Abstract
This study is to evaluate the association between the catechol-O-methyltransferase (COMT) gene val158met polymorphism and FM risk. We performed a meta-analysis of 8 case-control studies that included 589 FM cases and 527 case-free controls. We assessed the strength of the association, using odds ratios (ORs) with 95% confidence intervals (CIs). Overall, this meta-analysis showed that the COMT gene val158met polymorphism was not associated with FM risk in all genetic models, i.e., allele (met vs. val: OR=1.46, 95% CI=0.80-2.66, P heterpgeneity<0.001), homozygous (met/met vs. val/val: OR=1.72, 95% CI=0.61-4.87, P heterpgeneity<0.001), heterozygous (val/met vs. val/val: OR=1.25, 95% CI=0.82-1.92, P heterpgeneity=0.050), recessive (met/met vs. val/val+val/met: OR=1.52, 95% CI=0.60-3.86, P heterpgeneity<0.001) and dominant model (met/met+val/met vs. val/val: OR=1.52, 95% CI=0.80-2.90, P heterpgeneity<0.001). Similarly, there were no significant associations in the subgroup analyses by ethnicity and HWE. No publication bias was found in the present study. This meta-analysis suggests that the COMT gene val158met polymorphism is not associated with FM risk. Further large and well-designed studies are needed to confirm this association.
Keywords: Fibromyalgia, catechol-O-methyltransferase, polymorphism, meta-analysis
Introduction
Fibromyalgia (FM) syndrome is an idiopathic widespread persistent pain syndrome characterized by musculoskeletal pain, chronic diffuse tension and/or stiffness in joints and muscles, in the absence of inflammatory or structural musculoskeletal abnormalities, accompanied by a constellation of symptoms that include easy fatigue, poor sleep and mood disturbances, as well as a multitude of associated symptoms [1,2]. Fibromyalgia causes dysfunction of all age groups and adversely affects the quality of life [3,4]. Fibromyalgia is estimated to affect 2-4% of the population and it is dominated by women.
Although the definite etiology of fibromyalgia remains unclear, genetic and environmental factors have been considered as one of the potential causes in the development of fibromyalgia [5]. Significant familial aggregation, genetic linkages and associations demonstrate a potential genetic basis for fibromyalgia [6]. Catechol-O-methyltransferase (COMT) is an enzyme which metabolizes catecholamines. It has broad biological functions and has been implicated to be involved in the pathogenesis of neuropsychiatric disorders, migraine and Parkinson’s disease [7]. Recent studies have also demonstrated the involvement of COMT in the regulation of pain perception [8,9]. A common single nucleotide polymorphism (SNP) in codon 158 of the COMT gene (val158met), which affects COMT protein stability, resulting in reduced thermostability and activity of the enzyme. This polymorphism has been associated with cognitive function [10], affective moods [11], and the human experience of pain [12].
Recently, several studies have examined the potential contribution of the COMT gene val158met polymorphism to fibromyalgia susceptibility, but these studies have produced diverse results [8,9,13-17]. Given that a single study may be too underpowered to provide reliable conclusion owing to relatively small sample size, we performed this meta-analysis to estimate the association between COMT gene val158met polymorphism and FM susceptibility more precisely.
Materials and methods
Publication strategy
We searched for relevant studies up to May 2014 through the Pubmed and EMBASE database with the following terms and their combinations: “Catechol-O-methyltransferase/COMT”, “fibromyalgia” and “polymorphism or variant”. We tried to identify potential relevant studies from the whole reference lists by orderly reviewing title, abstract and full text.
Selection criteria
The inclusion criteria were as follows: a) Case-control studies focused on the association of COMT gene val158met polymorphism and fibromyalgia; b) Genotype and allele data available. Studies were excluded for following reasons: a) unpublished papers, reviews and duplication of publications; b) data unavailable for calculating genotype or allele frequencies. Additionally, if more than one article was published using the same case series, we selected the study with the largest sample size.
Data extraction
All the available data were extracted from each study by two investigators independently according to the inclusion criteria listed above. To ensure the accuracy of the information extracted, the two investigators checked the data extraction results and reached consensus on all of the items. If these two investigators could not reach a consensus, another author was consulted to resolve the dispute and a final decision was made by the majority of the votes. The following data were extracted: first author’s name, year of publication, country of origin, ethnicity, definition of study patients (cases), genotyping method, total number of cases and controls, and genotype distributions in cases and controls. Quality of studies was assessed according to the predefined criteria based on previous observational studies [18,19] (Table 1).
