Abstract
This study was performed to investigate the expression of reactive oxygen species (ROS)-related proteins and to analyze the implications for primary and metastatic breast cancer. We constructed a tissue microarray containing 143 metastatic breast cancers (52 lung metastases, 38 bone metastases, 37 brain metastases, and 16 liver metastases) and performed immunohistochemical staining for ROS-related proteins (catalase, GSTπ, TxNIP, and MnSOD). Analysis of ROS-related protein expression in metastatic breast cancers according to the metastatic sites revealed site-specific expression patterns. The expression of tumoral catalase was lower in bone metastases (P = 0.012), and stromal GSTπ expression was higher in bone and liver metastases (P < 0.001). The highest ROS activation status was observed for lung metastases, while non-activated ROS was observed for bone metastases (P = 0.001). Primary cancers were positive for stromal GSTπ, but a subset of lung metastases were negative (P = 0.021). Univariate analysis revealed that shorter overall survival (OS) was associated with negative catalase expression of the tumor (P = 0.026). Furthermore, univariate analyses according to the metastatic sites revealed that shorter OS was associated with TxNIP-positive tumors (P = 0.032) and the expression of stromal catalase (P = 0.032) in brain metastases. Tumors that were negative for MnSOD expression (P < 0.001) but positive for stromal catalase expression (P = 0.022) were associated with shorter OS in patients with liver metastases. In conclusion, cancer cells and stromal tissues showed different ROS-related protein expression patterns according to the metastatic site. In addition, the expression of ROS-related proteins is associated with patient prognosis.
Keywords: Breast cancer, metastasis, reactive oxygen species
Introduction
Among all cancers, breast cancer has one of the highest rates of morbidity and mortality due to the occurrence of distant metastases. The major organs affected in metastatic breast cancer are the lung, brain, liver, and bone [1,2]. However, most of the existing studies are performed for metastases to the brain and bone [3-8]. Not all cancers show similar metastatic patterns, raising the seed and soil hypothesis, which states that a specific tumor (the seed) grows in a specific visceral organ (the soil) [9]. Metastatic breast cancer also displays specific characteristics depending on the metastatic site. According to previous studies, the characteristics associated with brain metastases include young age, estrogen receptor (ER) negativity, prior lung metastasis, human epidermal growth factor receptor 2 (HER-2) overexpression, epidermal growth factor receptor (EGFR) overexpression, and basal subtype [5-7]. In contrast, the characteristics associated with bone metastases are lower histologic grade, ER positivity, ER positivity/progesterone receptor (PR) negativity, strand growth pattern, and the presence of fibrotic foci in invasive ductal carcinoma [4,10,11]. Therefore, the fact that different metastatic cancers display different characteristics depending on the metastatic site is well supported.
Neoplastic cells differ from normal cells; the most significant difference is that they receive constant stimulation from growth promoting signals. The resulting infinite cell proliferation allows for different metabolic activities of the neoplastic cells compared to the normal cells. Specifically, these changes lead to accelerated cell proliferation and impose increased oxidative stress, which results in an alteration of the normal redox balance.
A previous study showed that the redox buffering systems such as the thioredoxin, glutathione, and antioxidant systems (for example catalase and superoxide dismutase) are either deregulated or overexpressed in tumors [12-14]. The altered redox process can lead to oncogenic transformation or mutations within the tumor. Consequently, the ROS pathway was recently investigated as a new possible target for therapy [15]. Because targeted therapy can be utilized not only for primary cancer but also for metastatic cancer, it is important to understand the ROS status of both primary and metastatic cancers. It has previously been shown that the ER, PR, and HER-2 status of primary and metastatic breast cancers can be discordant. Therefore, the relationship between the ROS status of primary and metastatic breast cancers needs to be investigated. To address this discrepancy, this study investigates the expression of ROS-related proteins and its implications in primary and metastatic breast cancers.
Materials and methods
Patient selection
Patients with invasive primary breast cancer and metastases to distant organs (lung, bone, brain, and liver) were selected using their medical records at the Department of Pathology of Severance Hospital. Only patients with a diagnosis of invasive ductal carcinoma were included. In total, 143 patients were identified, and for 38 of those patients samples of primary and metastatic cancers were available. All slides were reviewed and the resulting pathologic diagnoses were approved by 2 pathologists (JSK and WHJ). The histological grade was assessed based on the Nottingham grading system [16]. This study was approved by the Institutional Review Board.
Immunohistochemistry
The antibodies used for immunohistochemistry (IHC) in this study are shown in Table 1. Formalin-fixed, paraffin-embedded (FFPE) tissue samples were used as follows: 3 μm-thick slices from the FFPE tissue block were deparaffinized and rehydrated in xylene and alcohol solutions and stained using the Ventana Discovery XT automated stainer (Ventana Medical System, Tucson, AZ, USA). Antigen retrieval was performed with Cell Conditioning 1 buffer (citrate buffer pH 6.0, Ventana Medical Systems). Appropriate positive and negative controls were used.
