Abstract
Background and purpose: To investigate the clinical significance of microRNA (miR)-23a and miR-23b expression in human gastric cancer (GC). Methods: Quantitative RT-PCR was performed to detect the expression changes of miR-23a and miR-23b in 160 human GC tissues and paired normal mucosa. The associations between miR-23a and miR-23b expression, and the selected clinicopathological characteristics and patients’ prognosis were also evaluated. Results: MiR-23a (GC vs. Normal: 3.98 ± 1.23 vs. 2.29 ± 1.12, P < 0.001) and miR-23b (GC vs. Normal: 3.70 ± 1.24 vs. 1.58 ± 1.18, P < 0.001) expression were both increased dramatically when compared with paired normal mucosa. Notably, the expression levels of miR-23a in GC tissues were positively correlated with those of miR-23b (Spearman correlation coefficient r = 0.77, P < 0.001). Then, the coexpression of miR-23a and miR-23b (miR-23a-high/miR-23b-high) in GC tissues was significantly associated with the advanced TNM stage (P < 0.001), the presence of lymph node metastasis (P = 0.008) and the great depth of invasion (P = 0.02). Furthermore, both univariate and multivariate analyses showed that miR-23a/miR-23b co-expression was an independent predictor for unfavorable overall survival. Conclusions: These results suggest that the dysregulation of miR-23a and miR-23b may be implicated in the progression of human GC. Combined expression of miR-23a and miR-23b appears to be a valuable marker for prognosis of this disease.
Keywords: Gastric cancer, microRNA-23a, microRNA-23b, clinicopathological feature, overall survival
Introduction
Gastric cancer (GC) is ranked as the fourth most common malignancy with an estimated one million new cases annually and the second leading cause of cancer mortality worldwide [1]. The geographic distribution of incidence and mortality of GC varies remarkably all through the world. Especially in China, its mortality rate accounts for 42% of the world total [2]. GC is affected by environmental and behavioral habits, including Helicobacter pylori infection, high-salted diet and smoking [3]. Despite significant advancements in diagnosis and treatment modalities, especially surgery, chemotherapy, and radiotherapy, the clinical outcome for GC patients remains poor. The 5-year survival rate of GC patients in China was lower than 40% [4]. Most GC patients are diagnosed at an advanced stage, when lymph node metastasis is as high as 50-75% [5]. As in all cancers, early diagnosis of GC remains the most promising approach to improve the long-term survival rate of patients with this malignancy. Although several clinicopatholgical features, such as TNM stage, histological differentiation and metastasis status, have been used to predict prognostic information of patients with GC. However, due to the patients’ heterogeneity, GC cases with the same clinicopatholgical features often have various survivals, implying that several complex molecular mechanisms may contribute to tumor progression and patients’ prognosis in GC. Therefore, it is a major goal to identify novel and efficient molecular markers for the early-stage diagnosis, for the treatment design and for the evaluation of prognosis in patients with GC.
MicroRNAs (miRNAs) comprise a subset of endogenous, small non-coding RNA molecules with 18-25 nucleotides in length. miRNAs exert their functions by base pairing between the seed region of miRNA and 3′’ un-translated regions of (3′’-UTR) of target mRNA, leading to the repression of protein translation of their targets mRNAs [6]. According to the data of the latest version of miRBase (Release 20: June 2013, http://www.mirbase.org/), there have been more than 2,578 miRNA sequences identified in the human genome. These miRNAs play crucial roles in various essential biological processes, including cell development, differentiation, proliferation, and apoptosis, which all affect major biological systems as stemness and immunity [7]. Since miRNAs have been specifically linked to critical developmental pathways, the dysregulation of many miRNAs has been shown to have functional significance for various human diseases such as cardiovascular disorders, inflammatory diseases, infections, developmental disorders muscular disorders, neurodegenerative diseases and many cancers [8]. In the context of cancers, dysregulated miRNAs have been reported to play either a tumor-suppressive or an oncogenic role in regulating tumor cell growth, cell cycles and cell migration, depending on functions of their target genes [9]. Tumor suppressive miRNAs usually repress oncogenes and oncogenic miRNAs usually silence tumor suppressor genes. Especially in human GC, Lu et al. [10] in 2005 originally reported the first miRNA expression profile of this cancer. After that, accumulating studies on miRNAs expression profile further found several significant deregulated miRNAs in GC cells versus normal gastric tissues. For example, miR-139 has been found to be expressed lowly in GC tissues compared with adjacent non-tumor tissues [11]; Overexpression of miR-139 could inhibit the proliferation of GC cell lines through inhibition of the gene expression of C-X-C chemokine receptor type 4 [12]; In contrast, miR-21 has been indicated to be an oncogenic miRNA in GC, and forced expression of miR-21 could significantly enhance cell proliferation and invasiveness in GC [13]. These findings imply that multiple miRNAs might have related functions in tumorigenesis of GC, and targeting them might be an effective therapy for this disease.
