of VEGF and Survivin with AD-AP2α infection in both cells (lower).
Artificial overexpression of AP-2α in T98G and U251 resulted in significant decreased expression of Bcl-2, Bcl-xl, c-IAP2 and survivin, together with increased Hrk mRNA level, and western blot displayed repression of survivin and VEGF after AD-AP2α treatment (Figure 3). Inconspicuous alterations were detected in the levels of other molecules such as Bik, BNIP3, c-IAP1 and CASP8 in T98G and U251 overexpressing AD-AP2α (data not shown).
AP-2α inhibited cell growth, promoted apoptosis, inhibited cell migration and HUVEC tube formation ability in glioma
Having confirmed the contributions of AP-2α to those tumor-associated genes, we then turned to show the biological effects of artificial AP-2α overexpression in glioma cells. Concomitant with AD-AP2α mediated AP-2α overexpression, glioma cells showed significantly reduced cell growth (Figure 4A), increased cell apoptosis (Figure 4B) and decreased cell migration (Figure 4C). Microtubule formation of HUVEC cells was decreased when stimulated with supernatant from glioma cells re-expressing AP-2α (AD-AP2α) (Figure 4C).
Figure 4.
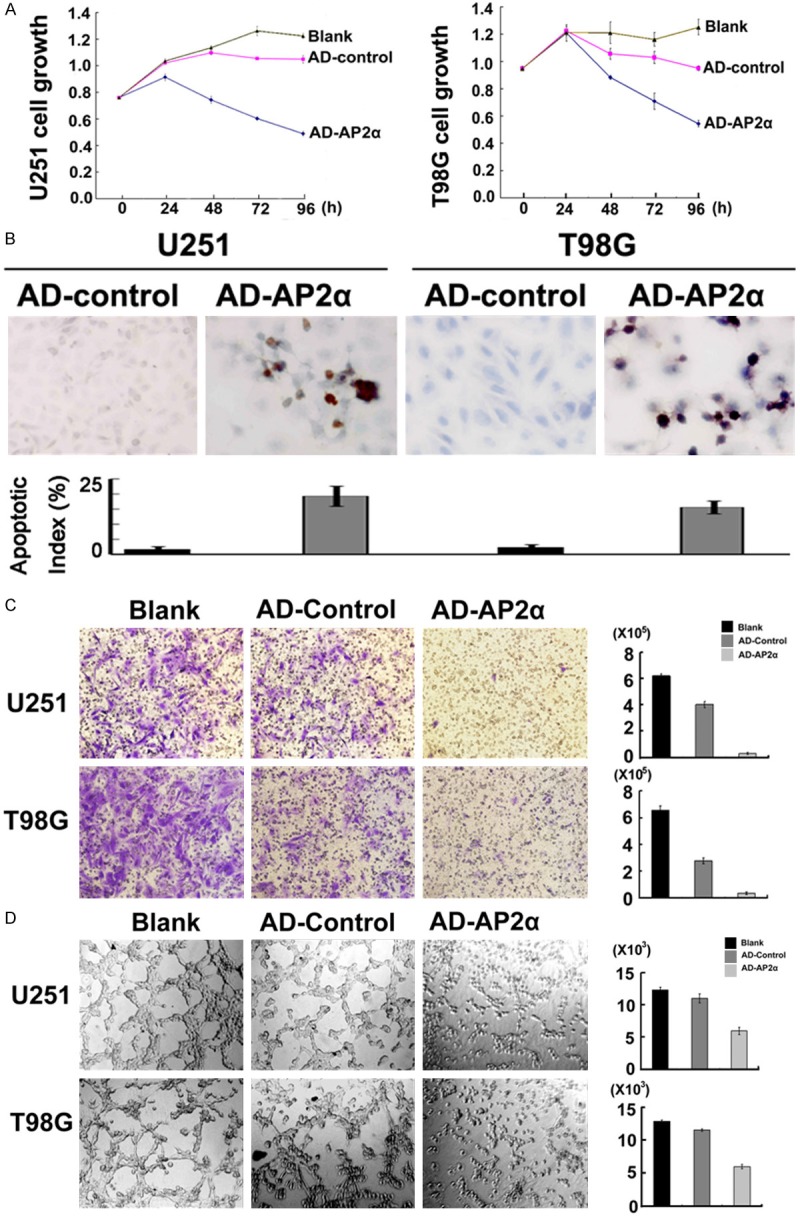
Effects on cell behavior by artificial overexpression of AP-2α in U251 and T98G cells. MTT assay (A) showed that glioma cell growth was significantly inhibited concomitant with the reintroduction of AP-2α. TUNEL assay (B) demonstrated increased apoptosis (shown by brown-staining granules in apoptotic cells) in glioma cells with AP-2α overexpression. Migration assay (C) revealed that artificial re-expression of AP-2α inhibited tumor cell migration. Microtube formation assay (D) showed suppressed HUVEC microtubule formation induced by AP-2α over expression in U251 and T98G cell supernatant. Values are means ± SD from three independent experiments. P < 0.05 compared to AD-control.
