Abstract
Objective: We investigate kidney injury caused by high dose bevacizumab to uncover the possible mechanisms involving in this process. Methods: Forty rats were divided into four groups: cisplation group (treated with 1 mg/kg cisplation), Bev-high group (treated with 5 mg/kg bevacizumab); Bev-low group (treated with 2.5 mg/kg bevacizumab) and control group (treated with saline). The urine microalbumin, serum cystatin C, blood urea nitrogen and serum creatinine were detected in the four group rats, respectively. The immunoglobulin of IgG, IgA and IgM and protein of VEGF (vascular endothelial growth factor) and nephrin were detected by immunohistochemical methods. Results: All the levels of microalbumin, cystatin C, serum creatinine and blood urea nitrogen in Bev-high group were significantly higher than those in normal control group (P < 0.05). The cystatin C was much more increased in kidney Bev-high group than cisplatin and Bev-low groups (P < 0.05). The light microscope showed a normal glomerular morphology in the four groups, while the electronic microscopy showed the podocytes were extensively fused in cisplatin group and Bev-high group. The two groups were found IgG and IgM deposition as well. The VEGF in kidney amples were down regulated in high dose bevacizumab group, whereas the nephrin and IgA showed no significant expression changes at all. Conclusion: Bevacizumab increases the risk of injury in glomerular filtration barrier in a dose dependent model. The injury may not only associate with the rising level of proteinuria but also with podocyte-dependent membrane structures.
Keywords: Kidney injury, bevacizumab, vascular endothelial growth factor, glomerular filtration, immunoglobulin deposition
Introduction
Since the hypothesis that antiangiogenesis would be a potential antitumor was proposed by Folkman [1], angiogenesis has become a common attractive drug target [2,3]. In particular, the vascular endothelial growth factor (VEGF) has been proved to be a critical regulator for antiangiogenic therapy [4]. Bevacizumab is a recombinant humanized monoclonal antibody that is directed against VEGF. By binding of the antibody to VEGF, bevacizumab prevents the activation of VEGF receptor and inhibits the angiogenesis, and eventually results in anti-tumor effects [5].
Despite the overall benefit in the treatment of various tumors, targeting to VEGF can bring a number of adverse effects [6,7]. The side effects, such as vascular disorders (embolism, bleeding and phlebitis) usually lead to cessation during the treatment [8]. Proteinuria is one of the most commonly observed side effects caused by bevacizumab therapy [9]. A significant increasing proteinuria risk ratio was detected up to 21%-63% [10], which would probably result in severe kidney injury [11]. In addition, there is a possibility that the poisonous effect of VEGF inhibitors also involving in poison on podocytes [12]. It was reported that inhibition of VEGF on the glomerular endothelium may alter the endothelial surface and induce the development of thrombotic microangiopathy [6]. It seems that, a risk factor in facilitating these side effects is the underlying kidney dysfunction. However, the mechanisms related to the pathogenesis of kidney injury caused by bevacizumab are unclear.
Bevacizumab is the first VEGF inhibitor that is approved by United States Food and Drug Administration (FDA) for systemic treatment in cancer in 2004 [13]. Since then, it has received more approval in long cancer [14], ovarian cancer [15], glioblastoma [16], etc. Cisplatin is another antineoplastic drug used in the treatment of many cancers, which also bring dose-limiting side effect such as nephrotoxicity [17]. Because of major and frequent results of nephrotoxicity in cisplatin-based therapy, the various doses of cisplatin were commonly used in inducing rat kidney failure model [17-19]. In the current study, we investigated the kidney function and pathology effects on administration of different doses of bevacizumab compared with cisplatin treated rats and untreated rats up to 4 weeks, trying to uncover the mechanisms involving in kidney injury. The results will be beneficial for rational use in clinical therapy.
Materials and methods
Animal and groups
Forty C57BL/6 rats (Shanghai SLRC experimental animal Co. Ltd) weighing 18-22 g (aged 6-8 months) were housed and used in experiments according to the National Institutes of Health guidelines approved by the Committee on the Ethics of Animal Experiments of the Chinese Academy of Medical Sciences. They were randomly divided into four groups (n = 10): (a) cisplation group: treated with 1 mg/kg cisplation (Qilu pharmaceutical factory, Jinan, China) intravenously 3 times a week; (b) Bev-high group: treated with 5 mg/kg bevacizumab (BMS359, eBioscience, California, USA) intravenously via caudal vein 5 times a week; (c) Bev-low group: treated with 2.5 mg/kg bevacizumab intravenously via caudal vein 5 times a week; (d) control group: treated with saline intravenously via caudal vein.
