Abstract
Fibrous dysplasia (FD) as an abnormal bone growth is one of the common fibro-osseous leasions (FOL) in oral and maxillofacial region, however, its etiology still remains unclear. Here, we performed gene expression profiling of FD using microarray analysis to explore the key molecule events in FD development, and develop potential diagnostic markers or therapeutic targets for FD. We found that 1,881 genes exhibited differential expression with more than two-fold changes in FD compared to normal bone tissues, including 1,200 upregulated genes and 681 downregulated genes. Pathway analysis indicated that obviously activated pathways are Ribosome and ECM-receptor interaction pathways; downregulated pathways are “Hepatitis C” and “cancer” signaling pathways. We further validated the expression of ADAMTS2, one of most differentiated expressed genes, by Immunohistochemistry (IHC) in 40 of FD cases. Results showed that ADAMTS2 was significantly overexpressed in FD tissues, but rarely expressed in normal bone tissues, suggesting that ADAMTS2 could be a potential biomarker for FD. Thus, this study uncovered differentially expressed candidate genes in FD, which provides pilot data for understanding FD pathogenesis, and developing novel biomarkers for diagnosis and targeting of FD.
Keywords: Fibrous dysplasia, gene expression profiling, ADAMTS2, marker
Introduction
Fibrous dysplasia (FD) is a fiber, bone tissue like proliferation, and belongs to a fibrous tissue hyperplasia lesions of the bone but not a form of cancer [18], which accounts for 2% of bone tumors and 7% of benign bone tumors [19]. FD is also a common benign fibro-osseous lesion (FOL) of oral and maxillofacial region, characterized by replacement of normal bone tissue with fibrous bone tissue, leading to abnormal growth or swelling of bone [5].
FD as the disorders of normal bone metabolism usually appears in childhood or adolescence, and its highest incidence age ranges between 11-30 years old, with clinical presentation of asymptomatic swelling of the affected bone [18]. It is categorized as monostotic, or polyostotic, or as part of the McCune-Albright syndrome (MAS). Monostotic fibrous dysplasia accounts for 75% to 80% of cases. Remarkably, FD can cause severe deformity and asymmetry of face and jaw bones by affecting the oral and maxillofacial region in children in their teenage years, resulting in devastating functional and aesthetic consequences for patients [8,14,15]. Kaban et al classified lesions of FD as quiescent, nonaggressive and aggressive according to its clinical behavior [9].
Increasing evidence shows several possible mechanisms causing FD, such as endocrine disorders, developmental abnormities of bone, and genetic factors, etc [13]. FD has generally been considering as a “dysplastic” process, but recent studies have identified that it is a neoplastic lesion [16], as evidenced by the presence of chromosomal aberrations, activating mutations in the GNAS1 gene, overexpression of the c-fos proto-oncogene and occasional malignant changes [25]. However, the etiology of FD remains largely unknown.
Here, we profiled mRNAs expression profiling in FD tissues and normal bone tissues using microarray analysis. We found that mRNA expression profiles are significantly different between normal bone tissues and FD tissues. We also validated ADAMTS2 overexpression in FD thus as a potential biomarker. These findings suggest that altered genes may contribute to the initiation and progression of FD and provide new strategies for its early diagnosis and targeting.
Materials and methods
Patient samples and RNA extraction
A total of 40 FD patients who underwent surgery between Jan 1993 and June 2012 at the Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (Shanghai, China) were investigated. Patients who received pre-operative chemotherapy were excluded. 40 FD (17 quiescent, 11 nonaggressive, 12 aggressive) paraffin blocks were available for immunohistochemistry. Ten normal bone biopsy samples were used as normal controls. Total RNA was extracted using TRIzol reagent (Invitrogen, CA, USA). RNA quantity and quality were measured by NanoDrop ND-1000. RNA integrity was assessed by standard denaturing agarose gel electrophoresis. The study was approved by the Institutional Ethics Committee, Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine.
DNA microarray
A pool of 3 fresh FD tissues and 1 pool of 3 normal bone tissues were randomly selected for gene expression microarray analysis. Microarray analysis of whole-genome gene expression profiling was performed using Human NimbleGen 12 × 135 K Gene Expression Microarrays (Roche Applied Science, India- napolis, IL, USA). About 45,033 genes were collected from the authoritative data source including NCBI with 135,000 probes targeting the latest genome builds.
