Abstract
Purpose: To investigate the expression of p15INK4b, p16INK4a and p21Waf1/Cip1 in specimens from cases of normal cervical epithelium (NCE), cervical intraepithelial neoplasia (CIN) and squamous cell carcinoma (SCC), and to evaluate whether there is evidence implicating oncogene-induced senescence (OIS) in cervical squamous cell cancer development. Methods: The immunohistochemical expression of p15INK4b, p16INK4a and p21Waf1/Cip1 were investigated in formalin-fixed paraffin-embedded specimens from 19 NCE, 51 CIN and 21 SCC cases, respectively. Comparisons among different groups for each marker were performed with Chi-square test. Results: The expression of p15INK4b, p16INK4a and p21Waf1/Cip1 were significantly higher in both CIN and SCC compared to NCE. Furthermore, the expression of p15INK4b and p21Waf1/Cip1 was significantly higher in CIN П compared to CIN І, and these expressions were statistically higher in CIN Ш compared to CIN П, respectively. The p16INK4a expression was significantly higher in CIN Ш compared to CIN І. Conclusions: The results suggested that the senescence programs mediated by p15INK4b, p16INK4a and p21Waf1/Cip1 were activated during the stage of CIN and SCC, and demonstrated that senescence may play important role in preventing from NCE to SCC.
Keywords: Cervical cancer, senescence, carcinogenesis
Introduction
Cervical cancer is the second only to breast cancer in women as the most common of gynecologic malignancies, and it remains one of the most important causes of mortality in women worldwide [1]. More than 90% of cervical cancer are SCC in pathologic classification. The direct precursor of cervical SCC is represented by CIN, that is usually detected and managed through the Papanicolaou (Pap) test cytological screening and/or high-risk human papillomavirus (HPV) DNA testing [2]. Most of CIN І has complete regression during the 2-year follow-up period. In contrast, high-grade CIN (CIN П and CIN Ш) carries a significant risk of progression to invasive carcinoma. So one of the focuses on cervical cancer research has always been the mechanism of the initiation and development of CIN and SCC.
It was recently demonstrated that cellular apoptosis and senescence are assumed to be two main mechanisms that prevent from cancer development for cells with accumulated somatic mutations. Senescence is defined by a process that keeps the stable form of cell cycle arrest at G1 phase [3], which can be subdivided into two distinct categories: replicative and premature senescence [4,5]. OIS, as one type of stress-induced senescence, has emerged as a barrier to carcinogenesis [6]. Senescent cells are characterized by a flat and large morphology with vacuoles, and with an increase in SAβ-gal [7]. Previous studies have revealed that the ARF/p53/p21 and p16/Rb/E2F pathways play important role in inducing cellular senescence [8]. The regulatory proteins involved in these pathways are cyclin-dependent kinases (CDKs) and cyclin-dependent kinase inhibitors (CDKIs). Among the CDKIs, p15INK4b, p16INK4a and p21Waf1/Cip1 have been identified to be important in maintaining senescence [7,9]. The p16INK4a negatively regulates the cell cycle through competitive binding of CDK4 and 6, thereby inhibiting their binding to cyclin D1. The p15INK4b is located centromeric to the p16/p14 gene locus p14ARF, which is a tumor suppressor and causes cell cycle arrest through transforming growth factor β [10]. The p21Waf1/Cip1 is involved in controlling CDKs activity, and results in cell cycle arrest at the G1- to S-phase transition. Its effector functions are predominantly induced by p53 and it is considered to be a mediator of the tumor-suppressor activity of p53. However, p21Waf1/Cip1 can also be induced in a p53-independent manner [11].
More recent evidence has revealed that senescence markers p15INK4b, p16INK4a and p21Waf1/Cip1 had different expression level in many types of premalignant lesions and cancers, indicating senescence may play important role in cancer development. However, these studies have reported conflicting results of senescence markers expression in different cancers [10,12,13]. In the present study, we investigate the expression of p15INK4b, p16INK4a and p21Waf1/Cip1 in specimens from cases of NCE, CIN (including CIN І, CIN П and CIN Ш) and SCC, and evaluate whether there is evidence implicating OIS in cervical squamous cell cancer development.
Materials and methods
The pathology database in the department of pathology, the Second Affiliated Hospital of Soochow University, was retrospectively reviewed. All investigations were approved by the local ethics committee, and waived the need for written informed consent. We recruited specimens from 19 cases of NCE, 51 cases of CIN and 21 cases of SCC. Furthermore, there were 18 CIN І, 16 CIN П, and 17 CIN Ш in total of 51 specimens of CIN.
