Abstract
We investigated the effects of the early phase of sepsis and prior treatment of Simvastatin on muscle structure and mitochondrial enzymes treated with lipopolysaccharide (LPS) in rats. We divided rats into control, LPS, simvastatin, simvastatin + LPS groups. Mitochondrial citrate synthase, complex I, II, I + III, II + III, cytochrome c oxidase (COX) activities were measured. Muscle tissue was stained using modified Gomori trichrome (MGT), succinic dehydrogenase (SDH) and cytochrome oxidase (COX). In all treated groups, complex I and citrate synthase activities were higher than in the controls. In the control and LPS groups, COX activity was increased when compared with simvastatins’. Complex II, II-III activities were higher in the LPS group than in the control group. Complex I-III activities were higher in the Simvastatin and Simvastatin + LPS groups than in the control and LPS groups (P < 0.05). Myopathic changes with LPS group were observed in MGT stained sections. Our findings showed improvements in the alterations of enzyme activities and muscle myofibrils after treating rats with LPS that had received a prior dose of simvastatin.
Keywords: Lipopolysaccharide, mitochondrial enzyme activites, rat, simvastatin, skeletal muscle
Introduction
Sepsis is a life threatening disease which affects many organ systems, if left untreated it can cause shock, organ dysfunction and multi-organ failure [1]. Patients with sepsis treated in Intensive Care Units often develop muscle weakness, muscle fatigue, and neuropathy resulting in proteolysis [2,3]. An incremental loss of proteins may appear in the presence of infection, which causes weight-loss and muscle fatigue and increases the risk of thromboembolic complications [4]. Many studies have shown that the release of cytokines such as TNF-α and IL-1 and increased ubiquitin mRNA during an infection cause loss of myofibrillary nitrogen and impairment of proteins, which activates proteolysis [5-8].
Skeletal tissue comprises 50%-60% of body cell mass and represents the largest organ affected by systemic inflammation. Pathologies such as ultrastructural mitochondrial alterations and oxidative mechanisms in sepsis and septic shock have attracted attention in recent years [9].
Statins are key precursor inhibitors of HMG-CoA reductase, which synthesizes mevalonate, in the cholesterol synthesis pathway. Some studies have suggested that prior statin treatment may prevent vascular hyporeactivity and inhibit the development of severe sepsis in patients with acute systemic inflammation [10,11]. However, using statins can also have dose-dependent adverse effects. High concentrations of statins can cause muscle damage and myalgia [12,13].
In this study we investigated the effects of the early phase of sepsis and prior simvastatin treatment on mitochondrial enzyme activities and histologic changes in skeletal muscle of rats treated with LPS.
Materials and methods
Experimenta l groups
This study was conducted at the University of Istanbul experimental research center, (Resolution No: 2012/138). We used male adult Wistar albino rats weighing 200-250 g in the experiments. The animals were fed with a commercial diet and tap water ad libitum, were housed in cages and kept at a controlled temperature (22±2°C) and humidity (55 to 60%) with a 12-h light/dark cycle.
The rats were divided into four groups, each composed of eight rats: (1) control group, (2) LPS group, (3) Simvastatin group, (4) Simvastatin + LPS group.
Experimental procedures
Lipopolysaccharide (LPS) from Escherichia coli O127: B8 (Sigma Aldrich, Product No: L5668) was dissolved in 1 ml of sterile saline solution and a single dose was injected intraperitoneally at a daily dose of 20 mg/kg. Simvastatin (20 mg/kg) (Sigma Aldrich, Product No: S0650000) were given p.o. via oral gavage for 5 days [14]. In the Simvastatin + LPS group, LPS was given 1.5 h after the fifth dose of simvastatin. Four hours after LPS treatment, animals (for both of LPS and Simvastatin + LPS groups) were sacrified. Gastrocnemius muscles were dissected in all groups at the end of 4th hours.
Biochemical procedures
At the end of the experimental period, blood samples were taken to determine serum concentrations of creatinine kinase (CK), aspartate aminotransferase (GOT), alanine aminotransferase (GPT), lactate dehydrogenase (LDH). Serum GOT, GPT and LDH, CK levels were measured using the corresponding kit in an autoanalyzer (Cobas autoanalyzer, DPC, Diagnostic products corporation, USA).
Cytokine levels
The blood samples were centrifuged at 2500 rpm for 20 minutes and the serum stored in eppendorf tubes at -20°C until analysis. The cytokine levels were measured using the ELISA method for TNF-α (BioSource, Invitrogen, USA, Cat No, KRC3012) and IL-10 (BioSource, Invitrogen, USA, Cat No, KRC0101). The absorbance values were measured at 450 nm using a micro-ELISA automatic analyzer. The sensitivity of the ELISA kits were < 4 pg/ml for TNF-α, < 5 pg/ml for IL-10.