Biochemistry index assay
Blood sample (280 mL) was obtained via caudal vein, and then all the rats were killed by cervical dislocation. The urine samples (500 mL) were collected by bladder puncture and kidney tissues were obtained from the four groups.
Microalbumin in urine was measured quantitatively using biuret method [20]. The serum cystatin C was detected by the homocysteine circulating enzymatic method. Blood urea nitrogen and serum creatinine levels were measured using picric acid creatinine reagent.
Histopathological examination of kidney tissue
Tissues in four groups were fixed with 4% neutral buffered formalin, embedded with paraffin, and stained with hematoxylin-eosin (HE). Then the histopathological change was observed under a light microscope (OLYMPUS BX51lF320H1, JEOL, Japan).
Immunostaining for IgG, IgA and IgM deposition
The kidney tissues were embedded in tissue OCT-freeze Medium, frozen in liquid nitrogen. The frozen sections (4 mm) at -20°C were air dried, washed using phosphate buffered saline (PBS) (3 ×, 5 min each) and blocked with PBS containing 5% FBS for 60 min. Then the sections were incubated overnight at 4°C with primary antibody (IgG1: BETHYL, USA; IgA: BETHYL, USA; IgM: BETHYL, USA). After washing with PBS 5 min for 3 times, sections were incubated with secondary antibody (cy3, Jackson ImmunoResearch, West Grove, PA, USA) for 60 min at room temperature. The cell nucleuses were stained by DAPI (Dojindo, Shanghai, China) for 5 min at 37°C, washed by PBS (3 ×, 5 min each), mounted using anti-quencher (Southern Biotech, USA), and subjected to a light microscope for IgG, IgA and IgM deposition.
Immunohistochemistry for VEGF expression
The expression of VEGF in the experimental group cells was detected by immunohistochemical sABC (streptavidin-biotin complex) method. The details of the method for dewaxing, antigen retrieval and blocking for the paraffin sections of kidney tissues have been published previously [21]. Then the sections were incubated for 60 min at room temperature with a rabbit polyclonal antibody against mouse VEGF (eBioscience, USA). After rinsing in PBS, they were incubated for 20 min at 37°C with the second biotinylated antibody (goat anti-rabbit IgG, eBioscience, USA). DBA (Dolichos biflorus agglutinin) staining was visualized using the ABC (avidin biotin peroxidase complex) method.
Immunohistochemistry for nephrin expression
The formalin-fixed, paraffin-embedded kidney tissue sections were dewaxed and inactivated with 3% H2O2, and submitted to antigen retrieval by microwave radiation for 15 min. After overnight incubation at 4°C, sections were reacted with a rabbit polyclonal anti-mouse nephrin (eBioscience, USA), and processed by adding polymer reinforced agent (reagent A) and enzyme labeled anti-rabbit polymer (reagent B). Immunoreactivity was visualized using DAB. Sections were then counterstained with haematoxylin (Beyotime Institute of Biotechnology, Shanghai, China), washed, dehydrated, and mount with permount (Amresco, USA).
Ultrastructure of kidney tissue
The ultrastructure of kidney tissue was observed under transmission electron microscope (JEM-1230, JEOL, Japan). Before observation, the specimens were fixed for 72 h in a solution of glutaraldehyde 2.5%. After rising 2 × 15 min in PBS, they were later fixed for 2 h in a soulution of osmic acid 1%. The specimens were dehydrated with graded acetone and were soaked in embedding medium. Ultra-thin slides were made with an ultramicrotome (LKB-NOVA, LKB, Sweden), and then subjected to transmission electron microscope.
Statistical methods
The experimental data were analyzed by SPSS 17 software (SPSS, Inc., Chicago IL). All quantitative data were reported as mean ± SEM (standard error of mean). Means among different groups were compared using ANOVA (analysis of variance) and multiple comparisons were performed by LSD (least significant difference) method. P value < 0.05 was considered to be statistically significant.
