Abstract
Prostatic acid phosphatase (PAP) is expressed in nociceptive dorsal root ganglion (DRG) neurons and functions as an ectonucleotidase that dephosphorylates extracellular adenosine monophosphate (AMP) to adenosine to suppress pain via activating A1-adenosine receptor (A1R) in dorsal spinal cord. However, the effect of peripheral nerve injury on the expression of PAP has not been reported until now. In the present study we found that PAP expression in DRG neurons is significantly decreased from the 2nd day after peripheral nerve injury and reaches the bottom at the 14th. In addition, intrathecal PAP injection can reduce mechanical allodynia induced by spared nerve injury. Our findings suggest that the decrease of PAP is involved in pathophysiological mechanisms of neuropathic pain.
Keywords: PAP, DRG, peripheral nerve injury, neuropathic pain
Introduction
Neuropathic pain occurs when the injury or disease affects the somatosensory system. It may be associated with abnormal sensations called dysesthesia, and pain produced by normally non-painful stimuli (allodynia) [1,2]. Therefore, it is essential to get a better understanding of pathophysiological mechanisms of neuropathic pain for developing more effective and specific new therapies. Animal models with sciatic nerve injury have been widely used to study the mechanisms of neuropathic pain. Many studies have proved that changes in gene expression in dorsal root ganglia (DRG) of peripherally axotomized animal may contribute to the generation and development of neuropathic pain [3-5].
Prostatic Acid Phosphatase (PAP) was originally identified as a secretory protein enriched in the prostate gland and has been used as a marker of diagnosis and therapy control of the prostate gland cancer for many years [6]. Recently the transmembrane isoform of PAP was reported to function as an ectonucleotidase that dephosphorylates extracellular adenosine monophosphate (AMP) to adenosine, a neuromodulator with anti-nociceptive properties, in nociceptive dorsal root ganglia neurons and therefore to suppress pain via activating A1-adenosine receptor (A1R) in dorsal spinal cord [7-9]. Intriguingly intraspinal injection of PAP protein has potent antinociceptive, antihyperalgesic, and antiallodynic effects that last longer than the opioid analgesic morphine [8].
Since then much efforts have been made to reveal molecular and physiological functions of PAP in nociception and to extend its application in antinociception. Sowa NA et al found that PAP inhibits noxious thermal sensitivity and sensitization that is associated with chronic pain through sustained activation of the A1R and phospholipase C-mediated depletion of phosphatidylinositol 4, 5-bisphosphate (PIP2) [10]. It was also reported that the antinociceptive effects of benzoylthiamine (BT), thiamine monophosphate (TMP) and thiamine-a compound that is not phosphorylated–are entirely dependent on PAP at the spinal level, which suggests a novel phosphatase-independent function for PAP [11]. In addition, injection of PAP into acupuncture points can mimic the antinociceptive effects of acupuncture in mouse models of acute and chronic pain for a longer period of time [12,13]. However, the change of PAP expression in DRG after peripheral nerve injury has not been reported until now. Here we examined the change of PAP expression after peripheral nerve injury to explore the physiological function of PAP in neuropathic pain.
Materials and methods
Animals
All interventions and animal care were performed in accordance with the policy of the Society for Neuroscience (USA) on the use of animals in neuroscience research and the guidelines of the Committee for Research and Ethic Issues of International Association for the Study of Pain. The experiments were approved by 117 Hospital of PLA Research Animal Care and Use Committee. All efforts have been made to minimize the number of animals used and their discomfort after peripheral nerve injury. The Sprague-Dawley male rats (200~250 g, Shanghai Center of Experimental Animals, CAS, Shanghai, China) used in this study were maintained under a 12-h light-dark cycle (lights on from 07:00 to 19:00) with room temperature at 22° ± 1°C. Food and water were available ad libitum.
Animal surgery and tissue preparation
SD male rats were anesthetized with chloral hydrate (300 mg/kg). For sciatic nerve transection (SNT) model, the sciatic nerve was exposed at mid-thigh level. A 5 mm portion of sciatic nerve was transected. For spared nerve injury (SNI) model, left tibial and common peroneal nerves were identified, ligated with 5-0 silk and transected after the exposure of sciatic nerve. After surgery, muscles and skin were sutured in layers, and the animals were allowed to recover from anesthesia in a warmed cage. Then the rats were allowed to survive for 2, 7, 14 and 28 days after nerve injury.
