Abstract
Aim: This study aimed to analyze the expression, clinical significance of stanniocalcin 2 in cervical carcinoma patients who were treated with radiotherapy. Methods: Stanniocalcin 2 expression was determined by real-time PCR in 10 pairs of cervical cancer and adjacent normal cervical tissues. Tumor samples from 92 patients diagnosed from 2004 to 2007 were studied. All samples were obtained prior to treatment start. All cases were clinically diagnosed and pathologically confirmed to be cervical carcinoma without distant metastasis, and have been treated with radical radiation therapy and followed-up for five years. The samples were immunohistochemically analyzed for stanniocalcin 2 expression and survival analyses were performed. Results: The tumors of cervical cancer patients had significantly increased expression of stanniocalcin 2 at mRNA level compared with adjacent normal cervical tissues. High levels of stanniocalcin 2 expression was correlated with shorter overall survival, whereas low levels of stanniocalcin 2 expression was correlated with longer overall survival (P = 0.003) and progression free survival (P = 0.001) after radiotherapy. Moreover, high expression of stanniocalcin 2 was correlated with lymph node metastasis (P = 0.002). Conclusion: Stanniocalcin 2 could be a useful marker for the prognosis of cervical cancer patients receiving radiotherapy. Stanniocalcin 2 may contribute to tumor development and radioresistance in cervical cancer.
Keywords: Stanniocalcin 2, radiotherapy, cervical cancer, prognosis
Introduction
Cervical carcinoma is one of the most common malignant tumors in the female reproductive system, accounting for 8% of the total cases and total deaths from cancer. The reported annual incidence of cervical carcinoma in the United States was about 15,000, and approximately 70% of cervical cancers occur in developing countries [1]. Suamous cell carcinomas account for about 70-80% of cervical cancers and adenocarcinomas and other pathological types for 10-15% [2]. In recent years, advances have been made in clinical treatment due to the development of radiotherapy (RT), however, tumor resistance to radiation remains a major therapeutic problem [3]. Therefore, it is essential to identify novel molecular markers to predict the prognosis of cervical cancer after RT, which will help us to explore additional prognostic factors to identify radioresistant cervical cancer patients and develop more effective therapeutic strategies.
Stanniocalcin 2 (STC2) is a secreted, homodimeric glycoprotein expressed in a wide variety of tissues [4]. It belongs to the stanniocalcin family and has 10 conserved cysteine residues, which are phosphorylated by casein kinase 2 [5]. The C-terminus of STC2 contains a cluster of histidine residues, which may coordinate with metal ions. It has been proposed that STC2 may play important roles in the regulation of cell metabolism, calcium/phosphate transport and homeostasis [6]. Overexpression of STC2 contributes to postnatal growth restriction, reduced skeletal muscle and bone growth in mice [7]. Phosphorylation of STC2 is catalyzed by the action of an ecto-protein kinase, and the ecto-kinase activity could be abolished by heparin [6]. Expression of STC2 is induced by hormone signaling such as E (2), P4 and RA signaling pathways in some cancers, and it can function in a paracrine/autocrine fashion to reduce cell proliferation in hormone receptor negative cell lines [8]. Recent studies have shown that STC2 expression was up-regulated in several cancers, including prostate cancer, breast cancer, gastric cancer, colon cancer, neuroblastoma, and esophageal squamous cell carcinoma [8-12], indicating its important roles in promoting tumor development and progression. A clinical retrospective study revealed that STC2 could be used as a biomarker of prognosis and response to radiation therapy in patients with nasopharyngeal carcinoma [13]. Thus, STC2 may be involved in radioresistance in cancer therapy. However, the role of STC2 in the response to radiation therapy in patients with cervical cancer has not been explored.
The aim of this study was to investigate the clinical significance of STC2 in cervical carcinoma patients undergoing RT. This study is the first to support interactions between STC2 expression and clinical outcome of this population.