Results
Biochemistry index analysis
The levels of biochemistry index in serum and urine were presented in Table 1. The levels of microalbumin, cystatin C and serum creatinine in all treatment groups were significantly higher than those in normal control group (P < 0.05). Treatment with cisplatin and low dose bevacizumab did not significantly affect the blood urea nitrogen level, whereas the blood urea nitrogen levels in Bev-high group were significantly higher than those in control and cisplatin groups (P < 0.05). The microalbumin levels in Bev-high group were significantly higher than those in cisplation and Bev-low groups (P < 0.05). The cystatin C was much more increased in kidney when treated with high dose bevacizumab than cisplatin and low dose bevacizumab treated groups (P < 0.05).
Table 1.
Biochemistry index in serum and urine
| Groups | Microalbumin (mg/L) | Cystatin C (μmol/L) | Blood urea nitrogen (mmol/L) | Serum creatinine (μmol/L) |
|---|---|---|---|---|
| Control group | 0.14 ± 0.25 | 5.7 ± 1.88 | 5.97 ± 0.96 | 10.59 ± 1.34 |
| Cisplatin group | 0.82 ± 0.66* | 10.01 ± 2.52*,Δ | 6.67 ± 1.22Δ | 12.24 ± 3.24* |
| Bev-high group | 0.90 ± 0.70* | 12.51 ± 2.69* | 10.50 ± 1.81* | 15.43 ± 2.38* |
| Bev-low group | 0.61 ± 0.66* | 8.28 ± 2.94*,Δ | 6.75 ± 1.46 | 12.04 ± 1.80* |
P < 0.05 versus control;
P < 0.05 versus Bev-high group.
Morphological changes of kidney tissue in light microscope and electron microscope
The results of HE staining showed a grossly normal glomerular morphology in the four groups (Figure 1A), with the legible glomeruli structure, the non-expanded mesangial matrix, regular intercapillary and epithelial cells growth. While the electronic microscopy showed the podocytes were extensively fused in cisplatin group and Bev-high group (Figure 1B). None abnormal effects were observed in all of the treated groups with regard to basement membrane and mesangial matrix.
Figure 1.
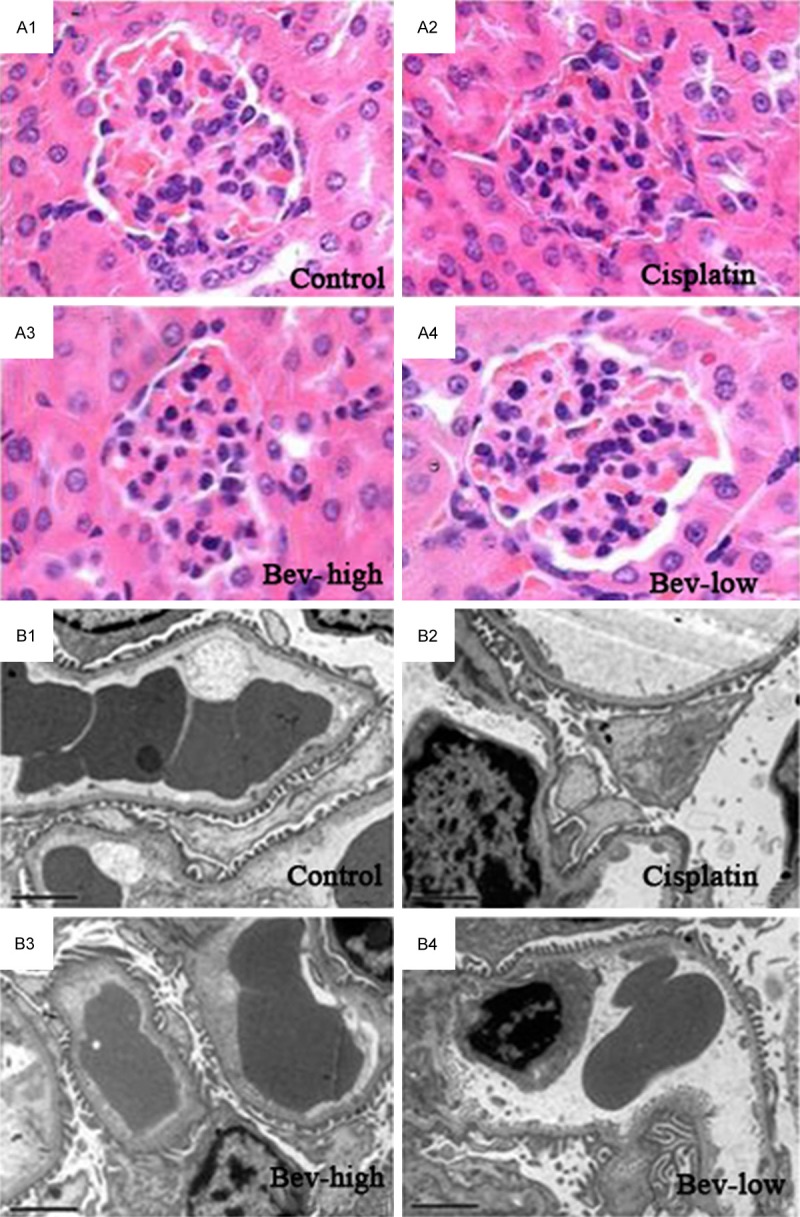
Glomerular structure of the control and treatment (cisplatin, Bev-high and Bev-low) groups. A: Under light microscope (× 200); B: Under electron microscope (× 5000).