Immunohistochemical staining
Four serial slides, each 5 um thick, were cut from paraffin-embedded tissue. One slide was used to give HE staining again. The remaining 3 slides was used to give immunohistochemical staining. The staining was performed by using the two-step procedure. The anti-human p15INK4b rabbit polyclonal antibody (ab53034) (Abcam, Cambridge, MA; diluted 1:500), anti-human p16INK4a rabbit monoclonal antibody (ab108349) (Abcam, Cambridge, MA; diluted 1: 250), and anti-human p21Waf1/Cip1 rabbit monoclonal antibody (2947) (Cell Signaling, Cambridge, MA; diluted 1:50) were used. After de-paraffinization and hydration, the slides were subjected to antigen retrieval by pressure-cooking for 30 minutes. Endogenous peroxidase activity was neutralized using peroxide block placement on the slides for 15 minutes at room temperature. The slides were then incubated with anti-p15INK4b, anti-p16INK4a, and anti-p21Waf1/Cip1 antibody for 30 minutes at 4°C, respectively. This was followed by incubation with peroxidase-conjugated polymer (ChemMate EnVision/HRP; Gene Tech, Shanghai, China) for 30 minutes at room temperature. The chromogen reaction was developed in 3, 3’-diaminobenzidine (DAB; Gene Tech, Shanghai, China) tetrahydrochloride for 10 minutes. Finally, hematoxylin was used as a light nuclear counterstain.
Assessment of p15INK4b, p16INK4a, and p21Waf1/Cip1 expression and statistical analysis
All slides were evaluated independently by two experienced pathologist (Zhang Y and Li F), and five high-power fields were selected randomly for each slide. The percentage of positive-staining cells were graded on a scale of 0-3, with less than 5% positive-staining cells as grade 0, 5-25% as grade 1, 26-50% as grade 2, and more than 50% as grade 3. The intensity of staining also graded on a scale of 0-2, with negative to weak intensity as grade 0, weak-moderate intensity as grade 1, and moderate to strong intensity as grade 2. For each marker, the score of percentage and intensity was multiplied. The final score between 0-2 was determined as low expression, and score higher than 2 was determined as high expression.
Comparisons among different groups for each marker were performed with Chi-square test. For all tests, a two-sided P < 0.05 was considered significant.
Results
Expression differences of p15INK4b, p16INK4a, and p21Waf1/Cip1 among NCE, CIN, and SCC
The expression of p21Waf1/Cip1 was predominantly within the nucleus, while the expression of p15INK4b and p16INK4a was predominantly within the cytoplasm. The p15INK4b expression level was low in all of NCE, and its expression was high in CIN (52.9%) and SCC (100.0%), respectively. The expression p16INK4a and p21Waf1/Cip1 were significantly higher in CIN and SCC compared to NCE. However, this expression was no statistically differences between CIN and SCC (Tables 1 and 2; Figure 1).
Table 1.
Expression of OIS markers in cases of NCE, CIN and SCC
| No. | p15INK4b | p16INK4a | p21Waf1/Cip1 | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Low (%) | High (%) | Low (%) | High (%) | Low (%) | High (%) | ||
| NCE | 19 | 19 (100.0) | 0 (0) | 13 (68.4) | 6 (31.6) | 19 (100.0) | 0 (0) |
| CIN | 51 | 24 (47.1) | 27 (52.9) | 16 (31.4) | 35 (68.6) | 21 (41.2) | 30 (58.8) |
| SCC | 21 | 0 (0) | 21 (100.0) | 4 (19.0) | 17 (81.0) | 5 (23.8) | 16 (76.2) |
Table 2.
Statistical results of expression differences of OIS markers among NCE, CIN and SCC
| p15INK4b | p16INK4a | p21Waf1/Cip1 | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| χ2 | P | χ2 | P | χ2 | P | |
| NCE vs. CIN | 16.375 | 0.000 | 8.429 | 0.004 | 19.559 | 0.000 |
| NCE vs. SCC | 40.000 | 0.000 | 9.950 | 0.002 | 24.127 | 0.000 |
| CIN vs. SCC | 14.824 | 0.000 | 0.905 | 0.341 | 1.945 | 0.163 |
Figure 1.