Discussion
The AP-2 family consists of five highly homologous members, namely AP-2α, AP-2β, AP-2γ, AP-2δ and AP-2ε [24], the expression of which is cell-type specific. The amine terminus of the AP-2α protein is responsible for transactivation activity, and a basic and helical region in the COOH terminus is responsible for dimerization and DNA binding [25,26].
AP-2α mediates programmed gene expression in embryonic morphogenesis and cell differentiation [27]. AP-2α knockout mice die perinatally with severe multiple congenital defects involving face, skull, and sensory organs. In situ hybridization showed that mouse embryos specifically expressed AP-2α in ectoderm-derived tissues, including craniofacial, gonad, kidney, and skin [27,28].
Also, AP-2α has been shown to be a tumor suppressor gene, the loss of which is linked to malignant transformation, tumor progression and elevated risk of metastasis in such tumors as melanoma [29-32], prostate cancer [33,34], breast cancer [35,36], pancreatic cancer [37,38], colorectal cancer [39] and ovarian cancer [13]. Notably, loss of AP-2α was strongly correlated with higher grade in human glioma, and appeared to be the most frequent molecular change in astrocytic tumor progression from lower to higher grade, although no significant effect of AP-2α on survival was observed [20].
In this research, significant reductions of AP-2α were also detected in glioma cells and tissues, which is in consistence with studies before. However, the role of AP-2α in the development/progression of glioma has not been fully defined. It has been reported that there are multitudinous genes regulated by AP-2α such as those involved in cell proliferation (Hoxa, IGF receptor type I and IGF-binding proteins 3/5) [40-43], cell cycle regulation (p21WAF1/CIP17 and HER-2) [44,45], apoptosis (c-KIT) [30,46,47], cell adhesion (E-cadherin and MCAM/MUC18) [48,49], and cell migration/angiogenesis (MMP-2, and PAR-1) [48,50,51]. Our data showed that AP-2α inhibited the expression of the anti-apoptotic genes Bcl-xl, Bcl-2, c-IAP2 and surviving and the pro-angiogenic gene VEGF, whereas promoted the expression of the pro-apoptotic gene HRK, thus enhanced glioma cell apoptosis and inhibited HUVEC tube formation ability. These finding are compatible with our earlier works [47,52,53]. Except for this, we also detected the influences of AP-2α on a series of genes involving in cell proliferation and migration (data not shown) but failed to get meaningful results. Nonetheless, AP-2α induced attenuated glioma cell proliferation and migration in biological experiments, the mechanism of which has not been well understood yet.
In summary, the results of this study showed that AP-2α, which is dramatically down-regulated in glioma, suppresses the progression of glioma by regulating a series of tumor-associated genes. These findings may shed some new lights on the AP-2α dysfunction in glioma progression and suggest AP-2α a potential therapeutic target in glioma.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of China (NSFC, 81272848, 81272820, 81101529, 81101628, 81302225) and Postdoctoral Fund of China (20100480076, 201104643, 2013M531970, 2014T70876).
Disclosure of conflict of interest
None.