miR-23a and miR-23b belong to the miR-23a/24/27a cluster which is located in chromosome 19p13.12 and the miR-23b/27b/24-1 cluster which is located in chromosome 9q22.32, respectively [14,15]. They have been reported to be enhanced in acute lymphoblastic leukemia, acute myeloid leukemia, glioblastoma, hepatocellular carcinoma, GC, pancreatic cancer and uterine leiomyoma [16-19]. Notably, both miR-23a and miR-23b have been observed to be upregulated in GC tissues and miR-23a has also been indicated to function as an oncogene in this malignancy [20-23]. However, the clinical significance of miR-23a and miR-23b aberrant expression in GC remains unclear. The aim of this study was to evaluate the associations of miR-23a and miR-23b expression with clinicopathological characteristics and prognosis of patients with GC.
Materials and methods
Patients and tissue samples
This study was approved by the Research Ethics Committee of Huai’an First People’s Hospital of Nanjing Medical University and Lianshui Third People’s Hospital, China. Written informed consent was obtained from all of the patients. All specimens were handled and made anonymous according to the ethical and legal standards.
One hundred and sixty fresh specimens from 160 patients with GC (108 male and 52 female; median age: 58 years, range: 28-86 years) were acquired from Department of Gastroenterology of Huai’an First People’s Hospital from January 2005 to December 2010. All specimens were stored at -80°C until use to detect relative expression levels of miR-23a and miR-23b by quantitative RT-PCR. None of the patients had received any radiotherapy or chemotherapy before the operation. Surrounding normal gastric mucosa was also obtained and studied. All GC patients were classified according to the World Health Organization Pathological Classification of Tumors. Of 160 cases, 58 (36.25%) were well or moderately differentiated tumors and 102 (63.75%) were poor or no differentiation. Histologically, there were 10 cases of papillary adenocarcinoma, 92 cases of tubular adenocarcinoma, 50 cases of mucinous adenocarcinoma, and 8 cases of signet-ring cell carcinoma. There were 61 cases of intestinal histological type and 99 cases of diffuse histological type according to the Lauren classification. According to 2002 tumor-nodes-metastases (TNM) classification system, 16 cases were TNM stage I, 40 cases were stage II, 42 cases were stage III, and 62 cases were stage IV. The detail information on the clinical features of all 160 patients with GC in this study was shown in Table 1.
Table 1.
Associations of combined miR-23a and miR-23b expression with the clinicopathological features of gastric cancer (GC)
| Clinical features | Case Number (%) | miR-23a-high/miR-23b-high | |
|---|---|---|---|
|
| |||
| (N, %) | P | ||
| Age (years) | |||
| < 58 | 50 (31.25) | 20 (40.00) | NS |
| ≥ 58 | 110 (68.75) | 40 (36.36) | |
| Gender | |||
| Male | 108 (67.50) | 42 (38.89) | NS |
| Female | 52 (32.50) | 18 (34.62) | |
| Tumor size (cm) | |||
| < 4 | 58 (36.25) | 20 (34.48) | NS |
| ≥ 4 | 102 (63.75) | 40 (39.22) | |
| Lauren classification | |||
| Diffuse type | 99 (61.88) | 35 (35.35) | NS |
| Intestinal type | 61 (38.12) | 25 (40.98) | |
| Differentiation | |||
| Well or moderate | 58 (36.25) | 22 (37.93) | NS |
| Poor | 102 (63.75) | 38 (37.25) | |
| Lymph node metastasis | |||
| Negative | 100 (62.50) | 20 (20.00) | 0.008 |
| Positive | 60 (37.50) | 40 (66.67) | |
| TNM stage | |||
| I | 16 (10.00) | 0 (0.00) | < 0.001 |
| II | 40 (25.00) | 6 (15.00) | |
| III | 42 (26.25) | 15 (35.71) | |
| IV | 62 (38.75) | 39 (62.90) | |
| Depth of invasion | |||
| Mucosa or submucosa | 26 (16.25) | 1 (3.85) | 0.02 |
| Muscularis or subserosa | 20 (12.50) | 3 (15.00) | |
| Serosa | 82 (51.25) | 35 (42.68) | |
| Adjacent structure | 32 (20.00) | 21 (65.63) | |
Note: ‘NS’ refers to the difference without statistical significance.