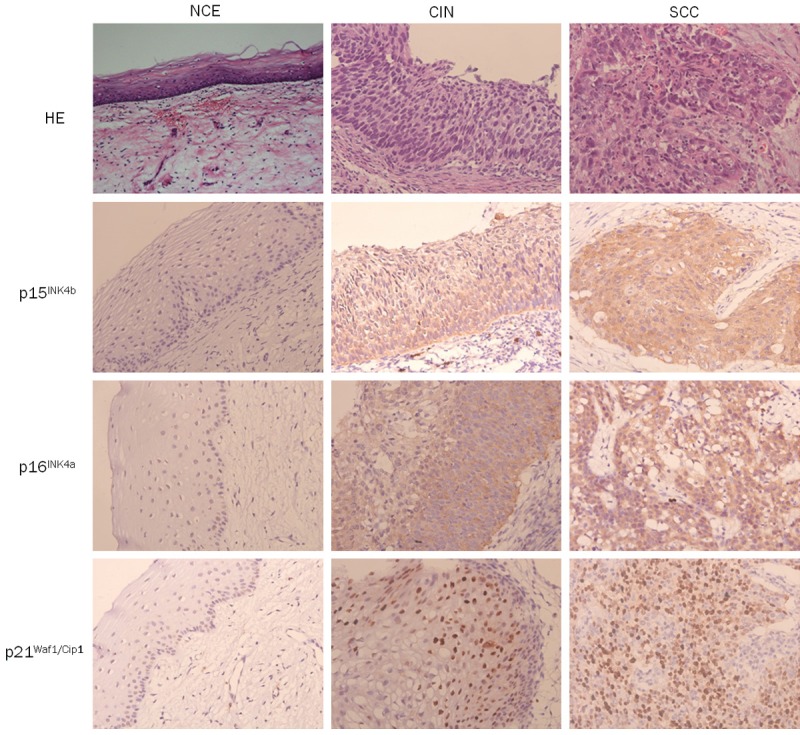
Expression of OIS markers in NCE, CIN and SCC (magnification × 200).
Expression differences of p15INK4b, p16INK4a, and p21Waf1/Cip1 among CIN І, CIN П, and CIN Ш
The expression of p15INK4b and p21Waf1/Cip1 was significantly higher in CIN П (62.5% and 62.5%) compared to CIN І (0% and 22.2%), and these expression were statistically higher in CIN Ш (100.0% and 94.1%) compared to CIN П, respectively. The p16INK4a expression was no significantly difference between CIN І (55.6%) and CIN П (62.5%) group, and between CIN П and CIN Ш (88.2%) group. However, its expression was significantly higher in CIN Ш compared to CIN І (Tables 3 and 4; Figure 2).
Table 3.
Expression of OIS markers in cases of CIN І, CIN П and CIN Ш
| No. | p15INK4b | p16INK4a | p21Waf1/Cip1 | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Low (%) | High (%) | Low (%) | High (%) | Low (%) | High (%) | ||
| CIN І | 18 | 18 (100.0) | 0 (0) | 8 (44.4) | 10 (55.6) | 14 (77.8) | 4 (22.2) |
| CIN П | 16 | 6 (37.5) | 10 (62.5) | 6 (37.5) | 10 (62.5) | 6 (37.5) | 10 (62.5) |
| CIN Ш | 17 | 0 (0) | 17 (100.0) | 2 (11.8) | 15 (88.2) | 1 (5.9) | 16 (94.1) |
Table 4.
Statistical results of expression differences of OIS markers among CIN І, CIN П and CIN Ш
| p15INK4b | p16INK4a | p21Waf1/Cip1 | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| χ2 | P | χ2 | P | χ2 | P | |
| CIN І vs. CIN П | 15.938 | 0.000 | 0.423 | 0.515 | 5.673 | 0.017 |
| CIN І vs. CIN Ш | 35.000 | 0.000 | 4.575 | 0.032 | 18.453 | 0.000 |
| CIN П vs. CIN Ш | 7.792 | 0.005 | 2.169 | 0.141 | 4.930 | 0.026 |
Figure 2.
