Abstract
Carcinoma of unknown primary (CUP) is a heterogeneous group of tumors with various clinical features causing diagnostic and therapeutic challenges. The aim of this study was to evaluate the ability of F-18 FDG PET/CT for localizing the primary tumor, disclosing additional metastases, and changing the treatment in patients with CUP. One hundred and twelve metastatic patients (female = 40, male = 72, median age = 60.5 years) in whom conventional diagnostic work-up failed to disclose the primary tumor were included in the study. F-18 FDG PET/CT imaging was performed in a standard protocol (patient supine, arms on patient’s side, vertex to thigh, 369.3 MBq (296-444 MBq) F-18 FDG, a 60-minute uptake period, 6-7 bed position). Histopathology was taken as the only reference standard. F-18 FDG PET/CT correctly detected primary tumor in 37 of 112 (33.03%) patients. The most common site of primary tumor detected by F-18 FDG PET/CT was lung (n = 18), which was followed by nasopharynx (n = 7), pancreas (n = 5), tonsil (n = 2), breast (n = 2), thyroid (n = 1), uterus (n = 1) and colon/rectum (n = 1). F-18 FDG PET/CT imaging disclosed additional previously undetected metastases in 32 (28.5%) and changed the treatment in 33 (29.4%) of 112 patients. There were false positive F-18 FDG PET/CT results in 21 (18.5%) patients. F-18 FDG PET/CT is able to disclose the primary tumor, disclose new metatases and change the treatment in about one third of patients with CUP.
Keywords: Carcinoma of unknown primary, F-18 FDG PET/CT, metastases, treatment change
Introduction
Carcinoma of unknown primary (CUP) is a group of malignant diseases in which the primary tumor cannot be disclosed by conventional diagnostic tools, and in a majorty of cases, even at autopsy [1]. CUP accounts for approximately 2% of all new cancer diagnoses, and most registries place it within the top 10 malignancies-usually 7th or 8th most common (more common than non-Hodgkin lymphoma) [2]. It represents a group of heterogeneous tumors with varying clinical features with respect to histopathology, biological nature and clinical characteristics. The prognosis is generally poor, and only approximately 50% of patients survive longer than 12 months [3]. The routine diagnostic work-up of a patient with CUP typically includes careful physical examination and anatomic imaging including computed tomography (CT) and magnetic resonance (MR) imaging, and endoscopic techniques.
F-18 FDG PET/CT may be a useful tool for diagnostic work-up of carcinoma of unknown primary (CUP) [4]. Despite its advantages over conventional anatomical imaging methods, F-18 FDG PET/CT is reimbursed only in a limited number of indications associated with CUP. The reluctancy for reimbursement is based on the argument that the number of patients employed in the clinical validation studies is small; the number of patients in the vast majority of the reported studies is smaller than 100 [5-10]. The aim of this study was to evaluate the ability of F-18 FDG PET/CT to detect the occult primary tumor and new metastases and to investigate its role in changing the treatment in a larger number of patients with CUP.
Material and methods
Patients
Between October 2009 and May 2014, 112 patients (female = 40, male = 72; median age = 60.5, age range = 24-90 years) with metastases from an unknown primary tumor were included in this retrospective study. This retrospective study was approved by the institutional review board at our institution. The patients’ files with initial diagnosis of cancer of unknown primary were retrieved from the archive. Those patients who underwent F-18 FDG PET/CT were included in the preliminary list. Among them, the patients without a histopathological diagnosis of malignancy through biopsy performed at least at one site were excluded from this study. Only the patients with a definite histopathological diagnosis and who survived long enough to be investigated thorougly by relevant imaging and laboratory tests were included in the study. Prior to F-18 FDG PET/CT imaging, all patients had metastatic disease, which was histopathologically confirmed, but, detailed physical examination, routine laboratory tests including serum tumor marker measurements, conventional diagnostic imaging procedures chest X-ray, abdominal ultrasonography, CT, magnetic resonance imaging, mammography and endoscopic procedures failed to identify the primary site.
