Abstract
Epithelial-mesenchymal transition (EMT) plays an important role in cancer invasion and metastasis by enabling cancer cells to depart from the primary tumor, invade surrounding tissue and disseminate to distant organs. The existence and function of EMT in cervical cancer is poorly understood. Placental growth factor (PLGF) has been shown to associate with EMT in various cancers. However, whether PLGF is involved in EMT in cervical cancer remains unclear. Thus the present study examined the relationship between PLGF expression and EMT-related proteins in 110 cervical lesions samples. We detected that PLGF was expressed in 61.8% cervical lesion sections. In addition, PLGF expression is positively correlated with low expression level of E-cadherin and high expression level of vimentin. Serum samples and cervical lavage samples were collected from patients with pre-invasive and invasive lesion of uterine cervix or normal control group, the PLGF levels were determined by enzyme-linked immunosorbent assay (ELISA). We found that a significantly high level of PLGF could be detected both in serum and vaginal lavage compared with normal women group, and there is no significant difference between serum and lavage in PLGF level. In addition, whatever in lavage or in serum, the PLGF level in stage I and II was significantly higher than it in CINIII or cancer in situ. However, there is no significant difference between the stage I and stage II; we also found that exogenous PLGF promotes molecular changes of epithelial-mesenchymal transition (EMT) in siha cells. In addition, application of a specific EKR1/2 inhibitor could reverse the effects of PLGF. These findings suggested that PLGF could regulate the expression of EMT-related proteins and promote migration of siha cells through ERK/MAPK signaling pathway. Therapies that targets PLGF/Flt-1/ERK/MAPK signaling pathway may be beneficial in treatment of cervical cancer.
Keywords: Placenta growth factor (PLGF), epithelial-mesenchymal transition (EMT), cervical cancer
Introduction
Cervical cancer is the leading cause of death among women in developing world and is the fourth most common cancer among women worldwide [1], with an estimated 528,000 new cases and 266,000 deaths in 2012 [1]. Over 85% of cases occur in less developed regions, where cervical cancer accounts for almost 12% of female cancers and stands out as a major cause of cancer-related deaths. In China, more than 60,000 women are diagnosed with cervical cancer contributing to about 30,000 deaths every year [1]. It is widely accepted that high-risk human papillomavirus (hr-HPV) serves as the main factor in cervical cancer [2]. Although two prophylactic HPV vaccines have been developed [2,3], However, these two vaccines have limited protection for women who have infected with high-risk HPV and in addition are out of reach of the majority of women in developing countries due to their high costs [4].
Cancer metastasis is the most tough problem in cancer therapy. In this process, polarized epithelial tumor cells converted into motile mesenchymal cells, invade the basement membrane beneath, enter the blood vessels, and disseminated to distant organs. The initial step of these processes is associated with morphological changes defined as epithelial-mesenchymal transition (EMT). EMT has been intensively studied recently, and has been implicated in tumor progression and metastasis [5], which is characterized by morphological changes from epithelial cells to fibroblast-like cells, loss of intercellular junctions and increased cell mobility [6,7]. Down-regulation of E-cadherin was the critical step in EMT process, which is achieved by up-regulation of transcription factors Snail, Slug, or Twist [8-10]. Although the role of EMT in tumor invasion and metastasis is a hot topic recently, its regulation mechanism is not fully understood.
Previous studies have provided convincing evidence for the activation of EMT in various primary epithelial tumors [11-13]. Interestingly, recent studies reveal a dynamic requirement of EMT in tumor metastasis: activation of EMT promotes local tumor invasion, while reversion of EMT is essential to establish metastasis in distant organs [14-16]. The “reversible” EMT model implies that EMT is unlikely to be regulated by permanent genetic and epigenetic changes in tumor cells; instead, EMT is dynamically controlled by various proinvasion signals from the tumor microenvironment (TME). The TME has been intensively studied as a dynamic system and is defined as the cellular and physical environment surrounding the primary tumor including endothelial, inflammatory and immune cells, fibroblasts, ECM components and soluble factors [5]. Cervical cancer is considered as a model of the relation occurring between the tumor micro-environment and tumor development [17]. The chronic inflammatory status of the cervix, sustained by the infection of HPV, as well as the production of cytokines and growth factors in the local cervix, lead to an intricate microenvironment [18-21]. Although the persistent infection of hr-HPV is the main pathogenic factor for cervical cancer, only a small fraction of women infected with hr-HPV develops into cervical cancer, indicating that HPV itself may not be sufficient to cause cervical cancer [22]. Other factors may contribute to the development of cervical cancer. How does the cancer cells acquire the ability to invade the surrounding tissue is not fully understood, accumulating evidence supports Epithelial-mesenchymal transition (EMT) plays a critical role in this process [17]. However, the detailed mechanism of EMT and its association with the progression of cervical cancer is unclear. Various growth factors secreted by the cancer cells and host cells in their local microenvironments may trigger the molecular events of the EMT program. Placenta growth factor (PLGF) has been reported as a potent stimulator in cancer invasion through activating angiogenesis. Previous researches indicated that infection of hr-HPV can induced the secretion of PLGF in cancer cells [23,24] and PLGF is overexpressed in cervical cancer tissues compared to adjacent normal tissues [25]. Recent studies suggested that PLGF not only has the ability to promote angiogenesis but also can activate EMT program [26]. However, how does PLGF regulate of EMT in cervical cancer is not fully understood.
The aim of this study was to evaluate the correlation between PLGF expression and EMT-related proteins in cervical lesions and detect the expression level of PLGF in serum and vaginal lavage of cervical lesion patients, and then analyze the correlation between them. Finally, we demonstrated that PLGF/Flt-1 signaling pathway could regulate the expression of EMT-related proteins, which also dependent on ERK/MAPK signaling pathway activation.
Methods
Cell line and reagents
Siha is a human cervical cancer cell line purchased from ATCC, and was cultured in Dulbecco’s modified Eagle’s media: Nutrient Mixture F-12 (DMEM/F12) supplemented with 10% fetal bovine serum (Hyclone, USA). All cells used in our experiments were at passages 3 to 15 after obtaining them from the suppliers. Cells were cultured in the absence of serum overnight prior to the treatment with PLGF-1 (PeproTech Inc.) for the indicated periods. Inhibitors PD98059, LY294002, and SP600125 were purchased from Sigma (USA).
Patients and samples
110 pathologically verified specimens of cervical lesions and the matched adjacent non-lesion samples were harvested from surgical resections in the Third Affiliated Hospital, third military medical university from January, 2011 to July, 2013. Among them, 34 cases were identified as cervical intraepithelial neoplasia (CIN) III or cancer in situ, and the remaining lesions were invasive, including 48 cases were stage I and 28 cases were stage II based on the staging system of the International Federation of Gynecology and Obstetrics. All patients did not undergo any chemotherapy or radiotherapy before surgery. The mean age of the patients was 51.8 years (23-79 years). Their clinicopathological parameters are summarized in Table 1. All the cases were individually categorized by independent pathologists. The procedure was approved by the Committee for the Conduct of Human Ethics Committee of the Third Affiliated Hospital, Third Military Medical University. Written informed consent was obtained from each patient enrolled in the study.
Table 1.