For real-time PCR, L4 and L5 DRGs of these rats and of normal rats were then dissected and frozen on dry ice. For in-situ hybridization, the rats were anesthetized and perfused via the ascending aorta with 50 ml of warm (37°C) saline followed by 50 ml of warm solution composed of 4% paraformaldehyde and 0.1% picric acid in 0.1 M phosphate buffer at pH 6.9. The perfusion was then followed by 200 ml of the same fixative (4°C) for another 5 min. L4 and L5 DRGs and the lumbar spinal cord were then dissected out. The tissues were post-fixed in the same fixative for 90 min at 4°C, and were then immersed in 20% sucrose in 0.1 M phosphate buffer for at least 1 day. Diethyl pyrocarbonate (DEPC) water was used for all solutions and appliances necessary for ISH.
Real-time PCR
Total RNA from DRGs (L4 & L5) at different time points was extracted with TRIzol reagent (Invitrogen, USA). The RNA (1 μg) was reverse transcribed (Superscript II, Invitrogen, USA) to cDNA. Quantitative PCR was performed with Premix Ex Taq (Takara) using a 7500 real-time PCR system (Applied Biosystems) according to the protocol of the manufacturer. The data were analyzed by 7500 System SDS Software 1.4.0 (Applied Biosystems) using the standard curve method. Gene expression level was normalized to the values for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The specific primers for detection of gene expression were listed below: for GAPDH 5’CCAGAACATCATCCCTGCAT3’ and 5’GCATGTCAGATCCACAACGG3’; for PAP 5’CTTCTTGCTCCTGCTATCT3’ and 5’TCCCGTATCTTCTCCTTAT3’.
In situ hybridization
A digoxigenin-labeled antisense cRNA riboprobe of PAP was generated. The primers for PAP were as following: 5’AACCTCGCAGCCCTGTTTCC3’ and 5’CTCGTTCTGGGTCTCATTCCG3’. DRG sections were fixed in 4% paraformaldehyde for 20 min, treated with proteinase K (10 μg/ml in DEPC water containing 50 mM Tris-HCl, pH 7.5 and 5 mM EDTA) for 20 min, acetylated in 0.25% acetic anhydride/0.1 M triethanolamine (pH 8.0) and prehybridized in hybridization buffer (50% formamide, 5 × SSC, 0.3 mg/ml yeast tRNA, 0.1 mg/ml heparin, 1 × Denhardt’s solution, 0.1% Tween-20, 5 mM EDTA in DEPC water) for 4 hours at 65°C. The prehybridization buffer was substituted by hybridization buffer with 1 μg/ml of the antisense probe in which the sections were incubated for 14 h at 67°C. After hybridization, excess probe was removed by washing three times with 2 × SSC at 67°C and once with RNase A (1 μg/ml) for 10 min. Sections were then incubated in alkaline phosphatase-conjugated sheep anti-digoxigenin antibodies (1:2,000; Roche Molecular Biochemicals), and then in NBT/BCIP in alkaline phosphatase buffer (100 mM Tris-HCl, pH 9.5, 50 mM MgCl2, 100 mM NaCl, 0.1% Tween-20 in distilled water). Control experiments were carried out using a digoxigenin-labeled sense riboprobe for PAP. The sense probes did not detect any specific signal (data not shown).
For ISH combined with immunohistochemical detection, sections were immunostained with anti-NF200 (mouse, Sigma) or fluorescein-labelled IB4 (1:100; Vector Laboratories) after ISH process. Images were captured with a Nikon microscope using Neurolucida software.
Immunohistochemistry
For all groups, 12 μm-thick sections of the fixed L4 and L5 DRGs, and L4-5 spinal cord segments were cut in series in a cryostat and mounted on same gelatin-coated slides. The sections were processed with indirect immunofluorescence histochemistry. The antibodies were diluted in phosphate-buffered saline with 1% bovine serum albumin and 0.3% Triton X-100. Briefly, the sections were incubated with a mixture of goat anti-PAP antibodies (1:500, Sigma) overnight at 4°C. After several rinses in PBS, the sections were incubated with biotin-conjugated goat anti-rabbit second antibodies (1:500; Vector labs) for 2 hours at room temperature, then incubated with VECTASTAIN Elite ABC Kit (1:1:500, Vector) at room temperature for 1 hour, then detected with DAB substrate (Vector). The sections were rinsed and mounted with a mixture of glycerol/PBS (9:1). Images were captured with a Nikon microscope using Neurolucida software.