F-18 FDG PET/CT scan
F-18 FDG PET/CT scans were performed with an integrated PET/CT scanner (Biograph 2, Siemens Medical Solutions, Knoxville, Tenn). Patients fasted for at least 6 hours before the intravenous administration of F18-FDG, and each patient’s blood glucose level was measured before tracer injection. Whole-body images were obtained approximately 60 minutes after intravenous injection of 369,3 MBq (296-444 MBq) F-18 FDG in a standard protocol (patient supine, arms on patient’s side, vertex to thigh, 6-7 bed position). Non-contrast-enhanced CT scanning was performed with the following settings: Peak x-ray tube voltage, 80 kV, current, 61 eff. mAs, slice width, 5.0 mm. Both PET and CT scans were performed with patients under normal tidal breathing. Attenuation-corrected PET images were reconstructed with an ordered subset expectation maximization iterative reconstruction algorithm. PET images and CT images were fused and displayed on a workstation.
Image analysis
F-18 FDG PET, CT and fusion F-18 FDG PET/CT images were examined in axial, coronal and sagittal planes on the manufacturer’s review station. On a transaxial, attenuation-corrected PET image, the maximum standardized uptake values (SUVmax) were obtained by placing regions of interest (ROIs) on the lesions that had been identified on visual analysis. All F-18 FDG PET/CT images were evaluated by two nuclear physicians. The presence, number, size, SUVmax, character, and precise location of suspected malignancy were recorded, and the number and the sites of metastases were compared with the known number and sites of metastases prior to F-18 FDG PET/CT imaging for each patient.
Results
The histopathology findings and the sites of metastases are summarized in Table 1.
Table 1.
Characteristics of Patients by Localization and Histopathology of Metastases at Initial Presentation
| Localization of Metastases | Squamous Cell Carcinoma | Adenocarcinoma (Differentiation) | Anaplastic | Undefined | Total | |
|---|---|---|---|---|---|---|
|
| ||||||
| Well to Moderately Differentiated | Poorly Differentiated | |||||
| Cervical LN Met. | 12 (10.7%) | 10 (8.9%) | 8 (7.1%) | 3 (2.6%) | 7 (6.2%) | 40 (35.7%) |
| Non-cervical LN Met. | 4 (3.5%) | 12 (10.7%) | 9 (8.0%) | 3 (2.6%) | 5 (4.4%) | 33 (29.4%) |
| Skeletal Metastases | 1 (0.8%) | 5 (4.4%) | 3 (2.6%) | 4 (3.5%) | 2 (1.7%) | 15 (13.3%) |
| Lung Metastases | 2 (1.7%) | 4 (3.5%) | 3 (2.6%) | 1 (0.8%) | 3 (2.6%) | 13 (11.6%) |
| Liver Metastases | 4 (3.5%) | 2 (1.7%) | 1 (0.8%) | 1 (0.8%) | 8 (7.1%) | |
| Other Met. | 1 (0.8%) | 1 (0.8%) | 1 (0.8%) | 3 (2.6%) | ||
Primary tumors and metastases
F-18 FDG PET/CT disclosed the primary tumor in 37 of 112 patients (33.03%, 37 of 112). Of these, 18 primaries were localized in the lungs, 7 in nasopharynx, 5 in pancreas, 2 in tonsil, 2 in breast, 1 in thyroid, 1 in uterus and 1 in colon/rectum (Figure 1). All previously known metastases were detected with F-18 FDG PET/CT in all patients, and in 32 (28.5%) of 112 patients, new sites of metastases were detected.
Figure 1.