Neuropathological procedures
The gastrocnemius muscle was taken while the animals were under anesthesia. The muscle samples were rapidly frozen in isopentane cooled by liquid nitrogen. We took serial cross-sections 8 μm thick and stained them with modified Gomori’s trichrome (MGT) method, succinic dehydrogenase (SDH) and cytochrome oxidase (COX) using standard protocols [15,16]. The stained sections were visualized and photographed using a Nikon microscope (ECLIPSE 80i Nikon Corporation, JAPAN).
Mitochondrial enzyme activities
Absorbances of all activities were recorded kinetically in tissue homogenate using a spectrophotometer (Shimadzu, Japan) at 30°C.
Citrate synthase
The reaction was initiated by adding of dithio-bis 2-nitrobenzoic acid into a mixture containing oxaloacetate, Acetyl-CoA, and tissue homogenate. Absorbance was measured at 412 nm [17].
Complex I (NADH dehydrogenase)
Complex I-oxidation of NAD-activity was monitored using first potassium phosphate, NADH, and potassium ferrisiyanide and then by adding tissue homogenate at 340 nm [18].
Complex II + III (succinate cytochrome c reductase)
Tissue homogenate was added to a mixture of fresh cytochrome c containing succinate, and potassium cyanide (KCN). Complex II + III activity was observed due to the increase in rate of reduction of cytochrome c. This was measured at 550 nm [18].
Complex I + III (NADH Cytochrome c Reductase)
Complex I + III activity was observed as a result of the rate of decrease in the addition cytochrome c homogenate including fresh prepared cytochrome c, NADH, and KCN alterations after adding rotenone at 550 nm [19].
Complex II (succinate dehydrogenase)
Complex II activity was determined adding tissue homogenate by the reduction of 2,6-dichlorophenolindophenol (DCIP) including succinate, KCN, and DCPIP at 600 nm [20].
Complex IV (cytochrome c oxidase)
Cytochrome c oxidase (COX) activity was measured as the oxidation rate of decrease in reduced cytochrome c at 550 nm at 30°C (CYTOCOX1, Sigma Aldrich, Saint Louise, USA) [21].
Statistical analyses
Data were expressed as mean ± standard deviation. Overall statistical significance between the groups was tested with oneway ANOVA or Kruskal-Wallis test, depending on normality of distribution. In all cases, P < 0.05 was set as limit of significance.
Results
Biochemical findings
Serum CK and GOT values were found to be increased in experimental groups as compared to those of controls (P < 0.05).
There were no significant changes in GPT levels in any groups (P > 0.05). However, levels of GPT were lower in the control than the other groups’. In the simvastatin group, LDH levels were observed to be higher in the Simvastatin group than in the other groups (P < 0.05) (Table 1).
Table 1.
Values of serum CK, GOT, GPT, LDH for groups
| Experiment Groups | CK (U/L) | GOT (U/L) | GPT (U/L) | LDH (U/L) |
|---|---|---|---|---|
| Control | 892.3±216.8 | 122.7±14.7 | 51.0±10.0 | 1176.75±310.36 |
| LPS | 1565.2±635.8* | 220.5±66.6* | 68.5±14.7 | 1267.20±630.65 |
| Simvastatin | 1908.8±412.7* | 218.8±32.2* | 58.2±16.96 | 2803.20±986.10* |
| Simvastatin + LPS | 1843±512.49* | 306.33±70.57* | 56.8±12.21 | 1913.4±252.95 |
P < 0.05.
Cytokine findings
Serum TNF-α and IL-10 values were found to be significantly higher in the LPS and Simvastatin + LPS groups compared to those of the controls (P < 0.01) (Figures 1, 2).
Figure 1.
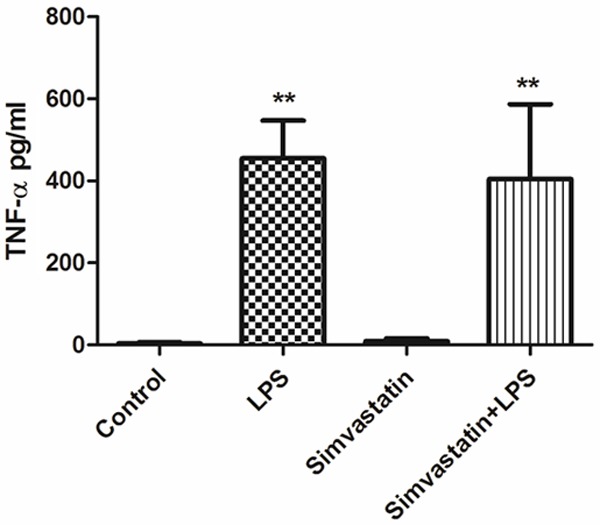
Serum TNF-α values in experimental groups. **Significant differences at P < 0.01, LPS and Simvastatin + LPS vs. other groups.
Figure 2.
