Abstract
Colorectal cancer (CRC) is one of the most common and fatal malignancies worldwide. Novel prognostic biomarkers are urgently warranted to help improve the treatment of CRC. Y-box-binding protein 1 (YB-1) has been identified as a multifunctional oncoprotein in various malignancies. Our previous study has suggested that YB-1 may promote malignant progression of CRC cells in vitro. However, its clinical and prognostic significance in CRC patients remains unclear. In this study, the expression of YB-1 was examined in 32 fresh CRC tissues using quantitative real-time polymerase chain reaction (qRT-PCR) and in 170 paraffin-embedded CRC tissues using immunohistochemistry. The result of qRT-PCR demonstrated mRNA expression of YB-1 was increased in 26 of 32 (81.25%) of CRC patients. The statistical analysis based on immunohistochemical staining suggested that YB-1 expression was significantly correlated with tumor differentiation, tumor invasion, lymph node metastasis and Dukes’ classification (all P<0.05). Furthermore, we found that patients with high YB-1 expression had a poorer prognosis and were more likely to undergo local recurrence, compared to those with low YB-1 expression. We also identified that YB-1 expression, together with lymph node metastasis and Dukes’ classification were independent prognostic factors for CRC patients. In conclusion, our study for the first time demonstrated the clinical and prognostic significance of YB-1 in CRC and suggested that YB-1 is of great potential to be an attractive therapeutic target as well as prognostic biomarker for CRC patients.
Keywords: YB-1, colorectal cancer, prognosis, local recurrence
Introduction
Colorectal cancer (CRC) is one of the most commonly diagnosed malignancies worldwide and the third leading cause of cancer-related death across both genders [1]. As projected by American Cancer Society in 2014, there will be 136,830 new cases diagnosed with CRC and 50,310 CRC deaths in United States [2]. Despite encouraging improvement in precancerous screening and surgical techniques, the prognosis of patients with CRC remains to be dissatisfied, especially for patients in advanced stages. For example, the 5-year survival rate of patients involved in regional invasion is 70.4%, while it drops to 12.5% in patients with distant metastasis [3]. Classical clinicopathologic parameters, such as Tumor-Node-Metastasis (TNM) staging and carcinoembryonic antigen level, have been widely used for predicting outcome of cancer patients, but most of them are reported to be insensitive for accurate individual prognosis [4]. Therefore, it is urgently hoped that novel and reliable molecular markers should be identified and applied in prediction of patient prognosis.
Multifunctional Y-box-binding protein-1 (YB-1) is a member of the cold-shock protein superfamily that participates in various DNA/RNA-dependent events, including transcription and translation [5]. Recent studies have regarded YB-1 as an important oncoprotein due to its facilitation in many hallmarks of cancer [6,7]. For example, over-expression of YB-1 was reported to accelerate lung carcinogenesis by transcriptionally regulating cyclin D1 [8]. In glioblastoma, YB-1 has been identified as a downstream target of mTOR pathway to promote migration and invasion of cancer cells [9]. Moreover, the evidence based on Immunohistochemical analysis has suggested that YB-1 may drive malignant progression of gastric cancer through inducing angiogenesis [10]. An interesting study by Jung et al has closely linked YB-1 with cancer stem cells that its high expression seems to be essential for maintaining stemness and tumorigenic properties in breast cancer cells [11].
We have previously found that YB-1 may promote the proliferation, apoptosis resistance, invasion and migration of CRC cells by regulating epithelial- mesenchymal transition (EMT), a well-known molecular mechanism of tumor progression and metastasis [12]. Furthermore, emerging studies have suggested a potential relationship between YB-1 and liver metastases of gastrointestinal malignancy [13,14]. These findings about YB-1 have strongly promoted us to explore whether it might act as an effective biomarker for predicting the prognosis of patients with CRC. In this study, quantitative real-time polymerase chain reaction (qRT-PCR) and immunohistochemistry were employed to exam the expression of YB-1 in CRC tissues and their matched normal tissues. The correlations of clinicopathological parameters and prognosis with YB-1 expression were statistically analyzed. As a result, we found high expression of YB-1 might be associated with local recurrence and act as an independent prognostic factor for CRC patients. Our study not only confirms the clinical and prognostic significance of YB-1 in CRC, but also suggests that targeting YB-1 is likely to be a novel therapeutic strategy for the treatment of CRC.