All 160 patients with GC were given a follow-up exam ranging from 3 to 6 years. Patients who died from diseases other than GC or from unexpected events were excluded from the case collection in this study. For the analysis of survival and follow-up, the date of surgery was used to represent the beginning of the follow-up period. Overall survival was an endpoint which was calculated as the amount of time between the date of surgery and the date of death, regardless of the cause.
Quantitative RT-PCR
Total RNA was isolated with Trizol (Invitrogen, Carlsbad, CA). A total of 2 μg RNA was reverse transcribed using the SuperScript II RNase-Reverse Transcriptase System (Invitrogen). The cDNA was then subjected to real-time PCR with primers specific for miR-23a, miR-23b and U6 which was used as an internal control. PCR primers were designed as follows: miR-23a forward, 5’-ATC ACA TTG CCA GGG ATT TCC-3’; miR-23a reverse, 5’-CCA GTG CAG GGT CCG AGG T-3’; miR-23b forward, 5’-CGC GGC CGC TAG TAT TAT GTT-3’; miR-23b reverse, 5’-CAC ATT TTA AAA AAC ATA-3’; U6 forward, 5’-TGC GGG TGC TCG CTT CGG CAG C-3’; U6 reverse, 5’-CCA GTG CAG GGT CCG AGG T-3’. PCR cycles were as follows: 94°C for 4 min, followed by 40 cycles of 95°C for 1 min, 60 °C for 1 min and 72 °C for 1 min. The SYBR Premix Ex TaqTM kit (TaKaRa, Otsu, Shiga, Japan) was used to measure the amplified DNA, and real-time PCR was performed using an iQ5 real-time PCR detection system (Bio-Rad). The amount of miR-23a or miR-23b relative to U6 was calculated as the average 2-ΔCt, where ΔCt = Ct-CtU6.
Statistical analysis
The statistical analysis was performed by the software of SPSS version13.0 for Windows (SPSS Inc, IL, USA). Continuous variables were expressed as Mean ± S.D. The differences of miR-23a and miR-23b expression between GC tissues and normal gastric mucosa were analyzed by the paired-t test. The correlation between miR-23a expression and miR-23b expression in GC tissues was determined by the Spearman correlation analysis. The associations of combined expression of miR-23a and miR-23b proteins with various clinicopathological features of patients with GC were analyzed by Fisher’s exact test for any 2 × 2 tables and Pearson χ2 test for non-2 × 2 tables. The survival analysis was estimated by the Kaplan-Meier method and was compared by using the log-rank test. Multivariate analysis was performed using the Cox proportional hazard model. A difference was considered significant when P < 0.05.