Materials and methods
Patients
The database of The Affiliated Suzhou Municipal Hospital of Nanjing Medical University was reviewed from 2004 to 2007, and a total of 92 cervical cancer patients. Characteristics of the patients, diseases and treatments are provided in Table 1. The mean age was 46.8 years, with an age range of 25-68 years. No patient had previously been treated with radiotherapy and chemotherapy at the time of original biopsy. Patients without distant metastasis were treated with a combination of 3-dimensional conformal external beam RT with concurrent weekly 30-mg/m2 cisplatin and high- dose rate (HDR) brachytherapy as previously described [14]. Total radiation doses ranged from 45-61.2 Gy (median, 50.4 Gy) in 1.8-2 Gy (median, 1.8 Gy) fractions. Intracavitary HDR brachytherapy of 20-25 Gy was conducted using a tandem and colpostat. Antiemetics and anticonvulsants drugs were administered if needed.
Table 1.
Patient characteristics
| Characteristic | No. of Patients | % |
|---|---|---|
| Age (y) | ||
| ≤ 50 | 41 | 44.6 |
| > 50 | 51 | 55.4 |
| Figo stage | ||
| IB2 | 10 | 10.9 |
| IIA1 | 4 | 4.4 |
| IIA2 | 2 | 2.2 |
| IIB | 52 | 56.4 |
| IIIA | 9 | 9.8 |
| IIIB | 15 | 16.3 |
| Tumor size | ||
| ≤ 4 cm | 13 | 14.1 |
| > 4 cm | 79 | 75.9 |
| Differentiation | ||
| Grade 1 and 2 | 25 | 27.2 |
| Grade 3 | 67 | 72.8 |
| Histology | ||
| SCC | 85 | 92.4 |
| AC | 7 | 7.6 |
| LN metastasis | ||
| Yes | 48 | 52.2 |
| No | 44 | 47.8 |
| Total No. of Patients | 92 | 100 |
SCC: squamous cell carcinoma; AC: adenocarcinoma.
RNA extraction and real-time PCR
Total RNA was isolated for Trizol reagent (Invitrogen, Carlsbad, CA) according to the instructions and cDNA was reversibly transcribed from the isolated mRNA using an M-MLV reagent kit (Clontech, USA) following the manufacturer’s instructions. The primers of STC2 and GAPDH genes were designed as follows: STC2 forward: forward primer, 5’-ATGCTACCTCAAGCACGACC-3’; reverse, 5’-TCTGCTCACACTGAACC TGC-3’; GAPDH forward: 5’-TGTTCGACACTCCTCCGTCAGC-3’, reverse: 5’-CAAATCCCCCAATACGACGTT-3’. The copy number of each sample was calculated and all the data were normalized to GAPDH prior to comparative analysis using 2-ΔCt method.
Immunohistochemical staining
Immunohistochemical staining was carried out to determine the expression pattern of STC2 in cervical cancer. Tissue sections of 5-μm thickness were cut from paraffin-embedded tissue blocks onto pre-coated slides and were then deparaffinized with xylene and rehydrated with ethanol. After washing in phosphate-buffered saline (PBS), slides were heated by microwave treatment in a 10-mM citrate buffer (pH 6.0) for 7 min for antigen retrieval. Then they were rinsed in peroxidase quenching solution (Invitrogen, USA) to block endogenous peroxidase, and then incubated with a primary mouse antibody targeting STC2 (1:200, Abcam, USA) at 4°C overnight. They were then incubated with an HRP-conjugated anti-mouse secondary antibody (1: 200, Abcam, USA) at room temperature for 30 min. Finally, a 3,3’-diaminobenzidine (DAB) solution was added, followed by counterstaining with hematoxylin.