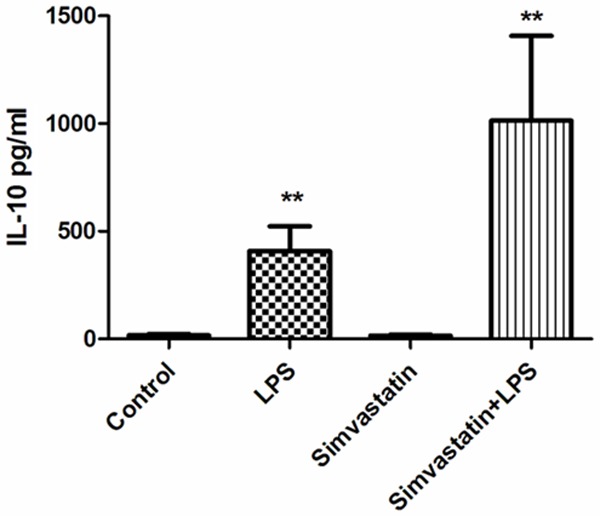
Serum IL-10 values in experimental groups. **Significant differences at P < 0.01, LPS and Simvastatin + LPS vs. other groups.
Mitochondrial enzyme activities
Complex I and citrate synthase activities were significantly increased in all experimental groups than those of the controls. The results were as follows: Control group; complex I 20.47±14.15 µmol/min/g tissue; LPS group 72.23±21.99 µmol/min/g tissue; Simvastatin group 58.20±16.76 µmol/min/g tissue; and the Simvastatin + LPS group 59.52±17.94 µmol/min/g tissue. For citrate synthase: Control group 22.8±1.8 µmol/min/g tissue; LPS group 31.0±4.2 µmol/min/g tissue; Simvastatin group 31.29±7.63 µmol/min/g tissue; and the Simvastatin + LPS group 36.02±6.33 µmol/min/g tissue (Figures 3 and 4).
Figure 3.
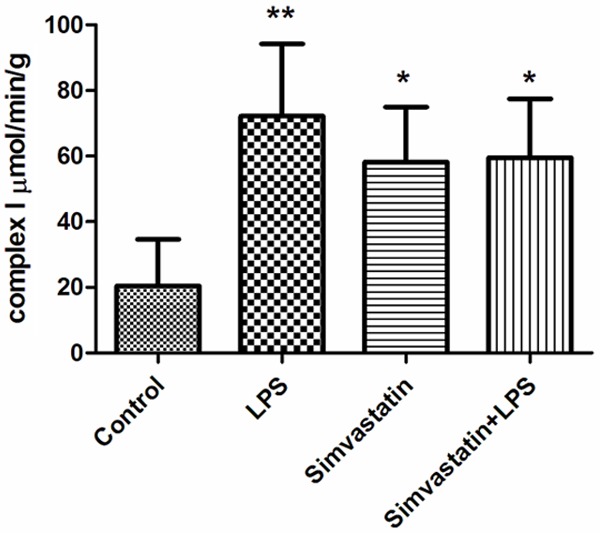
Activities of complex I in groups of muscle tissues, *: P < 0.05, Simvastatin and Simvastatin + LPS vs. control; **: P < 0.01 LPS vs. control.
Figure 4.
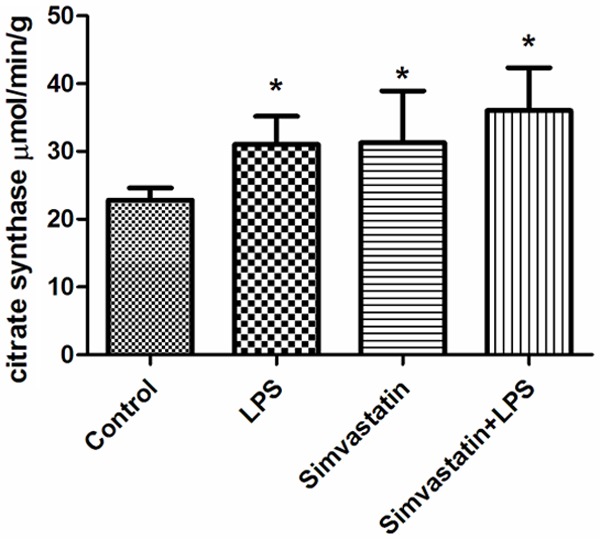
Activities of citrate synthase in groups of muscle tissues, *: P < 0.05, experimental groups vs. control.
COX activity in the control and LPS groups were observed to be significantly increased in comparison with the Simvastatin group: Control group 2.37±0.7 µmol/min/g tissue; LPS group 2.31±0.7 µmol/min/g tissue; Simvastatin group 1.25±0.15 µmol/min/g tissue; and the Simvastatin + LPS group 1.89±0.46 µmol/min/g tissue (Figure 5).
Figure 5.