Correlation analysis of clinicopathological features with expression levels of PLGF, Flt-1, E-cadherin and vimentin in 110 invasive cervical carcer samples
| Variable | PLGF | Flt-1 | E-cad | Vimentin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| low | high | P | low | high | P | low | high | P | low | high | P | |
| Age | ||||||||||||
| < 50 years | 17 | 27 | 0.936 | 16 | 28 | 0.632 | 23 | 21 | 0.092 | 21 | 23 | 0.235 |
| ≥ 50 years | 25 | 41 | 27 | 39 | 45 | 21 | 24 | 42 | ||||
| Histological grade | ||||||||||||
| HG1 | 3 | 9 | 0.006 | 5 | 7 | 0.172 | 8 | 4 | < 0.001 | 8 | 4 | < 0.001 |
| HG2 | 21 | 35 | 24 | 34 | 26 | 30 | 32 | 24 | ||||
| HG3 | 18 | 24 | 14 | 28 | 34 | 8 | 5 | 37 | ||||
| Staging | ||||||||||||
| CINIII & cancer in situ | 18 | 16 | 0.006 | 16 | 18 | 0.172 | 14 | 20 | < 0.01 | |||
| Stage I | 20 | 28 | 14 | 34 | 28 | 26 | 8 | 26 | < 0.01 | |||
| Stage II | 4 | 24 | 13 | 15 | 20 | 2 | 32 | 16 | ||||
| LN status | 5 | 23 | ||||||||||
| negative | 25 | 24 | 0.013 | 25 | 24 | 32 | 17 | 0.500 | 28 | 21 | 0.002 | |
| positive | 17 | 44 | 18 | 43 | 36 | 25 | 17 | 44 | ||||
HG: histological grade; CIN: cervical intraepithelial neoplasia; LN: lymph node. P < 0.05 was considered as statistically significant.
ELISA assay
For measurement of PLGF, vaginal lavage specimens were collected in 5 ml of normal saline and serum were collected as routine protocol, and both samples were frozen at -80°C until testing. Specimens were measured by enzyme-linked immuno-sorbent assay (BioHJ, Inc China) in triplicate according to the manufacturer’s instruction. The concentrations of PLGF were expressed as ng/ml.
Immunohistochemistry
Fresh tissue specimens were fixed and embedded, and placed on slides as 2-3 um. Immunohistochemistry was performed following the routine protocol. The slides were incubated with the following primary antibodies overnight at 4°C: a rabbit polyclonal PLGF antibody (1:50; Abcam); a rabbit polyclonal Flt-1 antibody (1:50; Abcam); a rabbit polyclonal E-cadherin antibody (1:100; Fantibody); a mouse polyclonal Vientin antibody (1:100; Fantibody). Following washes in PBS, sections were incubated with an appropriate secondary antibody (polymerized horseradish peroxidase [HRP]-anti rabbit IgG; ZSGB-BIO, Beijing, China) for 20 min at 37°C, followed by incubation with streptavidin-peroxidase (DAKO) for 15 min at 37°C. 3,3’- -diaminobenzidine (DAB; DAKO) was applied as the chromogenic agent. Then slides were counterstained with hematoxylin, dehydrated in graded ethanol, and coverslipped. The primary antibody was replaced with PBS or normal goat serum for the blank or negative control, respectively. The expression levels were independently evaluated by two investigators. The intensity of staining was recorded as follows: High grade (++) staining were defined as more than 10% of tumor cells showed immunoactivity or defined as low grade.
Generation of siha cells stably transfected with Flt-1 shRNA
Flt-1-shRNA and control shNC in eukaryotic lentiviral vectors/puromycin were obtained from Santa Cruz Biotechnology, Inc. 24 h before infection, cells were seeded onto 6-well culture plates. When cells grew to 40-70% confluence, remove the culture media and replace with 2 ml 6 μg/ml polybrene (Santa Cruz: sc-134220), adding the lentiviral particles to infect the cells, gently mix and incubate overnight, remove the culture and replace 2 ml of complete medium (without polybrene), incubate the cells overnight; 48 h post-infection, puromycin (Santa Cruz) was added to the cell culture media at a final concentration of 6 μg/ml for 3 weeks. Media was changed once every 3-4 days. After 3 weeks, puromycin -resistant colonies were isolated and cultured in 24-well culture plates for further experiments.
Cell migration assays
To assess the migration of siha cells, 24-well plates with 8.0 mm pore transwells (Millipore) were applied. The top chamber was planted with 2 × 104 cells (siha or siha/Flt-1-sh-RNA) in 200 ml of complete medium without FBS. In the lower chamber, 500 ml of complete medium containing 10% FBS with or without PLGF was added as a chemoattractant. The cells on the top surface of the membrane were removed. 4 h later, migrated cells on the underside of the membrane were fixed with methanol, stained with solution and counted in 10 random fields under a microscope.
Western blot analysis
Harvested cells were washed twice with PBS, the Cells were lysed in RIPA buffer with proteinase inhibitors for 30 min on ice, and then centrifuged at 12,000 rpm for 20 min at 4°C. The supernatant was collected as total cellular protein and its concentration was determined using the Bradford assay (Biotime Chemicals, Bangalore, India). Equivalent amounts of total cellular protein were subjected to SDS-PAGE (8-12%) followed by blotting on a polyvinylidene difluoride (PVDF) membrane. After blocking in 5% nonfat dry milk for 2 h, membranes were incubated with the following antibodies overnight at 4°C: a mouse polyclonal vimentin antibody (1:1,000; Fantibody); a rabbit polyclonal E-cadherin antibody (1:1000; Fantibody); a mouse monoclonal Flt-1 antibody (1:1000, Abcam) and a mouse monoclonal β-actin antibody (1:2000; ZSGB-BIO, Beijing, China). The membranes were then washed and incubated with a HRP-conjugated secondary antibody (Santa Cruz, CA, USA) for 2 h at room temperature and visualized by enhanced chemiluminescence (Biorad Bio- technology). β-actin expression was used as a loading control for whole cell lysates.
Statistical analysis
The SPSS13.0 statistical software package was applied in statistical analysis. All data were statistically analyzed using one-way ANOVA with a Bonferoni Correction. The values were depicted as mean ± standard and were considered significant if P < 0.05.
Results
Overexpression of PLGF and its receptor Flt-1 in cervical cancer tissues is associated with EMT-related proteins
We selected 110 cases of cervical lesions and matched adjacent normal tissues to examine the expression of PLGF and Flt-1 and its association with EMT-related proteins by Immunohistochemistry. Correlation analysis between expression levels of PLGF, Flt-1, Ecadherin and Vimentin and clinicopathological features are summarized in Table 1. Consistent with previous studies, the expression of PlGF protein was undetectable in normal tissues, and is significantly higher in the cancer tissues (shown in Figure 1A and 1B), the PLGF is mainly expressed in the cytoplasm of cancer cells (Figure 1B) in 61.8% (68/110) patients (Table 1). Also, the PLGF expression was detectable in 47.1% (16/34) of samples in CINIII or cancer in situ, 58.3% (28/48) in stage I and 85.7% (24/28) in stage II (Table 1). Moreover, PLGF expression was detected in 49.0% (24/49) of samples in the lymph node-negative group and 72.1% (44/61) of samples in the lymph node-positive group (Table 1). The cytoplasmic expression of PLGF is significantly associated with tumor stage and the node-positive tumor status (P = 0.006 and P = 0.013 respectively).
Figure 1.