Table 1.
Scale for quality assessment of molecular association studies of fibromyalgia
| Criteria | Score |
|---|---|
| Representativeness of cases | |
| Consecutive/randomly selected from case population with clearly defined sampling frame | 2 |
| Consecutive/randomly selected from case population without clearly defined sampling frame or with extensive inclusion/exclusion criteria | 1 |
| No method of selection described | 0 |
| Representativeness of controls | |
| Controls were consecutive/randomly drawn from the same sampling frame as cases | 2 |
| Controls were consecutive/randomly drawn from a different sampling frame as cases | 1 |
| Not described | 0 |
| Ascertainment of fibromyalgia | |
| Clearly described objective criteria for diagnosis of fibromyalgia | 2 |
| Diagnosis of asthma by patient self-report or by patient history | 1 |
| Not described | 0 |
| Quality control of genotyping methods | |
| Clearly described a different genotyping assay to confirm the data | 1 |
| Not described | 0 |
| Hardy-Weinberg equilibrium | |
| Hardy-Weinberg equilibrium in controls | 2 |
| Hardy-Weinberg disequilibrium in controls | 1 |
| No checking for Hardy-Weinberg disequilibrium | 0 |
| Association assessment | |
| Assess association between genotypes and fibromyalgia with appropriate statistics and adjustment for confounders | 2 |
| Assess association between genotypes and fibromyalgia with appropriate statistics without adjustment for confounders | 1 |
| Inappropriate statistics used | 0 |
Statistical analysis
The departure of frequencies of COMT gene val158met polymorphism from expectation under Hardy-Weinberg equilibrium (HWE) was assessed by the chi-square test in controls and a P<0.05 was considered as significant disequilibrium. The strength of the association between COMT gene val158met polymorphism and fibromyalgia was measured by odds ratios (ORs) with 95% confidence intervals (CIs). The significance of the pooled OR was determined by the Z-test, and P<0.05 was considered as statistically significant. For COMT gene val158met, the meta-analysis examined the association between met allele and FM risk compared with that for val allele (met versus val); co-dominant model (val/met versus val/val, met/met versus val/val), dominant model (val/met+met/met versus val/val) and recessive model (met/met versus val/met+ val/val) were also used. Subgroup analyses were done by ethnicity and HWE.
Heterogeneity among studies was assessed by using the chi-square-based Q test and I2 statistics [20]. When P>0.10, the pooled OR of each study was calculated by using the fixed-effects model [21]; otherwise, the random-effects model [22] was used. The Galbraith plot was used to detect the potential sources of heterogeneity, and re-analyses were conducted when the studies possibly causing the heterogeneity were excluded [23]. Relative influence of each study on the pooled estimate was assessed by omitting one study at a time for sensitivity analysis.
Publication bias was evaluated with the funnel plot, in which the standard error of log (OR) of each study was plotted against its log (OR). An asymmetric plot suggests a possible publication bias. Funnel plot asymmetry was assessed by the method of Egger’s linear regression test (P<0.05 was considered representative of statistically significant publication bias) [24]. All analyses were done using STATA software, version 11.0 (STATA Corp., College Station, TX, USA).
Results
Characteristics of the studies
There were 113 papers relevant to the search words. The flow chart of selection of studies and reasons for exclusion is presented in Figure 1. Overall, 7 publications with 8 case-control studies including 589 cases and 527 controls were available for this analysis. Study characteristics are summarized in Table 1. Among those 8 case-control studies, there were 5 studies about Caucasians, 3 studies about mixed, respectively. Genotyping methods included PCR-RFLP and TaqMan. The genotype distributions in the controls of all studies were in agreement with Hardy-Weinberg equilibrium except for three study [8,9,15] (Table 2).
Figure 1.