Table 1.
Clone, dilution, and source of antibodies used
| Antibody | Clone | Dilution | Source |
|---|---|---|---|
| Catalase | Polyclonal | 1:600 | Abcam, Cambridge, UK |
| GSTπ | Polyclonal | 1:500 | Assay Design, Michican, USA |
| TxNIP | JY2 | 1:100 | MBL International Corporation, Woburn, USA |
| MnSOD | Polyclonal | 1:100 | Abcam, Cambridge, UK |
GSTπ, Glutathione S-transferase π; TxNIP, Thioredoxin interacting protein; MnSOD, Manganese superoxide dismutase.
Interpretation of immunohistochemical results
A cut-off value of 1% or more was used to define ER and androgen receptor positivity in positively stained nuclei [17]. The HER-2 staining was analyzed according to the American Society of Clinical Oncology/College of American Pathologists guidelines using the following categories: (0) = no immunostaining; (1+) = weak and/or incomplete membrane staining in less than 10% of tumor cells; (2+) = complete membrane staining that is either uniform or weak in at least 10% of tumor cells; and (3+) = uniform, intense membrane staining in at least 30% of tumor cells [18]. The HER-2 immunostaining was considered positive when a strong (3+) membrane staining was observed, whereas it was considered negative when none or weak (0 to 1+) staining was seen.
IHC result interpretation was based on the product of the proportion of stained cells and the immunohistochemical staining intensity. A product between 0 and 1 was regarded as negative, a product between 2 and 4 as a low positive, and a product between 5 and 6 as a high positive [19]. The proportion of stained cells was scored as 0 for negative, 1 for positive with less than 30% of the cells stained, and 2 for positive with greater than or equal to 30% of cells stained. The staining intensity was scored as 0 for negative, 1 for weak, 2 for moderate, and 3 for strong.
Statistical analysis
Data were statistically processed using SPSS for Windows, version 12.0 (SPSS Inc., Chicago, IL, USA). Correlation analysis of the immunostaining results between primary and metastatic breast cancer was performed using the McNemar test. Comparative statistics were performed using chi-square analysis. Statistical significance was assumed for P < 0.05. Kaplan-Meier survival curves and log-rank statistics were used to evaluate the time until tumor metastasis and the time of survival.
Results
Basal characteristics of patients
Our study included a total of 143 patients. Of these, 52 (36.4%) had lung metastases, 38 (26.6%) had bone metastases, 37 (25.9%) had brain metastases, and 16 (11.2%) had liver metastases. The proportion of patients that were ER-positive and PR-positive was higher among those with bone and liver metastases compared to patients with metastases to other sites (P < 0.001). The proportion of patients that were HER-2 positive was higher in patients with brain metastases compared to patients with metastases to other sites (P = 0.035). Furthermore, luminal A type breast cancer was more common among patients with bone and liver metastases, while triple negative breast cancer (TNBC) was more common among patients with brain and lung metastases (P = 0.010) (Table 2).
Table 2.
Basal clinicopathological characteristics of breast cancer metastases according to the metastatic sites
| Parameters | Total n = 143 (%) | Bone metastasis n = 38 (%) | Brain metastasis n = 37 (%) | Liver metastasis n = 16 (%) | Lung metastasis n = 52 (%) | P-value |
|---|---|---|---|---|---|---|
| Age (years) | 0.169 | |||||
| ≤ 50 | 75 (52.4) | 22 (57.9) | 17 (45.9) | 5 (31.2) | 31 (59.6) | |
| > 50 | 68 (47.6) | 16 (42.1) | 20 (54.1) | 11 (68.8) | 21 (40.4) | |
| ER | < 0.001 | |||||
| Negative | 66 (46.2) | 7 (18.4) | 26 (70.3) | 4 (25.0) | 29 (55.8) | |
| Positive | 77 (53.8) | 31 (81.6) | 11 (29.7) | 12 (75.0) | 23 (44.2) | |
| PR | < 0.001 | |||||
| Negative | 100 (69.9) | 21 (55.3) | 36 (97.3) | 7 (43.8) | 36 (69.2) | |
| Positive | 43 (30.1) | 17 (44.7) | 1 (2.7) | 9 (56.2) | 16 (30.8) | |
| HER-2 | 0.035 | |||||
| Negative | 99 (69.2) | 31 (81.6) | 19 (51.4) | 12 (75.0) | 37 (71.2) | |
| Positive | 44 (30.8) | 7 (18.4) | 18 (48.6) | 4 (25.0) | 15 (28.8) | |
| Molecular subtypes | < 0.001 | |||||
| Luminal A | 54 (37.8) | 27 (71.1) | 3 (8.1) | 9 (56.2) | 15 (28.8) | |
| Luminal B | 24 (16.8) | 5 (13.2) | 8 (21.6) | 3 (18.8) | 8 (15.4) | |
| HER-2 | 29 (20.3) | 4 (10.5) | 12 (32.4) | 3 (18.8) | 10 (19.2) | |
| NBC | 36 (25.2) | 2 (5.3) | 14 (37.8) | 1 (6.2) | 19 (36.5) | |
| Patient death | 48 (33.6) | 21 (55.3) | 11 (29.7) | 4 (25.0) | 12 (23.1) | 0.010 |
ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2; TNBC, triple negative breast cancer.