For quantitative analysis, 3 sections from each DRG were representative for each rat and the data were collected from at least three animals at each time point. The same calculation for the percentage and the distribution of labelled neuron profiles each DRG was performed as in situ hybridization.
Behavioral analysis
All behavioral tests were performed by observers blinded to experimental conditions. Rodents were habituated to the testing environment daily for 3 d before baseline testing. For evaluating mechanical threshold, animals were placed in a plastic cage with a wire mesh floor and allowed to habituate for 30 min before the threshold testing. The hindpaw was pressed with one of a series of von Frey hairs with logarithmically incrementing stiffness (0.6, 1, 1.4, 2, 4, 6, 8, 10, 15, and 26 g) (Stoelting), presented perpendicular to the plantar surface (5~6 s for each hair). Dixon’s up-down method was used to determinate the 50% withdrawal threshold.
Purified PAP protein (Millipore) was injected intrathecally in L5-L6 intervertebral space by a microsyringe after rats were anesthetized with sevoflurane (3%, Hengrui Pharmaceutical CO.).
Quantitative analysis
All data were shown as mean ± S.E.M. Differences in changes of values between the intrathecal groups were tested using one-way ANOVA, followed by individual post hoc comparisons (Fisher’s exact test) or pairwise comparisons (t test). P value < 0.05 was considered to be significant.
Results
Decreased expression of PAP in DRG neurons after peripheral nerve injury
Decrease expression of PAP mRNA in the rat DRG after peripheral nerve injury was suggested by previous gene microarray analysis [3]. Real-time PCR was used to confirm the decreased expression of PAP mRNA in lumbar (L) 4 and L5 DRG after sciatic nerve transection (SNT). The level of PAP mRNA is markedly reduced in the DRG at the 2nd day after peripheral nerve injury (Figure 1). Such a decreased expression reaches the bottom at the 14th day after SNT (Figure 1). Then the level of PAP mRNA in DRG neurons becomes elevated at the 28th day after SNT but still significantly less than that of controls (Figure 1).
Figure 1.
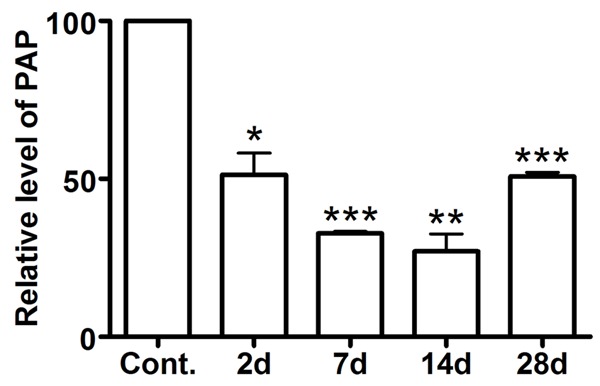
Down-regulation of PAP mRNA in rat DRGs after peripheral nerve injury. RT-PCR revealed that the level of PAP mRNA in L4 and L5 is markedly reduced in the DRG at the 2nd day after peripheral nerve injury, reaches the bottom at the 14th day and becomes elevated at the 28th day. *P < 0.05, **P < 0.01 versus control (day 0).
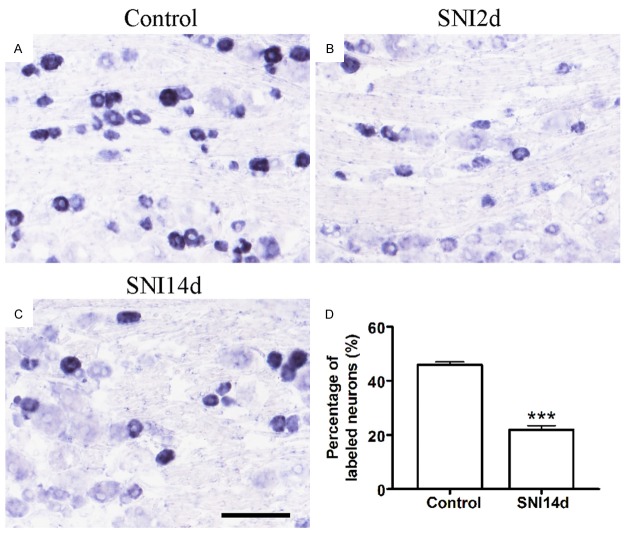
We intended to evaluate the effect of PAP on neuropathic pain induced by spared nerve injury (SNI) model. Then we measured the expression of PAP in rats after SNI. In situ hybridization experiments were performed to show the expression pattern of PAP mRNA in DRG. ISH data demonstrated that PAP mRNA was expressed in DRG neurons (Figure 2). Under normal condition, PAP mRNA was contained by ~45.8% DRG neurons (Figure 2A, 2D). The number of PAP mRNA-containing neurons was markedly decreased to ~20% of total neurons at the 14th day after SNI (Figure 2C, 2D). The immunohistochemistry also showed that the number of PAP+ neurons was markedly decreased from about 50% to ~20% of total neurons at the 14th day after SNI (Figure 3). These results revealed that the expression of PAP is reduced in DRG neurons after SNI.