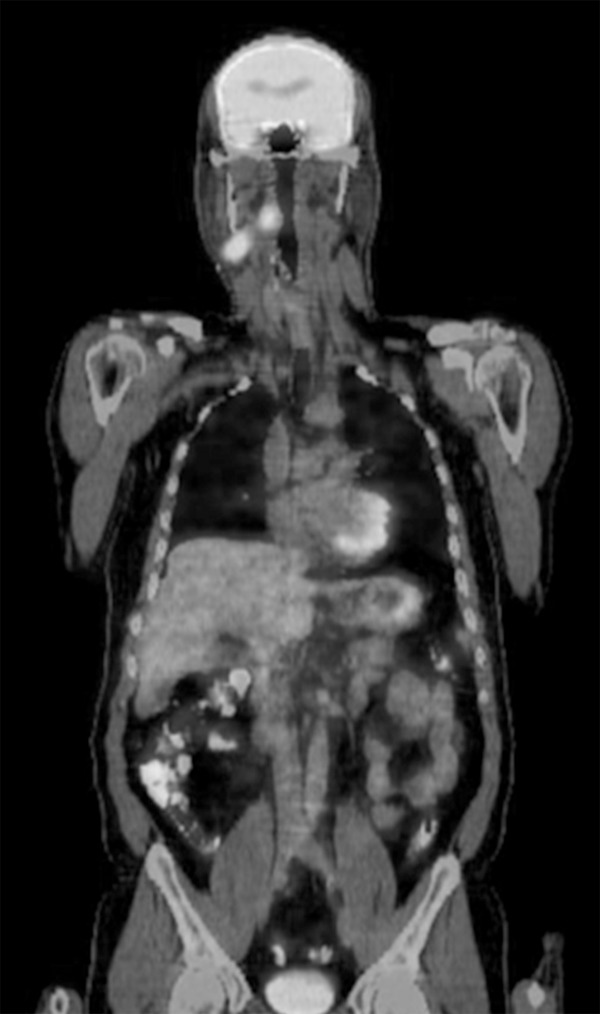
42-year old man presented with enlarged right cervical lymph nodes, epithelial tumor metastasis on biopsy, F18-FDG PET/CT showed increased uptake in the right tonsil (SUVmax 10.5) and right cervical lymph nodes; final diagnosis-squamous cell carcinoma of right tonsil.
False positive results
F-18 FDG PET/CT scan suggested a primary tumor in 21 of 112 patients, but histopathological examination revealed benign lesions in the gastrointestinal tract (atypical hyperplasia with sinus ventriculi in gastric mucosa in 4, colonic polyp in 3, rectal granuloma in 2 patients), head and neck (inflammation of nasopharynx in 5, submandibular gland in 2, thyroiditis in 1 patients), lungs (active tuberculosis in 1 and fungus infection in 1 patient), liver (benign hepatic tumor in 1 patient). In an additional patient, the lesion with increased F-18 FDG uptake (SUVmax: 6.5) suggesting the site for the primary tumor located in the lung which could not be confirmed histopathologically was decided to be benign by follow-up data (Table 2).
Table 2.
False Positive Results F-18 FDG PET/CT by Histopathology and Location
| Histopathological | Gastrointestinal tract | Head and Neck | Lungs | Liver |
|---|---|---|---|---|
| Atypical Hyperplasia | 4 | |||
| Polyp | 3 | |||
| Granuloma | 2 | |||
| Inflammation | 8 | 3 | ||
| Benign Tumor | 1 |
Change to patients’ treatment
The treatment was modified in 33 (29.4%) of 112 patients based on F-18 FDG PET/CT findings detecting primary tumor in 37 patients and additional metastases in 36 patients. Of these 33 patients, chemotherapy protocol was changed in 22, while surgical treatment which was considered prior to F-18 FDG PET/CT was cancelled and chemotherapy was initiated in 11 patients due to upstaging based on F-18 FDG PET/CT.
Discussion
Carcinoma of unkown primary is a distinct malignant disease that is different from metastatic stage of any malignant disease in which primary tumor is already known. Although its prevalence is estimated to be higher than non-Hodgkin lymphoma, the exact rate is not known since CUP is not usually reported as a separate entity by cancer registries. It is not uncommon to see that the metastatic stage of a malignancy with already known primary is confused with cancer of unknown primary during registration of the final diagnosis. It is generally an advanced stage disease and most of the patients have extensive metastatic disease at initial diagnosis. The presence of malignancy of unknown origin determined histopathologically in one or more metastatic site(s) and the failure to detect the primary tumor by routine diagnostic algortihm are the prerequisites for the diagnosis of CUP. The most common algorithm for the diagnostic workup of a patient with CUP includes physical examination, relevant laboratory tests, conventional X-ray studies, ultrasonography, computed tomography (CT) and/or magnetic resonance imaging (MRI) and endoscopic examinations when applicable. Further diagnostic studies such as mammography are included in the algorithm for some special cases. Despite the extensive use of these techniques, the primary tumor can be detected in a small portion of patients only.