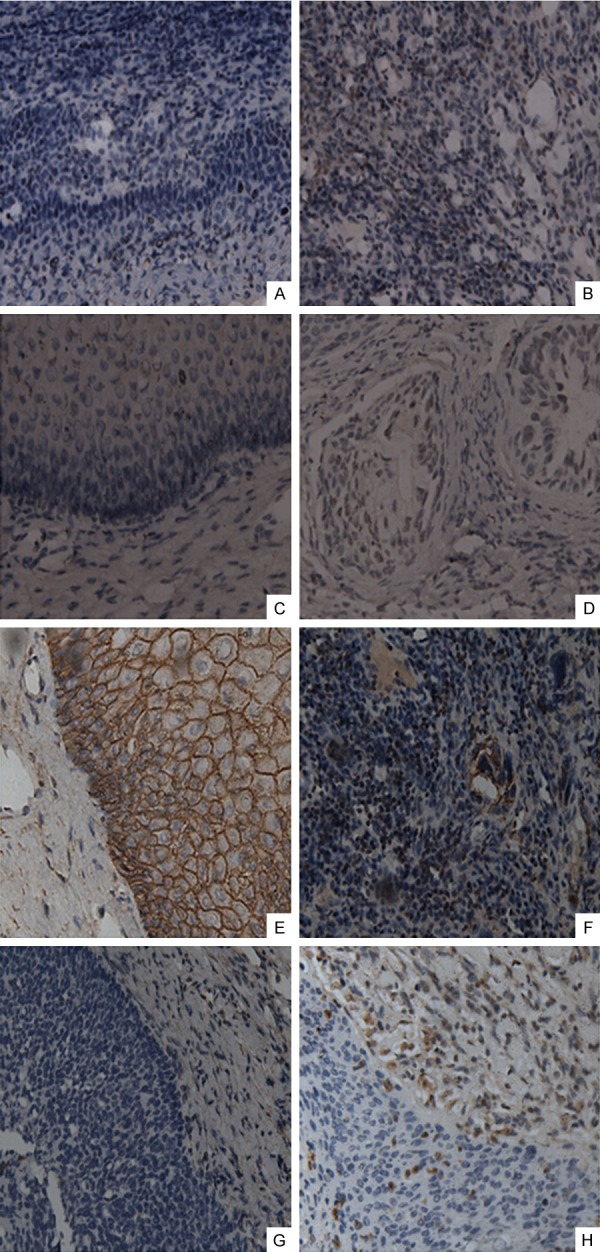
Immunohistochemical analysis of PLGF, Flt-1, E-cadherin and Vimentin in cervical carcinoma tissues and adjacent normal cervical tissues. PLGF (A), Flt-1 (C), E-cadherin (E), and vimentin (G) expression in normal cervix tissues, and PLGF (B), Flt-1 (D), E-cadherin (F) and vimentin (H) expression in tumor tissues (magnification: 200×).
The expression of Flt-1 protein could not be detected in normal cervix tissues (Figure 1C), and significantly high expressed in the cytoplasm of the tumor cells (Figure 1D) in 60.9% of patients (Table 1). Consistent with previous study, Flt-1 expression was detected in 49.0% of samples in the lymph node-negative group and 70.5% of samples in the lymph node-positive group (Table 1). A significant association was observed between the cytoplasmic expression of Flt-1 and the node-positive tumor status (P = 0.022). However, the Flt-1 expression has no significant association with the tumor stage (P = 0.172).
E-cadherin was normally present in the cell membranes of normal cervical tissues, but weakly expressed in tumor tissues (Figure 1E and 1F). Nearly 61.8% (68/110) of the tumor sections showed loss or reduction of E-cadherin expression (Table 1). The reduction of E-cadherin expression was detected in 41.2% (14/34) of the CINIII or cancer in situ and in 58.3% (28/48) and 92.9% (26/28) of the stage I and II tumors respectively. The reduction of E-cadherin expression is significantly associated and histological grade and tumor stage (both P < 0.01) (Table 1). However, the reduction of E-cadherin expression has no significant association with the node-positive tumor status (P = 0.5) (Table 1).
Vimentin was absent in normal cervical tissues, but highly expressed in tumor tissues (Figure 1G and 1H) in 59.1% of patients (Table 1). Vimentin expression was detected in 42.9% of samples with the lymph node-negative and 72.1% of samples with the lymph node-positive (Table 1), the Flt-1 expression is significant associated with the node-positive tumor status (P = 0.002). Moreover, the expression of vimentin was strongly associated with high-grade and late-stage tumors (both P < 0.01) (Table 1).
Correlation analysis between expression levels of PLGF, Flt-1, E-cadherin and Vimentin in cervical lesion samples
Then, we intended to analyze the correlation between the expression levels of PLGF and EMT-related proteins in cervical carcinoma tissues. In agreement with protein changes during EMT, the weak expression of E-cadherin was significantly correlated with high expression of vimentin (P < 0.01, r = -0.426) (Table 2). In addition, high PLGF expression was significantly correlated with the reduction of E-cadherin expression and the increase of vimentin expression (P < 0.01, r = -0.618; P < 0.01, r = 0.450, respectively) (Table 2). These analyses indicate that PLGF is associated with the EMT-related proteins expression, and may be an inducer in activating the EMT process. Particularly, we found that high PLGF expression was strongly associated with Flt-1 up-regulation (r = 0.828, P < 0.01), which suggested that PLGF may be involved in EMT process through activating its receptor Flt-1.
Table 2.
Association analysis between expression levels of PLGF, Flt-1, E-cadherin, vimentin in 110 invasive cervical carcinoma samples
| Definition | Flt-1 | E-cadherin | vimentin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Low | High | P | Low | High | P | Low | High | P | ||||
| PLGF | ||||||||||||
| Low | 38 | 4 | < 0.01 | 42 | 1 | < 0.01 | 29 | 13 | < 0.01 | |||
| High | 5 | 63 | 26 | 41 | 16 | 52 | ||||||
| Spearman correlation | 0.828 | -0.618 | 0.450 | |||||||||
| Flt-1 | ||||||||||||
| Low | 43 | 0 | < 0.01 | 34 | 11 | < 0.01 | ||||||
| High | 25 | 42 | 11 | 54 | ||||||||
| Spearman correlation | -0.630 | 0.622 | ||||||||||
| E-cadherin | ||||||||||||
| Low | 39 | 29 | < 0.01 | |||||||||
| High | 6 | 36 | ||||||||||
| Spearman correlation | -0.426 | |||||||||||
The significant difference was defined as P < 0.05.
The level of PLGF in serum and vaginal lavage of cervical cancer patients
Since the PLGF is over-expressed in cervical cancer tissues and significantly correlated with the expression level of EMT-related proteins. We further want to detect the level of PLGF in serum and vaginal lavage of cervical cancer patients. We chose 26 cases normal women, 22 cases of CINIII or cancer in situ patients and 36 cases of invasive cervical cancer patients, including 22 cases in stage I and 14 cases in stage II. And collected the serum and vaginal lavage samples before surgical excision of the cancer tissue. The mean PLGF levels are presented in Table 3.
Table 3.