Immunostaining results of IgG, IgA and IgM deposition
There was extensive staining of both IgG (Figure 2A) and IgM (Figure 2B) in the mesangial regions and prepapillary vascular loops for cisplatin (+++) and Bev-high (++) groups, whereas staining for IgG and IgM in other groups were negative. IgA (Figure 2C) staining in the four groups was negative as well, indicating IgA deposition was found in neither control group nor treatment groups.
Figure 2.
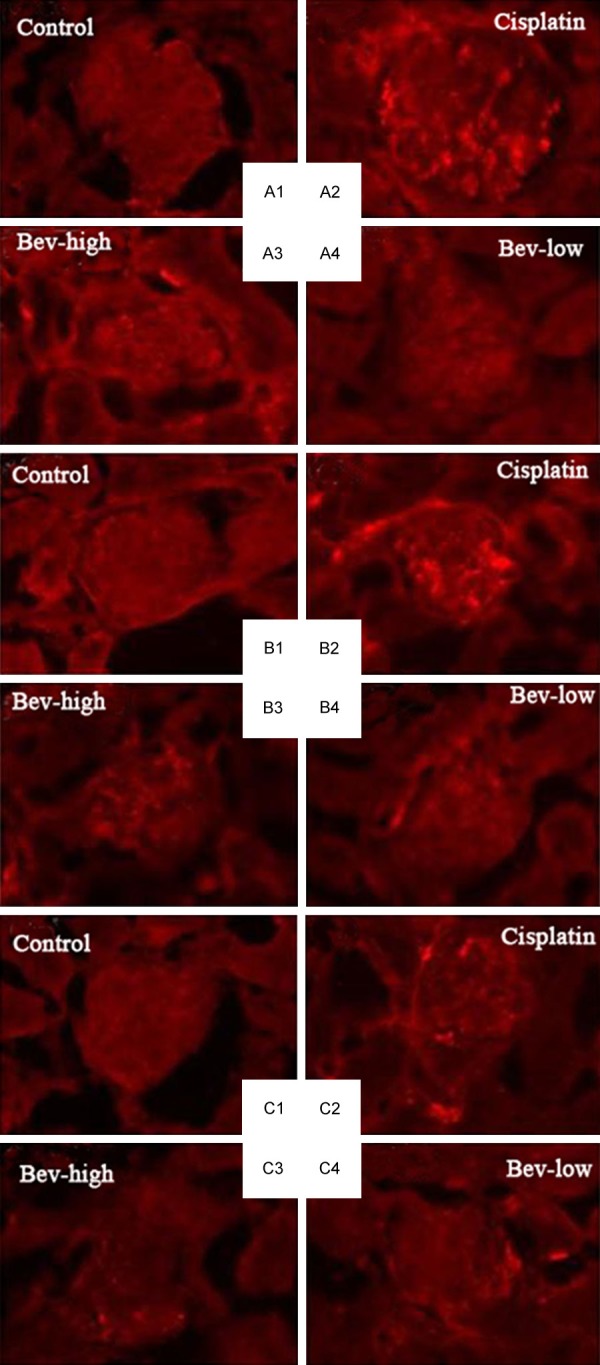
Immunofluorescence of deposition in the control and treatment (cisplatin, Bev-high and Bev-low) groups. A: IgG deposition; B: IgM deposition; C: IgA deposition.