The stained tissue slides were scored independently by two observers who were blinded to the clinical or clinicopathological status of the patients. An immunoreactivity scoring system was used. The proportion of tumor cell staining was evaluated in terms of four grades: 0, (no positive tumor cells); 1, (< 10% positive tumor cells); 2, (10-50% positive tumor cells); 3, (51-80% positive tumor cells); and 4, (> 80% positive tumor cells). The staining intensity was graded according to the following criteria: 0, no staining; 1, weak staining; 2, modest staining; and 3, strong staining. The final score was calculated as the product of staining intensity score and the proportion of positive tumor cells (0, 1, 2, 3, 4, 6, 8, 9 and 12). The cutoff value for high and low expression level was chosen based on a measure of heterogeneity with the log-rank test statistical analysis with respect to overall survival (OS) and progression free survival (PFS). An optimal cutoff value was identified: a staining score of ≤ 4 was used to define tumors with low STC2 expression and a staining score of > 4 was used to indicate high STC2 expression.
Statistical analysis
Overall survival (OS) and progression free survival (PFS) time were defined as the time from the diagnosis of cervical cancer to the date of death or progression. All of the survival curves were plotted using the Kaplan-Meier method. The Cox’s proportional hazard model was used to stepwise multivariate analysis. P < 0.05 in all cases was considered statistically significant. All statistical analyses were carried out using the SPSS17.0 statistical software package.
Results
Expression of STC2 mRNA in cervical cancer and adjacent normal tissues
We determined the levels of STC2 mRNA in 10 pairs of cervical tumor tissues and adjacent normal tissues by real time-PCR. The samples were obtained from randomly selected cervical cancer patients before RT. Figure 1 show that the expression of STC2 mRNA was significantly higher in the cervical cancer tissues than in the paired normal tissues.
Figure 1.
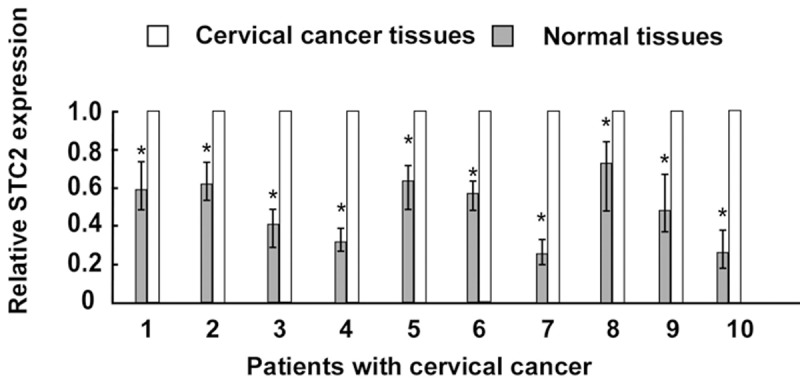
Analysis of STC2 mRNA in 10 pairs of cervical cancer and paired adjacent normal cervical tissues by Real time-PCR. asterisks, P < 0.05.
Immunoexpression of STC2 in cervical cancer and association with clinicopathological parameters
Immunohistochemistry was performed to detect the expression pattern of STC2 protein in cervical cancer tissues. The positive staining of STC2 wan mainly found in the cytoplasm of tumor cells. The representative immunostaining of STC2 in cervical cancer was shown in Figure 2A-D. Immunohistochemical staining of STC2 levels was statistically analyzed to determine their relationship with the clinical features of cervical cancer. As shown in Table 2, the expression of STC2 was significantly correlated with lymph node metastasis (P = 0.002). However, there was no association between the expression of STC2 protein and other factors including age, FIGO stage, tumor size, differentiation, or histological type.
Figure 2.