Materials and methods
Patients and specimens
For qRT-PCR assay, a total of 32 fresh primary CRC tissues and their matched normal tissues were collected between September 2013 and December 2013. For immunohistochemistry assay, a total of 170 paraffin-embedded primary CRC tissues and their matched normal tissues were collected from January 2003 to December 2008. All the specimens were collected from patients with CRC undergoing surgery at Department of General Surgery, The Sixth People’s Hospital affiliated to Shanghai Jiao Tong University. None of the patients have received preoperative chemotherapy or radiotherapy. The postoperative pathologic staging was determined according to the Dukes’ classification system and histological type was determined according to the criteria of the World Health Organization classification. For postoperative oncological follow-up, patients received laboratory tests every 3 months and radiological examination every 6 months to monitor local recurrence and/or distal metastasis. Patients who had incomplete follow-up records were excluded in our study. The study was conducted with the approval of the ethics committee of The Sixth People’s Hospital affiliated to Shanghai Jiao Tong University. The written informed consents from patients for using their tissue specimens were also obtained. The basic clinicopathologic parameters of patients were shown in Table 1.
Table 1.
Correlations between YB-1 expression and clinicopathological Characteristics
| Characteristics | Total | YB-1 expression | P value | |
|---|---|---|---|---|
|
| ||||
| High expression | Low expression | |||
| Gender | ||||
| male | 91 | 53 | 38 | 0.090 |
| female | 79 | 35 | 44 | |
| Age | ||||
| ≤60 | 77 | 37 | 40 | 0.441 |
| >60 | 93 | 51 | 42 | |
| Tumor location | ||||
| colon | 102 | 58 | 44 | 0.119 |
| rectal | 68 | 30 | 38 | |
| Tumor differentiation | ||||
| Well and moderate | 108 | 45 | 63 | 0.001 |
| poor | 62 | 43 | 19 | |
| Tumor size | ||||
| ≤5 cm | 101 | 51 | 50 | 0.755 |
| >5 cm | 69 | 37 | 32 | |
| Tumor invasion | ||||
| T1-T2 | 83 | 36 | 47 | 0.046 |
| T3-T4 | 87 | 52 | 35 | |
| Lymph node metastasis | ||||
| Absent | 85 | 36 | 49 | 0.021 |
| Present | 85 | 52 | 33 | |
| Duke’s classification | ||||
| A/B | 89 | 39 | 50 | 0.033 |
| C/D | 81 | 49 | 32 | |
Quantitative real-time polymerase chain reaction
Total RNA was isolated from 32 paired frozen CRC tissues and corresponding normal tissues using Trizol reagent (Invitrogen, USA). The obtained RNA was reverse-transcribed into cDNA by Superscript III Reverse Transcriptase (Promega, USA) according to the manufacturer’s instructions. The real time PCR assay was then carried out using SYBR Green mixes (TaKaRa, Japan) and StepOne Plus Real-time PCR System (Applied Biosystems, USA). The cycling conditions were programmed as follows: 95°C for 5 min, 95°C for 5 sec and 60°C for 30 sec, for 40 cycles. β-actin was used as the internal control and the 2-ΔΔCt Method was used for determining relative gene expression levels. The following primers were used in our study: YB-1: forward: 5’-TACCTTCGCAGTGTAGGAGAT-3’; reverse: 5’-CTGGCATTGGTACGGCTTCTCC-3’; β-actin: forward: 5’-AAGGTGACAGCAGTCGGTT-3’; reverse: 5’-TGTGTGGACTTGGGAGAGG-3’. Experiments were repeated three times independently.
Immunohistochemistry and staining evaluation
The paraffin-embedded tissue specimens were processed for 4 μm-thick sections routinely. The sections were then dewaxed in xylene and rehydrated with an alcohol gradient (100% alcohol, 95% alcohol, 90% alcohol 80% alcohol and 70% alcohol). Endogenous peroxidase activity was blocked by 25-min incubation in 0.3% hydrogen peroxidase and antigen retrieval was performed by 15-min heating in microwave. Subsequently, the sections were incubated with the primary antibody against YB-1 (1:200, Epitomics, USA) at 4°C overnight. Thereafter, the sections were washed with phosphate-buffered saline (PBS) and incubated with secondary antibody for 30 minutes. The diaminobenzidine solution was used for revealing antigen-antibody reactions. Finally, the sections were counterstained with hematoxylin, dehydrated and mounted. Negative controls were prepared by replacing primary antibody with PBS.