Expression of ROS-related proteins in breast cancer metastasis depending on the metastatic site
The analysis of ROS-related protein expression depending on the metastatic site in metastatic breast cancer revealed site-specific expression patterns (Figure 1). The expression of catalase in tumor cells was lower in bone metastases (P = 0.012), and the expression of stromal glutathione S-transferase π (GSTπ) was higher in bone and liver metastases (P < 0.001). The ROS status defined by the expression of ROS-related proteins differed depending on the metastatic site; the highest ROS activation status was observed for lung metastases, while non-activated ROS was observed in cancer cells derived from bone metastases (P = 0.001) (Table 3).
Figure 1.
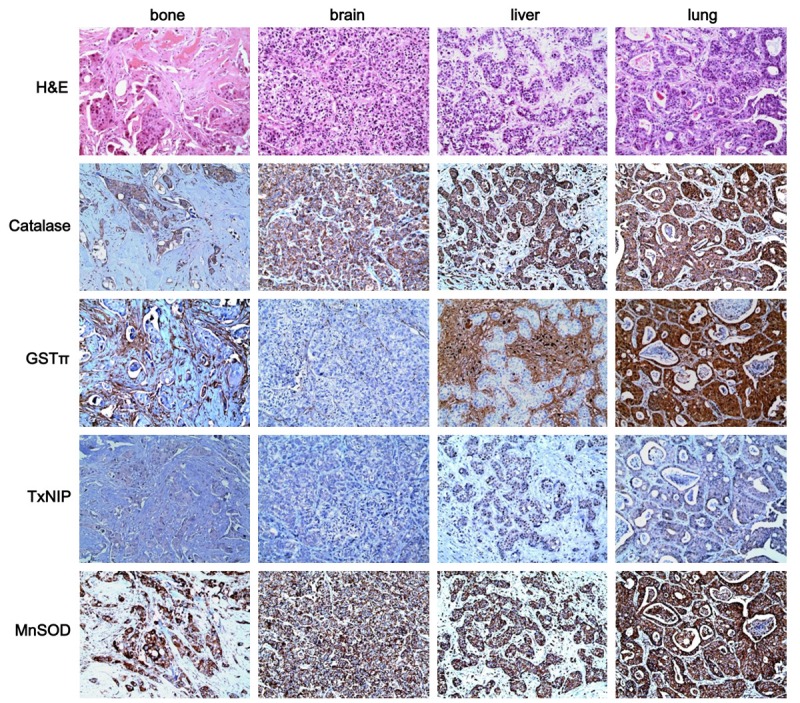
Expression of ROS-related proteins in metastatic breast cancers according to the metastatic sites. The expression of tumoral catalase was lower in bone metastasis, and that of stromal GSTπ was higher in bone and liver metastasis.
Table 3.