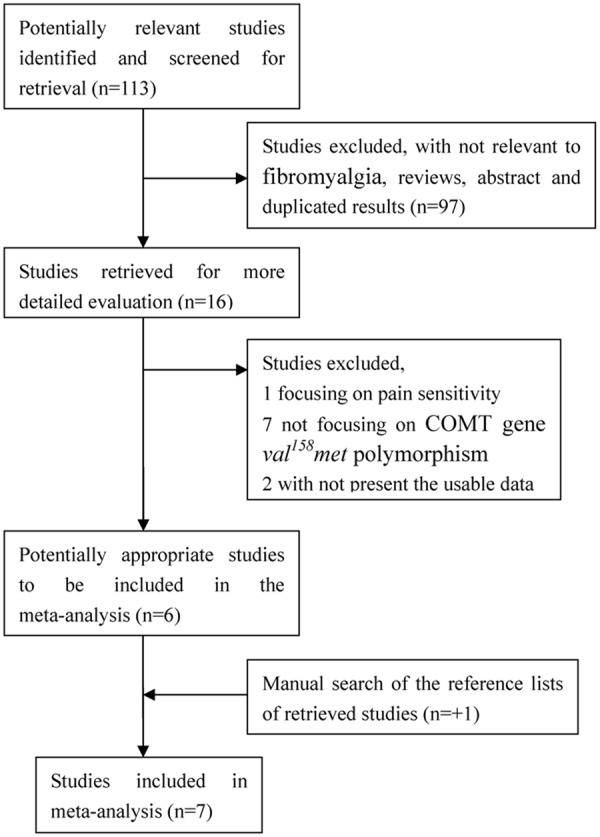
Flow chart of selection of studies and specific reasons for exclusion from the meta-analysis.
Table 2.
Characteristics of studies included in this meta-analysis
| Author | Year | Country | Ethnicity | Genotyping methods | Sample size (case/control) | Case | Control | Quality score | P HWE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Val/Val | Val/Met | Met/Met | Val/Val | Val/Met | Met/Met | ||||||||
| Gursoy | 2003 | Turkey | Caucasian | PCR-RFLP | 61/61 | 16 | 33 | 12 | 29 | 22 | 10 | 8 | 0.116 |
| Gilberto | 2007 | Spain | Caucasian | Taqman | 78/80 | 29 | 40 | 9 | 19 | 39 | 22 | 9 | 0.833 |
| Gilberto | 2007 | Mexico | Mixed | Taqman | 57/53 | 23 | 32 | 2 | 15 | 14 | 4 | 9 | 0.794 |
| Tander | 2008 | Turkey | Caucasian | PCR-RFLP | 80/91 | 26 | 32 | 22 | 30 | 34 | 27 | 9 | 0.016 |
| Potvin | 2009 | Cannda | Caucasian | PCR-RFLP | 37/36 | 8 | 23 | 6 | 9 | 21 | 6 | 7 | 0.294 |
| Matsuda | 2010 | Brazil | Mixed | PCR-RFLP | 51/51 | 9 | 23 | 19 | 15 | 31 | 5 | 7 | 0.059 |
| Martinez | 2012 | Spain | Caucasian | Taqman | 113/65 | 34 | 52 | 27 | 17 | 43 | 5 | 10 | 0.003 |
| Barbosa | 2012 | Brazil | Mixed | PCR-RFLP | 112/110 | 9 | 16 | 87 | 50 | 29 | 31 | 6 | < 0.001 |
PCR-RFLP: Polymerase Chain Reaction-restriction Fragment Length Polymorphism; HWE: Hardy-Weinberg Equilibrium.
Quantitative synthesis
We pooled all the eight studies together and it resulted into 527 controls and 589 FM cases, to review the overall association between COMT gene val158met polymorphism and FM risk. Overall pooled analysis did not suggest any correlation between COMT gene val158met polymorphism and FM risk in all the five genetic comparison models, i.e., allele (met vs. val: OR=1.46, 95% CI=0.80-2.66, P heterpgeneity<0.001), homozygous (met/met vs. val/val: OR=1.72, 95% CI=0.61-4.87, P heterpgeneity<0.001), heterozygous (val/met vs. val/val: OR=1.25, 95% CI=0.82-1.92, P heterpgeneity=0.050), recessive (met/met vs. val/val+val/met: OR=1.52, 95% CI=0.60-3.86, P heterpgeneity<0.001) and dominant model (met/met+val/met vs. val/val: OR=1.52, 95% CI=0.80-2.90, P heterpgeneity<0.001) (Table 3). To explore the sources of heterogeneity, we performed further subgroup analyses by ethnicity (Figure 2) and controls within/without HWE (Figure 3) respectively. Similarly, there were no significant associations in the subgroup analyses, and significant heterogeneity in most of the comparison models still existed. Table 3 showed the detailed results. To explorer the potential sources of heterogeneity further, we performed the Galbraith’s test and accordingly singled out two study of Barbosa et al. and Gilberto et al (Spain) [8,14] as the main contributors to heterogeneity (Figure 4). When excluding the two studies, the heterogeneity disappeared in dominant model, but no significantly association was found (OR=1.29, 95% CI=0.94-1.78, I 2=16.8%, P heterpgeneity=0.305).