References
- 1.Mohibullah N, Donner A, Ippolito JA, Williams T. SELEX and missing phosphate contact analyses reveal flexibility within the AP-2 [alpha] protein: DNA binding complex. Nucleic Acids Res. 1999;27:2760–9. doi: 10.1093/nar/27.13.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Britto R, Umesh S, Hegde AS, Hegde S, Santosh V, Chandramouli BA, Somasundaram K. Shift in AP-2alpha localization characterizes astrocytoma progression. Cancer Biol Ther. 2007;6:413. doi: 10.4161/cbt.6.3.3756. [DOI] [PubMed] [Google Scholar]
- 3.Heimberger AB, McGary EC, Suki D, Ruiz M, Wang H, Fuller GN, Bar-Eli M. Loss of the AP-2α transcription factor is associated with the grade of human gliomas. Clin Cancer Res. 2005;11:267. [PubMed] [Google Scholar]
- 4.Britto R, Umesh S, Hegde AS, Hegde S, Santosh V, Chandramouli BA, Somasundaram K. Shift in AP-2alpha localization characterizes astrocytoma progression. Cancer Biol Ther. 2007;6:413–8. doi: 10.4161/cbt.6.3.3756. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz M, Troncoso P, Bruns C, Bar-Eli M. Activator protein 2α transcription factor expression is associated with luminal differentiation and is lost in prostate cancer. Clin Cancer Res. 2001;7:4086. [PubMed] [Google Scholar]
- 6.Turner BC, Zhang J, Gumbs AA, Maher MG, Kaplan L, Carter D, Glazer PM, Hurst HC, Haffty BG, Williams T. Expression of AP-2 transcription factors in human breast cancer correlates with the regulation of multiple growth factor signalling pathways. Cancer Res. 1998;58:5466. [PubMed] [Google Scholar]
- 7.Friedrichs N, Jäger R, Paggen E, Rudlowski C, Merkelbach-Bruse S, Schorle H, Buettner R. Distinct spatial expression patterns of AP-2alpha and AP-2gamma in non-neoplastic human breast and breast cancer. Mod Pathol. 2004;18:431–438. doi: 10.1038/modpathol.3800292. [DOI] [PubMed] [Google Scholar]
- 8.Pellikainen JM, Kosma VM. Activator protein-2 in carcinogenesis with a special reference to breast cancer-A mini review. Int J Cancer. 2007;120:2061–2067. doi: 10.1002/ijc.22648. [DOI] [PubMed] [Google Scholar]
- 9.Gee JM, Robertson JF, Ellis IO, Nicholson RI, Hurst HC. Immunohistochemical analysis reveals a tumour suppressor-like role for the transcription factor AP-2 in invasive breast cancer. J Pathol. 1999;189:514–520. doi: 10.1002/(SICI)1096-9896(199912)189:4<514::AID-PATH463>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Ropponen KM, Kellokoski JK, Pirinen RT, Moisio KI, Eskelinen MJ, Alhava EM, Kosma VM. Expression of transcription factor AP-2 in colorectal adenomas and adenocarcinomas; comparison of immunohistochemistry and in situ hybridisation. J Clin Pathol. 2001;54:533. doi: 10.1136/jcp.54.7.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tellez CS, Davis DW, Prieto VG, Gershenwald JE, Johnson MM, McCarty MF, Bar-Eli M. Quantitative analysis of melanocytic tissue array reveals inverse correlation between activator protein-2α and protease-activated receptor-1 expression during melanoma progression. J Invest Dermatol. 2006;127:387–393. doi: 10.1038/sj.jid.5700539. [DOI] [PubMed] [Google Scholar]
- 12.Baldi A, Santini D, Battista T, Dragonetti E, Ferranti G, Petitti T, Groeger AM, Angelini A, Rossiello R, Baldi F, Natali PG, Paggi MG. Expression of AP-2 transcription factor and of its downstream target genes c-kit, E-cadherin and p21 in human cutaneous melanoma. J Cell Biochem. 2001;83:364–372. doi: 10.1002/jcb.1235. [DOI] [PubMed] [Google Scholar]