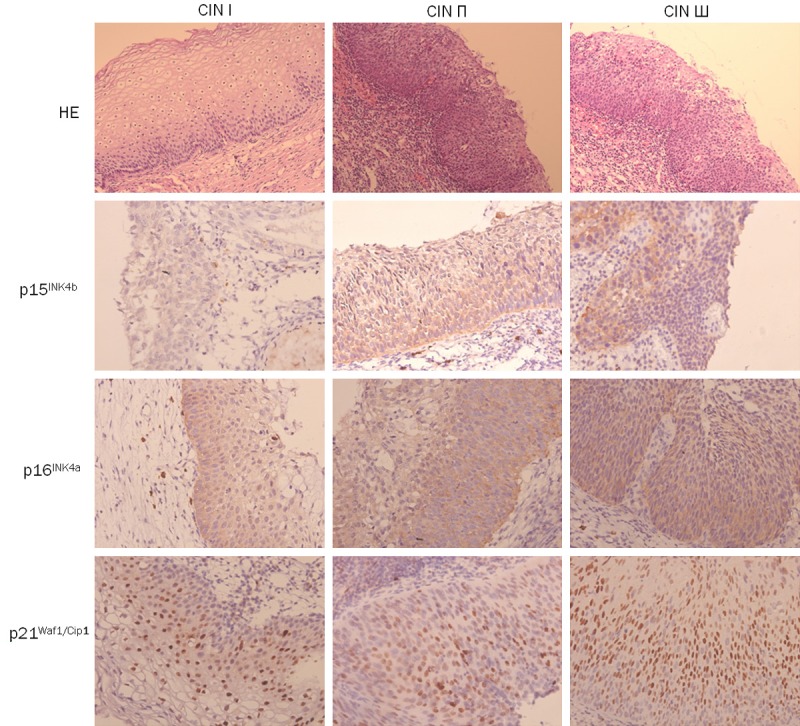
Expression of OIS markers in CIN І, CIN П and CIN Ш (magnification × 200).
Discussion
Recent studies have revealed that OIS plays important role in limiting the progression of premalignant lesions to invasive cancer during tumor initiation [6]. Elucidation of a number of potential biomarkers for detecting senescent cells has facilitated to evaluate the role of OIS in cancer development. Now SAβ-gal seems to be a reliable marker of senescent cells in culture [5,14], but it fails to demonstrate senescent cells in vivo models [15,16]. Other markers of senescence involving signaling pathway were studied.
Previous studies have revealed that the ARF/p53/p21 and p16/Rb/E2F pathways play important role in inducing cellular senescence [8]. The senescent-associated genes, including p15INK4b, p16INK4a and p21Waf1/Cip1, involve into these processes. Several studies showed that p15INK4b, p16INK4a and p21Waf1/Cip1 are upregulated in premalignat lesions and early stage of cancer, but widely downregulated in the corresponding cancers, including thyroid, hepatocellular, breast, pancreatic carcinoma and glioma [7,17,18]. However, Bai et al [10] found that the expression of p15INK4b and p16INK4a were almost completely negative in the normal esophageal epithelium. The p15INK4b and p16INK4a was found to be expressed in 73% and 73% of the esophageal intraepithelial dysplasia (EID), and 92% and 88% of the esophageal squamous cell carcinoma (ESCC). Similarly, Feng et al [13] found that p15INK4b and p16INK4a were also overexpressed in both CIN and cervical SCC. Van de Putte et al [12] found that p21Waf1/Cip1 had no expression in normal cervical squamous epithelium, while its high expression were detected in 20% cervical SCC. In the present study, p15INK4b, p16INK4a and p21Waf1/Cip1 expression were significantly higher in both CIN and SCC compared to NCE. Furthermore, the expression of p15INK4b and p21Waf1/Cip1 was significantly higher in CIN П compared to CIN І, and these expression were statistically higher in CIN Ш compared to CIN П, respectively. The p16INK4a expression was significantly higher in CIN Ш compared to CIN І. These results suggested that the senescence programs mediated by p15INK4b, p16INK4a and p21Waf1/Cip1 were also activated as reflected in the overexpression of these markers in cervical dysplasia and SCC, and the ARF/p53/p21 and p16/Rb/E2F pathways were activated during the dysplasia stage of cervical carcinogenesis and remained intact in most cervical SCC. In addition, these results suggested that the expression of these senescence markers may exist tissue-specific, and different cancer tissues have different expression level.
In conclusion, the results showed that the senescence programs mediated by p15INK4b, p16INK4a and p21Waf1/Cip1 were activated during the stage of CIN and SCC, and demonstrated that senescence may play important role in preventing from NCE to SCC. However, the exact mechanism is still unclear, and the further study is needed.
Acknowledgements
This study was supported by grants from Jiangsu Natural Science Funding (BK20141185) and Jiangsu Province’s Key Medical Person (RC2011144).
Disclosure of conflict of interest
None.