Figure 2.
Decreased number of PAP mRNA-containing neurons after peripheral nerve injury. (A-C) In situ hybridization of PAP in the control DRG (A), DRG at the 2nd day after SNI (B) and the 14th day after SNI (C). Statistical analysis revealed that the number of PAP mRNA-containing neurons was markedly decreased from about 50% to 20% of total neurons at the 14th day after SNI (D). **P < 0.01 versus control (day 0). Scale bars: 100 μm.
Figure 3.
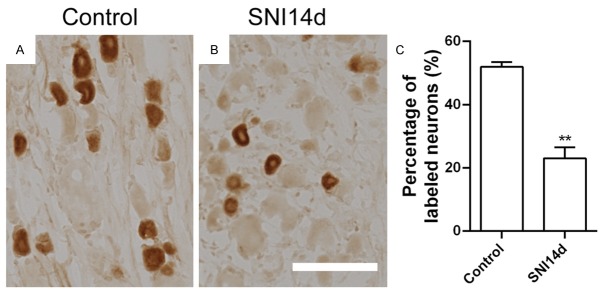
Decreased number of PAP+ neurons after peripheral nerve injury. Immunohistochemistry of PAP in the control DRG (A), DRG at the 14th day after peripheral nerve injury (B). Statistical analysis revealed that the number of PAP+ neurons was markedly decreased from about 50% to 20% of total neurons at the 14th day after SNI (C). **P < 0.01 versus control (day 0). Scale bars: 100 μm.
PAP mRNA is primarily expressed in nonpeptidergic DRG neurons
Furthermore, we measured the distribution of PAP mRNA within DRG neurons by ISH combined with immunolabeling for neurofilament 200 (NF200), a marker for myelinated sensory neurons [14], or isolectin B4 (IB4) that binds nonpeptidergic neurons [15] to identify the distribution of PAP in the DRG neurons. We found that only a few PAP (~10%) contained DRG neurons was colocalized with NF200 (Figure 4A-C), while ~15.6% PAP (~10%) contained DRG was not labeled with IB4 (Figure 4D-F). These results demonstrate that PAP is mainly expressed in nonpeptidergic small DRG neurons, which is consistent with previous reports [8].
Figure 4.
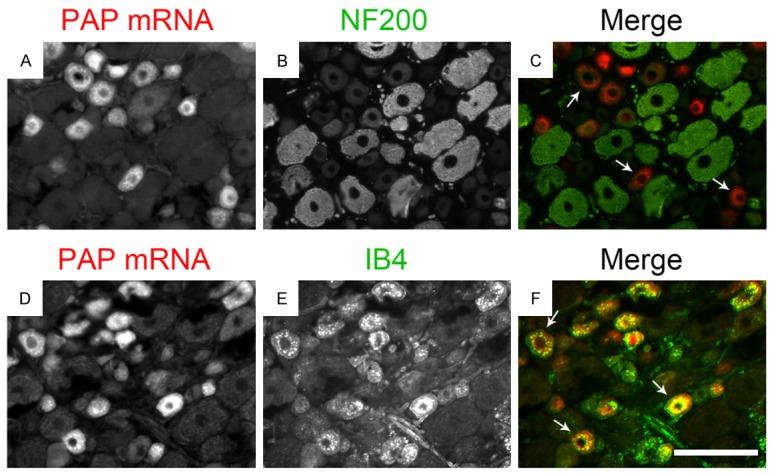
PAP mRNA is primarily expressed in nonpeptidergic DRG neurons. Data of ISH combined with IHC showed PAP mRNA and NF200 (A-C) or IB4 (D-F) revealed that just most PAP mRNA-containing neurons do not coexpress NF200 (arrows in C) and most PAP mRNA is colocalized with IB4 (arrows in F). Scale bars: 100 μm.