Table 3.
Quantitative analyses of the COMT gene val158met polymorphism on FM risk
| Variables | Na | Val/Met versus Val/Val | Met/Met versus Val/Val | Met versus Val | Val/Met + Met/Met versus Val/Val (dominant) | Met/Met versus Val/Met + Val/Val (recessive) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| OR (95% CI) | P b | OR (95% CI) | P b | OR (95% CI) | P b | OR (95% CI) | P b | OR (95% CI) | P b | ||
| Total | 8 | 1.25 (0.82-1.92) | 0.050 | 1.72 (0.61-4.87) | < 0.001 | 1.46 (0.80-2.66) | < 0.001 | 1.52 (0.80-2.90) | < 0.001 | 1.52 (0.60-3.86) | < 0.001 |
| Ethnicities | |||||||||||
| Caucasian | 5 | 1.05 (0.61-1.79) | 0.060 | 1.08 (0.48-2.44) | 0.015 | 1.03 (0.71-1.50) | 0.018 | 1.03 (0.62-1.74) | 0.048 | 1.04 (0.50-2.17) | 0.011 |
| Mixed | 3 | 1.80 (1.04-3.09) | 0.374 | 3.68 (0.51-26.48) | 0.001 | 2.57 (0.74-8.99) | < 0.001 | 2.88 (0.80-10.41) | 0.001 | 2.85 (0.55-14.73) | 0.001 |
| HWE in controls | |||||||||||
| Yes | 5 | 1.31 (0.79-1.08) | 0.170 | 1.09 (0.32-3.69) | 0.001 | 1.14 (0.67-1.93) | 0.001 | 1.29 (0.70-2.37) | 0.036 | 0.95 (0.33-2.72) | 0.001 |
| Caucasian | 3 | 1.30 (0.53-3.17) | 0.043 | 0.85 (0.22-3.34) | 0.013 | 0.98 (0.49-1.98) | 0.006 | 1.16 (0.42-3.23) | 0.011 | 0.72 (0.31-1.68) | 0.109 |
| Mixed | 2 | 1.37 (0.70-2.67) | 0.784 | 1.55 (0.08-28.29) | 0.009 | 1.45 (0.62-3.42) | 0.045 | 1.52 (0.80-2.87) | 0.484 | 1.30 (0.07-25.31) | 0.004 |
| No | 3 | 1.21 (0.51-2.86) | 0.025 | 3.41 (0.57-20.32) | < 0.001 | 2.14 (0.60-7.64) | < 0.001 | 1.98 (0.46-8.44) | < 0.001 | 3.11 (0.69-13.98) | < 0.001 |
The table given in bold indicate statistically significant values.
Number of comparisons.
P value of Q-test for heterogeneity test.
Random-effects model was used when P value for heterogeneity test, 0.10; otherwise, fix-effects model was used.
Figure 2.
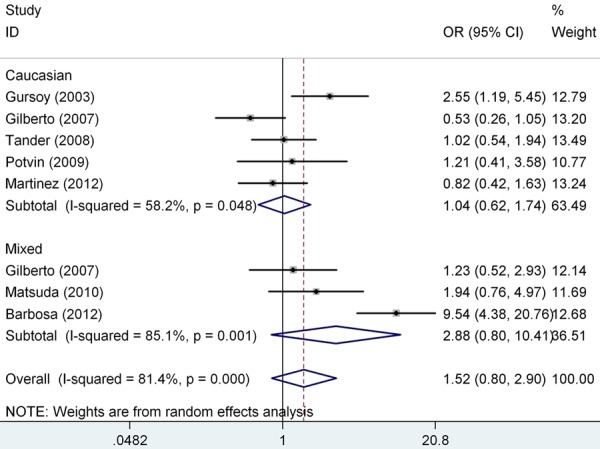
Odds ratios (OR) and 95% confidence interval (CI) of individual studies and pooled data for the association of the COMT gene val158met polymorphism and FM risk (met/met+val/met vs. val/val). I2, measure to quantify the degree of heterogeneity in meta-analyses.