Immunostaining results of VEGF expression
We investigated the effect of cisplatin and bevacizumab on expression of VEGF by immunostaining. Results (Figure 3) showed the VEGF immunoreaction was remarkable with claybank in the cytoplasm cells of control, cisplatin and Bev-low group, while the cells in Bev-high group displayed relatively light dyeing color.
Figure 3.
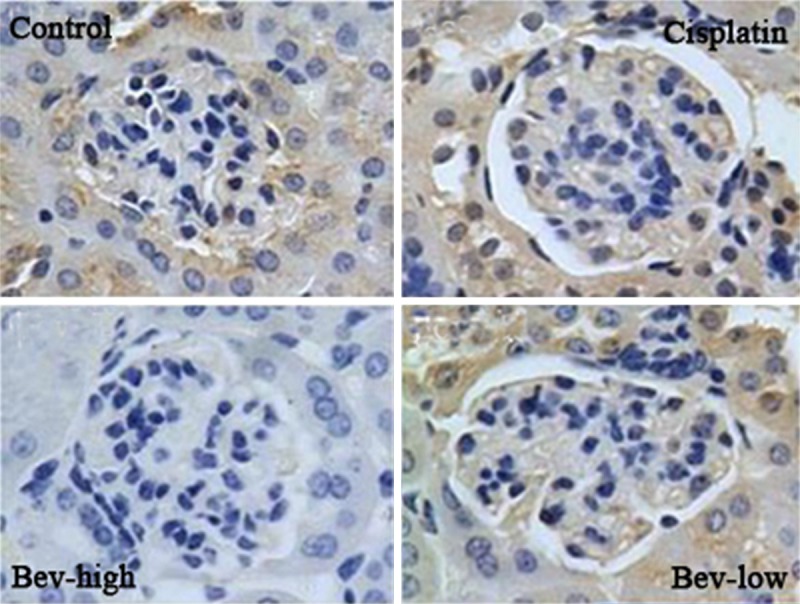
Immunohistochemical staining for VEGF (vascular endothelial growth factor) in kidney from control and treatment (cisplatin, Bev-high and Bev-low) groups (× 200).
Immunostaining results of nephrin expression
As shown in Figure 4, the immunohistochemical staining for nephrin in kidney exhibited a non-significant difference expression level in the treatment and control groups. That is, different dosages of bevacizumab, did not remarkably affect the nephrin expression level.
Figure 4.
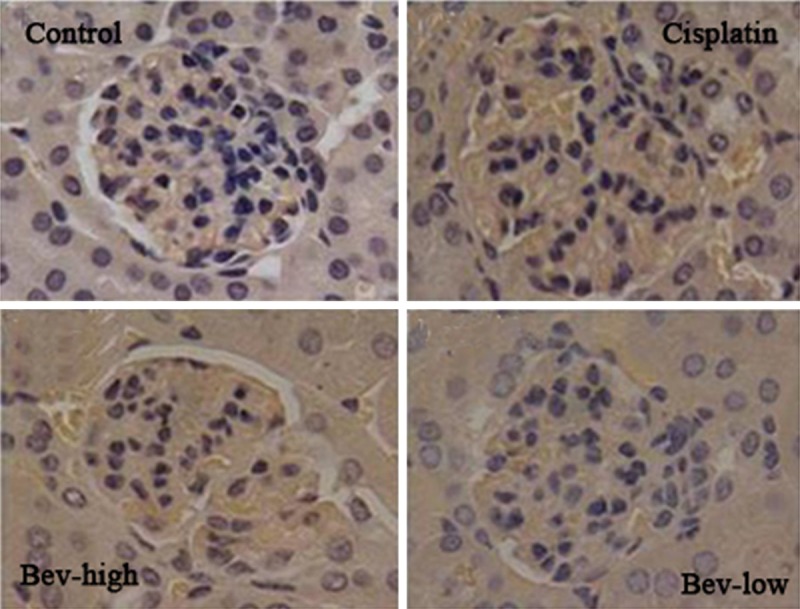
Immunohistochemical staining for nephrin in kidney from control and treatment (cisplatin, Bev-high and Bev-low) groups (× 200).