Data analysis
Double-strand cDNA (ds-cDNA) was synthesized from total RNA using a SuperScript ds-cDNA synthesis kit (Life Technologies Inc., Gaithersburg, Maryland, USA). ds-cDNA was cleaned and labeled according to the NimbleGen Gene Expression Analysis protocol (Roche Applied Science, Indianapolis, IL, USA). Microarrays were then hybridized with Cy3 labeled ds-cDNA in a hybridization chamber (Roche Applied Science, Indianapolis, IL, USA). After hybridization and washing, the slides were scanned using the Axon GenePix 4000B microarray scanner (Axon Instruments, Union City, CA, USA). Then, all gene level files were imported into Agilent GeneSpring GX software (version 11.5.1) for further analysis. Scanned images (TIFF format) were then imported into NimbleScan software (version 2.5) for grid alignment and expression data analysis. Expression data were normalized through quantile normalization and the Robust Multichip Average (RMA) algorithm included in the NimbleScan software. The Probe level (*_norm_RMA.pair) files and Gene level (*_RMA.calls) files were generated after normalization. Differentially expressed genes were identified through Fold Change filtering. Hierarchical clustering was performed using the Agilent GeneSpring GX software (version 11.5.1). GO analysis and Pathway analysis were performed using the standard enrichment computation method. The microarray analysis was performed by KangChen Bio-tech, Shanghai, China.
Functional group analysis
The Gene Ontology (GO) project aims to describe gene and gene product attributes (http://www.geneontology.org), which covers three domains: biological process, cellular component and molecular function. The p-value means the significance of the GO term enrichment in the DE genes FDR stranded for the false discovery rate. The lower the p-value is the more significant the GO term (a p-value of 60.05 was considered statistically significant). Pathway analysis is a functional analysis that maps genes to KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways (http://www.genome.jp/kegg/). The p-value (EASE-score, Fisher p value or Hypergeometric p value) means the significance of the pathway correlated to the conditions. The lower the p-value is the more significant the correlation (a p-value cut-off is 0.05.).
Quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) was performed to detected candidate genes, according to the manufacturer’s protocol (HT7500 system, Applied Biosytems). Total RNA extracted from FD samples and normal bone samples was treated with DNase I, and reverse transcripted as described previously [26]. SYBR Green master mix (Applied Biosystems, Grand Island, NY) was used. The sequences of primers used in quantitative RT-PCR will be provided upon request. GAPDH was used as an internal control.
Immunohistochemistry
Immunohistochemical staining was performed on 4 μm serial sections from formalin-fixed paraffin-embedded specimens. Monoclonal antibodies to ADAMTS2 (Abnova, Niehu, Taipei, Taiwan) was used for immunohistochemistry on formalin-fixed, paraffin-embedded sections. Antigen retrieval was achieved with 20 min incubation in 10 mmol/L sodium citrate buffer at pH 6.0. Overnight 4°C incubations with a 1:100 (ADAMTS2) dilution were done. Negative control slides were duplicate sections in the absence of primary antibody. For evaluating ADAMTS2 expression, an IRS scoring method was used: IRS = SI (staining intensity) × PP (percentage of positive cells). The percentage of ADAMTS2 positively stained cells (PP): 0 = no stained cells, 1 = > 0-25% stained cells, 2 = > 25%-50% stained cells, 3 = > 50%-75% stained cells, 4 = > 75%-100% stained cells. The intensity of ADAMTS2 staining (SI) was scored as follows: 0 = no, -; 1 = weak, +; 2 = moderate, ++; 3 = strong, +++. Statistical significance was evaluated by × 2 analysis and Fisher exact test. The stained tissues were scored blindly regarding clinical patient data. Scoring was done in a blinded fashion by two experienced pathologists.
Results
Differential gene expression profiling by microarray
The whole-genome of mRNA expression profiling was performed on FD and normal bone tissues. A total of 12,303 mRNAs were identified in FD samples through microarray analysis (Figure 1A). With SAM analysis, 1,881 genes with differential expression showed > 2-fold changes, of these, 1,200 genes were upregulated and 681 were downregulated (Figure 1B, Supplementary Table 1). We further utilized unsupervised two-way (genes and samples) hierarchical clustering analysis to separate differential expressed genes into two clusters for distinguishing FD from normal bone tissues (Figure 1C). Result showed that FD present a strikingly different gene expression profile compared with normal bone.
Figure 1.