Results
Upregulation of miR-23a and miR-23b in human GC tissues
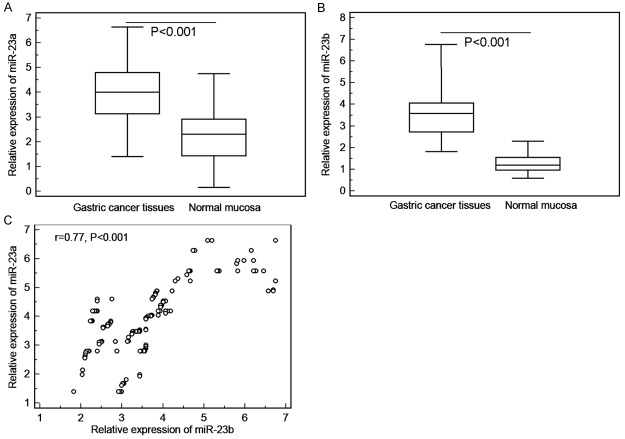
MiR-23a (GC vs. Normal: 3.98 ± 1.23 vs. 2.29 ± 1.12, P < 0.001, Figure 1A) and miR-23b (GC vs. Normal: 3.70 ± 1.24 vs. 1.58 ± 1.18, P < 0.001, Figure 1B) expression were both increased dramatically when compared with paired normal mucosa. Notably, the expression levels of miR-23a in GC tissues were positively correlated with those of miR-23b significantly (Spearman correlation coefficient r = 0.77, P < 0.001, Figure 1C).
Figure 1.
Relative expression of miR-23a (A) and miR-23b (B) in gastric cancer (GC) and paired normal mucosa. (C) Expression levels of miR-23a in GC tissues were positively correlated with those of miR-23b significantly (Spearman correlation coefficient r = 0.77, P < 0.001).
The median values (3.96 and 3.70, respectively) of miR-23a and miR-23b expression in GC tissues were used as the cutoff points for the classification of miR-23a-low/high and miR-23b-low/high groups. GC tissues with the relative expression levels of miR-23a and miR-23b exceeding median values for miR-23a or miR-23b were deemed to be miR-23a-high or miR-23b-high groups; all other tissues were considered to be miR-23a-low or miR-23b-low groups. Of 160 GC patients, 60 (37.50%) were both high expression of miR-23a and miR-23b, 56 (35.00%) were both low expression of miR-23a and miR-23b, 28 (17.50%) were miR-23a-high and miR-23b-low expression, and 16 (10.00%) were miR-23a-low and miR-23b-high expression.
Associations of miR-23a and miR-23b upregulation with the clinicopathological features of human GC
Since there was a significant correlation between miR-23a expression in GC tissues and miR-23b expression in GC tissues as shown above, we hypothesized that the combined expression of miR-23a and miR-23b might be associated with tumor progression of human GC. Table 1 summarized the associations of combined miR-23a and miR-23b upregulation with the clinicopathological features of GC patients. As a result, the coexpression of miR-23a and miR-23b (miR-23a-high/miR-23b-high) in GC tissues was significantly associated with the advanced TNM stage (P < 0.001, Table 1), the presence of lymph node metastasis (P = 0.008, Table 1) and the great depth of invasion (P = 0.02, Table 1).
Prognostic implications of combined miR-23a and miR-23b expression in GC
The associations of combined miR-23a and miR-23b expression with overall survival of patients with GC were analyzed using Kaplan-Meier method (Figure 2). The data showed that the overall survival of GC patients with miR-23a-high/miR-23b-high expression were shorter than any of other three groups (miR-23a-low/miR-23b-low, miR-23a-low/miR-23b-high, miR-23a-high/miR-23b-low) (P < 0.001), suggesting that patients in miR-23a-high/miR-23b-high expression group had the poorest prognosis in all four groups. In a multivariate Cox model, the data in Table 2 showed that TNM stage (P < 0.001), lymph node metastasis (P = 0.01), depth of invasion (P = 0.03) and combined miR-23a/miR-23b expression (P = 0.001) were all independent poor prognostic factors for the overall survival in patients with GC.
Figure 2.