- 13.Anttila MA, Kellokoski JK, Moisio KI, Mitchell PJ, Saarikoski S, Syrjänen K, Kosma VM. Expression of transcription factor AP-2alpha predicts survival in epithelial ovarian cancer. Br J Cancer. 2000;82:1974–83. doi: 10.1054/bjoc.2000.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oya M, Mikami S, Mizuno R, Miyajima A, Horiguchi Y, Nakashima J, Marumo K, Mukai M, Murai M. Differential expression of activator protein-2 isoforms in renal cell carcinoma. Urology. 2004;64:162–167. doi: 10.1016/j.urology.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 15.Yu L, Hitchler MJ, Sun W, Sarsour EH, Goswami PC, Klingelhutz AJ, Domann FE. AP-2α Inhibits c-MYC Induced Oxidative Stress and Apoptosis in HaCaT Human Keratinocytes. J Oncol. 2009;2009:780874. doi: 10.1155/2009/780874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wajapeyee N, Britto R, Ravishankar HM, Somasundaram K. Apoptosis induction by activator protein 2α involves transcriptional repression of Bcl-2. J Biol Chem. 2006;281:16207. doi: 10.1074/jbc.M600539200. [DOI] [PubMed] [Google Scholar]
- 17.Jean D, Gershenwald JE, Huang S, Luca M, Hudson MJ, Tainsky MA, Bar-Eli M. Loss of AP-2 Results in Up-regulation ofMCAM/MUC18 and an Increase in Tumor Growth and Metastasis of Human Melanoma Cells. J Biol Chem. 1998;273:16501. doi: 10.1074/jbc.273.26.16501. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz B, Melnikova VO, Tellez C, Mourad-Zeidan A, Blehm K, Zhao YJ, McCarty M, Adam L, Bar-Eli M. Loss of AP-2α results in deregulation of E-cadherin and MMP-9 and an increase in tumorigenicity of colon cancer cells in vivo. Oncogene. 2007;26:4049–4058. doi: 10.1038/sj.onc.1210193. [DOI] [PubMed] [Google Scholar]
- 19.Tellez C, McCarty M, Ruiz M, Bar-Eli M. Loss of activator protein-2α results in overexpression of protease-activated receptor-1 and correlates with the malignant phenotype of human melanoma. J Biol Chem. 2003;278:46632. doi: 10.1074/jbc.M309159200. [DOI] [PubMed] [Google Scholar]
- 20.Heimberger AB, McGary EC, Suki D, Ruiz M, Wang H, Fuller GN, Bar-Eli M. Loss of the AP-2alpha transcription factor is associated with the grade of human gliomas. Clin Cancer Res. 2005;11:267–72. [PubMed] [Google Scholar]
- 21.Chen X, Gong J, Zeng H, Chen N, Huang R, Huang Y, Nie L, Xu M, Xia J, Zhao F, Meng W, Zhou Q. MicroRNA145 targets BNIP3 and suppresses prostate cancer progression. Cancer Res. 2010;70:2728–38. doi: 10.1158/0008-5472.CAN-09-3718. [DOI] [PubMed] [Google Scholar]
- 22.Chen N, Chen X, Huang R, Zeng H, Gong J, Meng W, Lu Y, Zhao F, Wang L, Zhou Q. BCL-xL is a target gene regulated by hypoxia-inducible factor-1 {alpha} J Biol Chem. 2009;284:10004–12. doi: 10.1074/jbc.M805997200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan KC, Ko JM, Lung HL, Sedlacek R, Zhang ZF, Luo DZ, Feng ZB, Chen S, Chen H, Chan KW, Tsao SW, Chua DT, Zabarovsky ER, Stanbridge EJ, Lung ML. Catalytic activity of Matrix metalloproteinase-19 is essential for tumor suppressor and anti-angiogenic activities in nasopharyngeal carcinoma. Int J Cancer. 2011;129:1826–37. doi: 10.1002/ijc.25855. [DOI] [PubMed] [Google Scholar]
- 24.Eckert D, Buhl S, Weber S, Jäger R, Schorle H. The AP-2 family of transcription factors. Genome Biol. 2005;6:246. doi: 10.1186/gb-2005-6-13-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams T, Tjian R. Characterization of a dimerization motif in AP-2 and its function in heterologous DNA-binding proteins. Science. 1991;251:1067–71. doi: 10.1126/science.1998122. [DOI] [PubMed] [Google Scholar]
- 26.Williams T, Tjian R. Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev. 1991;5:670–82. doi: 10.1101/gad.5.4.670. [DOI] [PubMed] [Google Scholar]
- 27.Schorle H, Meier P, Buchert M, Jaenisch R, Mitchell PJ. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature. 1996;381:235–8. doi: 10.1038/381235a0. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Hagopian-Donaldson S, Serbedzija G, Elsemore J, Plehn-Dujowich D, McMahon AP, Flavell RA, Williams T. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature. 1996;381:238–41. doi: 10.1038/381238a0. [DOI] [PubMed] [Google Scholar]