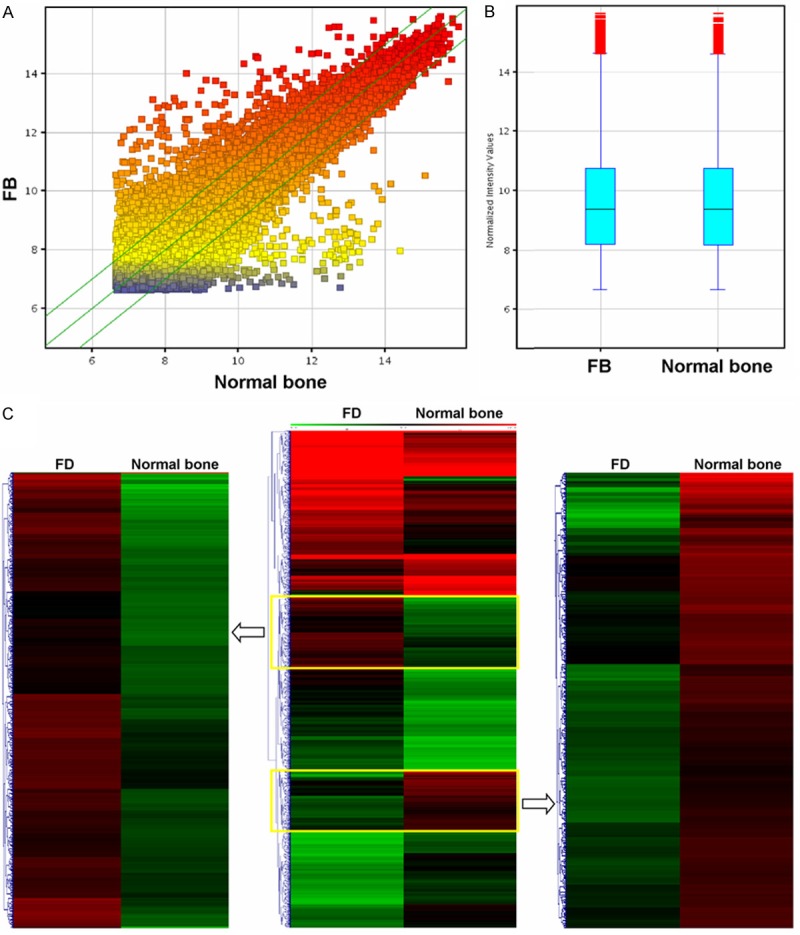
Comparison of mRNA expression profiles between fibrous dysplasia and normal bone samples. A. Distributions of a dataset for mRNA profiles after normalization as measured by box plot. B. mRNA expression variations between fibrous dysplasia and normal bone samples as measured by scatter plot. The values of the X and Y axes in the scatter plot are the averaged normalized signal values of the group (log2 scaled). The green lines are fold change lines (the default fold change given is 2.0). C. Hierarchical clustering of pooled fibrous dysplasia and normal bone samples using the 3973 differential gene sets.
GO and pathway analysis
Next, differentially expressed genes were further selected for subsequent Gene Otology (GO) terms in biological processes, cellular components and molecular functions. We found that the highest enriched GOs targeted by upregulated transcripts were extracellular matrix (ontology: cellular component), protein binding (ontology: molecular function), and extracellular matrix organization (ontology: biological process) (Figure 2, Supplementary Table 2); and that the highest enriched GOs targeted by downregulated transcripts were cytoplasm (ontology: cellular component), protein binding (ontology: molecular function), and macromolecule modification (ontology: biological process) (Figure 3).
Figure 2.