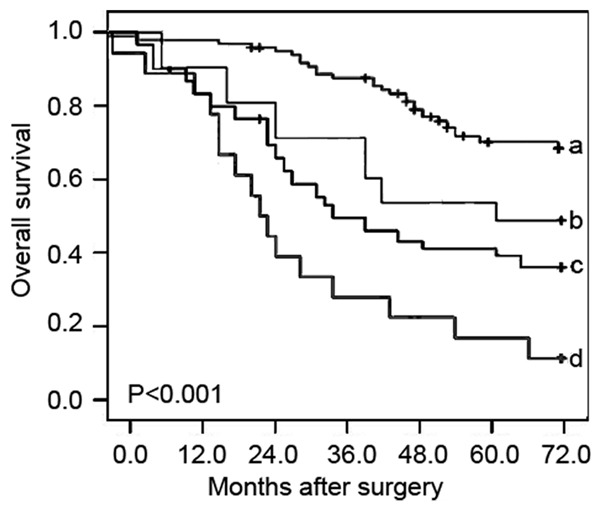
Kaplan-Meier survival curve of overall survival for combined expression miR-23a and miR-23b (miR-23a/miR-23b) in gastric cancer (GC). ‘a’ refers to miR-23a-low/miR-23b-low group; ‘b’ refers to miR-23a-low/miR-23b-high group; ‘c’ refers to miR-23a-high/miR-23b-low group; ‘d’ refers to miR-23a-high/miR-23b-high group.
Table 2.
Prognostic value of combined miR-23a and miR-23b expression for overall survival of patients with gastric cancer (GC) in univariate and multivariate analyses by Cox Regression
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
||||
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Age | 1.005 (0.101-2.008) | NS | 0.798 (0.122-1.689) | NS |
| Gender | 1.379 (0.303-2.743) | NS | 1.199 (0.336-2.306) | NS |
| Tumor size | 0.462 (0.202 -1.039) | NS | 0.729 (0.308 -1.692) | NS |
| Lauren classification | 2.232 (0.566-4.676) | NS | 1.928 (0.416-4.086) | NS |
| Differentiation | 1.019 (0.272-2.069) | NS | 1.012 (0.262-2.016) | NS |
| Lymph node metastasis | 4.658 (0.913-9.621) | 0.01 | 4.055 (0.839-8.228) | 0.01 |
| TNM stage | 10.039 (1.925-20.791) | < 0.001 | 9.462 (1.908-19.918) | < 0.001 |
| Depth of invasion | 3.939 (0.825-8.791) | 0.02 | 3.062 (0.768-6.918) | 0.03 |
| miR-23a/miR-23b | 8.698 (1.919-17.516) | < 0.001 | 6.869 (1.265-13.881) | 0.001 |
Discussion
As a major public health issue, GC results from the combined effects of environmental factors and genetic alterations, including oncogenes, tumor suppressor genes, cell adhesion molecules and growth factors [24]. Thus, it is of great significance to understand molecular mechanisms of this cancer. It is generally believed that clarifying and investigating the relationship between the expression of certain genes and tumor progression of GC may contribute to our understanding of the molecular mechanisms of gastric carcinogenesis and may help the identification of effective biomarkers for the diagnosis and prognosis for GC. miR-23a and miR-23b have been reported to play important roles in a wide variety of biological processes, including development, cell proliferation, apoptosis, differentiation, and carcinogenesis. In this retrospective study, we evaluated the expression of miR-23a and miR-23b in GC and the clinical implications of their combined expression. Our data showed that both miR-23a and miR-23b expression levels were upregulated in GC tissues as compared with that in paired normal gastric mucosae, and elevated miR-23a and miR-23b expression cooperated in tumor aggressive progression, including promoting TNM stage, enhancing depth of invasion and inducing lymph node metastasis. More importantly, miR-23a and miR-23b expression were both higher in the GC patients with shorter overall survival. These findings indicated that combined miR-23a and miR-23b overexpression was involved in the progression and prognosis of GC.