A semi-quantitative staining evaluation was performed independently by at least two observers, who were blind to the patient clinical data. A pathologist was employed to assess the discrepant cases and the consensus was reached. Five visual fields of each section were randomly selected for evaluation. The expression of YB-1 was evaluated according to the scoring system that we described preciously [15]. Briefly, the expression of YB-1 was scored according to the immunoreactive score (IRS), which was calculated as a product of Staining Intensity (SI) and Percentage of Positive cells (PP). SI was divided into four grades: score 0 (negative), score 1 (weak), score 2 (moderate) and score 3 (strong). PP was divided into five grades: score 0 (none), score 1 (≤10%), score 2 (11-50%), score 3 (51-80%) and score 4 (>80%). For statistical analyses, the cases scoring at least 3 points in our study were designated as high expression of YB-1.
Statistical analysis
The results were presented as mean ± SD. The statistical analysis was performed using 19.0 SPSS statistical software. Student’s t test was employed to analyze the data of qRT-PCR. The correlations between YB-1 expression and patient clinicopathologic parameters were assessed by Chi-square test. The survival and local recurrence curves were constructed using the Kaplan-Meier model and the log-rank test was applied to compare intergroup differences. The univariate and multivariate analysis based on the Cox proportional hazard model were performed to identify significant independent prognostic factors for CRC patients. A P-value <0.05 was considered statistically significant.
Results
Expression of YB-1 in CRC tissues and corresponding normal tissues
The quantitative real-time polymerase chain reaction was performed to detect the mRNA expression of YB-1 in CRC tissues and matched normal tissues. As a result, we found that 26 of 32 patients (81.25%) had an up-regulated mRNA expression of YB-1 in CRC tissues. On the whole, the mean relative expression level of YB-1 was significantly higher in CRC tissues than that in matched normal tissues (1.06 ± 0.41 vs 0.52 ± 0.26, P<0.001, Figure 1).
Figure 1.
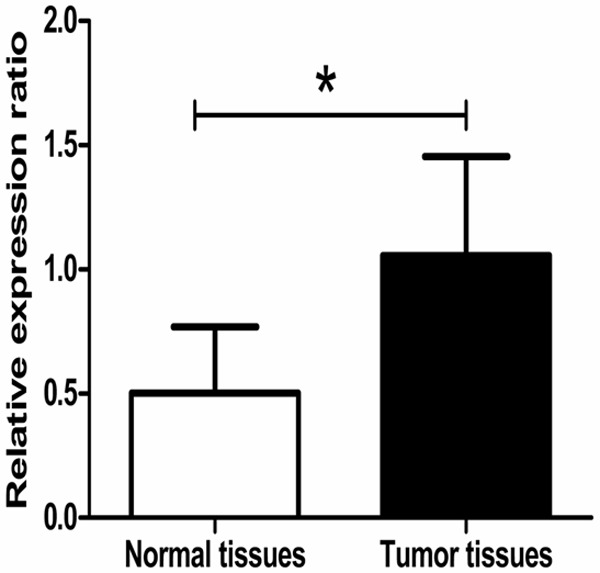
Up-regulation of YB-1 mRNA expression in CRC tissues. The mRNA expression of YB-1 in CRC and matched normal tissues was determined by quantitative real-time polymerase chain reaction. The result showed the mean relative expression level of YB-1 in CRC tissues was significantly higher than that in matched normal tissues (1.06 ± 0.41 vs 0.52 ± 0.26, P<0.001).
Immunohistochemistry was employed to detect the protein expression of YB-1 in CRC tissues and matched normal tissues. We found YB-1 was mainly expressed in the cytoplasm of tumor cells. According to the results of staining evaluation, high expression of YB-1 was found in 88 of 170 (51.8%) CRC tissues, compared with 33 of 170 (19.4%) matched normal tissues. The representative results of immunohistochemistry were shown in Figure 2.
Figure 2.
