Abstract
A compound that targets the Wnt signaling pathway in cancer moves into therapeutic trials, 30 years after Wnt was discovered.
Earlier this year, with little fanfare, one of the first small molecules targeting Wnt-mediated cellular signaling, called LGK974, entered a phase I clinical trial. Cell signaling that is controlled by secreted Wnt proteins is pivotal in animal development and tissue homeostasis, and has become a high-priority anticancer drug target given its essential role in colorectal cancer and its contribution to a broad range of other cancer types (1, 2). The importance of targeting this pathway was recently highlighted in a keynote address by Harold Varmus at a meeting in the Netherlands (3) marking the 30th anniversary of the discovery of the first Wnt molecule (there are 19 of them). With Roel Nusse, Varmus linked deviant activity of Wnt molecules to cancer. Although the target of LGK974—an acyltransferase called Porcupine (Porcn) that adds fatty acid to Wnt—has been well studied, few of the meeting participants were aware of the drug candidate, reflecting the remarkable speed and circumstances whereby this compound was nominated as a clinical candidate. The atypical path to the discovery of LGK974 may also signal changes in the approach to cancer drug discovery that include an increasing reliance on collaboration between government and industry to bring new drug targets to clinical testing.
Discovered in screens for genes that affect embryonic patterning in the fruit fly (4), PORCN is the founding member of a 16-gene family with predicted acyltransferase activity. Given their multiple membrane-spanning domains, the proteins encoded by these genes are called membrane-bound O-acyltransferases (MBOATs) to distinguish them from cytoplasmic acyltransferases such as those that modify the Ras proto-oncogene proteins. Two other family members have known protein substrates: Hhat modifies the signaling molecule Hedgehog, and Goat modifies the appetite-controlling hormone ghrelin. The fatty acyl modification of Wnt, Hedgehog, and ghrelin is essential to their activity. Palmiteoylation of Wnt proteins on a highly conserved serine residue precedes their engagement with a chaperone molecule (Wntless), which then shepherds them through the secretory pathway. Thus, in the absence of Porcn to catalyze this modification, Wnt proteins remain trapped inside the cell. This modification is also essential for Wnt binding to their cognate receptors (Frizzled proteins), as revealed in the crystal structure of Wnt and discussed at the recent Wnt anniversary meeting (5). The activity dependence of three major signaling molecules on fatty acyl adducts suggests that coordination of cellular behavior in metazoans may be directly influenced by metabolic status of ligand-producing cells.
The chemical tractability of Porcn was first revealed in a cell culture–based screen for small molecules that disable signaling by Wnt at the level of the transcriptional effector of the Wnt pathway, T cell factor (Tcf) (6). One class of small molecule identified through this strategy stabilizes Axin proteins by inhibiting Tankyrase (Tnks) enzymes (7). Axins scaffold a protein complex that destroys β-catenin, a downstream signaling molecule in the Wnt pathway that controls target gene expression. Another class identified by this approach disables Porcn and Wnt protein production (6). Porcn affords the more potent and selective means of inhibiting Wnt signaling given the superior activity of Porcn inhibitors (6) and the established roles of Tnks enzymes in non-Wnt–associated functions. Yet, with our limited understanding of MBOAT enzymology and cellular functions, and the remote prospect of acquiring structural information to guide the development of MBOAT antagonists, Porcn is in many ways an atypical drug target. In light of these challenges and the presumed lack of utility of Porcn inhibitors in the cancer type most strongly linked to Wnt signaling (colorectal cancer), the clinical testing of LGK974 represents a bold move but also reflects a confidence in the ultimate utility of achieving chemical control of key cell fate determination pathways in the management of disease.
Genetic approaches that abrogate Wnt signaling have indicated that the intense self-renewal process of the intestinal epithelium is absolutely dependent on Wnt signals (8). This has largely shaped opinion on the viability of therapeutic strategies targeting Wnt signaling. The initiation of dose-escalation studies (to determine how a drug is tolerated in patients) for a Porcn inhibitor should indicate whether toxicity issues associated with targeting Wnt-dependent cellular responses with drugs is more limited than anticipated. Indeed, genetic evidence derived from animals depleted of Paneth cells in the gut, which provide a Wnt signal to gut epithelial stem cells, reveal the adaptability of this stem cell compartment in the face of insults (9–11).