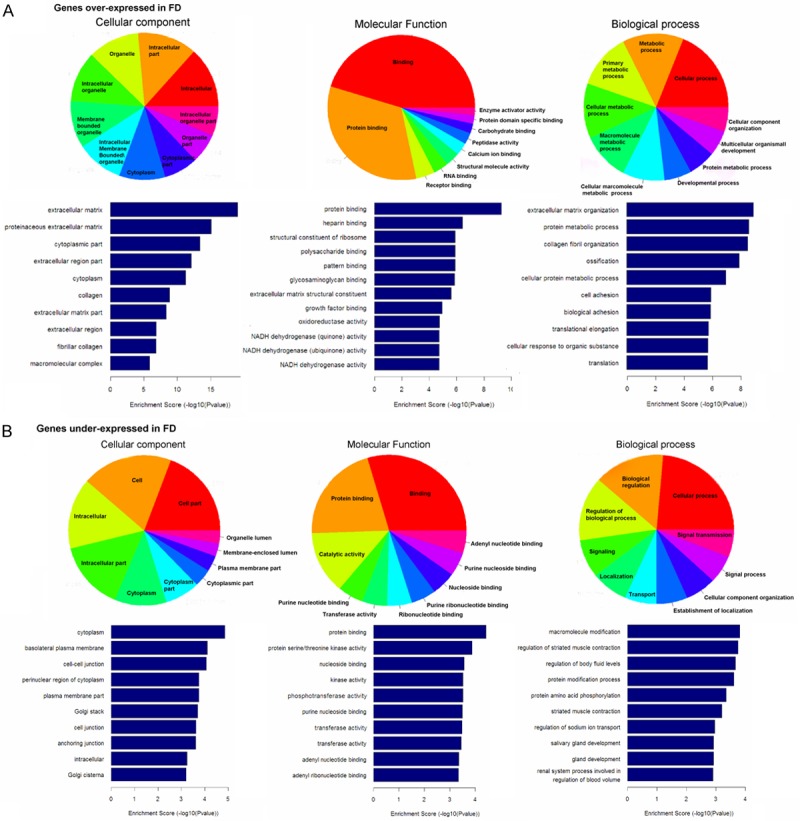
Functional annotation analysis of genes that were significantly differential genes including (A) overexpressed genes and (B) underexpressed genes between fibrous dysplasia tissues and normal bone tissues. The ontology covers three domains: biological process, cellular component and molecular function (p < 0.05).
Figure 3.
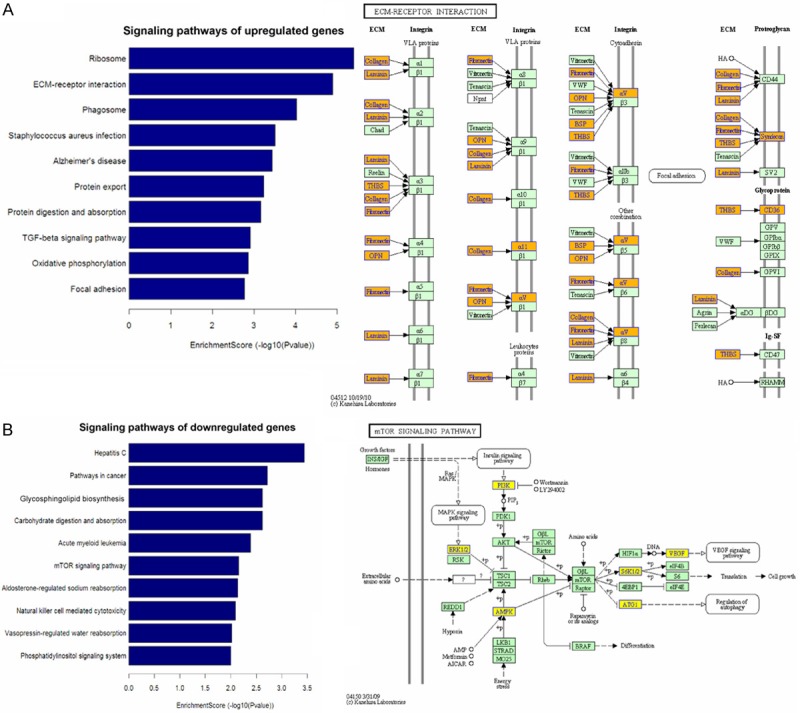
Pathway analysis maps differentially expressed genes including (A) upregulated genes and (B) downregulated genes to KEGG pathways (p < 0.05).
Pathway analysis indicated that 28 pathways mapping upregulated transcripts and the most two significantly disrupted pathways were “Ribosome” and “ECM-receptor interaction” signaling pathways. 18 pathways mapping downregulated transcripts and the most two significantly disrupted pathways were “Hepatitis C” and “cancer” signaling pathways. Other pathways and related components dysregulated in FD pathogenesis are shown in Figures 4 and 5 (Supplementary Table 3).
Figure 4.