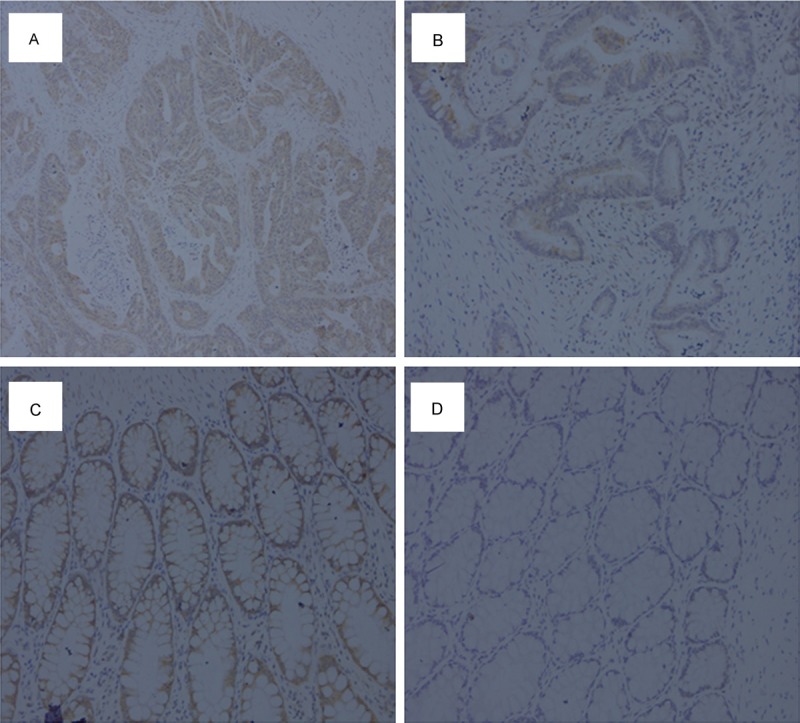
Representative results of immunohistochemical staining. A: High expression of YB-1 in CRC tissues. B: Low expression of YB-1 in CRC tissues. C: High expression of YB-1 in normal tissues. D: Low expression of YB-1 in normal tissues. Original magnification: ×200.
Correlations between YB-1 expression and CRC clinicopathologic characteristics
To further investigate the clinical significance of YB-1 in CRC, the correlations between YB-1 expression and CRC clinicopathologic characteristics were statistically analyzed. As shown in Table 1, the expression of YB-1 was significantly correlated with tumor differentiation (P=0.001), tumor invasion (P=0.046), lymph node metastasis (P=0.021) and Dukes’ classification (P=0.033). However, no statistically significant correlations were identified between YB-1 expression and other clinicopathologic characteristics, including gender (P=0.090), age (P=0.441), tumor location (P=0.119) and tumor size (P=0.755).
Prognostic significance of YB-1 expression in CRC
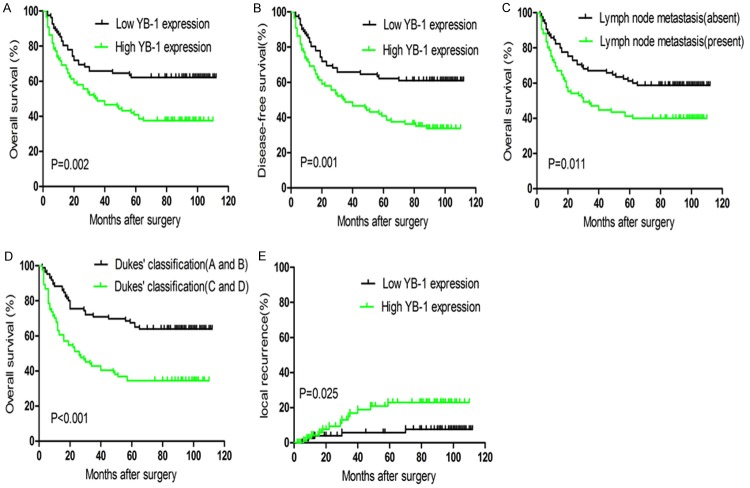
The prognostic significance of YB-1 expression was evaluated using Kaplan-Meier model. As shown in Figure 3A and 3B, patients with high expression of YB-1 had a significantly lower overall survival (OS) and disease free survival (DFS) rate than patients with low expression of YB-1 (P=0.002 and P=0.001). We also found the OS rate of CRC patients was significantly associated with lymph node metastasis (Figure 3C, P=0.011) and Dukes’ classification (Figure 3D, P<0.001). More importantly, the 5-year local recurrence rate of patients with high expression of YB-1 was 23.0%, compared to 5.8% of patients with low expression. As analyzed by log-rank test, this difference was considered statistically significant (Figure 3E, P=0.025).
Figure 3.
Kaplan-Meier curves for survival and local recurrence of CRC patients. A and B: Patients with high YB-1 expression had a poorer overall survival (OS) and disease-free survival (DFS) than those with low YB-1 expression (P=0.002 and P=0.001). C and D: Lymph node metastasis and Dukes’ classification as independent prognostic factors were correlated with OS of CRC patients (P=0.011 and P<0.001). E: Patients with high YB-1 expression had a higher local recurrence rate than patients with low YB-1 expression (P=0.025).