Discussion
Given the fact that VEGF inhibitor exhibits clinically side effect in therapeutic of various of tumor cells [22,23], we wished to reveal the toxicity mechanism of bevacizumab on kidney injury by administrated with low (2.5 mg/kg) and high (5 mg/kg) dosages of bevacizumab. Our study demonstrated that bevacizumab therapy significantly increased the proteinuria at the cost of its toxicity, especially at the higher dosage treatment level. Treatment with high dosage bevacizumab statistically increased the microalbumin, serum cystatin C, blood urea nitrogen and serum creatinine levels compared with control group rats. Immunohistochemical results showed the kidney function related proteins of IgG, IgM and VEGF were highly expressed when treated with high dose bevacizumab, whereas the IgA and nephrin were not significantly expressed at all. Podocytes were observed extensively fused in cisplatin group and Bev-high group. All these changes are considered to be the promising indicators for early kidney injury, and they are probably involving in injury mechanism by high dose of bevacizumab.
In the present clinical practice, early kidney injury is characterized as a rapid decline of glomerular filtration rate and sudden loss of kidney function, and clinically detected by an increase in serum creatinine and urea [24,25]. In our study, both of the serum creatinine and urea nitrogen were detected increased in all the treatment groups, indicating a kidney injury occurred in these rats after giving drugs (bevacizumab and cisplatin), while the urea nitrogen increase was not significant in cisplatin and Bev-low group. Previous results proved that creatinine did not accurately estimate the glomerular filtration rate attributing to the secretion and reabsorption of kidney tubule [26,27]. Compared with serum creatinine and blood urea nitrogen, our study showed microalbumin and cystatin C levels were more sensitive for early kidney injury detection because a more significantly dose dependent increase was found in these treatments. Our results were in coincidence with previous studies that microalbumin and cystatin C were considered to be the more promising and easier measurable markers for the progression of kidney injury [28,29]. Therefore, we strongly suggest that kidney functions should be carefully detected in patients who were treated with bevacizumab for cancer diseases, even without clinical signs.
Kidney injury caused by high dose of anti-VEGF drug bevacizumab is a process induced by subsequent signaling cascades. In the glomeruli, VEGF is continuously expressed and secreted by podocytes to protect the integrity of filtering membrane [30,31]. We found that, the expression of VEGF protein was significantly down-regulated in rats kidney after a period of bevacizumab administration. Moreover, the podocytes were extensively fused in rats injected with bevacizumab. The data indicates that the down-regulated VEGF is likely due to the disturbance of glomerular basement membrane. Further decline in VEGF expression was observed in rats kidney by higher dose of bevacizumab, which might be explained by podocytes apoptosis being induced by inhibition of VEGF activity [32]. In this process, the bevacizumab may partly activate alexin cascade, causing the formation of cell membrane attack complex and triggering cytolytic toxicity [33].
Bevacizumab caused podocytes damage may mediated by down-regulation of a member of immunoglobulin (Ig) superfamily-slit diaphragm nephrin [34]. Immunohistochemical analysis showed no significant changes in nephrin expression levels in the kidney biopsy specimens of treated rats at all. The expression of nephrin was not always consistent in other kidney injury [35,36]. These discrepant results may be explained by the differences in kidney injury degree and the methods in detecting protein expression. Patients who suffered from chemotherapy of anti-VEGF lead to proteinuria indicating injury of the glomerular filtration barrier [37,38]. Another immunoglobulin superfamilies, IgG and IgM were found higher deposited in high dose bevacizumab treated groups. So we concluded from our results that the proteinuria induced by bevacizumab was related to immunoglobulin deposition. Clearly, our data demonstrated that the perturbation of VEGF levels were not only associated with the rising level of proteinuria but also with adjustment of podocyte-dependent membrane structures.
In accordance with effects of high dose bevacizumab, the cisplatin injected rats kidneys also showed relative extensively podocytes, and immunoglobulin deposition in extracelluar matrix and vascular loops, indicating that high dose bevacizumab and cisplatin should not be treated in combination in patients for cancer diseases.
In summary, our results imply that the high dose bevacizumab for chemotherapy significantly increased the risk of injury in glomerular filtration barrier. The injury may not only associate with the rising level of proteinuria but also with podocyte-dependent membrane structures. It is important for doctors to recognize the risk with carefully detection in patients who are treated with bevacizumab for cancer diseases. Moreover, high dose bevacizumab and cisplatin should not be treated in combination, because they results in coincidence and severe kidney injury.
Acknowledgements
This study was supported by 2011 Key project of Hangzhou Medical Health Science and Technology Foundation (2011Z002) and 2012 Wu Jieping Medical Fund project (320.6752.12364). We wish to express our warm thanks to Fenghe (Shanghai) Information Technology Co., Ltd. Their ideas and help gave a valuable added dimension to our research.