CUP is predominantly seen between the fifth and seventh decades and slightly more prevalent in men than in women. Its reported prevalence varies between 1.3 to 4.2% of all newly diagnosed malignancies. Despite intensive diagnostic efforts, the primary tumor cannot be detected even at autopsy in almost 2/3 of patients with CUP. The detection of the primary tumor in patients with CUP is vitally important for the initiation of curative treatment. If the primary tumor detected after diagnostic workup is a hematologic malignancy, chemotherapy could dramatically saves the patient’s life. Also, if the primary tumors is detected in patients with solitary metastasis of unknown primary, surgery would be peformed in a significant number of these patients.
F-18 FDG PET/CT is useful in detecting the extent of metastatic disease in most majority of the patients and also able to detect the primary tumor in one third of the patients with CUP [4,11-16]. This is confirmed by the results of our current study; F-18 FDG PET/CT correctly detected primary tumor in 37 (33.03%) patients, disclosed additional previously undetected metastases in 32 (28.5%) and changed the treatment in 33 (29.4%) of 112 patients. The patient cohort in our study is one of the largest reported in the literature; the number of patients in most of the previously published studies is less than 100. Nevertheless, the success rate for detecting the primary tumor, additional metastases and change to the patients’ treatment is similar to previous reports (i.e. about one third of the patients).
Contrary to previous reports, one of the distinct features of our study is that there was no hematologic malignancy in our cohort. This is possibly due to the fact that the current more focused diagnostic techniques including bone marrow biopsy is used more frequently in routine clinical practice excluding the possibility of hematologic malignancy at an early stage of the diagnostic workup of patients with CUP.
Also, in most of the previously published reports, the highest number of primaries detected by F-18 FDG PET/CT was head and neck carcinomas while in our study the most common primary detected by F-18 FDG PET/CT is the lung carcinoma (18/37; 48.6%) which was followed by nasopharynx, pancreas, tonsil, breast, thyroid, uterus and colon/rectum. The rate of detection of lung carcinoma as the primary tumor in our study is higher than the rate in the prevously published postmortem series in which only 20% of patients with CUP had non-small-cell lung cancer [17].
The ability of F-18 FDG PET/CT in demonstrating increased metabolic uptake in the primary tumor localized in prostate is also noteworthy since the use of F-18 FDG PET/CT in the diagnostic workup of prostate carcinoma is associated with problems due to high urinary activity as reported in previous reports [18-20].
We believe that there are several reasons for the failure of F-18 FDG PET/CT in detecting the primary tumor in 2/3 of patients with CUP. First of all, F-18 FDG uptake can be influenced by tumor grade [21,22]. Low-grade tumors tend to have low or absent glucose consumption, and therefore have low F-18 FDG uptake [23]. High background signal, which results from the presence of physiological F-18 FDG uptake in nearby sites (especially in the gastrointestinal and urinary tract) may hide the primary lesion. The 5-mm spatial resolution limit of the PET/CT systems or the presence of small tumor foci, exhibiting low FDG uptake may also lead to failure of F-18 FDG PET/CT imaging in the diagnostic workup of CUP.
F-18 FDG FDG PET/CT may lead to changes in the treatment strategies due to the detection of previously unidentified primary tumor and/or new metastases. Detection of previously unrecognized regional disease can prompt inclusion of the relevant areas in radiotherapy target field and can also change the dose of radiation to be delivered by radiotherapy, especially for patients with cervical node metastasis [7,24]. In our present study, based on the identification of primary tumor by F-18 FDG PET/CT imaging, the chemotherapy protocol was modified in 22 (59.4%) of 37 patients in whom the primary tumor was correctly localized by F-18 FDG PET/CT. Also, F-18 FDG PET/CT detected the previously unrecognized distant metastases in 32 of patients (28.5%) of 112. This result contributed to treatment changes in 33 patients. A systematic review of 16 previously published studies covering 302 patients found that F-18 FDG-PET detected previously unrecognized metastases in 27.1% of patients (regional in 15.9%; distant in 11.2%), and led to changes in treatment in 24.7% of all patients in 6 studies [25]. Our findings and previously published results emphasize the impact of F-18 FDG PET/CT in the management of patients with CUP.