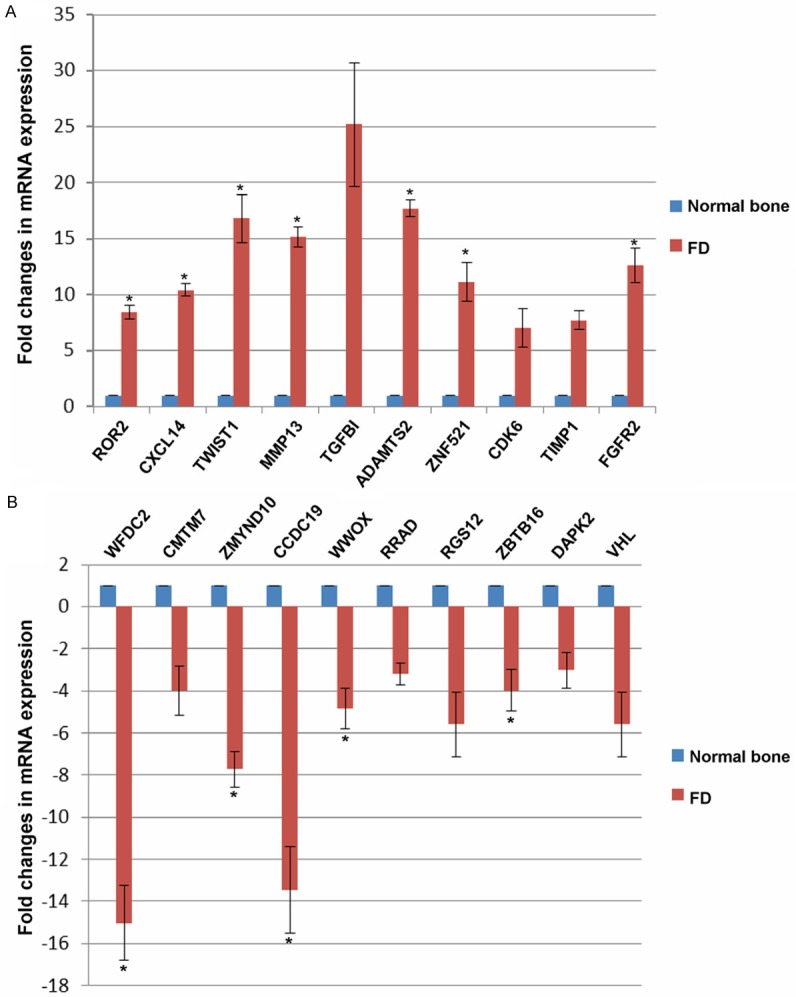
Detection of candidate genes selected from microarray by quantitative real time PCR in FD samples, and normal bone samples. *, p < 0.05.
Figure 5.
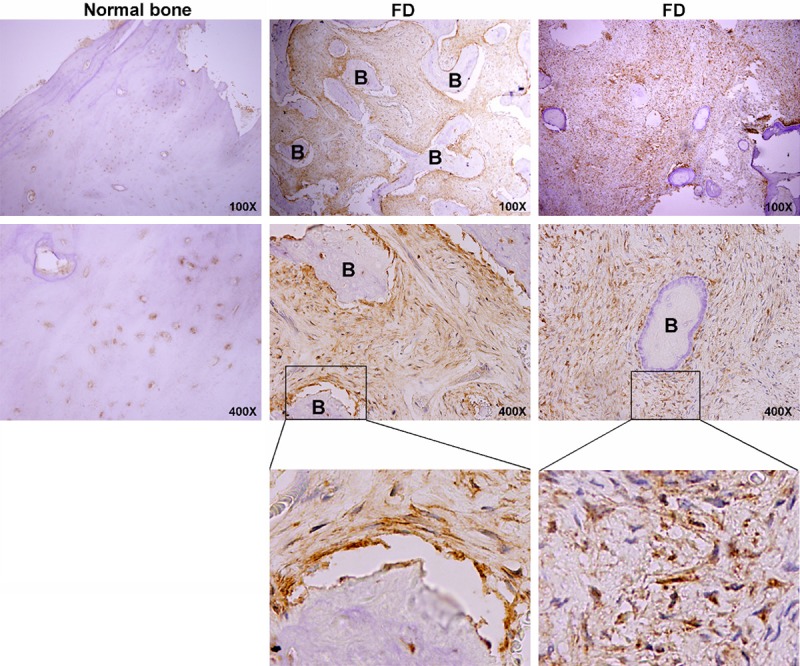
Representative images of immunostaining for ADAMTS2 on fibrous dysplasia tissues and normal bone tissues. B, bone.