Figure 3.
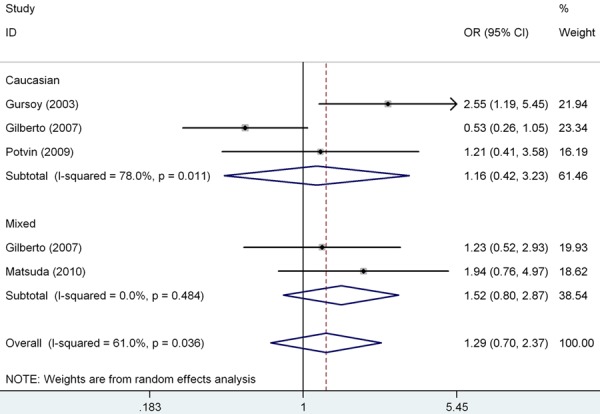
Forest plot showed the association of the COMT gene val158met polymorphism and FM risk, removing the study deviating from Hardy-Weinberg equilibrium (HWE) (met/met+val/met vs. val/val).
Figure 4.
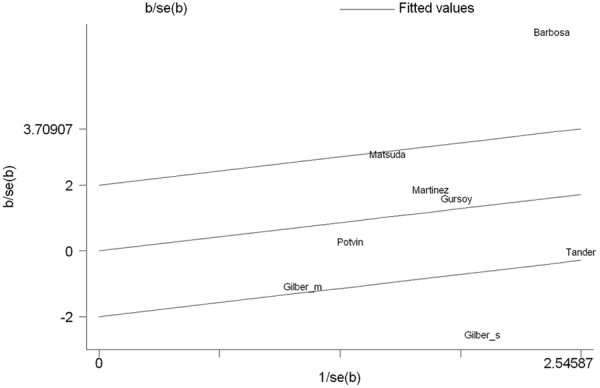
Galbraith plot of the COMT gene val158met polymorphism and FM risk. It indicated that two study was the potential source of heterogeneity.
Sensitivity analysis
Sensitivity analysis was performed by sequential omission of individual studies, and the result showed that no individual study affected the overall OR dominantly (Figure 5). This procedure confirmed the stability of our overall result.
Figure 5.
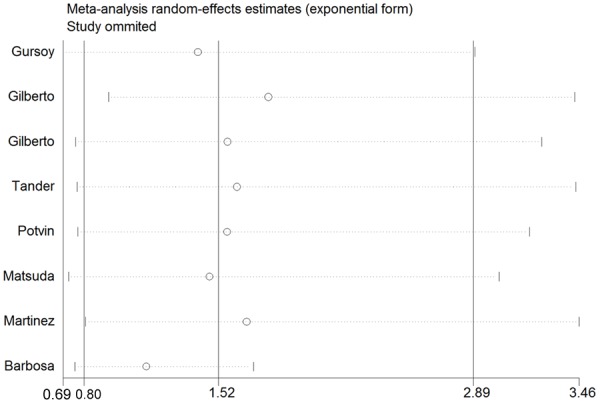
The influence of individual studies on the summary OR (met/met+val/met vs. val/val). The middle vertical axis indicates the overall OR and the two vertical axes indicate its 95% CI. Every hollow round indicates the pooled OR when the left study was omitted in this meta-analysis. The two ends of every broken line represent the 95% CI.
Publication bias
Begg’s funnel plot and Egger’s test were performed to assess publication bias among the literatures. No evidence of publication bias was observed in any comparison model (for met vs. val, Begg’s Test P=0.711, Egger’s test P=0.897; for met/met vs. val/val, Begg’s Test P=1.000, Egger’s test P=0.631; for val/met vs. val/val, Begg’s Test P=0.386, Egger’s test P=0.193; for met/met vs. val/val+val/met, Begg‘s Test P=0.902, Egger’s test P=0.371; for met/met+val/met vs. val/val, Begg’s Test P=0.386, Egger’s test P=0.506) (Figure 6).