- 29.Karjalainen JM, Kellokoski JK, Eskelinen MJ, Alhava EM, Kosma VM. Downregulation of transcription factor AP-2 predicts poor survival in stage I cutaneous malignant melanoma. J. Clin. Oncol. 1998;16:3584–91. doi: 10.1200/JCO.1998.16.11.3584. [DOI] [PubMed] [Google Scholar]
- 30.Baldi A, Santini D, Battista T, Dragonetti E, Ferranti G, Petitti T, Groeger AM, Angelini A, Rossiello R, Baldi F, Natali PG, Paggi MG. Expression of AP-2 transcription factor and of its downstream target genes c-kit, E-cadherin and p21 in human cutaneous melanoma. J Cell Biochem. 2001;83:364–72. doi: 10.1002/jcb.1235. [DOI] [PubMed] [Google Scholar]
- 31.Tellez CS, Davis DW, Prieto VG, Gershenwald JE, Johnson MM, McCarty MF, Bar-Eli M. Quantitative analysis of melanocytic tissue array reveals inverse correlation between activator protein-2alpha and protease-activated receptor-1 expression during melanoma progression. J Invest Dermatol. 2007;127:387–93. doi: 10.1038/sj.jid.5700539. [DOI] [PubMed] [Google Scholar]
- 32.Berger AJ, Davis DW, Tellez C, Prieto VG, Gershenwald JE, Johnson MM, Rimm DL, Bar-Eli M. Automated quantitative analysis of activator protein-2alpha subcellular expression in melanoma tissue microarrays correlates with survival prediction. Cancer Res. 2005;65:11185–92. doi: 10.1158/0008-5472.CAN-05-2300. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz M, Troncoso P, Bruns C, Bar-Eli M. Activator protein 2alpha transcription factor expression is associated with luminal differentiation and is lost in prostate cancer. Clin Cancer Res. 2001;7:4086–95. [PubMed] [Google Scholar]
- 34.Lipponen P, Aaltomaa S, Kellokoski J, Ala-Opas M, Kosma V. Expression of activator protein 2 in prostate cancer is related to tumor differentiation and cell proliferation. Eur Urol. 2000;37:573–8. doi: 10.1159/000020195. [DOI] [PubMed] [Google Scholar]
- 35.Pellikainen J, Kataja V, Ropponen K, Kellokoski J, Pietiläinen T, Böhm J, Eskelinen M, Kosma VM. Reduced nuclear expression of transcription factor AP-2 associates with aggressive breast cancer. Clin Cancer Res. 2002;8:3487–95. [PubMed] [Google Scholar]
- 36.Gee JM, Robertson JF, Ellis IO, Nicholson RI, Hurst HC. Immunohistochemical analysis reveals a tumour suppressor-like role for the transcription factor AP-2 in invasive breast cancer. J Pathol. 1999;189:514–20. doi: 10.1002/(SICI)1096-9896(199912)189:4<514::AID-PATH463>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Jonckheere N, Fauquette V, Stechly L, Saint-Laurent N, Aubert S, Susini C, Huet G, Porchet N, Van Seuningen I, Pigny P. Tumour growth and resistance to gemcitabine of pancreatic cancer cells are decreased by AP-2alpha overexpression. Br J Cancer. 2009;101:637–44. doi: 10.1038/sj.bjc.6605190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirono S, Yamaue H, Hoshikawa Y, Ina S, Tani M, Kawai M, Ushijima M, Matsuura M, Saiki Y, Saiura A, Yamamoto J, Miki Y, Noda T. Molecular markers associated with lymph node metastasis in pancreatic ductal adenocarcinoma by genome-wide expression profiling. Cancer Sci. 2010;101:259–66. doi: 10.1111/j.1349-7006.2009.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ropponen KM, Kellokoski JK, Pirinen RT, Moisio KI, Eskelinen MJ, Alhava EM, Kosma VM. Expression of transcription factor AP-2 in colorectal adenomas and adenocarcinomas; comparison of immunohistochemistry and in situ hybridisation. J Clin Pathol. 2001;54:533–8. doi: 10.1136/jcp.54.7.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding X, Yang Z, Zhou F, Wang F, Li X, Chen C, Li X, Hu X, Xiang S, Zhang J. Transcription factor AP-2alpha regulates acute myeloid leukemia cell proliferation by influencing Hoxa gene expression. Int J Biochem Cell Biol. 2013;45:1647–56. doi: 10.1016/j.biocel.