As one of the most famous members of the miR-23a/24/27a cluster, miR-23a functions as an oncomir in several types of human cancers and has diverse effects, including in cell proliferation, differentiation, metastasis and angiogenesis [25]. Cao et al. [17] revealed that miR-23a significantly promoted the migration and invasion of non-small cell lung cancer cells by targeting insulin receptor substrate-1 (IRS-1); Cheng et al. [18] provided evidence that miR-23a may be crucial in promoting neuroblastoma cell migration and invasion through targeting CDH1 and indicated that exogenous miR-23a may have therapeutic value in treating metastasis of this malignancy; miR-23a was also found to be up-regulated in the mouse liver tumors as well as in primary human HCC; It could enhance angiogenesis by promoting angiogenic signaling through targeting Sprouty2 and Sema6A proteins, which exert antiangiogenic activity [26]; Shang et al. [16] showed that miR-23a antisense could enhance 5-FU-induced apoptosis in colorectal cancer cells through the APAF-1/caspase-9 apoptotic pathway. Especially in GC, Zhu et al. [21] in 2010 showed the significant upregulation of miR-23a in gastric adenocarcinoma tissues, and further demonstrated that miR-23a could target IL6R and promote the growth activity of gastric adenocarcinoma cells in vitro; An et al. [22] in 2013 using the integration of CNV-miRNA-mRNA profiling demonstrated that miR-23a in amplified 19p13.13 loci promoted growth in gastric cancer cells by target metallothionein 2A; Zhu et al. [23] in the same year reported that the suppression of miR-23a with ASO-23a obviously inhibited cell growth, colony formation and invasiveness of MGC803 cells and significantly enhanced the cell apoptosis; Liu et al. [27] further indicated that miR-23a may suppress paclitaxel-induced apoptosis and promote cell viability and the colony formation ability of gastric adenocarcinoma cells by targeting IRF1 at the post-transcriptional level. These findings have shown great potential of miR-23a as a novel class of biomarkers for GC.
Different from miR-23a, miR-23b has the dual role in carcinogenesis. It has been found to be up-regulated or down-regulated in tumors compared with normal tissues, and functions as either tumor promoter or tumor suppressor [28]. In a variety of human tumors, downregulation of miR-23b have been demonstrated to promote cancer progression. For example, Li et al. [29] reported that decreased expression of miR-23b was significantly correlated with tumor aggressiveness and poor prognosis of patients with epithelial ovarian cancer and could suppress ovarian cancer progression by targeting runt-related transcription factor-2; Pellegrino et al. [30] demonstrated that miR-23b was involved in cytoskeletal remodelling through the enhancement of cell-cell interactions, reduction of cell motility and invasion during cancer progression; Majid et al. [31] revealed that miR-23b expression was dramatically reduced in bladder cancer cell lines and tumor tissues compared to the non-malignant counterparts, and conferred proliferative advantages, cell migration and invasion traits to these cells by regulating the expression of Zeb1, a direct target of this miRNA. Although the role of miR-23b as a tumor suppressor has been well established, many studies demonstrated that it can also act as an oncomir. For example, Chen et al. [32] observed the elevated expression of miR-23b in glioma tissues and different glioma cell lines and demonstrated that suppression of miR-23b upregulation could inhibit tumor survival, induce apoptosis and inhibit glioma invasion; Tian et al. [33] recently showed that a combination of four microRNAs, including miR-23b, could promote prostate cancer cell proliferation by regulating PTEN and its downstream signals. In human GC, there has been only one report indicating that miR-23b was one of 16 miRNAs upregulated in GC tissues, implying that this miRNA might be an oncomir for this cancer [20].
The previous findings that both miR-23a and miR-23b are upregulated in GC tissues and miR-23a is implicated in gastric carcinogenesis, allowed us to hypothesize that miR-23a/miR-23b combination might play an important role in GC. In the current study, we observed the overexpression of both miR-23a and miR-23b in GC tissues. The combined high expression of the two miRNAs was associated with aggressive tumor progression and patients’ prognosis. This differential effect has been confirmed by multivariate analysis and suggests that the co-expression of miR-23a and miR-23b may serve as a reliable prognostic factor. Determining the combined expression patterns of miR-23a and miR-23b might also help in further elucidating the risk of progression of patients with GC.