Figure 6.
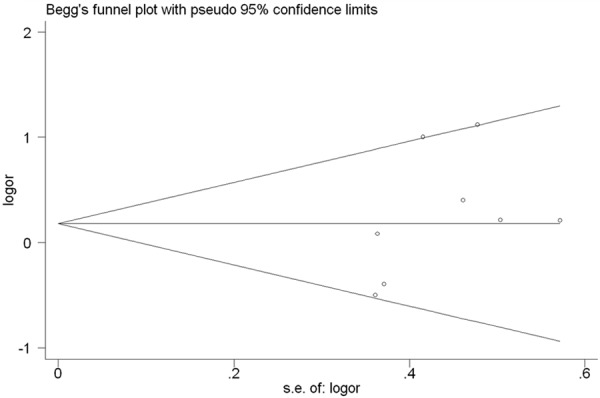
Begg’s funnel plot for publication bias test (val/met vs val/val). Each point represents a separate study for the indicated association.
Discussion
Fibromyalgia syndrome is characterized in part by abnormal central sensory processing of pain signals and is thought to arise from a combination of external pressure, hormones, neurotransmitters and the sympathetic nervous system [25]. Because of its painful and chronic character, the syndrome usually has a negative impact on the quality of life of patients. The prevalence of FM in the general population ranges from 0.66% to 4.4%, with the disease being 10 to 20 times more common among women than men [26,27]. Therefore, FM can be considered a major health problem among women [14]. Although the physiological mechanisms controlling fibromyalgia have not been fully established, neuroendocrine factors seem to play a key role. Increasing evidence suggests that genetic factors contribute significantly to individual differences in pain sensitivity, risk for developing clinical pain conditions and efficacy of pain treatments [28].
Single-nucleotide polymorphisms (SNPs) in the catecholamine-O-methyltransferase (COMT) enzyme gene have been extensively studied in association with pain perception and FM [8,9]. The well studied SNP (rs4680) occurs in codon 158 with a valine (val GTG) to methionine (met ATG) transition. The rs4680 may cause the three possible SNP genotypes: the H/H (GG val/val) genotype produces an effective enzyme, whereas the H/L (AG met/val) genotypes provide intermediate enzymatic activity, the L/L (AA met/met) genotype gives rise to a defective enzyme, which is unable to effectively remove catecholamines from the system [29]. Josep et al. [30] demonstrated that Spanish FM patients with met-158-met genotype have a more severe form of the disease when compared with affected individuals with the val-158-val genotype. Gilberto et al. [14] reported a significant association between three SNPs (rs6269, rs4818, and rs4680) and the presence of FM and high pain sensitivity in Spanish patients. However, this result was not observed in Mexican patients. Recently, several studies have focused on the role of the SNP rs4680 polymorphism in FM [8,9,13-17]. However, the data reported for individual study were limited and not able to support a convincible conclusion. Therefore, in the current study, we performed a meta-analysis to evaluate the influence of COMT gene val158met polymorphism on FM susceptibility. This study might help to explore a more robust estimate about the role of this polymorphism with FM risk, as combining data from many studies has the advantage of reduced random errors [31].
In this meta-analysis, no association of the COMT gene val158met polymorphism with FM risk was found under all comparisons and in subgroup analysis by ethnicity and HWE. The significant heterogeneity was found among studies in overall comparisons and also subgroup analyses. To explore the potential sources of heterogeneity further, we performed the Galbraith’s test and accordingly singled out two studies of Barbosa et al. and Gilberto et al (Spain) [8,14] as the main contributors to heterogeneity. When excluding the two studies, the heterogeneity disappeared in dominant model, but no significantly association was found. Therefore, our meta-analysis suggests that COMT gene val158met polymorphism is not associated with FM risk.