The univariate and multivariate analysis were conducted to identify the independent prognostic indicators for CRC patients. As shown in Table 2, the univariate analysis revealed that YB-1 expression, tumor differentiation, tumor invasion, lymph node metastasis and Dukes’ classification were significantly associated with OS of CRC patients (P=0.002, P=0.012, P=0.043, P=0.010 and P<0.001). The multivariate analysis of these factors suggested that only YB-1 expression, lymph node metastasis and Dukes’ classification were significantly independent prognostic indicators for OS of CRC patients (P=0.026, P=0.032 and P=0.002).
Table 2.
Univariate analysis and multivariate analysis for prognostic factors in CRC patients
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| RR | 95% CI | P value | RR | 95% CI | P value | |
| YB-1 expression | 1.988 | 1.280-3.090 | 0.002 | 1.719 | 1.066-2.771 | 0.026 |
| Age | 1.333 | 0.865-2.054 | 0.193 | |||
| Gender | 1.098 | 0.719-1.677 | 0.665 | |||
| Tumor location | 1.033 | 0.672-1.589 | 0.881 | |||
| Tumor differentiation | 1.721 | 1.125-2.632 | 0.012 | 1.240 | 0.791-1.945 | 0.348 |
| Tumor size | 1.336 | 0.874-2.042 | 0.180 | |||
| Tumor invasion | 1.561 | 1.015-2.401 | 0.043 | 1.260 | 0.808-1.965 | 0.308 |
| Lymph node metastasis | 1.765 | 1.147-2.717 | 0.010 | 1.636 | 1.042-2.569 | 0.032 |
| Dukes’ classification | 2.468 | 1.586-3.839 | <0.001 | 2.066 | 1.314-3.249 | 0.002 |
Discussion
Accumulating studies have demonstrated that YB-1 broadly participates in various malignant processes including uncontrolled proliferation [16], chemotherapy resistance [17], invasion and metastasis [18]. Given the fact that YB-1 plays a crucial role in cancer initiation and development, it is not surprising that YB-1 had an important clinical significance for cancer patients. In a latest study encompassing 204 patients with cervical cancer, positive nuclear YB-1 expression was reported to have a statistically significant association with poor progression-free survival and overall survival [19]. Using Immunohistochemistry on invasive breast cancer specimens, researchers found cytoplasmic YB-1 expression was correlated with an aggressive phenotype and patients with nuclear YB-1 expression seemed to have a high risk of recurrence [20]. In intestinal type of gastric cancer, the multivariate analysis indicated that YB-1 expression was an independent predictor of lymph node metastasis [21]. Furthermore, a novel study by Tacke et al has suggested that plasma detection of an 18 kDa YB-1 fragment might act as a useful tool for cancer screening independent of tumor origins, although it had statistically insignificant association with cancer patient prognosis [22]. Despite the fact that YB-1 expression has been described to be correlated with clinical parameters and patient outcome in numerous malignancies, its clinical and prognostic significance in CRC have rarely been discussed.
In this study, the mRNA expression of YB-1 in 32 paired primary CRC tissues and matched normal tissues were examined by qRT-PCR. The result demonstrated that most primary CRC tissues exhibited significantly higher mRNA expression of YB-1 than their matched normal tissues. This tendency was then confirmed by immunohistochemistry that 88 of 170 (51.8%) CRC tissues were found to have high protein expression of YB-1, compared with 33 of 170 (19.4%) matched normal tissues. Our finding was also in accordance with a recent review that overexpression of YB-1 was more frequently detected in malignant tissues than in normal tissues among most cancers [7]. Furthermore, the statistical analysis based on staining evaluation indicated that YB-1 expression was significantly associated with tumor differentiation, tumor invasion, lymph node metastasis and Dukes’ classification in 170 CRC patients, suggesting that YB-1 may be involved in the initiation and progression of CRC. Prognostic biomarkers for CRC patients are critical because they can provide useful information for the clinical management. For example, prognostic biomarkers may be helpful for identifying patients who are likely to undergone recurrence or be insensitive to the standard chemotherapy, leading to a more precise and tailored therapeutic strategy. However, to our knowledge, none of studies have validated whether YB-1 could act as a useful prognostic biomarker for CRC patients. In this study, we found patients with high YB-1 expression had a poorer OS and DFS than patients with low YB-1 expression. Using multivariate analysis, we identified that YB-1 expression, lymph node metastasis and Dukes’ classification were independent prognostic factors for CRC patients, suggesting that YB-1 detection combined with classic clinicopathological parameters might be beneficial to prognosis evaluation and personalized therapy of CRC. It has been well-known that local recurrence is a direct threat to patients with surgically treated CRC. Another important finding in our study was that patients with high YB-1 expression had a higher local recurrence rate than patients with low YB-1 expression. This result further corroborated the association between high YB-1 expression and poor prognosis of CRC patients. Similarly, Ardito et al recently found that high YB-1 expression may be correlated with liver recurrence in patients undergoing resection of CRC liver metastasis, also offering an indirect support to our results [14]. Considering previous studies about biology roles of YB-1 in tumors, we deduced that YB-1 may contribute to CRC recurrence partly by driving highly proliferative potential of CRC cells. However, its underlying molecular mechanisms need to be further investigated.