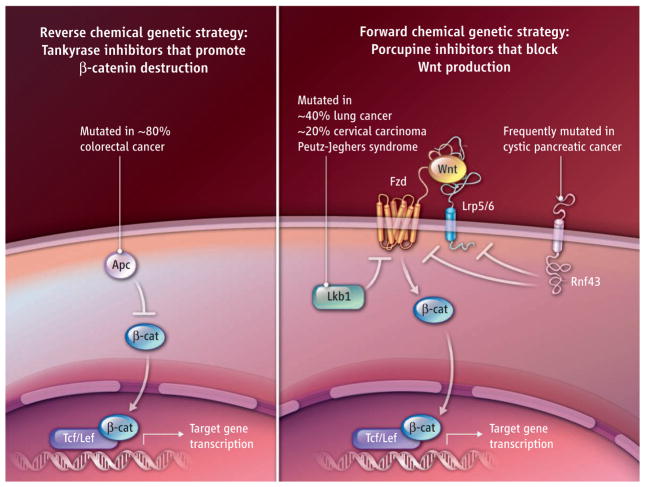
The Tnks inhibitors were identified by targeted disruption of the Wnt pathway in colorectal cancer cells that typically carry a mutation in the adenomatous polyposis coli (APC) gene. Such cells harbor an activated Wnt pathway in the absence of Wnt. By contrast, the Porcn inhibitors were identified through a reverse chemical genetics campaign wherein the discovery of compounds precedes their evaluation for therapeutic utility in different disease settings (see the figure). Two genetic indications for the potential use of Porcn inhibitors in cancerous settings have so far been identified from mining microarray and functional genomics data sets for regulators of Wnt signaling—the presence of loss-of-function mutations in the LKB1 tumor suppressor kinase gene that restrains the activity of Wnt receptors (Frizzled) and in the RNF43 transmembrane ubiquitin ligase gene that promotes the turnover of these receptors (12–14). Loss of either gene increases Wnt ligand-dependent signaling, which can be reversed by Porcn inhibitors. Given that mutations in RNF43 and LKB1 are frequently observed in cystic pancreatic and lung cancer, respectively, these efforts highlight two cancer types that may afford robust measures of LGK974 clinical utility should it prove well tolerated in humans (15, 16). In lung and pancreatic cancers, Wnt ligand-dependent signaling may also be essential in promoting distal metastasis (17, 18).
Figure. Blocking Wnt signaling.
Two chemical genetic strategies have identified small molecules that inhibit Wnt signaling. Certain cancers have mutations in constituents of the pathway. Apc, adenomatous polyposis coli; Tcf, T cell factor; Lef, lymphoid enhancer binding factor; Fzd, Frizzled; Lkb1, liver kinase B1; Rnf43, ring finger protein 43; Lrp5/6, LDL-related proteins 5/6; β-cat, β-catenin.
The unanticipated entry of a Porcn inhibitor into clinical testing this year is indicative of increased coordination between pharmaceutical companies and academic research centers to identify new clinical targets in response to decreased research funding in both private and government sectors. Thus, a need emerges to improve upon reverse chemical genetic strategies for matching disease to small molecules that increasingly originate from efforts to net chemical probes for basic research rather than for targeting specific genetic mutations. The advancement of a Porcn inhibitor for clinical testing also reflects a growing confidence in the anti-cancer promise of targeting cellular processes that have been long defined by their role in embryonic development. Indeed, this effort to target Wnt signaling follows on the approval earlier this year by the U.S. Food and Drug Administration for the use of Vismodegib, a small molecule that disrupts the Hedgehog signaling pathway, in the management of metastatic basal cell carcinoma. The attempt to target Porcn in cancer hopefully signifies a renewed courage to pursue nontraditional classes of drug targets and to rapidly expand the anticancer therapeutic arsenal.
Acknowledgments
L.L. has pending patents for both Porcn and Tnks inhibitors (2011/0136,813; filed 27 May 2009; published 9 June 2011). L.L. is supported by the Welch Foundation (I-1665) and CPRIT (RP100119).
Contributor Information
Lawrence Lum, Email: lawrence.lum@utsouthwestern.edu.
Hans Clevers, Email: h.clevers@hubrecht.eu.
References and Notes
- 1.The Cancer Genome Atlas Network. Nature. 2012;487:330. [Google Scholar]
- 2.Clevers H, Nusse R. Cell. 2012;149:1192. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 3.EMBO Conference: 30 Years of Wnt Signaling; 27 June to 1 July, 2012; Netherlands: Egmond aan Zee; [Google Scholar]
- 4.van den Heuvel M, Harryman-Samos C, Klingensmith J, Perrimon N, Nusse R. EMBO J. 1993;12:5293. doi: 10.1002/j.1460-2075.1993.tb06225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Science. 2012;337:59. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen B, et al. Nat Chem Biol. 2009;5:100. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang SM, et al. Nature. 2009;461:614. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 8.Pinto D, Gregorieff A, Begthel H, Clevers H. Genes Dev. 2003;17:1709. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durand A, et al. Proc Natl Acad Sci USA. 2012;109:8965. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim TH, Escudero S, Shivdasani RA. Proc Natl Acad Sci USA. 2012;109:3932. doi: 10.1073/pnas.1113890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato T, et al. Nature. 2011;469:415. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao HX, et al. Nature. 2012;485:195. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- 13.Jacob LS, et al. Sci Signal. 2011;4:ra4. doi: 10.1126/scisignal.2001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koo B-K, et al. Nature. 2012 doi: 10.1038/nature11308. [DOI] [Google Scholar]
- 15.Wu J, et al. Proc Natl Acad Sci USA. 2011;108:21188. [Google Scholar]
- 16.Ding L, et al. Nature. 2008;455:1069. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen DX, et al. Cell. 2009;138:51. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu M, et al. Nature. 2012;487:510. doi: 10.1038/nature11217. [DOI] [PMC free article] [PubMed] [Google Scholar]