Expression of ROS-related proteins in the tumor cell compartment of breast cancer metastasis according to the metastatic sites
| Parameters | Total n = 143 (%) | Bone metastasis n = 38 (%) | Brain metastasis n = 37 (%) | Liver metastasis n = 16 (%) | Lung metastasis n = 52 (%) | P-value |
|---|---|---|---|---|---|---|
| Cancer cell compartment | ||||||
| Catalase | 0.012 | |||||
| Negative | 75 (52.4) | 27 (71.1) | 21 (56.8) | 8 (50.0) | 19 (52.4) | |
| Positive | 68 (47.6) | 11 (28.9) | 16 (43.2) | 8 (50.0) | 68 (47.6) | |
| GSTπ | 0.051 | |||||
| Negative | 102 (71.3) | 31 (81.6) | 28 (75.7) | 13 (81.2) | 30 (57.7) | |
| Positive | 41 (28.7) | 7 (18.4) | 9 (24.3) | 3 (18.8) | 22 (42.3) | |
| TxNIP | 0.126 | |||||
| Negative | 126 (88.1) | 34 (89.5) | 36 (97.3) | 14 (87.5) | 42 (80.8) | |
| Positive | 17 (11.9) | 4 (10.5) | 1 (2.7) | 2 (12.5) | 10 (19.2) | |
| MnSOD | 0.198 | |||||
| Negative | 5 (3.5) | 1 (2.6) | 3 (8.1) | 1 (6.2) | 0 (0.0) | |
| Positive | 138 (96.5) | 37 (97.4) | 34 (91.9) | 15 (93.8) | 52 (100.0) | |
| ROS status | 0.001 | |||||
| Non-activated | 113 (79.0) | 35 (92.1) | 32 (86.5) | 14 (87.5) | 32 (61.5) | |
| Activated | 30 (21.0) | 3 (7.9) | 5 (13.5) | 2 (12.5) | 20 (38.5) | |
| Stromal cell compartment | ||||||
| Catalase | 0.080 | |||||
| Negative | 130 (90.9) | 31 (81.6) | 36 (97.3) | 14 (87.5) | 49 (94.2) | |
| Positive | 13 (9.1) | 7 (18.4) | 1 (2.7) | 2 (12.5) | 3 (5.8) | |
| GST π | < 0.001 | |||||
| Negative | 99 (69.2) | 21 (55.3) | 32 (86.5) | 4 (25.0) | 42 (80.8) | |
| Positive | 44 (30.8) | 17 (44.7) | 5 (13.5) | 12 (75.0) | 10 (19.2) | |
| TxNIP | 0.623 | |||||
| Negative | 142 (99.3) | 38 (100.0) | 37 (100.0) | 16 (100.0) | 51 (98.1) | |
| Positive | 1 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.9) | |
| MnSOD | 0.110 | |||||
| Negative | 118 (82.5) | 30 (78.9) | 34 (91.9) | 15 (93.8) | 39 (75.0) | |
| Positive | 25 (17.5) | 8 (21.1) | 3 (8.1) | 1 (6.2) | 13 (25.0) | |
| ROS status | 0.426 | |||||
| Non-activated | 136 (95.1) | 35 (92.1) | 37 (100.0) | 15 (93.8) | 49 (94.2) | |
| Activated | 7 (4.9) | 3 (7.9) | 0 (0.0) | 1 (6.2) | 3 (5.8) | |
GSTπ, Glutathione S-transferase π; TxNIP, Thioredoxin interacting protein; MnSOD, Manganese superoxide dismutase; ROS, Reactive oxygen species.
Correlation of expression of ROS-related proteins between primary and metastatic breast cancer depending on the metastatic site
We analyzed the expression levels of ROS-related proteins in primary and metastatic cancers in 38 patients from whom both samples were available, and found that the expression levels were not different. However, in metastatic cancer patients, primary cancers were positive for stromal GSTπ, while a subset of lung metastases were negative (P = 0.021) (Table 4).
Table 4.
Correlation of ROS-related protein expression in cancer cells between primary and metastatic breast cancers with respect to the metastatic sites
| Parameters | Total | Bone metastasis | Brain metastasis | Liver metastasis | Lung metastasis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| n = 38 (%) | P-value | n = 7 (%) | P-value | n = 7 (%) | P-value | n = 3 (%) | P-value | n = 21 (%) | P-value | |
| Cancer cell compartment | ||||||||||
| Catalase | 0.118 | 1.000 | 0.500 | 1.000 | 0.453 | |||||
| (+) → (+) | 8 (21.1) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 7 (33.3) | |||||
| (+) → (-) | 5 (13.2) | 2 (28.6) | 0 (0.0) | 0 (0.0) | 3 (14.3) | |||||
| (-) → (+) | 11 (28.9) | 3 (42.9) | 2 (28.6) | 1 (33.3) | 5 (23.8) | |||||
| (-) → (-) | 14 (36.8) | 2 (28.6) | 5 (71.4) | 1 (33.3) | 6 (28.6) | |||||
| GSTπ | 0.453 | 1.000 | 1.000 | 1.000 | 0.375 | |||||