Validation of candidate genes by quantitative real-time PCR (qRT-PCR)
To validate altered mRNAs in FD patients, we randomly selected 20 genes, ten (ROR2, CXCL14, TWIST1, MMP13, TGFBI, ADAMTS2, ZNF521, CDK6, TIMP1 and FGFR2) for upregulation, ten (WFDC2, CMTM7, ZMYND10, CCDC19, WWOX, RRAD, RGS12, ZBTB16, DAPK2 and VHL) for downregulation, to check their mRNA expression levels by qRT-PCR using 10 FD samples and 5 normal bone tissues. We found significant upregulated ROR2, CXCL14, TWIST1, MMP13, ADAMTS2, ZNF521, FGFR2 (Figure 4A) and downregulated WFDC2, ZMYND10, CCDC19, WWOX, ZBTB16 in FD samples, compared with normal bone samples, which was consistent with the microarray data.
Overexpression of ADAMTS2 in FD by immunochemistry
We then examined ADAMTS2 expression, one of the differential expression genes, in 10 normal bone tissues and 40 FD tissues by immunochemistry to validate the microarray data. Representative immunostaining of normal bone tissues and FD tissues was shown in Figure 5. ADAMTS2 was mainly localized to the fibrous areas, with highly expressed on the interstitial fibers, while sporadically expressed in bone matrix. ADAMTS2 was differentially expressed between normal bone tissues and FD tissues (P = 0.0165) (Figure 5, Table 1).
Table 1.
Summary of ADAMTS2 expression by immunohistochemistry in normal bone and FD samples
| Scoring Method | Normal bone | FD | P value | |
|---|---|---|---|---|
|
|
||||
| (n = 10) | (n = 40) | |||
| Positive rate | (-) | 2 | 4 | |
| (+) | 4 | 4 | ||
| (++) | 3 | 16 | ||
| (+++) | 1 | 16 | ||
| Mean score | 16.3 | 27.8 | P = 0.0187 | |
| Density | (+) | 3 | 6 | |
| (++) | 6 | 13 | ||
| (+++) | 1 | 21 | ||
| Mean score | 16.9 | 27.7 | P = 0.0232 | |
| Positive rate+density | Mean score | 15.8 | 27.9 | P = 0.0165 |
We next analyzed the correlation between ADAMTS2 expression and clinicopathological features of FD patients, including gender, age, location and stage. However, no significant correlation between ADAMTS2 expression and clinicopathological features was found (Supplementary Table 4). These data suggest that ADAMTS2 overexpression might be a potential biomarker during FD progression.
Discussion
FD as a benign fibrous bone disease was firstly reported by Lichenstein in 1938 [6]. Its incidence and clinical features are distinct in different populations. A retrospective review with a small sample size in Chinese population was conducted by our group to assess its clinical features, radiographic findings and the association with serum alkaline phosphotase (ALP). We found that FD has no sex predilection, and elevated ALP is closely related to the extent of FD lesion [2]. Another retrospective review with a larger sample size in Indian population showed that FD has a definite male predominance with diffuse borders, which is mainly composed of fibrous stroma and woven bone [21]. Other sporadical FD cases in different population have also been reported [1,11,17,24].
FD is caused by an abnormal proliferation of fibrous tissue in bone, thus exhibiting a rapid growth feature [3,25]. However, the important molecular events for FD development are largely unclear. Most of studies have been focused on GNAS1 mutations [13,22], which have been considered to be a hallmark of FD. In this study, we investigated the mRNA expression profile of FD using microarray analysis to explore the molecular basis of FD development. From the microarray expression profile, we found that 12,303 mRNAs were differentially expressed in FD, in which 1,200 genes upregulated and 681 genes downregulated (P > 2.0-fold) in FD samples. We further selected 10 upreguated genes and 10 downregulated genes from microarray data for validation using qRT-PCR, and the results were consistent.
FD is characterized by abnormal matrix overproduction and fibrous bone cell accumulation. Extracellular matrix (ECM) plays a crucial role in multiple cellular processes including growth, wound healing, and fibrosis, through regulating cell dynamic behavior and sequestering cellular growth factors [7]. The enzymes involving ECM destruction include serine proteases, threonine proteases, and matrix metalloproteinases, etc. MMP9, VEGF [10] and FGF23 [23] have been identified to be involved in FD initiation and progression. We also uncovered differentially expressed GO terms and signaling pathways in the development of FD. Of note, ECM was identified to be one of the highest enriched GOs, resulting in significant deregulation of ECM-receptor interaction signaling pathway, supporting the importance of ECM-receptor interaction signaling in FD pathogenesis.