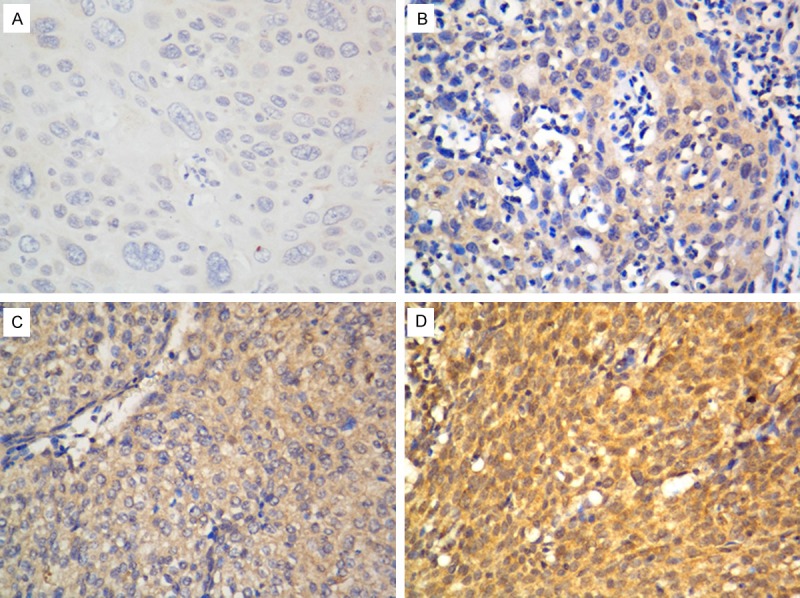
Representative immunostaining of STC2 protein expression in cervical cancer tissues. The immunostaining of STC2 protein was mainly located in the cytoplasm of tumor cells. A. Negative expression; B. Weak expression; C. Moderate expression; D. Strong expression. Magnification: 400 x.
Table 2.
STC2 expression in cervical cancer patients according to clinicopathologic features
| Characteristic | No. | STC2 | P | |
|---|---|---|---|---|
|
| ||||
| high | low | |||
| Age (y) | 0.458 | |||
| ≤ 50 | 41 | 29 | 12 | |
| > 50 | 51 | 20 | 31 | |
| FIGO Stage | 0.710 | |||
| ≤ IIB | 68 | 37 | 31 | |
| > IIB | 24 | 12 | 12 | |
| Tumor size | 0.213 | |||
| ≤ 4cm | 13 | 9 | 4 | |
| > 4cm | 79 | 40 | 39 | |
| Differentiation | 0.073 | |||
| Grade 1/2 | 25 | 9 | 16 | |
| Grade 3 | 67 | 40 | 27 | |
| Histology | 0.830 | |||
| SCC | 85 | 45 | 40 | |
| AC | 7 | 4 | 3 | |
| LN Metastasis | 0.002 | |||
| Yes | 48 | 33 | 15 | |
| No | 44 | 16 | 28 | |
| Total NO. | 92 | 49 | 43 | |
SCC: squamous cell carcinoma; AC: adenocarcinoma.
Correlation between STC2 expression and patients’ survival
The correlation between the STC2 expression and patients’ survival was explored by the Kaplan-Meier analysis. Patients with high STC2 levels had a significantly shorter overall and progression free survival than those with low levels (Figure 3, P = 0.003, and 0.001, respectively). Then, multivariate regression analysis was performed with the Cox proportional hazards regression model to analyze the independent factors related to prognosis. As shown in Table 3, high STC2 expression was an independent prognostic factor for overall and progression free survival (P = 0.013, and 0.004, respectively).
Figure 3.
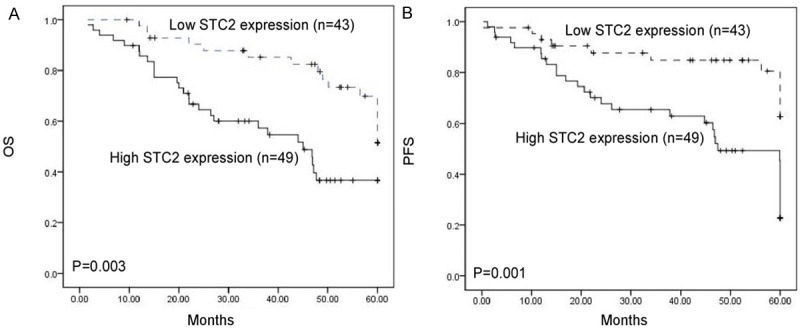
Kaplan-Meier overall survival (A) and progression free survival (B) of patients with cervical cancer who had STC2-high and STC2-low character. A significant difference was observed between two groups.