Disclosure of conflict of interest
None.
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 3.Grothey A, Galanis E. Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol. 2009;6:507–518. doi: 10.1038/nrclinonc.2009.110. [DOI] [PubMed] [Google Scholar]
- 4.Arao T, Matsumoto K, Maegawa M, Nishio K. Target Therapy for Cancer: Anti-cancer Drugs Targeting Growth-Factor Signaling Molecules. Biol Pharm Bull. 2011;34:1789–1793. [Google Scholar]
- 5.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–4. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 6.Machado FG, Kuriki PS, Fujihara CK, Fanelli C, Arias SC, Malheiros DM, Camara NO, Zatz R. Chronic VEGF blockade worsens glomerular injury in the remnant kidney model. PLoS One. 2012;7:e39580. doi: 10.1371/journal.pone.0039580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer G. Capillary rarefaction, hypoxia, VEGF and angiogenesis in chronic renal disease. Nephrol Dial Transplant. 2011;26:1132–1137. doi: 10.1093/ndt/gfq832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA. 2011;305:487–494. doi: 10.1001/jama.2011.51. [DOI] [PubMed] [Google Scholar]
- 9.Izzedine H, Massard C, Spano JP, Goldwasser FO, Khayat D, Soria JC. VEGF signalling inhibition-induced proteinuria: Mechanisms, significance and management. Eur J Cancer. 2010;46:439–48. doi: 10.1016/j.ejca.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, Li H, Wang M. Acute renal failure in a patient receiving anti-VEGF therapy for advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:1185–1187. doi: 10.1097/JTO.0b013e3181b2362f. [DOI] [PubMed] [Google Scholar]
- 12.Guan F, Villegas G, Teichman J, Mundel P, Tufro A. Autocrine VEGF-A system in podocytes regulates podocin and its interaction with CD2AP. Am J Physiol Renal Physiol. 2006;291:F422–428. doi: 10.1152/ajprenal.00448.2005. [DOI] [PubMed] [Google Scholar]
- 13.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 14.Baty F, Rothschild S, Fruh M, Betticher D, Droge C, Cathomas R, Rauch D, Gautschi O, Bubendorf L, Crowe S, Zappa F, Pless M, Brutsche M. EGFR exon-level biomarkers of the response to bevacizumab/erlotinib in non-small cell lung cancer. PLoS One. 2013;8:e72966. doi: 10.1371/journal.pone.0072966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato S, Itamochi H. Bevacizumab and ovarian cancer. Curr Opin Obstet Gynecol. 2012;24:8–13. doi: 10.1097/GCO.0b013e32834daeed. [DOI] [PubMed] [Google Scholar]
- 16.Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14:1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 17.Aleksunes LM, Augustine LM, Scheffer GL, Cherrington NJ, Manautou JE. Renal xenobiotic transporters are differentially expressed in mice following cisplatin treatment. Toxicology. 2008;250:82–88. doi: 10.1016/j.tox.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M, Chien CC, Burne-Taney M, Molls RR, Racusen LC, Colvin RB, Rabb H. A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J Am Soc Nephrol. 2006;17:765–74. doi: 10.1681/ASN.2005010102. [DOI] [PubMed] [Google Scholar]
- 19.Singh AP, Junemann A, Muthuraman A, Jaggi AS, Singh N, Grover K, Dhawan R. Animal models of acute renal failure. Pharmacol Rep. 2012;64:31–44. doi: 10.1016/s1734-1140(12)70728-4. [DOI] [PubMed] [Google Scholar]
- 20.Shigeru U. A modified method for estimation of fish muscle protein by Biuret method. Bull Jpn Soc Fish. 1996;32:427–435. [Google Scholar]
- 21.Merz H, Malisius R, Mannweiler S, Zhou R, Hartmann W, Orscheschek K, Moubayed P, Feller A. ImmunoMax. A maximized immunohistochemical method for the retrieval and enhancement of hidden antigens. Lab Invest. 1995;73:149–156. [PubMed] [Google Scholar]