ADAMTS (A Disintegrin And Metalloproteinase with Thrombospondin Motifs) family, one of the key components in ECM-receptor interaction signaling, plays a crucial role in extracellular matrix degradation and turn over, and participates in various human biological processes, including fibrosis, angiogenesis, cell invasion and migration [20]. We thus chose ADAMTS2, one of the ADAMTS members, to validate microarray data by immunochemistry. We found that ADAMTS2 expression was significant upregulated in FD compared to normal bone tissues, although no significant correlation between ADAMTS2 expression and clinicopathological features was found, which needs to be further confirmed by large sample size study. ADAMTS2 as a procollagen N-proteinase can remove the aminopropeptide of type I, type II, and type III procollagen. Its mutations result in Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis, through generating stop codons and decreasing its mRNA expression level [4,12]. Further studies are needed to investigate the biological function and underlying mechanism of ADAMTS2 in FD pathogenesis.
In conclusion, we report for the first time that mRNAs are differentially expressed in FD compared with normal bone tissues. With some key molecules and related signaling pathways are uncovered, it will help in comprehending the complex dynamics of FD development and developing novel strategies for the early diagnosis and treatment of FD. Further work is needed to understand the molecular mechanisms and biological functions of differentially expressed mRNAs in FD.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (No. 81202133) and Training Subsidy Scheme of Young College Teachers in Shanghai (jdy11025).
Disclosure of conflict of interest
The authors declare no conflict of interest.
Supplementary Tables 1-3
Supplementary Table 4
References
- 1.Assaf AT, Benecke AW, Riecke B, Zustin J, Fuhrmann AW, Heiland M, Friedrich RE. Craniofacial fibrous dysplasia (CFD) of the maxilla in an 11-year old boy: a case report. J Craniomaxillofac Surg. 2012;40:788–792. doi: 10.1016/j.jcms.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Cheng J, Wang Y, Yu H, Wang D, Ye J, Jiang H, Wu Y, Shen G. An epidemiological and clinical analysis of craniomaxillofacial fibrous dysplasia in a Chinese population. Orphanet J Rare Dis. 2012;7:80. doi: 10.1186/1750-1172-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MM Jr, Howell RE. Etiology of fibrous dysplasia and McCune-Albright syndrome. Int J Oral Maxillofac Surg. 1999;28:366–371. [PubMed] [Google Scholar]
- 4.Colige A, Nuytinck L, Hausser I, van Essen AJ, Thiry M, Herens C, Ades LC, Malfait F, Paepe AD, Franck P, Wolff G, Oosterwijk JC, Smitt JH, Lapiere CM, Nusgens BV. Novel types of mutation responsible for the dermatosparactic type of Ehlers-Danlos syndrome (Type VIIC) and common polymorphisms in the ADAMTS2 gene. J Invest Dermatol. 2004;123:656–663. doi: 10.1111/j.0022-202X.2004.23406.x. [DOI] [PubMed] [Google Scholar]
- 5.DiCaprio MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am. 2005;87:1848–1864. doi: 10.2106/JBJS.D.02942. [DOI] [PubMed] [Google Scholar]
- 6.Garlock JH. The Differential Diagnosis of Hyperparathyroidism: With Special Reference to Polyostotic Fibrous Dysplasia. Ann Surg. 1938;108:347–361. doi: 10.1097/00000658-193809000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 8.Kolomvos N, Theologie-Lygidakis N, Christopoulos P, Iatrou I. Benign fibro-osseous lesions of the jaws in children. A 12-year retrospective study. J Craniomaxillofac Surg. 2013;41:574–80. doi: 10.1016/j.jcms.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Koury ME, Regezi JA, Perrott DH, Kaban LB. “Atypical” fibro-osseous lesions: diagnostic challenges and treatment concepts. Int J Oral Maxillofac Surg. 1995;24:162–169. doi: 10.1016/s0901-5027(06)80094-9. [DOI] [PubMed] [Google Scholar]