Table 3.
Multivariate analysis of prognostic factors for OS and PFS
| Variables | OS | PFS | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (> 50 vs. ≤ 50) | 1.07 (0.16-5.32) | 0.403 | 1.18 (0.142-6.056) | 0.433 |
| FIGO Stage (> IIB vs. ≤ IB) | 2.19 (0.87-6.98) | 0.141 | 2.75 (1.782-8.219) | 0.194 |
| Tumor size (> 4 cm vs. ≤ 4 cm) | 154 (0.73-4.93) | 0.443 | 1.46 (0.775-5.278) | 0.578 |
| Differentiation (Grade 3 vs. 1/2) | 1.87 (0.25-5.36) | 0.876 | 2.10 (0.624-7.928) | 0.712 |
| Histology (SCC vs. AC) | 1.15 (0.26-4.18) | 0.264 | 1.53 (0.244-6.258) | 0.357 |
| LN Metastasis (Yes vs. No) | 2.65 (1.07-6.49) | 0.035 | 2.41 (1.175-7.526) | 0.056 |
| STC2 expression (High vs. Low) | 6.12 (2.491-9.347) | 0.013 | 5. 35 (2.342-7.163) | 0.004 |
Discussion
In this study we found that the tumors of cervical cancer patients had significantly increased expression of STC2 at the mRNA level relative to adjacent normal cervical tissues. In addition, our survival analysis of cervical cancer patients after RT indicated that STC2 expression was significantly associated with poor overall and progression free survival. Moreover, STC2 expression was significantly correlated lymph node metastasis in those patients. Thus, there is a possibility that STC2 overexpression is related to radioresistance and tumor progression in cervical cancer.
Radioresistance is the basic biological character which is related to progression and also effect on the survival of cervical cancer patients. The prognosis of these patients is usually poor. The reported 5-year survival rates of patients who recur after radiotherapy or surgery are between 3.2% and 13% [15]. Thus, identifying prognostic biomarker may facilitate the prediction of response radiotherapy, thus help guiding the choice of treatment. Previous RT studies indicated that the radiation sensitivity had correlation with cell cycle regulation, and STC2 plays an important role in cell cycle regulation. In particular, ectopic expression of STC2 promoted cancer cell proliferation and colony formation, while silencing of endogenous STC2 resulted in a reduced cell growth by cell cycle delay in G0/G1 phase [16]. Tumor radioresistance could also be governed by CSC resistance to reactive oxygen species and micro environmental factors such as hypoxia [17], and STC2 also has been found to be upregulated and promotes cancer cell proliferation under hypoxia [18]. Recently, it has been suggested that the STC2 gene may play a role in human carcinogenesis [19]. In our study, we found that STC2 expression in cervical cancer tissues was overexpressed in comparison with those in adjacent normal tissues, suggesting that STC2 may play a vital role in cervical cancer development.
Previous studies have shown that STC2 overexpression is associated with poor prognosis or cancer progression in several cancers. Arigami et al. reported that the 5-year survival rate was significantly lower in patients with STC2 expression compared to patients without STC2 expression, and that its expression was significantly correlated with age, depth of tumor invasion, lymph node metastasis, stage and venous invasion [20]. Law et al. demonstrated that STC2 promotes epithelial-mesenchymal transition and invasiveness in hypoxic human ovarian cancer cells [21]. Yokobori et al. proposed that high expression level of STC2 in gastric cancer tissues could be a very powerful marker of poor prognosis [9]. These studies indicate the role of STC2 in predicting the clinical outcome of cancers. In line with these studies, our study revealed the relationship between STC2 expression and the outcome of cervical patients receiving radiotherapy, which further indicate the crucial role of STC2 in radioresistace.