Intrathecal PAP injection can reduce mechanical allodynia
We intended to test the effect of PAP on the development of neuropathic pain. We used SNI model since rats after SNI demonstrated a stable and long-lasting mechanical allodynia (citation). At the 4th day of SNI, rodents showed an obvious decrease in mechanical threshold as reported. Then rats were injected intrathecally with PAP protein (500 ng, or 432 mU). We found that PAP could significantly attenuate mechanical allodynia induced by SNI. The analgesic effect lasted for up to 3 days (Figure 5).
Figure 5.
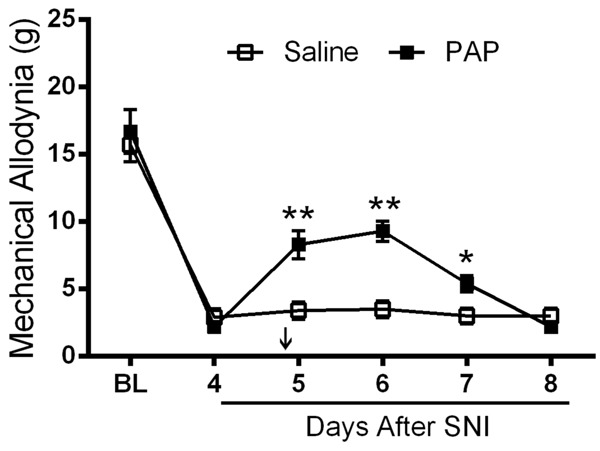
A single intrathecal injection of PAP protein has anti-allodynic effect. The sural and common peroneal branches of the sciatic nerve were ligated and then transected. Then PAP was injected 4 days later. Injured and noninjured (control) hindpaws were tested for mechanical sensitivity.
Discussion
Here we found PAP mRNA expression in DRG neurons is significantly decreased from the 2nd day after peripheral nerve injury and intrathecal PAP injection can reduce mechanical allodynia, which suggest that PAP is involved in pathophysiological mechanisms of neuropathic pain.
The mechanisms of PAP function in anti-nociception and anti-neuropathic pain are very complicated. It was firstly reported to be an ectonucleotidase in nociceptive circuits and dephosphorylate AMP to adenosine [8]. Adenosine activates A1R and acute A1R activation inhibits neurotransmitter release from nociceptive neurons, voltage-gatedcalcium channels, and postsynaptic neurons in spinal cord [16,17]. In addition, PAP acts via A1R to reduce the levels of PIP2 in cultured cells and in vivo. In turn, this reduction in PIP2 inhibits signaling and sensitization through diverse pronociceptive GPCR [10,18]. More recently PAP was found to be required for antinociceptive effects of BT, TMP and thiamine-a compounds [11]. But this function is not dependent on the ectonucleotidase activity of PAP, but a novel phosphatase independent function. Therefore to examine the expression pattern of PAP mRNA after peripheral nerve injury will help us to understand the antinociceptive mechanisms of PAP. Our study demonstrated that peripheral nerve injury could induce the decrease of PAP expression in DRG, which might result in the decrease of extracellular adenosine. Then the effect of adenosine was reduced. Thus, pathological pain induced by peripheral nerve injury became long-lasting.
Besides PAP, it was found that another Ecto-5-nucleotidase (NT5E, CD73) also contributes to AMP hydrolysis in nociceptive neurons [19]. PAP only locates on peptidergic nociceptive neurons, while NT5E locates on both peptidergic and nonpeptidergic nociceptive neurons in DRG. In PAP/NT5E double knockout (dKO) mice, AMP hydrolysis, when measured histochemically, was nearly abolished in DRG neurons and lamina II of spinal cord [20]. In addition, the antinociceptive effects of AMP, when combined with nucleoside transport inhibitors (dipyridamole or 5-iodotubericidin), were reduced by 80-100% in dKO mice [20]. Our findings is consistent with the previous report that NT5E protein is reduced in lamina II of spinal cord following nerve injury, which supports the view that PAP and NT5E are the main ectonucleotidases that generate adenosine in nociceptive circuits.
Acknowledgements
This work was supported by Science Foundation of Health and Family Planning Commission of Zhejiang Province Grant No. 2013KYB229.
Disclosure of conflict of interest
None.