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 41.DiGiovanni J, Kiguchi K, Frijhoff A, Wilker E, Bol DK, Beltrán L, Moats S, Ramirez A, Jorcano J, Conti C. Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc Natl Acad Sci U S A. 2000;97:3455–60. doi: 10.1073/pnas.97.7.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohick WS, Wang B, Verma P, Boisclair YR. Insulin-Like growth factor I (IGF-I) and cyclic adenosine 3’, 5’-monophosphate regulate IGF-binding protein-3 gene expression by transcriptional and posttranscriptional mechanisms in mammary epithelial cells. Endocrinology. 2000;141:4583–91. doi: 10.1210/endo.141.12.7854. [DOI] [PubMed] [Google Scholar]
- 43.Duan C, Clemmons DR. Transcription factor AP-2 regulates human insulin-like growth factor binding protein-5 gene expression. J Biol Chem. 1995;270:24844–51. doi: 10.1074/jbc.270.42.24844. [DOI] [PubMed] [Google Scholar]
- 44.Zeng YX, Somasundaram K, el-Deiry WS. AP2 inhibits cancer cell growth and activates p21WAF1/CIP1 expression. Nat Genet. 1997;15:78–82. doi: 10.1038/ng0197-78. [DOI] [PubMed] [Google Scholar]
- 45.Bosher JM, Williams T, Hurst HC. The developmentally regulated transcription factor AP-2 is involved in c-erbB-2 overexpression in human mammary carcinoma. Proc Natl Acad Sci U S A. 1995;92:744–7. doi: 10.1073/pnas.92.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang S, Jean D, Luca M, Tainsky MA, Bar-Eli M. Loss of AP-2 results in downregulation of c-KIT and enhancement of melanoma tumorigenicity and metastasis. EMBO J. 1998;17:4358–69. doi: 10.1093/emboj/17.15.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu M, Chen X, Chen N, Nie L, Li X, Li Q, Zeng H, Zhou Q. Synergistic silencing by promoter methylation and reduced AP-2alpha transactivation of the proapoptotic HRK gene confers apoptosis resistance and enhanced tumor growth. Am J Pathol. 2013;182:84–95. doi: 10.1016/j.ajpath.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Zhang L, Jia L, Cui S, Shi Y, Chang A, Zeng X, Wang P. AP-2alpha suppresses invasion in BeWo cells by repression of matrix metalloproteinase- 2 and -9 and up-regulation of E-cadherin. Mol Cell Biochem. 2013;381:31–9. doi: 10.1007/s11010-013-1685-8. [DOI] [PubMed] [Google Scholar]
- 49.Jean D, Gershenwald JE, Huang S, Luca M, Hudson MJ, Tainsky MA, Bar-Eli M. Loss of AP-2 results in up-regulation of MCAM/MUC18 and an increase in tumor growth and metastasis of human melanoma cells. J Biol Chem. 1998;273:16501–8. doi: 10.1074/jbc.273.26.16501. [DOI] [PubMed] [Google Scholar]
- 50.Gille J, Swerlick RA, Caughman SW. Transforming growth factor-alpha-induced transcriptional activation of the vascular permeability factor (VPF/VEGF) gene requires AP-2-dependent DNA binding and transactivation. EMBO J. 1997;16:750–9. doi: 10.1093/emboj/16.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tellez C, McCarty M, Ruiz M, Bar-Eli M. Loss of activator protein-2alpha results in overexpression of protease-activated receptor-1 and correlates with the malignant phenotype of human melanoma. J Biol Chem. 2003;278:46632–42. doi: 10.1074/jbc.M309159200. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Chen N, Wang X, He Y, Chen X, Huang Y, Yin W, Zhou Q. Apoptosis and proliferation markers in diffusely infiltrating astrocytomas: profiling of 17 molecules. J Neuropathol Exp Neurol. 2006;65:905–13. doi: 10.1097/01.jnen.0000235857.79502.c3. [DOI] [PubMed] [Google Scholar]
- 53.Yin W, Chen N, Zhang Y, Zeng H, Chen X, He Y, Wang X, Zhou Q. Survivin nuclear labeling index: a superior biomarker in superficial urothelial carcinoma of human urinary bladder. Mod Pathol. 2006;19:1487–97. doi: 10.1038/modpathol.3800675. [DOI] [PubMed] [Google Scholar]