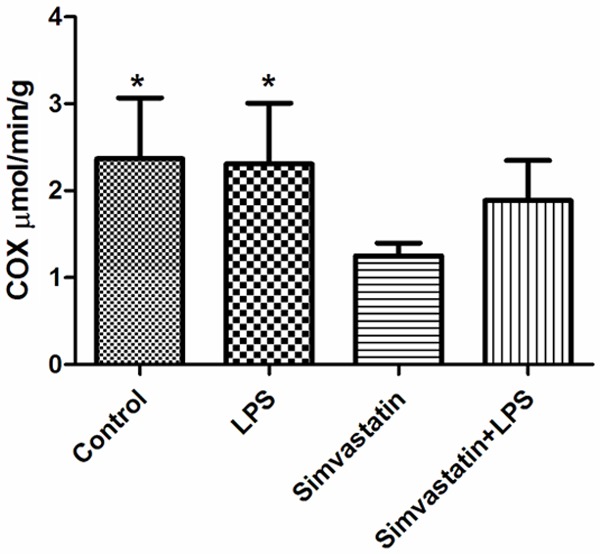
Activities of COX in groups of muscle tissues, *: P < 0.05, LPS and control vs. simvastatin.
Complex II activity was higher in the LPS and control groups than the Simvastatin + LPS groups: Control group 1.18±0.18 µmol/min/g tissue; LPS group 2.90±0.88 µmol/min/g tissue; Simvastatin group 2.09±0.86 µmol/min/g tissue; and Simvastatin + LPS 1.64±0.66 µmol/min/g tissue (Figure 6).
Figure 6.
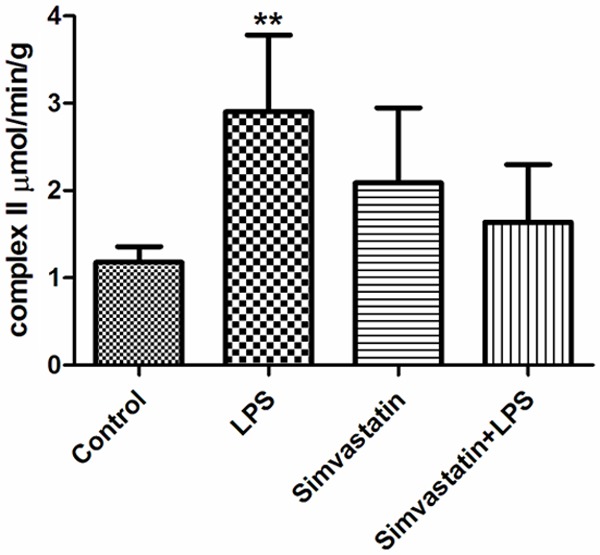
Activities of complex II in groups of muscle tissues, **: P < 0.01, LPS vs. control and Simvastatin + LPS.
Complex I-III activity was higher in the Simvastatin and Simvastatin + LPS groups than the LPS and control groups: Control group 1.23±0.25 µmol/min/g tissue; LPS group 0.57±0.34 µmol/min/g tissue; Simvastatin group 3.52±1.06 µmol/min/g tissue; and the Simvastatin + LPS group 4.85±2.04 µmol/min/g tissue (Figure 7).
Figure 7.
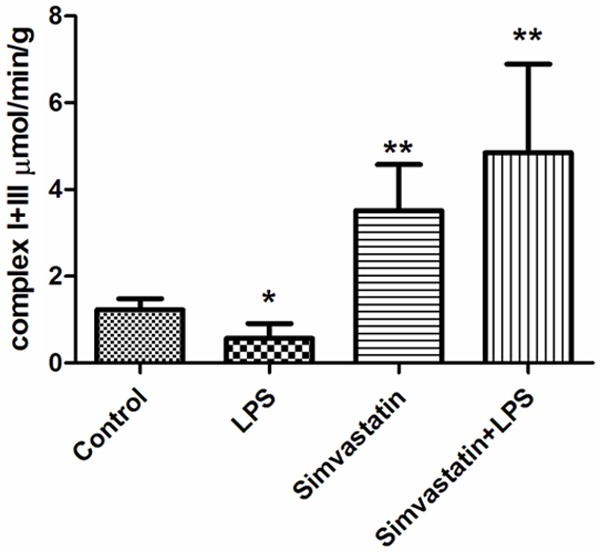
Activities of complex I + III in groups of muscle tissues, *: P < 0.05, LPS vs. control. **: P < 0.01, Simvastatin and Simvastatin + LPS vs. LPS and control.
Complex II-III activity was higher in the LPS and control groups than the Simvastatin and Simvastatin + LPS groups: Control group 0.75±0.09 µmol/min/g tissue; LPS group 1.18±0.35 µmol/min/g tissue; Simvastatin group 0.13±0.04 µmol/min/g tissue; and Simvastatin + LPS group 0.32±0.19 µmol/min/g tissue (P < 0.05) (Figure 8).
Figure 8.