According to previous studies and our present results, we can conclude that several reasons may be responsible for the association between high YB-1 expression and poor patient prognosis. Firstly, YB-1 may be involved in invasion and metastasis of CRC by driving EMT program. It is well known that patients with invasive/metastatic CRC usually have unfavorable outcome and EMT is a key molecular event in cell invasion and metastasis [23]. Recently, increasing studies have regarded YB-1 as an important EMT mediator in various cancers including prostate cancer [24], hepatocellular cancer [25] and cervical cancer [26]. Moreover, our previous study in vitro has proved that YB-1 may promote malignant progression of CRC cells by inducing EMT [12]. Thus, we assume YB-1 mediated EMT to the most possible reason for explaining why high YB-1 expression was associated with poor prognosis in CRC patients. Secondly, YB-1 may be involved in drug resistance of CRC. Acquired drug resistance is almost clinically inevitable for a majority of cancer patients. YB-1 rose to prominence in chemotherapy resistance following the first report about its association with Multiple Drug Resistance gene 1 in breast cancer cells [27]. In prostate cancer, researchers even found YB-1 activation may induce resistance to endocrinotherapy as well as cytotoxic chemotherapy [17,28]. With regard to CRC, YB-1 has already been reported to induce resistance to oxaliplatin (a first-line chemotherapeutics for CRC) in SW480 and HT-29 colon adenocarcinoma cell lines [29]. Taken together, these studies suggest that drug resistance induced by YB-1 may also contribute to poor outcome of CRC patients. Finally, YB-1 may be associated with cancer stem cells (CSC) in CRC. CSC are a small group of cancer cells that persist for years after surgery or chemoradiotherapy, but erupt suddenly to threaten patient life [30]. The association between YB-1 expression and CSC phenotype in breast cancer has been comprehensively studied [11,31]. More importantly, YB-1 dependent virotherapy has been proved to effectively eradicate glioma CSC in vitro and in vivo [32]. Although none of studies have clearly linked YB-1 with CSC in CRC, it is reasonable to speculate that YB-1 may also be implicated in CSC phenotype of CRC, which results in unfavorable patient prognosis.
In conclusion, our results demonstrated that YB-1 expression was increased in CRC tissues compared to adjacent normal tissues, and might be associated with pathological development of CRC. In addition, we found high expression of YB-1 was correlated with local recurrence and could serve as an independent prognostic factor for CRC patients. These convincing evidences not only confirm our previous study in vitro, but also suggest that YB-1 is of great potential to be an attractive therapeutic target as well as prognostic biomarker for patients with CRC.