Interestingly, our study demonstrated that STC2 overexpression is associated with lymph nodes metastasis, while is not associated with age, tumor stage, tumor size, differentiation, or histological type in cervical cancer patients. These results was in consistent with the report by Lin and colleagues, who revealed that STC2 overexpression correlates to poor prognosis for nasopharyngeal carcinomas after RT and could be used as a novel marker to predict tumor responses to RT [13]. Our results indicate that STC2 may be a factor in predicting responses to RT in cervical cancer patients.
There are several limitations in this study. The cervical cancer specimens were not necessarily from the same date before RT, although most were within 1 week. Moreover, the data of this retrospective study was from one single institution, but involving different radiologists, and there might be a lack of uniformity. Further, we included patients combined with chemotherapy, which may have influenced the results.
In summary, STC2 had a high correlative relationship with the clinical outcome in patients with cervical cancer treated with RT, and STC2 is a very useful factor for predicting survival in those patients. Therefore, we suggest that the STC2 be included in the routine assessment of cervical cancer patients before radiotherapy.
Disclosure of conflict of interest
None.
References
- 1.Colombo N, Carinelli S, Colombo A, Marini C, Rollo D, Sessa C. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii27–32. doi: 10.1093/annonc/mds268. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Forman D, O’Brien M, Ferlay J, Center M, Parkin DM. Cancer burden in Africa and opportunities for prevention. Cancer. 2012;118:4372–4384. doi: 10.1002/cncr.27410. [DOI] [PubMed] [Google Scholar]
- 3.Kim TJ, Lee JW, Song SY, Choi JJ, Choi CH, Kim BG, Lee JH, Bae DS. Increased expression of pAKT is associated with radiation resistance in cervical cancer. Br J Cancer. 2006;94:1678–1682. doi: 10.1038/sj.bjc.6603180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang AC, Reddel RR. Identification of a second stanniocalcin cDNA in mouse and human: stanniocalcin 2. Mol Cell Endocrinol. 1998;141:95–99. doi: 10.1016/s0303-7207(98)00097-5. [DOI] [PubMed] [Google Scholar]
- 5.Ishibashi K, Miyamoto K, Taketani Y, Morita K, Takeda E, Sasaki S, Imai M. Molecular cloning of a second human stanniocalcin homologue (STC2) Biochem Biophys Res Commun. 1998;250:252–258. doi: 10.1006/bbrc.1998.9300. [DOI] [PubMed] [Google Scholar]
- 6.Jellinek DA, Chang AC, Larsen MR, Wang X, Robinson PJ, Reddel RR. Stanniocalcin 1 and 2 are secreted as phosphoproteins from human fibrosarcoma cells. Biochem J. 2000;350:453–461. [PMC free article] [PubMed] [Google Scholar]
- 7.Gagliardi AD, Kuo EY, Raulic S, Wagner GF, DiMattia GE. Human stanniocalcin-2 exhibits potent growth-suppressive properties in transgenic mice independently of growth hormone and IGFs. Am J Physiol Endocrinol Metab. 2005;288:E92–105. doi: 10.1152/ajpendo.00268.2004. [DOI] [PubMed] [Google Scholar]
- 8.Raulic S, Ramos-Valdes Y, DiMattia GE. Stanniocalcin 2 expression is regulated by hormone signalling and negatively affects breast cancer cell viability in vitro. J Endocrinol. 2008;197:517–529. doi: 10.1677/JOE-08-0043. [DOI] [PubMed] [Google Scholar]