The mean serum and lavage PLGF levels in normal control subjects and cervical cancer patients before surgical excision of tumor
| Groups | PLGF levels | Mean ± SD (ng/ml) | ||
|---|---|---|---|---|
|
| ||||
| lavage | P | serum | P | |
| Normal control subjects (n = 26) | 11.29 ± 0.9036 | 9.926 ± 1.049 | ||
| CINIII or. cancer in situ (n = 22) | 21.91 ± 1.110 | P < 0.05 | 18.89 ± 1.400 | P < 0.05 |
| CCI (n = 22) | 26.77 ± 0.6499 | P < 0.05 | 24.50 ± 1.211 | P < 0.05 |
| CCII (14) | 26.25 ± 1.959 | P < 0.05 | 26.95 ± 1.579 | P < 0.05 |
CIN: cervical intraepithelial neoplasia; CC: cervical cancer. P < 0.05 was defined as significant.
As show in Table 3, we found that a significantly high level of PLGF could be detected both in serum and vaginal lavage compared with normal women group (P < 0.05), and there is no significant difference between serum and lavage in PLGF level (P = 0.405, data not show). In addition, the mean serum PLGF level was significantly higher in cervical cancer (CC)patients (22.96 ± 0.90 ng/ml) than in normal control individuals (9.926 ± 1.049 ng/ml, P<0.001) (Table 3). Also, the serum PLGF level is positively associated with the tumor stage (P < 0.001, Figure 2), however, there is no significant difference between the stage I and stage II. These phenomena could also be observed in the lavage PLGF levels. In the lavage, the PLGF level was significantly higher in CC patients (24.80 ± 0.73 ng/ml) than in normal control individuals (11.29 ± 0.90 ng/ml, P < 0.001) (Table 3). Whatever in vaginal lavage or in serum, the PLGF level in stage I and II was significantly higher than it in CINIII or cancer in situ group. However, there is no significant difference between the stage I and stage II (Figure 2).
Figure 2.
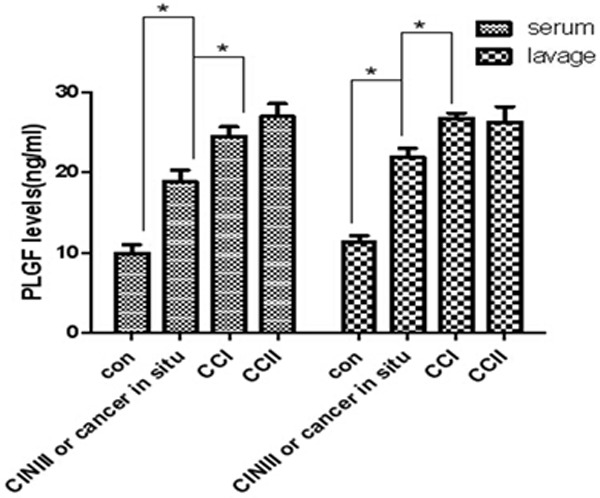
The mean PLGF level in serum and lavage of CC patients and normal control individuals. Data were shown as mean ± SD; Con: normal control group; CIN: cervical intraepithelial neoplasia; CC: cervical cancer. *P < 0.05 was defined as significant.
Our data suggested that PLGF is overexpressed in cervical lesions, and secreted in serum and lavage, which is an important element in cervical microenvironment. And the high PLGF level is significantly associated with tumor stage, which may plays an important role in the progression of cervical cancer.
PLGF promoted the migration in Siha cells through activating Flt-1 signal pathway
Now that, the PLGF is over-expressed in serum and vaginal lavage of the CC patients, we next want to see exogenous PLGF could activated migration in Siha cells, we obtained the PLGF from Peprotech, Inc. and the siha cells were treated with PLGF (40 ng/ml for 4 h). After treated with PLGF, the migration of siha cells increased nearly 2-fold compared with cells without PLGF (P < 0.05) (Figure 3B); Since, PLGF could promote the migration in siha cells, from the literature, we know that Flt-1 is its specific receptor, so we want to know whether PLGF promote the migration of siha cells through activation of Flt-1 signaling pathway. The siha cells were transfected with Flt-1-shRNA lentiviral vector which express shRNAs against Flt-1 or a control sequence (NC). Puromycin-resistant cells expressed low level of Flt-1 analyzed by western blot using anti-Flt-1 antibody, as shown in Figure 3A, Flt-1 protein expression was significantly decreased in siha cells expressing Flt-1-shRNA. In addition, down-regulation of Flt-1 expression blocked PlGF-induced migration of siha cells (Figure 3B).These findings indicated that the migration ability of siha is in dependence of PLGF/Flt-1 signaling pathway activation.
Figure 3.
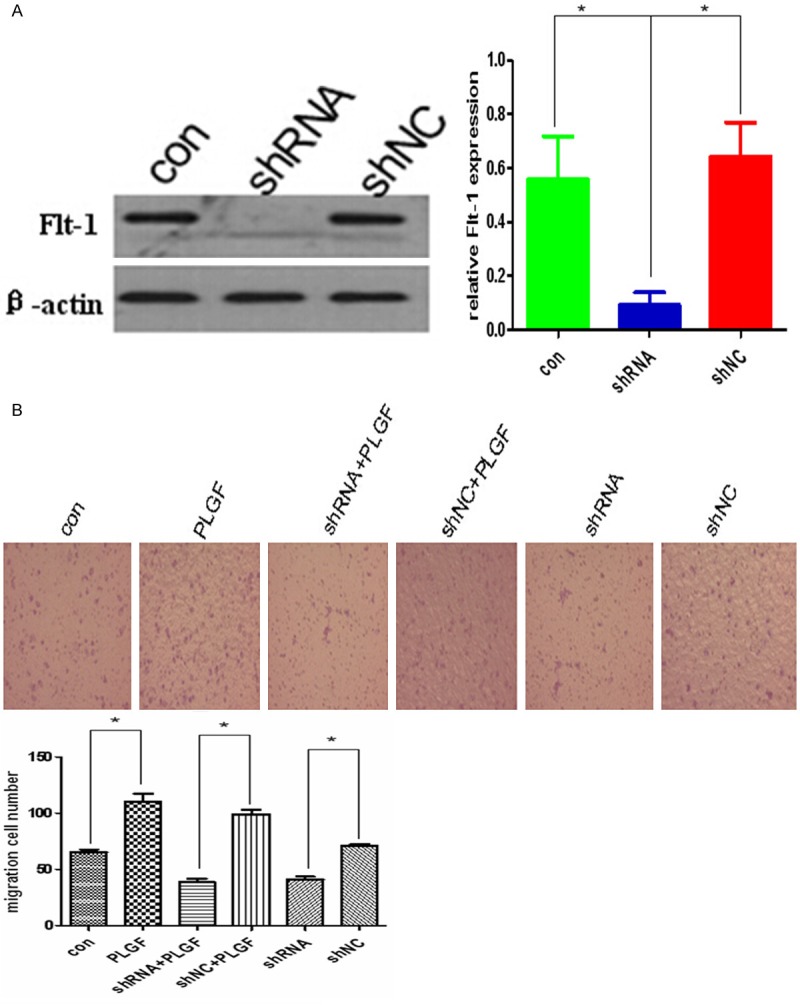
PLGF promote migration in siha cells through activating Flt-1 signaling pathway. A. Western blot analysis of Flt-1 expression in siha cells infected with a shRNA lentivirus against Flt-1. Flt-1 expression was significantly decreased in siha cells expressing Flt-1-shRNA. The bar graph shows the relative protein expression levels among groups. β-actin was used as a loading control. Data are presented as mean ± SD. for three independent experiments. *P < 0.05. B. PLGF promoted the migration of siha cells in vitro through Flt-1 signaling pathway. PLGF induced a 2-fold increase in migration of siha cells compared with the controls. Reduction of Flt-1 expression inhibited the PLGF-mediated migration of siha cells. *P < 0.05.