References
- 1.Truini A, Cruccu G. Pathophysiological mechanisms of neuropathic pain. Neurol Sci. 2006;27(Suppl 2):S179–182. doi: 10.1007/s10072-006-0597-8. [DOI] [PubMed] [Google Scholar]
- 2.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 3.Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, Cheng XP, Yan Q, Zhu ZD, Zhang X, Chen Z, Han ZG, Zhang X. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci U S A. 2002;99:8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thacker MA, Clark AK, Marchand F, McMahon SB. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg. 2007;105:838–847. doi: 10.1213/01.ane.0000275190.42912.37. [DOI] [PubMed] [Google Scholar]
- 5.Huang B, Zhao X, Zheng LB, Zhang L, Ni B, Wang YW. Different expression of tissue inhibitor of metalloproteinase family members in rat dorsal root ganglia and their changes after peripheral nerve injury. Neuroscience. 2011;193:421–428. doi: 10.1016/j.neuroscience.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Ostrowski WS, Kuciel R. Human prostatic acid phosphatase: selected properties and practical applications. Clin Chim Acta. 1994;226:121–129. doi: 10.1016/0009-8981(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 7.Quintero IB, Araujo CL, Pulkka AE, Wirkkala RS, Herrala AM, Eskelinen EL, Jokitalo E, Hellstrom PA, Tuominen HJ, Hirvikoski PP, Vihko PT. Prostatic acid phosphatase is not a prostate specific target. Cancer Res. 2007;67:6549–6554. doi: 10.1158/0008-5472.CAN-07-1651. [DOI] [PubMed] [Google Scholar]
- 8.Zylka MJ, Sowa NA, Taylor-Blake B, Twomey MA, Herrala A, Voikar V, Vihko P. Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron. 2008;60:111–122. doi: 10.1016/j.neuron.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawynok J. Adenosine receptor activation and nociception. Eur J Pharmacol. 1998;347:1–11. doi: 10.1016/s0014-2999(97)01605-1. [DOI] [PubMed] [Google Scholar]
- 10.Sowa NA, Street SE, Vihko P, Zylka MJ. Prostatic acid phosphatase reduces thermal sensitivity and chronic pain sensitization by depleting phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2010;30:10282–10293. doi: 10.1523/JNEUROSCI.2162-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurt JK, Coleman JL, Fitzpatrick BJ, Taylor-Blake B, Bridges AS, Vihko P, Zylka MJ. Prostatic acid phosphatase is required for the antinociceptive effects of thiamine and benfotiamine. PLoS One. 2012;7:e48562. doi: 10.1371/journal.pone.0048562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman N, Chen M, Fujita T, Xu Q, Peng W, Liu W, Jensen TK, Pei Y, Wang F, Han X, Chen JF, Schnermann J, Takano T, Bekar L, Tieu K, Nedergaard M. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 2010;13:883–888. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurt JK, Zylka MJ. ACPPupuncture has localized and long-lasting antinociceptive effects in mouse models of acute and chronic pain. Mol Pain. 2012;8:28. doi: 10.1186/1744-8069-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol. 1991;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu ZL, Huang Y, Tao XR, Qi ZH, Chen JY, Ding YQ. Inducible Prrxl1-CreER(T2) recombination activity in the somatosensory afferent pathway. Genesis. 2012;50:552–560. doi: 10.1002/dvg.22020. [DOI] [PubMed] [Google Scholar]
- 16.Eisenach JC, Rauck RL, Curry R. Intrathecal, but not intravenous adenosine reduces allodynia in patients with neuropathic pain. Pain. 2003;105:65–70. doi: 10.1016/s0304-3959(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 17.Sawynok J. Adenosine and ATP receptors. Handb Exp Pharmacol. 2007:309–328. doi: 10.1007/978-3-540-33823-9_11. [DOI] [PubMed] [Google Scholar]
- 18.Dale C, Vergnolle N. Protease signaling to G protein-coupled receptors: implications for inflammation and pain. J Recept Signal Transduct Res. 2008;28:29–37. doi: 10.1080/10799890801941913. [DOI] [PubMed] [Google Scholar]
- 19.Sowa NA, Taylor-Blake B, Zylka MJ. Ecto-5’-nucleotidase (CD73) inhibits nociception by hydrolyzing AMP to adenosine in nociceptive circuits. J Neurosci. 2010;30:2235–2244. doi: 10.1523/JNEUROSCI.5324-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Street SE, Walsh PL, Sowa NA, Taylor-Blake B, Guillot TS, Vihko P, Wightman RM, Zylka MJ. ACPP and NT5E inhibit nociceptive neurotransmission by rapidly hydrolyzing nucleotides to adenosine. Mol Pain. 2011;7:80. doi: 10.1186/1744-8069-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]