In conclusion, our data suggest that the dysregulation of miR-23a and miR-23b may be implicated in the aggressive progression of human GC. Combined expression of miR-23a and miR-23b appears to be a valuable marker for prognosis of this disease.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko S, Yoshimura T. Time trend analysis of gastric cancer incidence in Japan by histological types, 1975-1989. Br J Cancer. 2001;84:400–405. doi: 10.1054/bjoc.2000.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdecchia A, Corazziari I, Gatta G. Explaining gastric cancer survival differences among European countries. Int J Cancer. 2004;109:737–741. doi: 10.1002/ijc.20047. [DOI] [PubMed] [Google Scholar]
- 5.Alberts SR, Cervantes A, de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14(Suppl 2):ii31–6. doi: 10.1093/annonc/mdg726. [DOI] [PubMed] [Google Scholar]
- 6.Winter J, Jung S, Keller S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 7.Garzon R, Fabbri M, Cimmino A. MicroRNA expression and function in cancer. Trend Mol Med. 2006;12:580–587. doi: 10.1016/j.molmed.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 9.Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 10.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 11.Guo J, Miao Y, Xiao B, Huan R, Jiang Z, Meng D, Wang Y. Differential expression of microRNA species in human gastric cancer versus non-tumorous tissues. J Gastroenterol Hepatol. 2009;24:652–657. doi: 10.1111/j.1440-1746.2008.05666.x. [DOI] [PubMed] [Google Scholar]
- 12.Bao W, Fu HJ, Xie QS, Wang L, Zhang R, Guo ZY, Zhao J, Meng YL, Ren XL, Wang T, Li Q, Jin BQ, Yao LB, Wang RA, Fan DM, Chen SY, Jia LT, Yang AG. HER2 interacts with CD44 to up-regulate CXCR4 via epigenetic silencing of microRNA-139 in gastric cancer cells. Gastroenterology. 2011;141:2076–2087. e6. doi: 10.1053/j.gastro.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Zhou L, Li Y, Lin S, Tomuleasa C. MicroRNA-21 stimulates gastric cancer growth and invasion by inhibiting the tumor suppressor effects of programmed cell death protein 4 and phosphatase and tensin homolog. J BUON. 2014;19:228–236. [PubMed] [Google Scholar]
- 14.Jahid S, Sun J, Edwards RA, Dizon D, Panarelli NC, Milsom JW, Sikandar SS, Gümüs ZH, Lipkin SM. miR-23a promotes the transition from indolent to invasive colorectal cancer. Cancer Discov. 2012;2:540–553. doi: 10.1158/2159-8290.CD-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donadelli M, Dando I, Fiorini C, Palmieri M. Regulation of miR-23b expression and its dual role on ROS production and tumour development. Cancer Lett. 2014;349:107–113. doi: 10.1016/j.canlet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Shang J, Yang F, Wang Y, Wang Y, Xue G, Mei Q, Wang F, Sun S. MicroRNA-23a antisense enhances 5-fluorouracil chemosensitivity through APAF-1/caspase-9 apoptotic pathway in colorectal cancer cells. J Cell Biochem. 2014;115:772–784. doi: 10.1002/jcb.24721. [DOI] [PubMed] [Google Scholar]
- 17.Cao M, Seike M, Soeno C, Mizutani H, Kitamura K, Minegishi Y, Noro R, Yoshimura A, Cai L, Gemma A. MiR-23a regulates TGF-β-induced epithelial-mesenchymal transition by targeting E-cadherin in lung cancer cells. Int J Oncol. 2012;41:869–875. doi: 10.3892/ijo.2012.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng L, Yang T, Kuang Y, Kong B, Yu S, Shu H, Zhou H, Gu J. MicroRNA-23a promotes neuroblastoma cell metastasis by targeting CDH1. Oncol Lett. 2014;7:839–845. doi: 10.3892/ol.2014.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang N, Zhu M, Tsao SW, Man K, Zhang Z, Feng Y. MiR-23a-mediated inhibition of topoisomerase 1 expression potentiates cell response to etoposide in human hepatocellular carcinoma. Mol Cancer. 2013;12:119. doi: 10.1186/1476-4598-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Zhang Y, Zhang H, Liu X, Gong T, Li M, Sun L, Ji G, Shi Y, Han Z, Han S, Nie Y, Chen X, Zhao Q, Ding J, Wu K, Daiming F. miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol Cancer Res. 2011;9:824–833. doi: 10.1158/1541-7786.MCR-10-0529. [DOI] [PubMed] [Google Scholar]