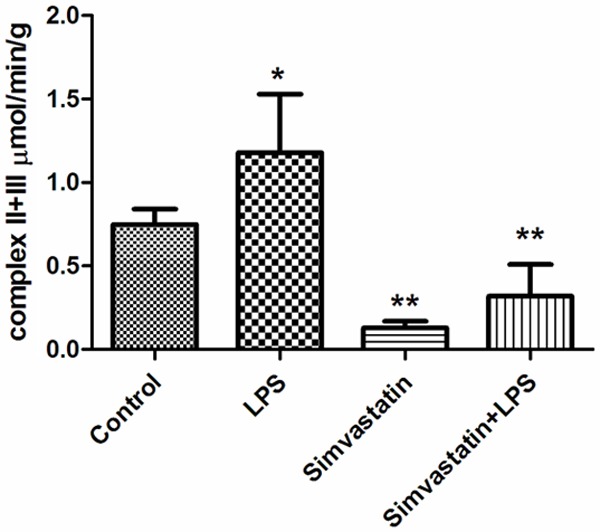
Activities of complex II + III in groups of muscle tissues. *: P < 0.05 LPS vs. control. **: P < 0.01 Simvastatin and Simvastatin + LPS vs. control.
Histopathological findings
More than half of the animals that were treated with LPS showed myopathic changes characterized by rounding of the muscle fibers and fiber size variation. In only one animal, the fibers of some fasicles showed mitochondrial aggregates. Cytochrome c oxidase activity was found normal in the LPS group as compared to the control group.
In the Simvastatin group, myopathic changes were seen in all rats. In addition, there were no changes with COX and SDH staining in any animal.
In the Simvastatin + LPS groups, MGT and COX results were the same as those of the controls, but in less of the animals, mild myopathic changes were seen (Figures 9, 10 and 11).
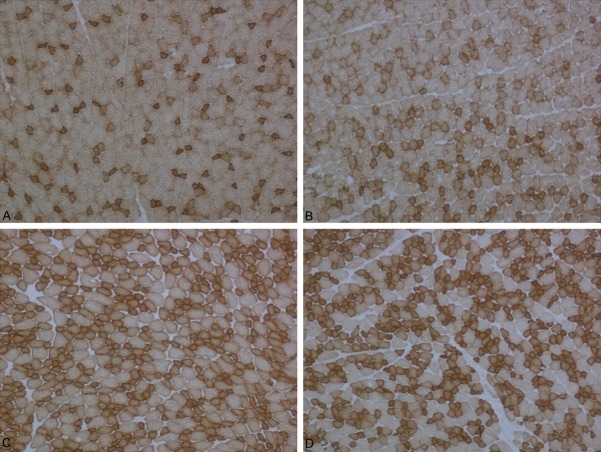
Figure 9.
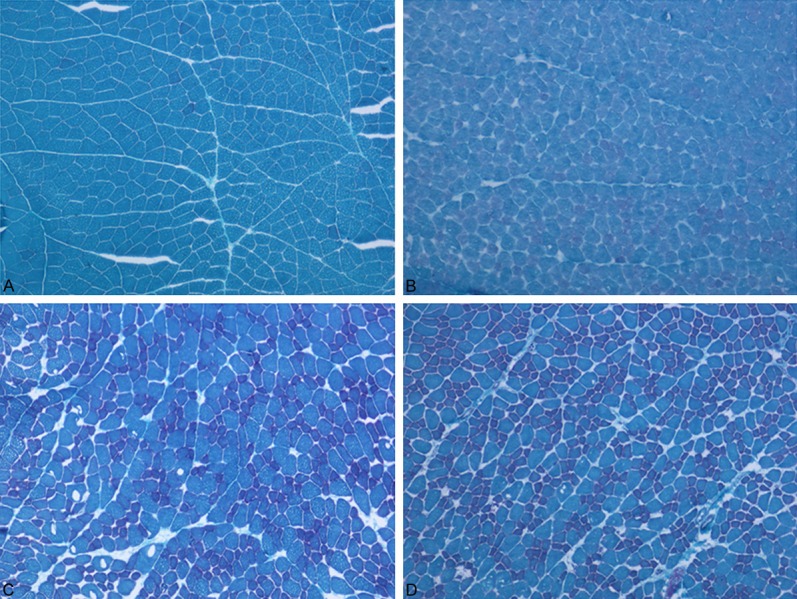
A: Muscle tissues were stained control group with Modified Gomori Trichrome. B: Muscle tissues were stained LPS group with Modified Gomori Trichrome. C: Muscle tissues were stained Simvastatin group with Modified Gomori Trichrome. D: Muscle tissues were stained Simvastatin + LPS group with Modified Gomori Trichrome.
Figure 10.
A: Muscle tissues were stained control group with Cytochrome Oxidase. B: Muscle tissues were stained LPS group with Cytochrome Oxidase. C: Muscle tissues were stained Simvastatin group with Cytochrome Oxidase. D: Muscle tissues were stained Simvastatin + LPS group with Cytochrome Oxidase.
Figure 11.