Acknowledgements
This work was partly supported by the funding of Science and Technology Commission of Shanghai Municipality (NO. 124119a7202). We thank Professor Yuping Gao (Department of Pathology, Renji Hospital Affiliated to Shanghai Jiao Tong University) for her crucial guidance in immunohistochemistry assay. We also thank Dr. Jiayuan Peng and Qingchao Zhu (Department of General Surgery, The Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University) for their kind assistance in our follow-up study.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 4.Ribero D, Vigano L, Amisano M, Capussotti L. Prognostic factors after resection of colorectal liver metastases: from morphology to biology. Future Oncol. 2013;9:45–57. doi: 10.2217/fon.12.159. [DOI] [PubMed] [Google Scholar]
- 5.Lyabin DN, Eliseeva IA, Ovchinnikov LP. YB-1 protein: functions and regulation. Wiley Interdiscip Rev RNA. 2014;5:95–110. doi: 10.1002/wrna.1200. [DOI] [PubMed] [Google Scholar]
- 6.Lasham A, Print CG, Woolley AG, Dunn SE, Braithwaite AW. YB-1: oncoprotein, prognostic marker and therapeutic target? Biochem J. 2013;449:11–23. doi: 10.1042/BJ20121323. [DOI] [PubMed] [Google Scholar]
- 7.Kosnopfel C, Sinnberg T, Schittek B. Y-box binding protein 1--a prognostic marker and target in tumour therapy. Eur J Cell Biol. 2014;93:61–70. doi: 10.1016/j.ejcb.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Harada M, Kotake Y, Ohhata T, Kitagawa K, Niida H, Matsuura S, Funai K, Sugimura H, Suda T, Kitagawa M. YB-1 promotes transcription of cyclin D1 in human non-small-cell lung cancers. Genes Cells. 2014;19:504–516. doi: 10.1111/gtc.12150. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Ding Z, Mo J, Sang B, Shi Q, Hu J, Xie S, Zhan W, Lu D, Yang M, Bian W, Zhou X, Yu R. GOLPH3 promotes glioblastoma cell migration and invasion via the mTOR-YB1 pathway in vitro. Mol Carcinog. 2014 doi: 10.1002/mc.22197. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Wang KY, Li Z, Liu YP, Izumi H, Yamada S, Uramoto H, Nakayama Y, Ito K, Kohno K. Y-box binding protein 1 expression in gastric cancer subtypes and association with cancer neovasculature. Clin Transl Oncol. 2014 doi: 10.1007/s12094-014-1208-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Jung K, Wu F, Wang P, Ye X, Abdulkarim BS, Lai R. YB-1 regulates Sox2 to coordinately sustain stemness and tumorigenic properties in a phenotypically distinct subset of breast cancer cells. BMC Cancer. 2014;14:328. doi: 10.1186/1471-2407-14-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan XB, Zhu QC, Chen HQ, Peng JY, Chao HL, Du HX, Wang ZG, Jin ZM. Knockdown of Yboxbinding protein1 inhibits the malignant progression of HT29 colorectal adenocarcinoma cells by reversing epithelialmesenchymal transition. Mol Med Rep. 2014;10:2720–2728. doi: 10.3892/mmr.2014.2545. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y, Yamada S, Izumi H, Li Z, Shimajiri S, Wang KY, Liu YP, Kohno K, Sasaguri Y. Strong YB-1 expression is associated with liver metastasis progression and predicts shorter disease-free survival in advanced gastric cancer. J Surg Oncol. 2012;105:724–730. doi: 10.1002/jso.23030. [DOI] [PubMed] [Google Scholar]
- 14.Ardito F, Arena V, Vellone M, Grande G, Pennacchia I, Majellaro F, Giovannini I, Vecchio FM, Nuzzo G, Giuliante F. Strong YB-1 Expression Predicts Liver Recurrence Following Resection for Colorectal Metastases. J Gastrointest Surg. 2014;18:1987–93. doi: 10.1007/s11605-014-2657-3. [DOI] [PubMed] [Google Scholar]
- 15.Yan X, Yan L, Su Z, Zhu Q, Liu S, Jin Z, Wang Y. Zinc-finger protein X-linked is a novel predictor of prognosis in patients with colorectal cancer. Int J Clin Exp Pathol. 2014;7:3150–3157. [PMC free article] [PubMed] [Google Scholar]
- 16.Kotake Y, Ozawa Y, Harada M, Kitagawa K, Niida H, Morita Y, Tanaka K, Suda T, Kitagawa M. YB1 binds to and represses the p16 tumor suppressor gene. Genes Cells. 2013;18:999–1006. doi: 10.1111/gtc.12093. [DOI] [PubMed] [Google Scholar]
- 17.Shiota M, Yokomizo A, Takeuchi A, Itsumi M, Imada K, Kashiwagi E, Inokuchi J, Tatsugami K, Uchiumi T, Naito S. Inhibition of RSK/YB-1 signaling enhances the anti-cancer effect of enzalutamide in prostate cancer. Prostate. 2014;74:959–969. doi: 10.1002/pros.22813. [DOI] [PubMed] [Google Scholar]