- 21.Zhu L, Jin L, Jiang R, Wang Q, Jiang J, Mao C, Chen D. Correlations between miRNAs and TGF-β1 in tumor microenvironment of esophageal squamous cell cancer. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2013;29:524–528. [PubMed] [Google Scholar]
- 22.An J, Pan Y, Yan Z, Li W, Cui J, Yuan J, Tian L, Xing R, Lu Y. MiR-23a in amplified 19p13.13 loci targets metallothionein 2A and promotes growth in gastric cancer cells. J Cell Biochem. 2013;114:2160–2169. doi: 10.1002/jcb.24565. [DOI] [PubMed] [Google Scholar]
- 23.Zhu LH, Liu T, Tang H, Tian RQ, Su C, Liu M, Li X. MicroRNA-23a promotes the growth of gastric adenocarcinoma cell line MGC803 and downregulates interleukin-6 receptor. FEBS J. 2010;277:3726–3734. doi: 10.1111/j.1742-4658.2010.07773.x. [DOI] [PubMed] [Google Scholar]
- 24.Ishiguro H, Kimura M, Takeyama H. Role of microRNAs in gastric cancer. World J Gastroenterol. 2014;20:5694–5699. doi: 10.3748/wjg.v20.i19.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Yh, Yu Sn, Lu Zh, Chen J. Construction of a miR-23a-27a cluster expression plasmid: a preliminary study of its function. Zhonghua Bing Li Xue Za Zhi. 2012;41:470–474. doi: 10.3760/cma.j.issn.0529-5807.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Liu X, Xu W, Zhou P, Gao P, Jiang S, Lobie PE, Zhu T. c-MYC-regulated miR-23a/24-2/27a cluster promotes mammary carcinoma cell invasion and hepatic metastasis by targeting Sprouty2. J Biol Chem. 2013;288:18121–18133. doi: 10.1074/jbc.M113.478560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Ru J, Zhang J, Zhu LH, Liu M, Li X, Tang H. miR-23a targets interferon regulatory factor 1 and modulates cellular proliferation and paclitaxel- induced apoptosis in gastric adenocarcinoma cells. PLoS One. 2013;8:e64707. doi: 10.1371/journal.pone.0064707. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Li B, Sun M, Gao F, Liu W, Yang Y, Liu H, Cheng Y, Liu C, Cai J. Up-regulated expression of miR-23a/b targeted the pro-apoptotic Fas in radiation- induced thymic lymphoma. Cell Physiol Biochem. 2013;32:1729–1740. doi: 10.1159/000356607. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Liu Z, Chen L, Zhou L, Yao Y. MicroRNA-23b is an independent prognostic marker and suppresses ovarian cancer progression by targeting runt-related transcription factor-2. FEBS Lett. 2014;588:1608–1615. doi: 10.1016/j.febslet.2014.02.055. [DOI] [PubMed] [Google Scholar]
- 30.Pellegrino L, Stebbing J, Braga VM, Frampton AE, Jacob J, Buluwela L, Jiao LR, Periyasamy M, Madsen CD, Caley MP, Ottaviani S, Roca-Alonso L, El-Bahrawy M, Coombes RC, Krell J, Castellano L. miR-23b regulates cytoskeletal remodeling, motility and metastasis by directly targeting multiple transcripts. Nucleic Acids Res. 2013;41:5400–5412. doi: 10.1093/nar/gkt245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majid S, Dar AA, Saini S, Arora S, Shahryari V, Zaman MS, Chang I, Yamamura S, Tanaka Y, Deng G, Dahiya R. miR-23b represses proto-oncogene Src kinase and functions as methylation-silenced tumor suppressor with diagnostic and prognostic significance in prostate cancer. Cancer Res. 2012;72:6435–6446. doi: 10.1158/0008-5472.CAN-12-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Han L, Zhang K, Shi Z, Zhang J, Zhang A, Wang Y, Song Y, Li Y, Jiang T, Pu P, Jiang C, Kang C. VHL regulates the effects of miR-23b on glioma survival and invasion via suppression of HIF-1α/VEGF and β-catenin/Tcf-4 signaling. Neuro Oncol. 2012;14:1026–1036. doi: 10.1093/neuonc/nos122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian L, Fang YX, Xue JL, Chen JZ. Four microRNAs promote prostate cell proliferation with regulation of PTEN and its downstream signals in vitro. PLoS One. 2013;8:e75885. doi: 10.1371/journal.pone.0075885. [DOI] [PMC free article] [PubMed] [Google Scholar]