As far as we know, this is the first comprehensive meta-analysis exploring the association between COMT gene val158met polymorphism and FM risk up to now, which involved 589 cases and 527 controls from 8 case-control studies. Our meta-analysis also has some advantages. First, the search and selection studies were conducted strictly. Second, no evidence of publication bias was found by Begg’s funnel plot and Egger’s test, indicating that the whole pooled results may be unbiased. Despite of the advantages mentioned above, the current study has some inevitable limitations that should be acknowledged. First, only published studies were included in this meta-analysis, unpublished data and ongoing studies were not sought, which may have biased our results. Second, there was significant heterogeneity among included studies. Even though we used the random-effect model to calculate pool ORs, the precision of outcome would be affected. Third, our results were based on an unadjusted estimated, a more precise analysis would have been conducted if more detailed individual data were available.
In conclusion, this meta-analysis suggests that the COMT gene val158met polymorphism is not associated with FM risk. However, future well designed large studies, particularly stratified by gene-gene and gene-environment interactions might be necessary to clarify the possible role of the COMT gene val158met polymorphism in the susceptibility to FM.
Disclosure of conflict of interest
None.
References
- 1.Belt NK, Kronholm E, Kauppi MJ. Sleep problems in fibromyalgia and rheumatoid arthritis compared with the general population. Clin Exp Rheumatol. 2009;27:35–41. [PubMed] [Google Scholar]
- 2.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 3.Da Costa D, Dobkin PL, Fitzcharles MA, Fortin PR, Beaulieu A, Zummer M, Senécal JL, Goulet JR, Rich E, Choquette D, Clarke AE. Determinants of health status in fibromyalgia: a comparative study with systemic lupus erythematosus. J Rheumatol. 2000;27:365–72. [PubMed] [Google Scholar]
- 4.Mas AJ, Carmona L, Valverde M, Ribas B. Prevalence and impact of fibromyalgia on function and quality of life in individuals from the general population: results from a nationwide study in Spain. Clin Exp Rheumatol. 2008;26:519–26. [PubMed] [Google Scholar]
- 5.Buskila D, Sarzi-Puttini P. Biology and therapy of fibromyalgia. Genetic aspects of fibromyalgia syndrome. Arthritis Res Ther. 2006;8:218. doi: 10.1186/ar2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ablin JN, Cohen H, Buskila D. Mechanisms of Disease: genetics of fibromyalgia. Nat Clin Pract Rheumatol. 2006;2:671–8. doi: 10.1038/ncprheum0349. [DOI] [PubMed] [Google Scholar]
- 7.Zhu BT. Catechol-O-methyltransferase (COMT)-mediated methylation metabolism of endogenous bioactive catechols and modulation by endobiotics and xenobiotics: importance in pathophysiology and pathogenesis. Curr Drug Metab. 2002;3:321–49. doi: 10.2174/1389200023337586. [DOI] [PubMed] [Google Scholar]
- 8.Barbosa FR, Matsuda JB, Mazucato M, de Castro França S, Zingaretti SM, da Silva LM, Martinez-Rossi NM, Júnior MF, Marins M, Fachin AL. Influence of catechol-O-methyltransferase (COMT) gene polymorphisms in pain sensibility of Brazilian fibromialgia patients. Rheumatol Int. 2012;32:427–30. doi: 10.1007/s00296-010-1659-z. [DOI] [PubMed] [Google Scholar]
- 9.Martínez-Jauand M, Sitges C, Rodríguez V, Picornell A, Ramon M, Buskila D, Montoya P. Pain sensitivity in fibromyalgia is associated with catechol-O-methyltransferase (COMT) gene. Eur J Pain. 2013;17:16–27. doi: 10.1002/j.1532-2149.2012.00153.x. [DOI] [PubMed] [Google Scholar]
- 10.Winterer G, Goldman D. Genetics of human prefrontal function. Brain Res Brain Res Rev. 2003;43:134–63. doi: 10.1016/s0165-0173(03)00205-4. [DOI] [PubMed] [Google Scholar]