- 18.Wu K, Chen K, Wang C, Jiao X, Wang L, Zhou J, Wang J, Li Z, Addya S, Sorensen PH, Lisanti MP, Quong A, Ertel A, Pestell RG. Cell fate factor DACH1 represses YB-1-mediated oncogenic transcription and translation. Cancer Res. 2014;74:829–839. doi: 10.1158/0008-5472.CAN-13-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishio S, Ushijima K, Yamaguchi T, Sasajima Y, Tsuda H, Kasamatsu T, Kage M, Ono M, Kuwano M, Kamura T. Nuclear Y-box-binding protein-1 is a poor prognostic marker and related to epidermal growth factor receptor in uterine cervical cancer. Gynecol Oncol. 2014;132:703–708. doi: 10.1016/j.ygyno.2014.01.045. [DOI] [PubMed] [Google Scholar]
- 20.Mylona E, Melissaris S, Giannopoulou I, Theohari I, Papadimitriou C, Keramopoulos A, Nakopoulou L. Y-box-binding protein 1 (YB1) in breast carcinomas: relation to aggressive tumor phenotype and identification of patients at high risk for relapse. Eur J Surg Oncol. 2014;40:289–296. doi: 10.1016/j.ejso.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Guo T, Yu Y, Yip GW, Baeg GH, Thike AA, Lim TK, Tan PH, Matsumoto K, Bay BH. Y-Box Binding Protein 1 is correlated with lymph node metastasis in intestinal type of gastric cancer. Histopathology. 2014 doi: 10.1111/his.12570. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Tacke F, Galm O, Kanig N, Yagmur E, Brandt S, Lindquist JA, Eberhardt CS, Raffetseder U, Mertens PR. High prevalence of Y-box protein-1/p18 fragment in plasma of patients with malignancies of different origin. BMC Cancer. 2014;14:33. doi: 10.1186/1471-2407-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinestel K, Eder S, Schrader AJ, Steinestel J. Clinical significance of epithelial-mesenchymal transition. Clin Transl Med. 2014;3:17. doi: 10.1186/2001-1326-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan MI, Adhami VM, Lall RK, Sechi M, Joshi DC, Haidar OM, Syed DN, Siddiqui IA, Chiu SY, Mukhtar H. YB-1 expression promotes epithelial-to-mesenchymal transition in prostate cancer that is inhibited by a small molecule fisetin. Oncotarget. 2014;5:2462–2474. doi: 10.18632/oncotarget.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Cheng S, Zhang M, Ma X, Zhang L, Wang Y, Rong R, Ma J, Xia S, Du M, Shi F, Wang J, Yang Q, Bai X, Leng J. Prostaglandin E2 promotes hepatocellular carcinoma cell invasion through upregulation of YB-1 protein expression. Int J Oncol. 2014;44:769–780. doi: 10.3892/ijo.2013.2234. [DOI] [PubMed] [Google Scholar]
- 26.Kim YM, Cho M. Activation of NADPH oxidase subunit NCF4 induces ROS-mediated EMT signaling in HeLa cells. Cell Signal. 2014;26:784–796. doi: 10.1016/j.cellsig.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Bargou RC, Jurchott K, Wagener C, Bergmann S, Metzner S, Bommert K, Mapara MY, Winzer KJ, Dietel M, Dorken B, Royer HD. Nuclear localization and increased levels of transcription factor YB-1 in primary human breast cancers are associated with intrinsic MDR1 gene expression. Nat Med. 1997;3:447–450. doi: 10.1038/nm0497-447. [DOI] [PubMed] [Google Scholar]
- 28.Shiota M, Itsumi M, Yokomizo A, Takeuchi A, Imada K, Kashiwagi E, Inokuchi J, Tatsugami K, Uchiumi T, Naito S. Targeting ribosomal S6 kinases/Y-box binding protein-1 signaling improves cellular sensitivity to taxane in prostate cancer. Prostate. 2014;74:829–838. doi: 10.1002/pros.22799. [DOI] [PubMed] [Google Scholar]
- 29.Tsofack SP, Garand C, Sereduk C, Chow D, Aziz M, Guay D, Yin HH, Lebel M. NONO and RALY proteins are required for YB-1 oxaliplatin induced resistance in colon adenocarcinoma cell lines. Mol Cancer. 2011;10:145. doi: 10.1186/1476-4598-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 31.To K, Fotovati A, Reipas KM, Law JH, Hu K, Wang J, Astanehe A, Davies AH, Lee L, Stratford AL, Raouf A, Johnson P, Berquin IM, Royer HD, Eaves CJ, Dunn SE. Y-box binding protein-1 induces the expression of CD44 and CD49f leading to enhanced self-renewal, mammosphere growth, and drug resistance. Cancer Res. 2010;70:2840–2851. doi: 10.1158/0008-5472.CAN-09-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantwill K, Naumann U, Seznec J, Girbinger V, Lage H, Surowiak P, Beier D, Mittelbronn M, Schlegel J, Holm PS. YB-1 dependent oncolytic adenovirus efficiently inhibits tumor growth of glioma cancer stem like cells. J Transl Med. 2013;11:216. doi: 10.1186/1479-5876-11-216. [DOI] [PMC free article] [PubMed] [Google Scholar]