PLGF regulates EMT-related proteins expression in siha cells
To investigate whether PLGF promote cervical cancer progression through activating EMT process, we next examined the expression of EMT-related protein by western blot in siha after treated with PLGF. Siha cells normally expressed a high level of E-cadherin and a weak expression level of vimentin. Cells treated with PLGF was significantly reduced E-cadherin expression and increased vimentin expression. However, down-regulation of Flt-1, which is a receptor of PLGF, reduced the vimentin and increased the E-cadherin in siha cells. Furthermore, down-regulation of Flt-1 rescued the PLGF-mediated expression changes (Figure 4), which conversely increased the expression of E-cadherin and decreased the expression of vimentin.
Figure 4.
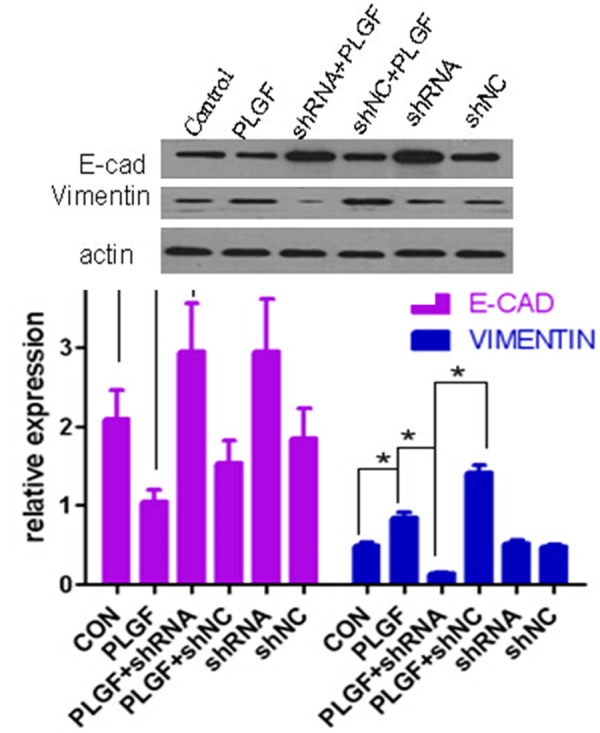
PLGF regulates EMT-related proteins expression in siha cells. Western blot analysis of mesenchymal cell markers and epithelial cell markers in siha cells when Flt-1 was activated by PlGF or down-regulated by shRNA. Western blot analysis of mesenchymal cell markers and epithelial cell markers in siha cells when Flt-1 was activated by PlGF or down-regulated by shRNA. β-actin was used as a loading control for whole cell lysate, The bar graphs show the relative expression of proteins among each treatment groups. Data are presented as mean ± SD. for three independent experiments. *P < 0.05.
PLGF regulated EMT-related proteins expression through ERK/MAPK signaling pathway
We next analyze the signaling pathway through which PLGF regulate the EMT-related protein expression. Cells were treated with a specific ERK/MAPK signaling pathway inhibitor, PD98059 (10 μmol/l), or application of a specific PI3k/Akt signaling pathway inhibitor, LY294002 (20 μmol/l), or a specific JNK pathway inhibitor, SP600125 (10 μmol/l) for 24 hr before treated with PLGF. Then the expression of E-cadherin was detect by Western blot (Figure 5A), Moreover, in a transwell migration assay, application of only PD98059 significantly decreased the migration of PLGF-treated siha cells (Figure 5B). These data suggested that PLGF may regulate the expression of E-cadherin protein via ERK/MAPK signaling pathway and inhibiting ERK/MAPK signaling pathway may decrease the migration of cervical cancer cells. Taken together, our data suggest that PLGF/Flt-1 signaling pathway plays an important role in regulating EMT-related proteins and promoting migration of cervical cancer cells, which is in dependence of ERK/MAPK signaling pathway.
Figure 5.
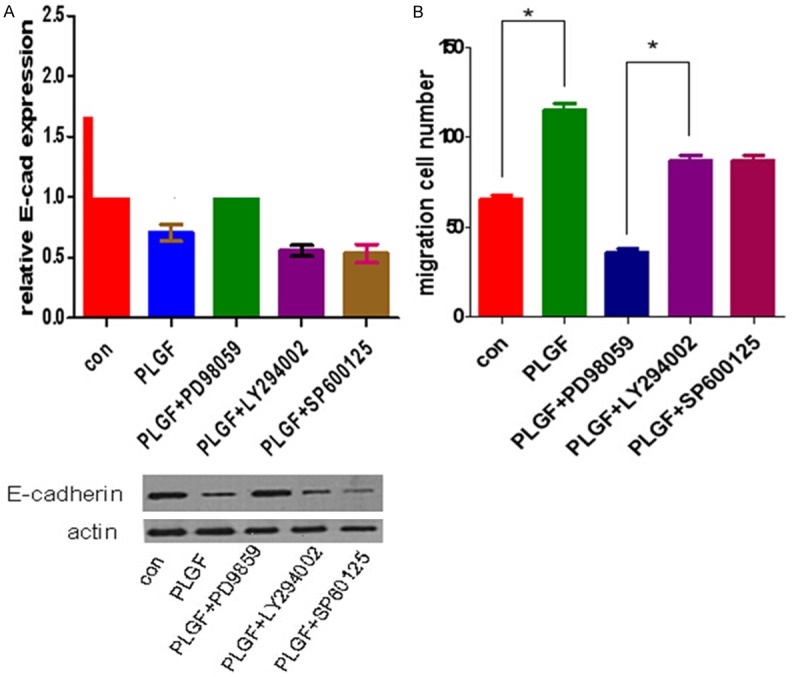
PLGF regulated the EMT-related protein expression through ERK/MAPK signaling pathway. A. Application of a specific ERK/MAPK signaling pathway inhibitor, PD98059 (10 μmol/l), or application of a specific PI3k/Akt signaling pathway inhibitor, LY294002 (20 μmol/l), or a specific JNK pathway inhibitor, SP600125 (10 μmol/l) to siha cells before treated with PLGF. PD98059 substantially abolished the decrease of E-cadherin expression by Western blot. B. Application of only PD98059 significantly decreased the migration of siha cells in a transwell assay. *P < 0.05.
Discussion
PLGF was initially identified as an angiogenic factor, which present in several types of tumor [27], suggesting that PLGF plays a role in tumor invasiveness. Previous studies reported that high level of PLGF could be detected in cancer microenvironment and the expression of PLGF is elevated after HPV infection [24,28-31]. And the expression of PLGF is up-regulated in cervical cancer tissues compared to adjacent normal tissues [25]. However, the percentage of positive PLGF expression in cervical cancer and the PLGF level in body fluid of cervical cancer patients is unclear. Since, Studies with tumor samples or experimental tumor xenograft models have provided convincing evidence that PLGF was abnormally expressed in many hypostatic tumors such as brain cancer [32,33], renal cell cancer [34,35], lung cancer [36-38], breast cancer and gastric cancer [39-42]. Furthermore, it has been reported that the expression of PLGF mRNA was founded elevated in cervical cancer tissues [25]. The purpose of this study was to examine the expression of PLGF in cervical lesions samples and detect the PLGF expression level in serum and vaginal lavage of cervical lesion suffers, and to explore its roles in the migration of cervical cancer cells.