- 11.Funke B, Malhotra AK, Finn CT, Plocik AM, Lake SL, Lencz T, DeRosse P, Kane JM, Kucherlapati R. COMT genetic variation confers risk for psychotic and affective disorders: a case control study. Behav Brain Funct. 2005;1:19. doi: 10.1186/1744-9081-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–3. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 13.Gürsoy S, Erdal E, Herken H, Madenci E, Alaşehirli B, Erdal N. Significance of catechol-O-methyltransferase gene polymorphism in fibromyalgia syndrome. Rheumatol Int. 2003;23:104–7. doi: 10.1007/s00296-002-0260-5. [DOI] [PubMed] [Google Scholar]
- 14.Vargas-Alarcón G, Fragoso JM, Cruz-Robles D, Vargas A, Vargas A, Lao-Villadóniga JI, García-Fructuoso F, Ramos-Kuri M, Hernández F, Springall R, Bojalil R, Vallejo M, Martínez-Lavín M. Catechol-O-methyltransferase gene haplotypes in Mexican and Spanish patients with fibromyalgia. Arthritis Res Ther. 2007;9:R110. doi: 10.1186/ar2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tander B, Gunes S, Boke O, Alayli G, Kara N, Bagci H, Canturk F. Polymorphisms of the serotonin-2A receptor and catechol-O-methyltransferase genes: a study on fibromyalgia susceptibility. Rheumatol Int. 2008;28:685–91. doi: 10.1007/s00296-008-0525-8. [DOI] [PubMed] [Google Scholar]
- 16.Potvin S, Larouche A, Normand E, de Souza JB, Gaumond I, Grignon S, Marchand S. DRD3 Ser9Gly polymorphism is related to thermal pain perception and modulation in chronic widespread pain patients and healthy controls. J Pain. 2009;10:969–75. doi: 10.1016/j.jpain.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda JB, Barbosa FR, Morel LJ, França Sde C, Zingaretti SM, da Silva LM, Pereira AM, Marins M, Fachin AL. Serotonin receptor (5-HT 2A) and catechol-O-methyltransferase (COMT) gene polymorphisms: triggers of fibromyalgia? Rev Bras Reumatol. 2010;50:141–9. [PubMed] [Google Scholar]
- 18.Thakkinstian A, D’Este C, Eisman J, Nguyen T, Attia J. Meta-analysis of molecular association studies: vitamin D receptor gene polymorphisms and BMD as a case study. J Bone Miner Res. 2004;19:419–28. doi: 10.1359/JBMR.0301265. [DOI] [PubMed] [Google Scholar]
- 19.Thakkinstian A, McEvoy M, Minelli C, Gibson P, Hancox B, Duffy D, Thompson J, Hall I, Kaufman J, Leung TF, Helms PJ, Hakonarson H, Halpi E, Navon R, Attia J. Systematic review and meta-analysis of the association between {beta}2-adrenoceptor polymorphisms and asthma: a HuGE review. Am J Epidemiol. 2005;162:201–11. doi: 10.1093/aje/kwi184. [DOI] [PubMed] [Google Scholar]
- 20.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 21.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med. 1988;7:889–94. doi: 10.1002/sim.4780070807. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace DJ, Linker-Israeli M, Hallegua D, Silverman S, Silver D, Weisman MH. Cytokines play an aetiopathogenetic role in fibromyalgia: a hypothesis and pilot study. Rheumatology (Oxford) 2001;40:743–9. doi: 10.1093/rheumatology/40.7.743. [DOI] [PubMed] [Google Scholar]
- 26.Assumpção A, Cavalcante AB, Capela CE, Sauer JF, Chalot SD, Pereira CA, Marques AP. Prevalence of fibromyalgia in a low socioeconomic status population. BMC Musculoskelet Disord. 2009;10:64. doi: 10.1186/1471-2474-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yunus MB, Inanici F, Aldag JC, Mangold RF. Fibromyalgia in men: comparison of clinical features with women. J Rheumatol. 2000;27:485–90. [PubMed] [Google Scholar]
- 28.Martínez-Jauand M, Sitges C, Rodríguez V, Picornell A, Ramon M, Buskila D, Montoya P. Pain sensitivity in fibromyalgia is associated with catechol-O-methyltransferase (COMT) gene. Eur J Pain. 2013;17:16–27. doi: 10.1002/j.1532-2149.2012.00153.x. [DOI] [PubMed] [Google Scholar]
- 29.Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 30.Josep García-Fructuoso F, Ignacio Lao-Villadóniga J, Beyer K, Santos C. Relationship between COMT gene genotypes and severity of fibromyalgia. Reumatol Clin. 2006;2:168–72. doi: 10.1016/S1699-258X(06)73042-X. [DOI] [PubMed] [Google Scholar]
- 31.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–76. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]