References
- 1.Sun Y, Liu JH, Jin L, Lin SM, Yang Y, Sui YX, Shi H. Over-expression of the Beclin1 gene upregulates chemosensitivity to anti-cancer drugs by enhancing therapy-induced apoptosis in cervix squamous carcinoma CaSki cells. Cancer Lett. 2010;294:204–210. doi: 10.1016/j.canlet.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Origoni M, Salvatore S, Perino A, Cucinella G, Candiani M. Cervical intraepithelial neoplasia (CIN) in pregnancy: the state of the art. Eur Rev Med Pharmacol Sci. 2014;18:851–860. [PubMed] [Google Scholar]
- 3.Stein GH, Dulic V. Origins of G1 arrest in senescent human fibroblasts. Bioessays. 1995;17:537–543. doi: 10.1002/bies.950170610. [DOI] [PubMed] [Google Scholar]
- 4.Flores JM, Martin-Caballero J, Garcia-Fernandez RA. p21 and p27 a shared senescence history. Cell Cycle. 2014;13:1–2. doi: 10.4161/cc.29147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsson L. Oncogene- and tumor suppressor gene-mediated suppression of cellular senescence. Semin Cancer Biol. 2011;21:367–376. doi: 10.1016/j.semcancer.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell ME, DeNicola GM, Martins CP, Jacobetz MA, Maitra A, Hruban RH, Tuveson DA. Cellular features of senescence during the evolution of human and murine ductal pancreatic cancer. Oncogene. 2012;31:1599–1608. doi: 10.1038/onc.2011.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vizioli MG, Possik PA, Tarantino E, Meissl K, Borrello MG, Miranda C, Anania MC, Pagliardini S, Seregni E, Pierotti MA, Pilotti S, Peeper DS, Greco A. Evidence of oncogene-induced senescence in thyroid carcinogenesis. Endocr Relat Cancer. 2011;18:743–757. doi: 10.1530/ERC-11-0240. [DOI] [PubMed] [Google Scholar]
- 8.Bascones-Martinez A, Lopez-Duran M, Cano-Sanchez J, Sánchez-Verde L, Díez-Rodríguez A, Aguirre-Echebarría P, Alvarez-Fernández E, González-Moles MA, Bascones-Ilundain J, Muzio LL, Campo-Trapero J. Differences in the expression of five senescence markers in oral cancer, oral leukoplakia and control samples in humans. Oncol Lett. 2012;3:1319–1325. doi: 10.3892/ol.2012.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguría A, Zaballos A, Flores JM, Barbacid M, Beach D, Serrano M. Tumor Biology: senescence in premalignant tumors. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 10.Bai P, Xiao X, Zou J, Cui L, Bui Nguyen TM, Liu J, Xiao J, Chang B, Wu J, Wang H. Expression of p14(ARF), p15(INK4b), p16(INK4a) and skp2 increases during esophageal squamous cell cancer progression. Exp Ther Med. 2012;3:1026–1032. doi: 10.3892/etm.2012.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lampejo T, Kavanagh D, Clark J, Goldin R, Osborn M, Ziprin P, Cleator S. Prognostic biomarkers in squamous cell carcinoma of the anus: a systematic review. Br J Cancer. 2010;103:1858–1869. doi: 10.1038/sj.bjc.6605984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van de Putte G, Holm R, Lie AK, Tropé CG, Kristensen GB. Expression of p27, p21, and p16 protein in early squamous cervical cancer and its relation to prognosis. Gynecol Oncol. 2003;89:140–7. doi: 10.1016/s0090-8258(03)00010-6. [DOI] [PubMed] [Google Scholar]
- 13.Feng W, Xiao J, Zhang Z, Rosen DG, Brown RE, Liu J, Duan X. Senescence and apoptosis in carcinogenesis of cervical squamous carcinoma. Mod Path. 2007;20:961–966. doi: 10.1038/modpathol.3800927. [DOI] [PubMed] [Google Scholar]
- 14.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saab R, Rodriguez-Galindo C, Matmati K, Rehg JE, Baumer SH, Khoury JD, Billups C, Neale G, Helton KJ, Skapek SX. p18Ink4c and p53 act as tumor suppressors in cyclin D1-driven primitive neuroectodermal tumor. Cancer Res. 2009;69:440–448. doi: 10.1158/0008-5472.CAN-08-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dankort D, Filenova E, Collado M, Serrano M, Jones K, McMahon M. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 2007;21:379–384. doi: 10.1101/gad.1516407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin M, Piao Z, Kim NG, Park C, Shin EC, Park JH, Jung HJ, Kim CG, Kim H. p16 is a major inactivation target in hepato-celluar carcinoma. Cancer. 2000;89:60–68. doi: 10.1002/1097-0142(20000701)89:1<60::aid-cncr9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Forbes S, Clements J, Dawson E, Bamford S, Webb T, Dogan A, Flanagan A, Teague J, Wooster R, Futreal PA, Stratton MR. COSMIC 2005. Br J Cancer. 2006;94:318–322. doi: 10.1038/sj.bjc.6602928. [DOI] [PMC free article] [PubMed] [Google Scholar]