| (+) → (+) | 2 (5.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (9.1) | |||||
| (+) → (-) | 2 (5.1) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 1 (4.5) | |||||
| (-) → (+) | 5 (12.8) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 4 (18.2) | |||||
| (-) → (-) | 30 (76.9) | 6 (85.7) | 6 (85.7) | 3 (100.0) | 15 (68.2) | |||||
| TxNIP | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | |||||
| (+) → (+) | 2 (5.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (9.1) | |||||
| (+) → (-) | 3 (7.7) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 2 (9.1) | |||||
| (-) → (+) | 3 (7.7) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 2 (9.1) | |||||
| (-) → (-) | 31 (79.5) | 5 (71.4) | 7 (100.0) | 3 (100.0) | 16 (72.7) | |||||
| MnSOD | 1.000 | 1.000 | 0.500 | 1.000 | 0.500 | |||||
| (+) → (+) | 34 (87.2) | 6 (85.7) | 5 (71.4) | 3 (100.0) | 20 (90.9) | |||||
| (+) → (-) | 2 (5.1) | 0 (0.0) | 2 (28.6) | 0 (0.0) | 0 (0.0) | |||||
| (-) → (+) | 3 (7.7) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 2 (9.1) | |||||
| (-) → (-) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| Stromal cell compartment | ||||||||||
| Catalase | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | |||||
| (+) → (+) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| (+) → (-) | 5 (12.8) | 1 (14.3) | 1 (33.3) | 1 (33.3) | 2 (9.1) | |||||
| (-) → (+) | 4 (10.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (13.6) | |||||
| (-) → (-) | 30 (76.9) | 6 (85.7) | 2 (66.7) | 2 (66.7) | 17 (77.3) | |||||
| GSTπ | 0.096 | 1.000 | 1.000 | 1.000 | 0.021 | |||||
| (+) → (+) | 5 (12.8) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 4 (18.2) | |||||
| (+) → (-) | 13 (33.3) | 2 (28.6) | 2 (28.6) | 0 (0.0) | 9 (40.9) | |||||
| (-) → (+) | 5 (12.8) | 2 (28.6) | 1 (14.3) | 1 (33.3) | 1 (4.5) | |||||
| (-) → (-) | 16 (41.0) | 3 (42.9) | 4 (57.1) | 1 (33.3) | 8 (36.4) | |||||
| TxNIP | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | |||||
| (+) → (+) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| (+) → (-) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| (-) → (+) | 1 (2.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.5) | |||||
| (-) → (-) | 38 (97.4) | 7 (100.0) | 7 (100.0) | 3 (100.0) | 21 (95.5) | |||||
| MnSOD | 1.000 | 1.000 | 1.000 | 1.000 | 0.688 | |||||
| (+) → (+) | 3 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (13.6) | |||||
| (+) → (-) | 5 (12.8) | 1 (14.3) | 2 (28.6) | 0 (0.0) | 2 (9.1) | |||||
| (-) → (+) | 6 (15.4) | 1 (14.3) | 1 (14.3) | 0 (0.0) | 4 (18.2) | |||||
| (-) → (-) | 25 (64.1) | 5 (71.4) | 4 (57.1) | 3 (100.0) | 13 (59.1) | |||||
GSTπ, Glutathione S-transferase π; TxNIP, Thioredoxin interacting protein; MnSOD, Manganese superoxide dismutase.
The impact of autophagy-related protein expression on patient prognosis
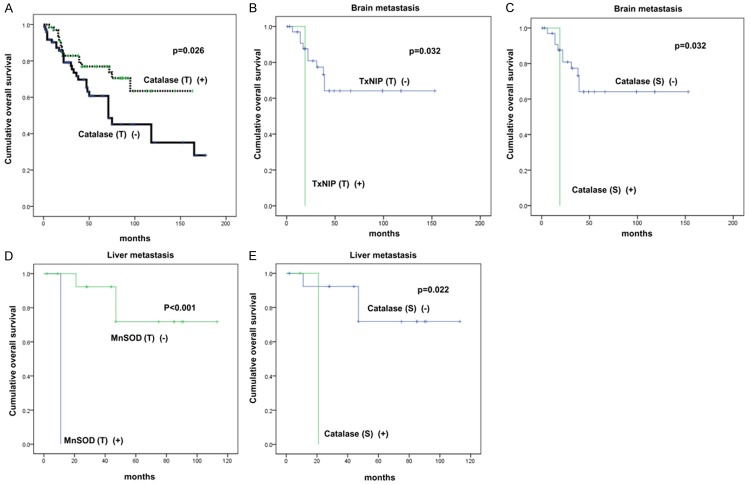
Using univariate analysis, we analyzed the association of ROS-related protein expression with respect to patient prognosis (Table 5 and Figure 2). We found that the factor associated with shorter overall survival (OS) was a tumor with negative catalase expression (P = 0.026). Using univariate analysis with respect to the metastatic site, we found that the factors associated with shorter OS in patients with brain metastases were tumors with positive Thioredoxin Interacting Protein (TxNIP) staining (P = 0.032) and stromal catalase expression (P = 0.032). Tumors with negative manganese superoxide dismutase (MnSOD) staining (P < 0.001) and positive stromal catalase expression (P = 0.022) were associated with shorter OS in patients with liver metastases.
Table 5.