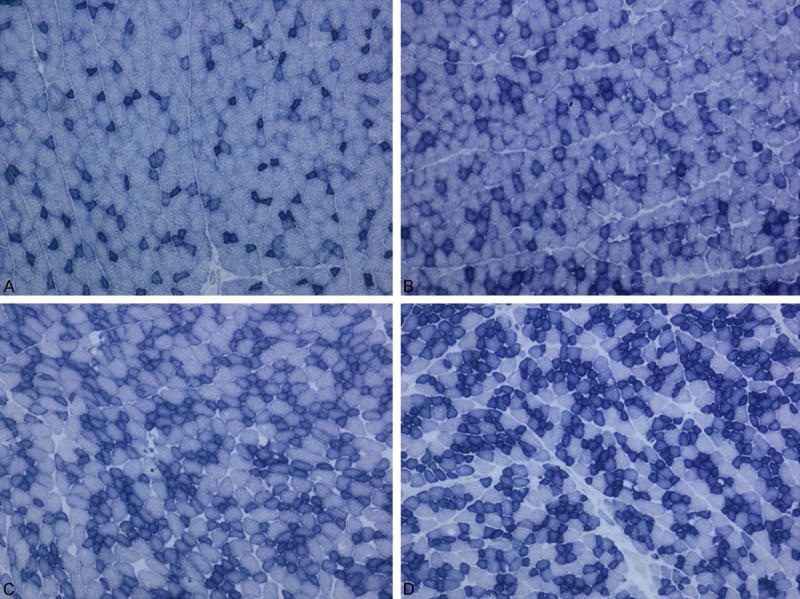
A: Muscle tissues were stained control group with Succinate Dehydrogenase. B: Muscle tissues were stained LPS group with Succinate Dehydrogenase. C: Muscle tissues were stained Simvastatin group with Succinate Dehydrogenase. D: Muscle tissues were stained Simvastatin + LPS group with Succinate Dehydrogenase.
Discussion
In our study, we searched whether it would be possible to induce sepsis by 20 mg/kg i.p. 4 hours LPS in rats and if this effect is reduced by prior simvastatin (20 mg/kg p.o. via oral gavage) treatment. The effects were tested on muscle tissue, which represents the largest organ affected by systemic inflammation. Mitochondrial structure, changes in cytokine levels, and enzyme activities in the first hours of sepsis in all groups were evaluated.
With this method, we observed that sepsis developed in the LPS treated animals and the effect of LPS was less by prior symvastatin treatmet. The elevated CK levels in all experimental groups evidenced involvement of muscle tissue.
We found that TNF-α and IL10 were higher than controls in the early phase of sespsis as well as in the pre-treated groups. While the increase in the proinflammatory cytokine TNF--α was more evident in the sepsis (LPS) group, increase in the anti-inflammatory cytokine IL-10 was more prominent in the pre-treated (SL) group. It has been reported that proinflammatory cytokines such as TNF-α and interleukin-6 cause loss of myofibrillary nitrogen and the destruction of proteins during sepsis [6,8,22]. Likewise, Almendro et al. demonstrated the increments of protein catabolism and DNA break-out that occurred in muscles of rats with inducing cecal ligation and puncture [23]. Our results were in agreement with the previous studies and showed that increase in cytokine levels were compatible with the effects of LPS and simvastatin.
In human and experimental animal models of sepsis, there are relationships between the severity of sepsis, mitochondrial damage and bioenergetic dysfunction [24]. Brealey et al. (2004) demonstrated that the analysis of muscle mitochondria may be an indicator of mitochondrial changes during long-term sepsis [25]. It has been reported that using statins causes mitochondrial dysfunction and alterations in both mitochondrial swelling and membrane potential [26]. However, reports on sepsis models show variable results in changes in mitochondrial enzyme activities [27-29]. While Brealey et al showed that complex I activity was at higher levels, Fredriksson et al. implicated the activities of citrate synthase and complex I when they reported a 53% and 60% decrease respectively, in intercostal muscle compared with controls in patients with sepsis [27]. This variability may depend on the variability of the method in inducing sepsis, and the variability in methods of measurement of the enzyme levels. We found alterations in the activities of complex-I, -II, -I-III, -II-III, Citrate synthase and COX.
Complex-I and citrate synthase activities were significantly higher in all experimental groups than those in the controls. As complex-I is the first enzyme in the electron transport system it may be possible that increase in its expression may be a response to the chemicals used, rather than being a sepsis response. This is supported by the explanation of Fredriksson et al. who showed increament in complex-I levels in muscle tissue of healthy volunteers was due to a response to acute endotoxin challenge [29]. In another study the group concluded that increased complex I activity is a compensator mechanism, which effects mitochondrial membrane potential [30].