In conclusion, F-18 FDG PET/CT is an efficient method for detecting the occult primary tumor in patients with CUP, as well as detecting previously unrecognized metastases. Also, F-18 FDG PET/CT imaging can change the treatment in a significant number of patients.
Disclosure of conflict of interest
None.
References
- 1.Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 2003;39:1990–2005. doi: 10.1016/s0959-8049(03)00547-1. [DOI] [PubMed] [Google Scholar]
- 2.Plot L, Dovrish Z, Hadari R, Weisenberg N, Zehavi T, Nisenbaum B, Amital H. Cancer of unknown primary site origin--advances in diagnosis and therapy. Harefuah. 2008;147:294–298. [PubMed] [Google Scholar]
- 3.Fernandez-Cotarelo MJ, Guerra-Vales JM, Colina F, De la Cruz J. Prognostic factors in cancer of unknown primary site. Tumori. 2010;96:111–116. doi: 10.1177/030089161009600118. [DOI] [PubMed] [Google Scholar]
- 4.Kwee TC, Basu S, Alavi A. PET and PET/CT for unknown primary tumors. Methods Mol Biol. 2011;727:317–333. doi: 10.1007/978-1-61779-062-1_17. [DOI] [PubMed] [Google Scholar]
- 5.Delgado-Bolton RC, Fernandez-Perez C, Gonzalez-Mate A, Carreras JL. Meta-analysis of the performance of 18F-FDG PET in primary tumour detection in unknown primary tumors. J Nucl Med. 2003;44:1301–1314. [PubMed] [Google Scholar]
- 6.Rades D, Kuhnel G, Wildfang I, Börner AR, Schmoll HJ, Knapp W. Localised disease in cancer of unknown primary (CUP): the value of positron emission tomography (PET) for individual therapeutic management. Ann Oncol. 2001;12:1605–1659. doi: 10.1023/a:1013107732572. [DOI] [PubMed] [Google Scholar]
- 7.Bohuslavizki KH, Klutmann S, Kroger S, Sonnemann U, Buchert R, Werner JA, Mester J, Clausen M. FDG PET detection of unknown primary tumors. J Nucl Med. 2000;41:816–822. [PubMed] [Google Scholar]
- 8.Regelink G, Brouwer J, De Bree R, Pruim J, Van der Laan BF, Vaalburg W, Hoekstra OS, Comans EF, Vissink A, Leemans CR, Roodenburg JL. Detection of unknown primary tumours and distant metastases in patients with cervical metastases: value of FDG-PET versus conventional modalities. Eur J Nucl Med Mol Imaging. 2002;29:1024–1030. doi: 10.1007/s00259-002-0819-0. [DOI] [PubMed] [Google Scholar]
- 9.Alberini JL, Belhocine T, Hustinx R, Daenen F, Rigo P. Whole-body positron emission tomography using fluorodeoxyglucose in patients with metastases of unknown primary tumours (CUP syndrome) Nucl Med Commun. 2003;24:1081–1086. doi: 10.1097/00006231-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Demir H, Berk F, Raderer M, Plowman PN, Lassen U, Daugaard G, Clausen M, Bohuslavizki KH, Peters M, Harmer C, Malamitsi J, Aktolun C. The role of nuclear medicine in the diagnosis of cancer of unknown origin. Q J Nucl Med Mol Imaging. 2004;48:164–173. [PubMed] [Google Scholar]
- 11.Moller AK, Loft A, Berthelsen AK, Damgaard Pedersen K, Graff J, Christensen CB, Perell K, Petersen BL, Daugaard G. 18F-FDG PET/CT as a diagnostic tool in patients with extracervical carcinoma of unknown primary site: a literature review. Oncologist. 2011;16:445–451. doi: 10.1634/theoncologist.2010-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pak K, Kim SJ, Kim IJ, Nam HY, Kim BS, Kim K, Kim YK. Clinical implication of (18)F-FDG PET/CT in carcinoma of unknown primary. Neoplasma. 2011;58:135–139. doi: 10.4149/neo_2011_02_135. [DOI] [PubMed] [Google Scholar]