Univariate analysis of the impact of ROS-related protein expression in metastatic breast cancers on overall survival using the log-rank test
| Parameters | Total n = 143 (%) | Bone metastasis n = 38 (%) | Brain metastasis n = 7 (%) | Liver metastasis n = 16 (%) | Lung metastasis n = 52 (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Mean survival(95% CI) months | P-value | Mean survival(95% CI) months | P-value | Mean survival(95% CI) months | P-value | Mean survival(95% CI) months | P-value | Mean survival(95% CI) months | P-value | |
| Cancer cell compartment | ||||||||||
| Catalase | 0.026 | 0.940 | 0.390 | 0.940 | 0.139 | |||||
| Negative | 94 (75-114) | 81 (57-105) | 96 (64-128) | 66 (47-84) | 111 (68-153) | |||||
| Positive | 120 (102-137) | 60 (39-82) | 87 (62-112) | 85 (52-117) | 124 (95-153) | |||||
| GSTπ | 0.177 | 0.653 | 0.659 | 0.248 | 0.052 | |||||
| Negative | 116 (100-133) | 87 (62-113) | 105 (79-132) | 91 (70-112) | 144 (117-171) | |||||
| Positive | 99 (72-126) | 73 (29-116) | 75 (39-111) | 22 (13-31) | 90 (35-145) | |||||
| TxNIP | 0.383 | 0.467 | 0.032 | n/a | 0.061 | |||||
| Negative | 114 (99-129) | 80 (58-102) | 107 (84-130) | n/a | 146 (124-167) | |||||
| Positive | 67 (48-85) | 79 (73-85) | 19 (19-19) | n/a | 66 (42-90) | |||||
| MnSOD | 0.970 | 0.171 | n/a | <0.001 | n/a | |||||
| Negative | 126 (34-218) | 165 (165-165) | n/a | 11 (11-11) | n/a | |||||
| Positive | 110 (96-125) | 71 (56-86) | n/a | 92 (72-112) | n/a | |||||
| ROS status | 0.340 | 0.499 | 0.427 | n/a | 0.306 | |||||
| Non-activated | 108 (91-124) | 80 (58-102) | 99 (72-125) | n/a | 142 (117-167) | |||||
| Activated | 88 (72-104) | 79 (73-85) | 98 (63-132) | n/a | 72 (55-90) | |||||
| Stromal cell compartment | ||||||||||
| Catalase | 0.140 | 0.094 | 0.032 | 0.022 | n/a | |||||
| Negative | 112 (97-127) | 87 (65-110) | 107 (84-130) | 91 (70-112) | n/a | |||||
| Positive | 91 (49-134) | 37 (15-60) | 19 (19-19) | 21 (21-21) | n/a | |||||
| GSTπ | 0.551 | 0.317 | 0.824 | n/a | 0.215 | |||||
| Negative | 114 (97-131) | 82 (65-100) | 105 (81-128) | n/a | 126 (100-152) | |||||
| Positive | 100 (74-126) | 76 (37-116) | 72 (29-115) | n/a | 130 (81-153) | |||||
| TxNIP | n/a | n/a | n/a | n/a | n/a | |||||
| Negative | n/a | n/a | n/a | n/a | n/a | |||||
| Positive | n/a | n/a | n/a | n/a | n/a | |||||
| MnSOD | 0.466 | 0.687 | n/a | n/a | 0.968 | |||||
| Negative | 108 (92-123) | 83 (60-106) | n/a | n/a | 130 (103-158) | |||||
| Positive | 120 (90-151) | 50 (31-69) | n/a | n/a | 123 (84-163) | |||||
| ROS status | 0.716 | 0.264 | n/a | n/a | n/a | |||||
| Non-activated | 109 (94-124) | 84 (62-105) | n/a | n/a | n/a | |||||
| Activated | 117 (62-173) | 32 (0-65) | n/a | n/a | n/a | |||||
GSTπ, Glutathione S-transferase π; TxNIP, Thioredoxin interacting protein; MnSOD, Manganese superoxide dismutase; ROS, Reactive oxygen species.
Figure 2.
The impact of autophagy-related proteins on patient prognosis in metastatic breast cancer (A), brain metastasis (B, C), and liver metastasis (D, E).
Discussion
In this study, differences in the expression of ROS-related proteins were observed among patients with metastatic breast cancer depending on the metastatic site. Our analysis revealed site-specific expression patterns of catalase in tumor cells, with lower expression levels in patients with bone metastases (P = 0.012). Furthermore, higher expression levels of stromal GSTπ were observed in patients with bone and liver metastases (P < 0.001). A comparison based on previous studies was not possible due to the lack of ROS-related protein expression data of metastatic breast cancers. ROS is widely known to play an important role in tumor metastasis [20]. ROS promotes metastasis by activating the signaling pathway that is involved in tumor metastasis. Mechanistically, ROS activates key signal transduction proteins through oxidative posttranslational modifications of redox-sensitive proteins [21]. Therefore, metastatic cancers are expected to show an increase in the expression of ROS-related proteins. In this study, the expression of ROS-related proteins differed depending on the metastatic site. Previous studies reported different expression patterns of the most important biomarkers of breast cancer (ER, PR, and HER-2) according to the metastatic site. Most brain metastases are TNBC or HER-2 positive [6], whereas most bone metastases are hormone receptor positive [4]. These results were in agreement with our study. The proportion of hormone receptor positive cancer was high in liver metastases, and the proportion of TNBC was high in lung metastases. Our study showed different biological characteristics depending on the metastatic site. Previous studies showed a reverse correlation between ER and ROS. Specifically, the suppression of ER resulted in increased ROS production in breast cancer cells [22]. In this study, patients with bone metastases showed a high proportion of ER positivity and non-activated ROS status, while patients with lung metastases showed a low proportion of ER positivity and activated ROS status. Thus, the difference of ROS status according to the metastatic site could be the result of the hormone receptor status. Further research is required to substantiate this hypothesis.