In our study, we found that the PLGF is mainly expressed in the cytoplasm of cancer cells in 61.8% patients, and the high level of PLGF expression is significantly associated with tumor stage and the node-positive tumor status. This is agreement with previous studies showing that the high expression level of PLGF was presented in many kinds of tumors and is correlated with high recurrence risk and poor prognosis [43,44]. Furthermore, we examine the expression level of Flt-1 in cervical lesion tissues, which is a specific receptor of PLGF. We found that the expression of Flt-1 protein could not be detected in normal cervix tissues, and significantly high expressed in the cytoplasm of the tumor cells in 60.9% of patients. Consistent with previous study, a significant association was observed between the cytoplasmic expression of Flt-1 and the node-positive tumor status [45,46]. However, there is no significant association between Flt-1 expression and the tumor stage. These studies implied that in addition to its canonical role in angiogenesis, PLGF may play a role in tumor growth and metastasis though activating its receptor Flt-1 and may be an unfavorable progression indicator in cervical cancer.
The tumor microenvironment (TME) is defined as the cellular and physical environment surrounding the primary tumor including endothelial, inflammatory and immune cells, fibroblasts, ECM components and soluble factors [47]. Diverse growth factors secreted by the cancer cells and host cells in vaginal-cervix local microenvironments may promote the progression of cancer. The chronic inflammatory status of the cervix, sustained by the infection of HPV, as well as the production of cytokines and growth factors in the local cervix, lead to an intricate microenvironment [18-21], so cervical cancer is considered as a model of the relation occurring between the tumor micro-environment and tumor development [17]. Abundance evidence indicated that high expression level of PLGF could be observed in various tumors [27]. However, the expression level of PLGF in cervical cancer is unclear. Previous research indicated that high level of PLGF could be detected in diverse tumor microenvironments. Here, we want to know whether PLGF is also up-regulated in vaginal-cervical microenvironment and serum of cervical lesions suffers. We found that a significantly high level of PLGF could be detected both in serum and vaginal lavage compared with normal women group, and there is no significant difference between serum and lavage in PLGF level. In addition, whatever in lavage or in serum, the PLGF level in stage I and II was significantly higher than it in CINIII or cancer in situ. However, there is no significant difference between the stage I and stage II. These data suggested that high level of PLGF may associate with more advanced clinical stages and may be a surrogate biomarker for predicting recurrence risk and prognosis in cervical cancer. However, due to our limited sample size and hasty time, we did not compare the PLGF level in post-surgery patients, to deep understand the correlation between PLGF level and the prognosis and recurrence of cervical cancer needs further research.
During the progression of epithelial cancer, cells usually lose epithelial characteristic features and gain a mesenchymal phenotype, which is defined as Epithelial-mesenchymal transition (EMT) [12]. Cervical cancer is a common female malignancy worldwide. Despite the generally good prognosis for early-stage cervical cancer patients, many patients still die as a result of metastasis and recurrence. Epithelial-mesenchymal transition (EMT) has been implicated in the metastasis of primary tumors and provides molecular mechanisms for cervical cancer metastasis [11]. Through this complex process, epithelial-derived tumor cells lose intercellular tight adhesion and acquire a mesenchymal phenotype with increased migratory behavior. Down-regulation of epithelial cadherins (e.g., E-cadherin) and up-regulation of mesenchymal markers (e.g., vimentin.) are the most important characteristics for EMT [14]. However, evidence is lacking that PLGF plays a role in the EMT of uterine cervical cancer. Among the 110 cervical lesions in our study, we found that nearly 61.8% of the tumor sections showed loss or reduction of E-cadherin, while 59.1% showed positive vimentin expression. Correlation analysis indicated that the weak expression of E-cadherin was siginficantly correlated with high expression of Vimentin. In addition, high PLGF expression was significantly correlated with the reduction of E-cadherin expression and the increase of Vimentin expression. These analyses indicate that the process of EMT occurs in the progression of cervical and PLGF is associated with the EMT-related proteins expression. These data imply that PLGF may be an inducer in initiating the EMT process. Although PLGF was able to induce EMT in human pancreatic carcinoma cells and breast cancer cells, to our best knowledge, no studies have reported that PLGF is a possible mediator for EMT in cervical cancer. In this study, since PLGF/Flt-1 expression was strongly associated with expression of EMT-related proteins, we hypothesized that PLGF could regulate EMT of cervical cancer cells, leading to cervical cancer progression and metastasis.
Here, we found PLGF and its receptor are highly expressed in cervical cancer tissues, suggesting that PLGF could promote EMT though activating Flt-1 signaling pathway. The regulation of EMT in cervical cancer is not fully understood. Up to date, only membrane KCl cotransporter-3, human papillomavirus, and transforming growth factor-β1 and epithelial growth factor (EGF) were reported as EMT inducers in cervical cancer cell lines [48-52]. PLGF was mainly reported as an angiogenesis factor. Here, we found chronic PLGF treatments could decrease the expression of E-cadherin and increase the expression of vimentin in siha cells, the most important characteristic of EMT, which could be abolished by repressing the expression of Flt-1, suggesting that PLGF acts as an autocrine factor to activate the Flt-1 signaling pathway and regulate the EMT-related proteins expression. Furthermore, we found that PLGF-mediated Flt-1 activation promoted the migration in cervical cancer cell lines, and inhibit the expression of Flt-1 could reverse the effect of PLGF, which is consistent with the report using a breast cancer model [26]. However, it remains to be elucidated how PlGF activates cytoplasmic Flt-1. Also the down stream signaling pathway of Flt-1 is unclear. Our data suggest that PLGF may act as an autocrine factor to activate the Flt-1 signaling pathway and regulate the EMT-related proteins expression.
We next want to know the down stream signaling pathway of Flt-1. ERK/MAPK signaling pathways are highly conserved in all eukaryotes and are involved in numerous cellular responses [53]. Extracellular stimuli, like diverse growth factors, lead to activation of the ERK/MAPK through a MAPK signaling cascade [54]. Deregulation of the ERK/MAPK signaling pathway is often associated with many human diseases, including cancers [55,56]. Therefore, we applied a specific inhibitor of ERK1/2 signaling pathway. Surprisely, we found that use the ERK/MAPK pathway inhibitor could abolish the effect of PLGF on E-cadherin reduction and migration promotion in siha cells. However, using the AKT or other inhibitor doesn’t have such effects. These data indicated that ERK/MAPK signaling pathway may also participated in the process of PLGF activating EMT program.
In conclusion, we provide evidence that PLGF was overexpressed in cervical lesions samples and high PLGF level could be observed in the vaginal lavage and serum of cervical lesions suffers. In addition, the expression of PLGF in cervical lesions sections is significantly correlated with weak E-cadherin expression and high vimentin expression. Furthermore, exogenous PLGF could promote cervical cancer cell migration and regulate the EMT-related proteins expression, which is dependent on the ERK/MAPK signaling pathway. These findings suggest that PLGF/Flt-1 signaling pathway might be a potential target for neoadjuvant therapy in patients with cervical cancer. Therapies targeting PLGF/Flt-1/ERK/MAPK signaling may be a novel therapeutic approach for invasive cervical cancer patients. However, to fully understand the mechanism of EMT in cervical cancer, additional studies should be needed to further elucidate how PLGF regulates EMT and who are the factors regulating PLGF expression.