- 10.Kumta SM, Huang L, Cheng YY, Chow LT, Lee KM, Zheng MH. Expression of VEGF and MMP-9 in giant cell tumor of bone and other osteolytic lesions. Life Sci. 2003;73:1427–1436. doi: 10.1016/s0024-3205(03)00434-x. [DOI] [PubMed] [Google Scholar]
- 11.Kurra S, Reddy DS, Gunupati S, K S, Reddy MS. Fibrous dysplasia and central giant cell granuloma: a report of hybrid lesion with its review and hypotheticated pathogenesis. J Clin Diagn Res. 2013;7:954–958. doi: 10.7860/JCDR/2013/5533.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Goff C, Somerville RP, Kesteloot F, Powell K, Birk DE, Colige AC, Apte SS. Regulation of procollagen amino-propeptide processing during mouse embryogenesis by specialization of homologous ADAMTS proteases: insights on collagen biosynthesis and dermatosparaxis. Development. 2006;133:1587–1596. doi: 10.1242/dev.02308. [DOI] [PubMed] [Google Scholar]
- 13.Lietman SA, Levine MA. Fibrous dysplasia. Pediatr Endocrinol Rev. 2013;2:389–96. [PubMed] [Google Scholar]
- 14.MacDonald-Jankowski DS. Fibro-osseous lesions of the face and jaws. Clin Radiol. 2004;59:11–25. doi: 10.1016/j.crad.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Menon S, Venkatswamy S, Ramu V, Banu K, Ehtaih S, Kashyap VM. Craniofacial fibrous dysplasia: Surgery and literature review. Ann Maxillofac Surg. 2013;3:66–71. doi: 10.4103/2231-0746.110088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikami M, Koizumi H, Ishii M, Nakajima H. The identification of monoclonality in fibrous dysplasia by methylation-specific polymerase chain reaction for the human androgen receptor gene. Virchows Arch. 2004;444:56–60. doi: 10.1007/s00428-003-0907-y. [DOI] [PubMed] [Google Scholar]
- 17.Nair PP, Bhargava D, Thomas S, Shreenivas K. Immature fibrous dysplasia: a mixed radio-opaque radiolucent lesion. BMJ Case Rep. 2013;3:2013. doi: 10.1136/bcr-2012-007934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papadaki ME, Troulis MJ, Kaban LB. Advances in diagnosis and management of fibro-osseous lesions. Oral Maxillofac Surg Clin North Am. 2005;17:415–434. doi: 10.1016/j.coms.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Pinsolle V, Rivel J, Michelet V, Majoufre C, Pinsolle J. [Treatment of fibrous dysplasia of the cranio-facial bones. Report of 25 cases] . Ann Chir Plast Esthet. 1998;43:234–239. [PubMed] [Google Scholar]
- 20.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prabhu S, Sharanya S, Naik PM, Reddy A, Patil V, Pandey S, Mishra A, Rekha K. Fibro-osseous lesions of the oral and maxillo-facial region: Retrospective analysis for 20 years. J Oral Maxillofac Pathol. 2013;17:36–40. doi: 10.4103/0973-029X.110707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puranik RS, Puranik SR, Vanaki SS, Hosur MB. GNAS1 mutations are hallmark expressions of fibrous dysplasia. J Oral Maxillofac Surg. 2012;8:1768–9. doi: 10.1016/j.joms.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 23.Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron Robey P. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–692. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suarez-Soto A, Baquero-Ruiz de la Hermosa MC, Minguez-Martinez I, Floria-Garcia LM, Barea-Gamiz J, Delhom-Valero J, Risueno-Mata P. Management of fibro-osseous lesions of the craniofacial area. Presentation of 19 cases and review of the literature. Med Oral Patol Oral Cir Bucal. 2013;18:e479–485. doi: 10.4317/medoral.18289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toyosawa S, Yuki M, Kishino M, Ogawa Y, Ueda T, Murakami S, Konishi E, Iida S, Kogo M, Komori T, Tomita Y. Ossifying fibroma vs fibrous dysplasia of the jaw: molecular and immunological characterization. Mod Pathol. 2007;20:389–396. doi: 10.1038/modpathol.3800753. [DOI] [PubMed] [Google Scholar]
- 26.Zhou S, Qu X, Yu Z, Zhong L, Ruan M, Ma C, Wang M, Zhang C, Jian X. Survivin as a potential early marker in the carcinogenesis of oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;4:575–81. doi: 10.1016/j.tripleo.2009.10.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