Stromal tissues showed different expression patterns of ROS-related proteins; stromal GSTπ showed higher expression levels in bone and liver metastases. The possible theory, which can explain the expression of ROS-related proteins in stromal tissue, is the reverse-Warburg effect theory. According to this theory, cancer cells generate ROS (such as nitric oxide), which can cause oxidative stress and trigger glycolysis, autophagy (mitophagy), and mitochondrial dysfunction through NFκB and HIF-1α in stromal cells. Ketone bodies and lactate are formed through glycolysis in stromal cells that enter the cancer cells and produce ATP effectively through oxidative phosphorylation in the mitochondria of the cancer cells, which as a whole contributes to their survival and growth [23-27]. According to the reverse Warburg effect theory, to maintain the metabolic support of cancer cells, stromal cells should withstand the oxidative stress caused by ROS secreted from cancer cells. If detoxifying enzymes such as GSTπ are expressed at high levels, stromal cells could maintain the metabolic support of cancer cells under such oxidative stress environments. Previous studies reported that the reverse Warburg effect occurs in a high proportion of hormone receptor-positive luminal type breast cancers [28]. In this study, bone and liver metastases, of which a high proportion was of the luminal type, showed high stromal GSTπ expression. This can be because most bone and liver metastases display a reverse Warburg effect. However, further validation is required to generalize the findings of this study.
The clinical significance of this study is that ROS regulators may be a treatment modality. Previous studies reported that the substance increasing ROS caused ROS-induced apoptosis in cancer cells, while normal cells were not affected [29,30]. However, the substance decreasing ROS accelerated tumor growth [31]. Therefore, ROS modulators can be considered as potential therapeutic agents that cause cancer cell death by further increasing ROS levels in targeted metastatic cancers with already increased ROS levels. However, more research is required to validate this hypothesis.
In conclusion, our study showed different expression patterns of ROS-related proteins in cancer cells and stromal tissues in metastatic breast cancers depending on the metastatic site. The expression of catalase was lower in bone metastases and that of stromal GSTπ was higher in bone and liver metastases.
Acknowledgements
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1420080).
Disclosure of conflict of interest
None.
References
- 1.Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005;167:913–920. doi: 10.1016/S0002-9440(10)61180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80:1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Abali H, Celik I. High incidence of central nervous system involvement in patients with breast cancer treated with epirubicin and docetaxel. Am J Clin Oncol. 2002;25:632–633. doi: 10.1097/00000421-200212000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Colleoni M, O’Neill A, Goldhirsch A, Gelber RD, Bonetti M, Thurlimann B, Price KN, Castiglione-Gertsch M, Coates AS, Lindtner J, Collins J, Senn HJ, Cavalli F, Forbes J, Gudgeon A, Simoncini E, Cortes-Funes H, Veronesi A, Fey M, Rudenstam CM. Identifying breast cancer patients at high risk for bone metastases. J. Clin. Oncol. 2000;18:3925–3935. doi: 10.1200/JCO.2000.18.23.3925. [DOI] [PubMed] [Google Scholar]
- 5.Evans AJ, James JJ, Cornford EJ, Chan SY, Burrell HC, Pinder SE, Gutteridge E, Robertson JF, Hornbuckle J, Cheung KL. Brain metastases from breast cancer: identification of a high-risk group. Clin Oncol (R Coll Radiol) 2004;16:345–349. doi: 10.1016/j.clon.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Gaedcke J, Traub F, Milde S, Wilkens L, Stan A, Ostertag H, Christgen M, von Wasielewski R, Kreipe HH. Predominance of the basal type and HER-2/neu type in brain metastasis from breast cancer. Mod Pathol. 2007;20:864–870. doi: 10.1038/modpathol.3800830. [DOI] [PubMed] [Google Scholar]
- 7.Hicks DG, Short SM, Prescott NL, Tarr SM, Coleman KA, Yoder BJ, Crowe JP, Choueiri TK, Dawson AE, Budd GT, Tubbs RR, Casey G, Weil RJ. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am J Surg Pathol. 2006;30:1097–1104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 8.Lorincz T, Toth J, Badalian G, Timar J, Szendroi M. HER-2/neu genotype of breast cancer may change in bone metastasis. Pathol Oncol Res. 2006;12:149–152. doi: 10.1007/BF02893361. [DOI] [PubMed] [Google Scholar]
- 9.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–572. [PubMed] [Google Scholar]