Acknowledgements
This work was supported by Grant No. 714-1690 from the National Natural Science Foundation of China. We thank Prof. Yuanguo Zhou for his advice for modification of this paper.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Munoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 3.Chang L, Ci P, Shi J, Zhai K, Feng X, Colombara D, Wang W, Qiao Y, Chen W, Wu Y. Distribution of genital wart human papillomavirus genotypes in China: a multi-center study. J Med Virol. 2013;85:1765–74. doi: 10.1002/jmv.23646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen Y, Pan XF, Zhao ZM, Chen F, Fu CJ, Li SQ, Zhao Y, Chang H, Xue QP, Yang CX. Knowledge of human papillomavirus (HPV) infection, cervical cancer, and HPV vaccine and its correlates among medical students in Southwest China: a multi-center cross-sectional survey. Asian Pac J Cancer Prev. 2014;15:5773–9. doi: 10.7314/apjcp.2014.15.14.5773. [DOI] [PubMed] [Google Scholar]
- 5.Jung HY, Fattet L, Yang J. Molecular Pathways: Linking Tumor Microenvironment to Epithelial-Mesenchymal Transition in Metastasis. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-3173. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–84. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavallaro U, Schaffhauser B, Christofori G. Cadherins and the tumour progression: is it all in a switch? Cancer Lett. 2002;176:123–8. doi: 10.1016/s0304-3835(01)00759-5. [DOI] [PubMed] [Google Scholar]
- 9.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Micalizzi DS, Ford HL. Epithelial-mesenchymal transition in development and cancer. Future Oncol. 2009;5:1129–43. doi: 10.2217/fon.09.94. [DOI] [PubMed] [Google Scholar]
- 12.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–34. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiery JP, Chua K, Sim WJ, Huang R. [Epithelial mesenchymal transition during development in fibrosis and in the progression of carcinoma] . Bull Cancer. 2010;97:1285–95. doi: 10.1684/bdc.2010.1206. [DOI] [PubMed] [Google Scholar]
- 14.Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39:305–18. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 15.Ito Y, Iwase T, Hatake K. Eradication of breast cancer cells in patients with distant metastasis: the finishing touches? Breast Cancer. 2012;19:206–11. doi: 10.1007/s12282-011-0266-5. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Zhou BP. Epithelial-mesenchymal transition in breast cancer progression and metastasis. Chin J Cancer. 2011;30:603–11. doi: 10.5732/cjc.011.10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qureshi R, Arora H, Rizvi MA. EMT in cervical cancer: Its role in tumour progression and response to therapy. Cancer Lett. 2014 doi: 10.1016/j.canlet.2014.09.021. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Wei XM, Wang Q, Gao SJ, Sui L. [Relationship between nitric oxide in cervical microenvironment and different HPV types and effect on cervical cancer cells] . Zhonghua Fu Chan Ke Za Zhi. 2011;46:260–5. [PubMed] [Google Scholar]
- 19.Ali KS, Ali HY, Jubrael JM. Concentration levels of IL-10 and TNFalpha cytokines in patients with human papilloma virus (HPV) DNA(+) and DNA(-) cervical lesions. J Immunotoxicol. 2012;9:168–72. doi: 10.3109/1547691X.2011.642419. [DOI] [PubMed] [Google Scholar]
- 20.Deligeoroglou E, Giannouli A, Athanasopoulos N, Karountzos V, Vatopoulou A, Dimopoulos K, Creatsas G. HPV infection: immunological aspects and their utility in future therapy. Infect Dis Obstet Gynecol. 2013;2013:540850. doi: 10.1155/2013/540850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeCarlo CA, Rosa B, Jackson R, Niccoli S, Escott NG, Zehbe I. Toll-like receptor transcriptome in the HPV-positive cervical cancer microenvironment. Clin Dev Immunol. 2012;2012:785825. doi: 10.1155/2012/785825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castle PE. Beyond human papillomavirus: the cervix, exogenous secondary factors, and the development of cervical precancer and cancer. J Low Genit Tract Dis. 2004;8:224–30. doi: 10.1097/00128360-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Hsu HW, Wall NR, Hsueh CT, Kim S, Ferris RL, Chen CS, Mirshahidi S. Combination antiangiogenic therapy and radiation in head and neck cancers. Oral Oncol. 2014;50:19–26. doi: 10.1016/j.oraloncology.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Xi L, Wang S, Wang C, Xu Q, Li P, Tian X, Wu P, Wang W, Deng D, Zhou J, Ma D. The pro-angiogenic factors stimulated by human papillomavirus type 16 E6 and E7 protein in C33A and human fibroblasts. Oncol Rep. 2009;21:25–31. [PubMed] [Google Scholar]
- 25.Yang S, Cheng H, Cai J, Cai L, Zhang J, Wang Z. PlGF expression in pre-invasive and invasive lesions of uterine cervix is associated with angiogenesis and lymphangiogenesis. APMIS. 2009;117:831–8. doi: 10.1111/j.1600-0463.2009.02538.x. [DOI] [PubMed] [Google Scholar]
- 26.Ning Q, Liu C, Hou L, Meng M, Zhang X, Luo M, Shao S, Zuo X, Zhao X. Vascular endothelial growth factor receptor-1 activation promotes migration and invasion of breast cancer cells through epithelial-mesenchymal transition. PLoS One. 2013;8:e65217. doi: 10.1371/journal.pone.0065217. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Dewerchin M, Carmeliet P. Placental growth factor in cancer. Expert Opin Ther Targets. 2014:1–16. doi: 10.1517/14728222.2014.948420. [DOI] [PubMed] [Google Scholar]
- 28.Giordano G, Febbraro A, Venditti M, Campidoglio S, Olivieri N, Raieta K, Parcesepe P, Imbriani GC, Remo A, Pancione M. Targeting angiogenesis and tumor microenvironment in metastatic colorectal cancer: role of aflibercept. Gastroenterol Res Pract. 2014;2014:526178. doi: 10.1155/2014/526178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagley RG, Ren Y, Weber W, Yao M, Kurtzberg L, Pinckney J, Bangari D, Nguyen C, Brondyk W, Kaplan J, Teicher BA. Placental growth factor upregulation is a host response to antiangiogenic therapy. Clin Cancer Res. 2011;17:976–88. doi: 10.1158/1078-0432.CCR-10-2687. [DOI] [PubMed] [Google Scholar]
- 30.Oude Munnink TH, Tamas KR, Lub-de Hooge MN, Vedelaar SR, Timmer-Bosscha H, Walenkamp AM, Weidner KM, Herting F, Tessier J, de Vries EG. Placental growth factor (PlGF)-specific uptake in tumor microenvironment of 89Zr-labeled PlGF antibody RO5323441. J Nucl Med. 2013;54:929–35. doi: 10.2967/jnumed.112.112086. [DOI] [PubMed] [Google Scholar]
- 31.Sands M, Howell K, Costello CM, McLoughlin P. Placenta growth factor and vascular endothelial growth factor B expression in the hypoxic lung. Respir Res. 2011;12:17. doi: 10.1186/1465-9921-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nomura M, Yamagishi S, Harada S, Yamashima T, Yamashita J, Yamamoto H. Placenta growth factor (PlGF) mRNA expression in brain tumors. J Neurooncol. 1998;40:123–30. doi: 10.1023/a:1006198422718. [DOI] [PubMed] [Google Scholar]