In our study, COX (complex IV) activity in the control and LPS groups were significantly increased in comparison with the Simvastatin groups. Fredriksson et al. observed a decreased protein content of complex IV in patients with sepsis when compared with control subjects; this was an unexpected finding. The activity of complex IV was 30% lower in leg muscles of patients with sepsis but not in intercostal muscle. Morever, the activity of complex IV decreased to 30% in leg muscle [27]. In our study, we found higher activity of complex II in the LPS group than in the control and Simvastatin + LPS groups being more prominent in the LPS group. This suggested that the increase in complex II was due to sepsis. Our finding is consistent with the Larsen et al study who reported the simvastatin induced decrement of complex II was due to a reduction of mitochondrial volume [30]. Complex I + III activity was higher in the Simvastatin and Simvastatin + LPS groups than in the control and LPS groups. It has been shown that high levels of LPS leads to a 42% lower activity of complex I + III and a 23% reduction of pyruvate oxidation in the mitochondria of skeletal muscle [31]. We found that complex II + III activity was higher in the LPS group than the control group, and was lower in the Simvastatin and Simvastatin + LPS groups than in the control group. In agreement with our findings, Nadanaciva et al. reported that use of simvastatin or lovastatin causes mitochondrial toxicity and inhibits complex II + III activities by affecting uncoupled proteins [26]. It has been suggested that the use of statins reduces ubiquinone, which decreases activities of certain mitochondrial enzymes along with muscle damage in skeletal tissue [32,33].
Myopathic changes in muscle sections in the majority of animals in the LPS and Simvastatin groups were evident in our study. However, mitochondrial pathology in the muscle sections was very scarce. In this study we have shown that sepsis can be induced by a single dose of 20 mg/kg LPS i.p, the effects are less prominent with pre-treatment with 5 days oral simvastatin at 20 mg/kg/day. The effects at early phase of sepsis were evidenced by the profiles of changes in cytokines, and in activities of electron transport chain enzymes.
Acknowledgements
Our study was granted from Istanbul University Research Projects (Project No: 42019). We would like to thank Ozgun Chemical Company and Roche Diagnostics for analyzing biochemical parameters in autoanalyzer. We would like also thank Mr. David F Chapman for editing of English and Ms. Hatice Tasli for preparing of muscle section.
Disclosure of conflict of interest
None.
References
- 1.Balk RA. Severe sepsis and septic shock. Crit Care Clin. 2000;16:179–92. doi: 10.1016/s0749-0704(05)70106-8. [DOI] [PubMed] [Google Scholar]
- 2.Bolton CF. Sepsis and the systemic inflammatory response syndrome: neuromuscular manifestations. Crit Care Med. 1996;24:1408–16. doi: 10.1097/00003246-199608000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Larsson L, Li X, Edstrom L, Eriksson LI, Zackrisson H, Argentini C, Schiaffino S. Acute quadriplegia and loss of muscle myosin in patients treated with nondepolarizing neuromuscular blocking agents and corticosteroids: mechanisms at the cellular and molecular levels. Crit Care Med. 2000;28:34–45. doi: 10.1097/00003246-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Theologides A. Cancer cachexia. Cancer. 1979;43:2004–12. doi: 10.1002/1097-0142(197905)43:5+<2004::aid-cncr2820430708>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Evans RD, Argilés JM, Williamson DH. Metabolic effects of tumour necrosis factor-alpha (cachectin) and interleukin-1. Clin Sci. 1989;77:357. doi: 10.1042/cs0770357. [DOI] [PubMed] [Google Scholar]
- 6.Argilés JM, García-Martínez C, Llovera M, López-Soriano FJ. The role of cytokines in muscle wasting: its relation with cancer cachexia. Med Res Rev. 1992;12:637–52. doi: 10.1002/med.2610120605. [DOI] [PubMed] [Google Scholar]
- 7.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–45. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem. 1996;271:26690–97. doi: 10.1074/jbc.271.43.26690. [DOI] [PubMed] [Google Scholar]
- 9.Crouser ED, Julian MW, Blaho DV, Pfeiffer DR. Endotoxin-induced mitochondrial damage correlates with impaired respiratory activity. Crit Care Med. 2002;30:276–84. doi: 10.1097/00003246-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Pleiner J, Schaller G, Mittermayer F, Zorn S, Marsik C, Polterauer S, Kapiotis S, Wolzt M. Simvastatin prevents vascular hyporeactivity during inflammation. Circulation. 2004;110:3349–54. doi: 10.1161/01.CIR.0000147774.90396.ED. [DOI] [PubMed] [Google Scholar]