- 10.Hasebe T, Imoto S, Yokose T, Ishii G, Iwasaki M, Wada N. Histopathologic factors significantly associated with initial organ-specific metastasis by invasive ductal carcinoma of the breast: a prospective study. Hum Pathol. 2008;39:681–693. doi: 10.1016/j.humpath.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Wei B, Wang J, Bourne P, Yang Q, Hicks D, Bu H, Tang P. Bone metastasis is strongly associated with estrogen receptor-positive/progesterone receptor-negative breast carcinomas. Hum Pathol. 2008;39:1809–1815. doi: 10.1016/j.humpath.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Pennington JD, Wang TJ, Nguyen P, Sun L, Bisht K, Smart D, Gius D. Redox-sensitive signaling factors as a novel molecular targets for cancer therapy. Drug Resist Updat. 2005;8:322–330. doi: 10.1016/j.drup.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Woolston CM, Deen S, Al-Attar A, Shehata M, Chan SY, Martin SG. Redox protein expression predicts progression-free and overall survival in ovarian cancer patients treated with platinum-based chemotherapy. Free Radic Biol Med. 2010;49:1263–1272. doi: 10.1016/j.freeradbiomed.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 16.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 17.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 19.Won KY, Kim GY, Kim YW, Song JY, Lim SJ. Clinicopathologic correlation of beclin-1 and bcl-2 expression in human breast cancer. Hum Pathol. 2010;41:107–112. doi: 10.1016/j.humpath.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Lei Y, Huang K, Gao C, Lau QC, Pan H, Xie K, Li J, Liu R, Zhang T, Xie N, Nai HS, Wu H, Dong Q, Zhao X, Nice EC, Huang C, Wei Y. Proteomics identification of ITGB3 as a key regulator in reactive oxygen species-induced migration and invasion of colorectal cancer cells. Mol Cell Proteomics. 2011;10:M110.005397. doi: 10.1074/mcp.M110.005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang W, Zou L, Huang C, Lei Y. Redox regulation of cancer metastasis: molecular signaling and therapeutic opportunities. Drug Dev Res. 2014;75:331–341. doi: 10.1002/ddr.21216. [DOI] [PubMed] [Google Scholar]
- 22.Cook KL, Clarke PA, Parmar J, Hu R, Schwartz-Roberts JL, Abu-Asab M, Warri A, Baumann WT, Clarke R. Knockdown of estrogen receptor-alpha induces autophagy and inhibits antiestrogen-mediated unfolded protein response activation, promoting ROS-induced breast cancer cell death. FASEB J. 2014 doi: 10.1096/fj.13-247353. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, Frank PG, Flomenberg N, Howell A, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Ketones and lactate “fuel” tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9:3506–3514. doi: 10.4161/cc.9.17.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, Whitaker-Menezes D, Daumer KM, Lin Z, Witkiewicz AK, Flomenberg N, Howell A, Pestell RG, Knudsen ES, Sotgia F, Lisanti MP. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9:3256–3276. doi: 10.4161/cc.9.16.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Loss of stromal caveolin-1 leads to oxidative stress, mimics hypoxia and drives inflammation in the tumor microenvironment, conferring the “reverse Warburg effect”: a transcriptional informatics analysis with validation. Cell Cycle. 2010;9:2201–2219. doi: 10.4161/cc.9.11.11848. [DOI] [PubMed] [Google Scholar]
- 26.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 27.Witkiewicz AK, Whitaker-Menezes D, Dasgupta A, Philp NJ, Lin Z, Gandara R, Sneddon S, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Using the “reverse Warburg effect” to identify high-risk breast cancer patients: stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell Cycle. 2012;11:1108–1117. doi: 10.4161/cc.11.6.19530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi J, Kim do H, Jung WH, Koo JS. Metabolic interaction between cancer cells and stromal cells according to breast cancer molecular subtype. Breast Cancer Res. 2013;15:R78. doi: 10.1186/bcr3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, Stern AM, Mandinova A, Schreiber SL, Lee SW. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Wu XJ, Hua X. Targeting ROS: selective killing of cancer cells by a cruciferous vegetable derived pro-oxidant compound. Cancer Biol Ther. 2007;6:646–647. doi: 10.4161/cbt.6.5.4092. [DOI] [PubMed] [Google Scholar]
- 31.Salganik RI. The benefits and hazards of antioxidants: controlling apoptosis and other protective mechanisms in cancer patients and the human population. J Am Coll Nutr. 2001;20:464S–472S. doi: 10.1080/07315724.2001.10719185. discussion 473S-475S. [DOI] [PubMed] [Google Scholar]