- 33.Gaal EI, Tammela T, Anisimov A, Marbacher S, Honkanen P, Zarkada G, Leppänen VM, Tatlisumak T, Hernesniemi J, Niemelä M, Alitalo K. Comparison of vascular growth factors in the murine brain reveals placenta growth factor as prime candidate for CNS revascularization. Blood. 2013;122:658–65. doi: 10.1182/blood-2012-07-441527. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto K, Suzuki K, Koike H, Okamura K, Tsuchiya K, Uchida T, Takezawa Y, Kobayashi M, Yamanaka H. Prognostic significance of plasma placental growth factor levels in renal cell cancer: an association with clinical characteristics and vascular endothelial growth factor levels. Anticancer Res. 2003;23:4953–8. [PubMed] [Google Scholar]
- 35.Rini BI, Michaelson MD, Rosenberg JE, Bukowski RM, Sosman JA, Stadler WM, Hutson TE, Margolin K, Harmon CS, DePrimo SE, Kim ST, Chen I, George DJ. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J. Clin. Oncol. 2008;26:3743–8. doi: 10.1200/JCO.2007.15.5416. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Ye L, Zhang L, Jiang WG. Placenta growth factor, PLGF, influences the motility of lung cancer cells, the role of Rho associated kinase, Rock1. J Cell Biochem. 2008;105:313–20. doi: 10.1002/jcb.21831. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Chen J, Ke Y, Mansel RE, Jiang WG. Expression of Placenta growth factor (PlGF) in non-small cell lung cancer (NSCLC) and the clinical and prognostic significance. World J Surg Oncol. 2005;3:68. doi: 10.1186/1477-7819-3-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li B, Wang C, Zhang Y, Zhao XY, Huang B, Wu PF, Li Q, Li H, Liu YS, Cao LY, Dai WM, Fang WG, Shang DS, Cao L, Zhao WD, Chen YH. Elevated PLGF contributes to small-cell lung cancer brain metastasis. Oncogene. 2013;32:2952–62. doi: 10.1038/onc.2012.313. [DOI] [PubMed] [Google Scholar]
- 39.Taylor AP, Goldenberg DM. Role of placenta growth factor in malignancy and evidence that an antagonistic PlGF/Flt-1 peptide inhibits the growth and metastasis of human breast cancer xenografts. Mol Cancer Ther. 2007;6:524–31. doi: 10.1158/1535-7163.MCT-06-0461. [DOI] [PubMed] [Google Scholar]
- 40.Taylor AP, Leon E, Goldenberg DM. Placental growth factor (PlGF) enhances breast cancer cell motility by mobilising ERK1/2 phosphorylation and cytoskeletal rearrangement. Br J Cancer. 2010;103:82–9. doi: 10.1038/sj.bjc.6605746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parr C, Watkins G, Boulton M, Cai J, Jiang WG. Placenta growth factor is over-expressed and has prognostic value in human breast cancer. Eur J Cancer. 2005;41:2819–27. doi: 10.1016/j.ejca.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 42.Chen CN, Hsieh FJ, Cheng YM, Cheng WF, Su YN, Chang KJ, Lee PH. The significance of placenta growth factor in angiogenesis and clinical outcome of human gastric cancer. Cancer Lett. 2004;213:73–82. doi: 10.1016/j.canlet.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Cheng SJ, Lee JJ, Cheng SL, Chen HM, Chang HH, Wang YP, Kok SH, Kuo MY, Chiang CP. Increased serum placenta growth factor level is significantly associated with progression, recurrence and poor prognosis of oral squamous cell carcinoma. Oral Oncol. 2012;48:424–8. doi: 10.1016/j.oraloncology.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Cheng SJ, Cheng SL, Lee JJ, Chen HM, Chang HH, Kok SH, Chiang ML, Kuo MY. Increased placenta growth factor mRNA level is significantly associated with progression, recurrence and poor prognosis of oral squamous cell carcinoma. J Formos Med Assoc. 2013;112:253–8. doi: 10.1016/j.jfma.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Karaca Z, Tanriverdi F, Unluhizarci K, Ozturk F, Gokahmetoglu S, Elbuken G, Cakir I, Bayram F, Kelestimur F. VEGFR1 expression is related to lymph node metastasis and serum VEGF may be a marker of progression in the follow-up of patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2011;164:277–84. doi: 10.1530/EJE-10-0967. [DOI] [PubMed] [Google Scholar]
- 46.Fujita K, Nakayama M, Nakai Y, Takayama H, Nishimura K, Ujike T, Nishimura K, Aozasa K, Okuyama A, Nonomura N. Vascular endothelial growth factor receptor 1 expression in pelvic lymph nodes predicts the risk of cancer progression after radical prostatectomy. Cancer Sci. 2009;100:1047–50. doi: 10.1111/j.1349-7006.2009.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leonardi GC, Candido S, Cervello M, Nicolosi D, Raiti F, Travali S, Spandidos DA, Libra M. The tumor microenvironment in hepatocellular carcinoma (review) Int J Oncol. 2012;40:1733–47. doi: 10.3892/ijo.2012.1408. [DOI] [PubMed] [Google Scholar]
- 48.Cheng YM, Chou CY, Hsu YC, Chen MJ, Wing LY. The role of human papillomavirus type 16 E6/E7 oncoproteins in cervical epithelial-mesenchymal transition and carcinogenesis. Oncol Lett. 2012;3:667–671. doi: 10.3892/ol.2011.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ha GH, Kim JL, Breuer EK. TACC3 is essential for EGF-mediated EMT in cervical cancer. PLoS One. 2013;8:e70353. doi: 10.1371/journal.pone.0070353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou XM, Zhang H, Han X. Role of epithelial to mesenchymal transition proteins in gynecological cancers: pathological and therapeutic perspectives. Tumour Biol. 2014;35:9523–30. doi: 10.1007/s13277-014-2537-1. [DOI] [PubMed] [Google Scholar]
- 51.Hsu YM, Chen YF, Chou CY, Tang MJ, Chen JH, Wilkins RJ, Ellory JC, Shen MR. KCl cotransporter-3 down-regulates E-cadherin/beta-catenin complex to promote epithelial-mesenchymal transition. Cancer Res. 2007;67:11064–73. doi: 10.1158/0008-5472.CAN-07-2443. [DOI] [PubMed] [Google Scholar]
- 52.Qureshi R, Arora H, Rizvi MA. EMT in cervical cancer: Its role in tumour progression and response to therapy. Cancer Lett. 2014 doi: 10.1016/j.canlet.2014.09.021. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Rubinfeld H, Seger R. The ERK cascade as a prototype of MAPK signaling pathways. Methods Mol Biol. 2004;250:1–28. doi: 10.1385/1-59259-671-1:1. [DOI] [PubMed] [Google Scholar]
- 54.Kyriakis JM. Making the connection: coupling of stress-activated ERK/MAPK (extracellular-signal-regulated kinase/mitogen-activated protein kinase) core signalling modules to extracellular stimuli and biological responses. Biochem Soc Symp. 1999;64:29–48. [PubMed] [Google Scholar]
- 55.Wang X, Cao X. Regulation of metastasis of pediatric multiple myeloma by MMP13. Tumour Biol. 2014;35:8715–20. doi: 10.1007/s13277-014-2147-y. [DOI] [PubMed] [Google Scholar]
- 56.Wang J, Su H, Han X, Xu K. Inhibition of fibroblast growth factor receptor signaling impairs metastasis of hepatocellular carcinoma. Tumour Biol. 2014 doi: 10.1007/s13277-014-2384-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