- 13.Fencl P, Belohlavek O, Skopalova M, Jaruskova M, Kantorova I, Simonova K. Prognostic and diagnostic accuracy of [18F] FDG-PET/CT in 190 patients with carcinoma of unknown primary. Eur J Nucl Med Mol Imaging. 2007;34:1783–1792. doi: 10.1007/s00259-007-0456-8. [DOI] [PubMed] [Google Scholar]
- 14.Ambrosini V, Nanni C, Rubello D, Moretti A, Battista G, Castellucci P, Farsad M, Rampin L, Fiorentini G, Franchi R, Canini R, Fanti S. 18F-FDG PET/CT in the assessment of carcinoma of unknown primary origin. Radiol Med. 2006;111:1146–1155. doi: 10.1007/s11547-006-0112-6. [DOI] [PubMed] [Google Scholar]
- 15.Sève P, Billotey C, Broussolle C, Dumontet C, Mackey JR. The role of 2-deoxy-2-[F-18] fluoro-D-glucose positron emission tomography in disseminated carcinoma of unknown primary site. Cancer. 2007;109:292–299. doi: 10.1002/cncr.22410. [DOI] [PubMed] [Google Scholar]
- 16.Kolesnikov-Gauthier H, Levy E, Merlet P, Kirova J, Syrota A, Carpentier P, Meignan M, Piedbois P. FDG PET in patients with cancer of an unknown primary. Nucl Med Commun. 2005;26:1059–1066. doi: 10.1097/00006231-200512000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Wu ZJ, Zhang YX, Wei H, Jia Q. The role of whole body 2-[fluorine-18] -fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in the management of unknown primary tumors. Natl Med J China. 2007;87:2253–2256. [PubMed] [Google Scholar]
- 18.Avril N, Dambha F, Murray I, Shamash J, Powles T, Sahdev A. The clinical advances of fluorine-2-D-deoxyglucose--positron emission tomography/computed tomography in urological cancers. Int J Urol. 2010;17:501–511. doi: 10.1111/j.1442-2042.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- 19.Sanz G, Rioja J, Zudaire JJ, Berián JM, Richter JA. PET and prostate cancer. World J Urol. 2004;22:351–352. doi: 10.1007/s00345-004-0418-8. [DOI] [PubMed] [Google Scholar]
- 20.Hofer C, Kübler H, Hartung R, Breul J, Avril N. Diagnosis and monitoring of urological tumors using positron emission tomography. Eur Urol. 2001;40:481–487. doi: 10.1159/000049823. [DOI] [PubMed] [Google Scholar]
- 21.Karantanis D, Allen-Auerbach M, Czernin J. Relationship among glycolytic phenotype, grade, and histological subtype in ovarian carcinoma. Clin Nucl Med. 2012;37:49–53. doi: 10.1097/RLU.0b013e3182291e03. [DOI] [PubMed] [Google Scholar]
- 22.Groheux D, Giacchetti S, Moretti JL, Porcher R, Espié M, Lehmann-Che J, De Roquancourt A, Hamy AS, Cuvier C, Vercellino L, Hindié E. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–435. doi: 10.1007/s00259-010-1640-9. [DOI] [PubMed] [Google Scholar]
- 23.Wong TZ, Van der Westhuizen GJ, Coleman RE. Positron emission tomography imaging of brain tumors. Neuroimaging Clin N Am. 2002;12:615–626. doi: 10.1016/s1052-5149(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 24.Basu S, Alavi A. FDG-PET in the clinical management of carcinoma of unknown primary with metastatic cervical lymphadenopathy: shifting gears from detecting the primary to planning therapeutic strategies. Eur J Nucl Med Mol Imaging. 2007;34:427–428. doi: 10.1007/s00259-006-0313-1. [DOI] [PubMed] [Google Scholar]
- 25.Rusthoven KE, Koshy M, Paulino AC. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer. 2004;10:2641–2649. doi: 10.1002/cncr.20687. [DOI] [PubMed] [Google Scholar]