- 9.Yokobori T, Mimori K, Ishii H, Iwatsuki M, Tanaka F, Kamohara Y, Ieta K, Kita Y, Doki Y, Kuwano H, Mori M. Clinical significance of stanniocalcin 2 as a prognostic marker in gastric cancer. Ann Surg Oncol. 2010;17:2601–2607. doi: 10.1245/s10434-010-1086-0. [DOI] [PubMed] [Google Scholar]
- 10.Volland S, Kugler W, Schweigerer L, Wilting J, Becker J. Stanniocalcin 2 promotes invasion and is associated with metastatic stages in neuroblastoma. Int J Cancer. 2009;125:2049–2057. doi: 10.1002/ijc.24564. [DOI] [PubMed] [Google Scholar]
- 11.Ieta K, Tanaka F, Yokobori T, Kita Y, Haraguchi N, Mimori K, Kato H, Asao T, Inoue H, Kuwano H, Mori M. Clinicopathological significance of stanniocalcin 2 gene expression in colorectal cancer. Int J Cancer. 2009;125:926–931. doi: 10.1002/ijc.24453. [DOI] [PubMed] [Google Scholar]
- 12.Tamura K, Furihata M, Chung SY, Uemura M, Yoshioka H, Iiyama T, Ashida S, Nasu Y, Fujioka T, Shuin T, Nakamura Y, Nakagawa H. Stanniocalcin 2 overexpression in castration-resistant prostate cancer and aggressive prostate cancer. Cancer Sci. 2009;100:914–919. doi: 10.1111/j.1349-7006.2009.01117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin S, Guo Q, Wen J, Li C, Lin J, Cui X, Sang N, Pan J. Survival analyses correlate stanniocalcin 2 overexpression to poor prognosis of nasopharyngeal carcinomas. J Exp Clin Cancer Res. 2014;33:26. doi: 10.1186/1756-9966-33-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onal C, Reyhan M, Parlak C, Guler OC, Oymak E. Prognostic value of pretreatment 18F-fluorodeoxyglucose uptake in patients with cervical cancer treated with definitive chemoradiotherapy. Int J Gynecol Cancer. 2013;23:1104–1110. doi: 10.1097/IGC.0b013e3182989483. [DOI] [PubMed] [Google Scholar]
- 15.Lai CH. Management of recurrent cervical cancer. Chang Gung Med J. 2004;27:711–717. [PubMed] [Google Scholar]
- 16.Wang H, Wu K, Sun Y, Li Y, Wu M, Qiao Q, Wei Y, Han ZG, Cai B. STC2 is upregulated in hepatocellular carcinoma and promotes cell proliferation and migration in vitro. BMB Rep. 2012;45:629–634. doi: 10.5483/BMBRep.2012.45.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sattler UG, Meyer SS, Quennet V, Hoerner C, Knoerzer H, Fabian C, Yaromina A, Zips D, Walenta S, Baumann M, Mueller-Klieser W. Glycolytic metabolism and tumour response to fractionated irradiation. Radiother Oncol. 2010;94:102–109. doi: 10.1016/j.radonc.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Law AY, Wong CK. Stanniocalcin-2 is a HIF-1 target gene that promotes cell proliferation in hypoxia. Exp Cell Res. 2010;316:466–476. doi: 10.1016/j.yexcr.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Law AY, Wong CK. Stanniocalcin-1 and -2 promote angiogenic sprouting in HUVECs via VEGF/VEGFR2 and angiopoietin signaling pathways. Mol Cell Endocrinol. 2013;374:73–81. doi: 10.1016/j.mce.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 20.Arigami T, Uenosono Y, Ishigami S, Yanagita S, Hagihara T, Haraguchi N, Matsushita D, Hirahara T, Okumura H, Uchikado Y, Nakajo A, Hokita S, Natsugoe S. Clinical significance of stanniocalcin 2 expression as a predictor of tumor progression in gastric cancer. Oncol Rep. 2013;30:2838–2844. doi: 10.3892/or.2013.2775. [DOI] [PubMed] [Google Scholar]
- 21.Law AY, Wong CK. Stanniocalcin-2 promotes epithelial-mesenchymal transition and invasiveness in hypoxic human ovarian cancer cells. Exp Cell Res. 2010;316:3425–3434. doi: 10.1016/j.yexcr.2010.06.026. [DOI] [PubMed] [Google Scholar]