- 11.Gao F, Linhartova L, Johnston AM, Thickett DR. Statins and sepsis. Br J Anaesth. 2008;100:288–98. doi: 10.1093/bja/aem406. [DOI] [PubMed] [Google Scholar]
- 12.Tiwari A, Bansal V, Chugh A, Mookhtiar K. Statins and myotoxicity: atherapeutic limitation. Expert Opin Drug Saf. 2006;5:651–66. doi: 10.1517/14740338.5.5.651. [DOI] [PubMed] [Google Scholar]
- 13.Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–81. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Nezić L, Skrbić R, Dobrić S, Stojiljković MP, Satara SS, Milovanović ZA, Stojaković N. Effect of simvastatin on proinflammatory cytokines production during lipopolysaccharide-induced inflammation in rats. Gen Physiol Biophys. 2009;28:119–26. [PubMed] [Google Scholar]
- 15.Engel WK, Cunningham GG. Rapid examinaiıon of muscle tissue an improved trichrome method for fresh-frozen biopsy sections. Neurology. 1963;13:919–23. doi: 10.1212/wnl.13.11.919. [DOI] [PubMed] [Google Scholar]
- 16.Tanji K, Bonilla E. Light microscopic methods to visualize mitochondria on tissue sections. Methods. 2008;46:274–80. doi: 10.1016/j.ymeth.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 17.Srere PA. Citrate synthase. Methods Enzymol. 1969;13:3–11. [Google Scholar]
- 18.King TE, Howard RL. Preparation and properties of soluble NADH dehydrogenase from cardiac muscle. Methods Enzymol. 1967;10:275–94. [Google Scholar]
- 19.Scottocosa GL, Kuylenstierna B, Ernster L, Bergstrand A. An electron transport system associated with the outer membrane of the mitochondria. J Cell Biol. 1967;32:415–38. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King TE. Preparation of succinate dehydrogenase and reconstitution of succinate oxidase. Methods Enzymol. 1967;10:322–31. [Google Scholar]
- 21.Trounce IA, Kim YL, Jun AS, Wallace DC. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 1996;264:484–509. doi: 10.1016/s0076-6879(96)64044-0. [DOI] [PubMed] [Google Scholar]
- 22.Pajak B, Orzechowska S, Pijet B, Pijet M, Pogorzelska A, Gajkowska B, Orzechowski A. Crossroads of cytokine signaling: the chase to stop muscle cachexia. J Physiol Pharmacol. 2008;59:251–264. [PubMed] [Google Scholar]
- 23.Almendro V, Carbó N, Busquets S, Figueras M, Tessitore L, López-Soriano FJ, Argilés JM. Sepsis induces DNA fragmentation in rat skeletal muscle. Eur Cytokine Netw. 2003;14:256–9. [PubMed] [Google Scholar]
- 24.Zang Q, Maass DL, Tsai SJ, Horton JW. Cardiac mitochondrial damage and inflammation responses in sepsis. Surg Infect (Larchmt) 2007;8:41–54. doi: 10.1089/sur.2006.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brealey D, Karyampudi S, Jacques TS, Novelli M, Stidwill R, Taylor V, Smolenski RT, Singer M. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol. 2004;286:R491–7. doi: 10.1152/ajpregu.00432.2003. [DOI] [PubMed] [Google Scholar]
- 26.Nadanaciva S, Dykens JA, Bernal A, Capaldi RA, Will Y. Mitochondrial impairment by PPAR agonists and statins identified via immunocaptured OXPHOS complex activities and respiration. Toxicol Appl Pharmacol. 2007;223:277–87. doi: 10.1016/j.taap.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Fredriksson K, Hammarqvist F, Strigard K, Hultenby K, Ljungqvist O, Wernerman J, Rooyackers O. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis induced multiple organ failure. Am J Physiol Endocrinol Metab. 2006;291:E1044–50. doi: 10.1152/ajpendo.00218.2006. [DOI] [PubMed] [Google Scholar]
- 28.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–23. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 29.Fredriksson K, Fläring U, Guillet C, Wernerman J, Rooyackers O. Muscle mitochondrial activity increases rapidly after an endotoxin challenge in human volunteers. Acta Anaesthesiol Scand. 2009;53:299–304. doi: 10.1111/j.1399-6576.2008.01851.x. [DOI] [PubMed] [Google Scholar]
- 30.Larsen S, Stride N, Hey-Mogensen M, Hansen CN, Bang LE, Bundgaard H, Nielsen LB, Helge JW, Dela F. Simvastatin effects on skeletal muscle: relation to decreased mitochondrial function and glucose intolerance. J Am Coll Cardiol. 2013;61:44–53. doi: 10.1016/j.jacc.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 31.Trumbeckaite S, Opalka JR, Neuhof C, Zierz S, Gellerich FN. Different sensitivity of rabbit heart and skeletal muscle to endotoxin-induced impairment of mitochondrial function. Eur J Biochem. 2001;268:1422–29. doi: 10.1046/j.1432-1327.2001.02012.x. [DOI] [PubMed] [Google Scholar]
- 32.Brealey DA, Singer M, Terblanche M. Potential metabolic consequences of statins in sepsis. Crit Care Med. 2011;39:1514–20. doi: 10.1097/CCM.0b013e31820eb74f. [DOI] [PubMed] [Google Scholar]
- 33.Sirvent P, Mercier J, Vassort G, Lacampagne A. Simvastatin triggers mitochondria- induced Ca2+ signaling alteration in skeletal muscle. Biochem Biophys Res Commun. 2005;329:1067–75. doi: 10.1016/j.bbrc.2005.02.070. [DOI] [PubMed] [Google Scholar]