- 22.Mailliez A, Baldini C, Van J, Servent V, Mallet Y, Bonneterre J. Nasal septum perforation: a side effect of bevacizumab chemotherapy in breast cancer patients. Br J Cancer. 2010;103:772–775. doi: 10.1038/sj.bjc.6605828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pellé G, Shweke N, Van Huyen JPD, Tricot L, Hessaïne S, Frémeaux-Bacchi V, Hiesse C, Delahousse M. Systemic and kidney toxicity of intraocular administration of vascular endothelial growth factor inhibitors. Am J Kidney Dis. 2011;57:756–759. doi: 10.1053/j.ajkd.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 24.Miura N, Mizuno N, Aoyama R, Kitagawa W, Yamada H, Nishikawa K, Imai H. Massive proteinuria and acute renal failure after oral bisphosphonate (alendronate) administration in a patient with focal segmental glomerulosclerosis. Clin Exp Nephrol. 2009;13:85–88. doi: 10.1007/s10157-008-0078-x. [DOI] [PubMed] [Google Scholar]
- 25.Hu JY, Meng XC, Han J, Xiang F, Fang YD, Wu J, Peng YZ, Wu YZ, Huang YS, Luo QZ. Relation between proteinuria and acute kidney injury in patients with severe burns. Crit Care. 2012;16:R172. doi: 10.1186/cc11649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: physiological principles. Intensive Care Med. 2004;30:33–7. doi: 10.1007/s00134-003-2078-3. [DOI] [PubMed] [Google Scholar]
- 27.Premaratne E, Macisaac RJ, Tsalamandris C, Panagiotopoulos S, Smith T, Jerums G. Renal hyperfiltration in type 2 diabetes: effect of age-related decline in glomerular filtration rate. Diabetologia. 2005;48:2486–2493. doi: 10.1007/s00125-005-0002-9. [DOI] [PubMed] [Google Scholar]
- 28.Mogensen CE. Early glomerular hyperfiltration in insulin-dependent diabetics and late nephropathy. Scand J Clin Lab Invest. 1986;46:201–6. doi: 10.3109/00365518609083660. [DOI] [PubMed] [Google Scholar]
- 29.Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, Philipp T, Kribben A. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66:1115–22. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 30.Ku CH, White KE, Dei Cas A, Hayward A, Webster Z, Bilous R, Marshall S, Viberti G, Gnudi L. Inducible overexpression of sFlt-1 in podocytes ameliorates glomerulopathy in diabetic mice. Diabetes. 2008;57:2824–2833. doi: 10.2337/db08-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eremina V, Quaggin SE. Biology of anti-angiogenic therapy-induced thrombotic microangiopathy. Semin Nephrol. 2010;30:582–90. doi: 10.1016/j.semnephrol.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Hara A, Wada T, Furuichi K, Sakai N, Kawachi H, Shimizu F, Shibuya M, Matsushima K, Yokoyama H, Egashira K. Blockade of VEGF accelerates proteinuria, via decrease in nephrin expression in rat crescentic glomerulonephritis. Kidney Int. 2006;69:1986–1995. doi: 10.1038/sj.ki.5000439. [DOI] [PubMed] [Google Scholar]
- 33.Zhang A, Huang S. Progress in pathogenesis of proteinuria. Int J Nephrol. 2012;2012:314251. doi: 10.1155/2012/314251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugimoto H, Hamano Y, Charytan D, Cosgrove D, Kieran M, Sudhakar A, Kalluri R. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-vegf antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem. 2003;278:12605–8. doi: 10.1074/jbc.C300012200. [DOI] [PubMed] [Google Scholar]
- 35.Bignami E, Casamassima N, Frati E, Lanzani C, Corno L, Alferi O, Gottlieb S, Simonini M, Shah KB, Mizzi A. Preoperative endogenous ouabain predicts acute kidney injury in cardiac surgery patients. Crit Care Med. 2013;41:744. doi: 10.1097/CCM.0b013e3182741599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guan N, Ding J, Zhang J, Yang J. Expression of nephrin, podocin, alpha-actinin, and WT1 in children with nephrotic syndrome. Pediatr Nephrol. 2003;18:1122–7. doi: 10.1007/s00467-003-1240-z. [DOI] [PubMed] [Google Scholar]
- 37.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel TV, Morgan JA, Demetri GD, George S, Maki RG, Quigley M, Humphreys BD. A preeclampsia-like syndrome characterized by reversible hypertension and proteinuria induced by the multitargeted kinase inhibitors sunitinib and sorafenib. J Natl Cancer Inst. 2008;100:282–284. doi: 10.1093/jnci/djm311. [DOI] [PubMed] [Google Scholar]


