Abstract
Study Design
Prospective cross-sectional study.
Objectives
To compare knee muscle morphology and voluntary neuromuscular control in individuals who sustained an anterior cruciate ligament (ACL) injury and were identified as being capable of avoiding surgery (potential copers) and those who were recommended for surgery (noncopers), within 6 months of injury.
Background
Quadriceps atrophy and poor neuromuscular control have been found in noncopers. However, the reasons why some noncopers may be able to avoid surgery remain elusive.
Methods
Twenty participants (10 ACL-deficient noncopers and 10 ACL-deficient potential copers) were included in this study. Axial spin-echo, T1-weighted magnetic resonance imaging data of the lower extremities were captured. The volume and maximum cross-sectional area (CSA) of each muscle of the quadriceps and hamstrings were calculated following digital reconstruction. In addition, voluntary neuromuscular control was evaluated using an established target-matching task that required participants to produce static isometric loads across the knee joint. Electromyography was acquired from 5 muscles as participants performed the target-matching task. Circular statistics were used to calculate a specificity index to describe how well focused each muscle was activated toward its primary direction of muscle action. The ACL-deficient limb was then compared to the uninvolved limb of the noncopers and potential copers.
Results
The vasti (vastus medialis and vastus intermedius) of the involved limb of the noncopers were significantly smaller (P<.031) in comparison to those of their uninvolved limb. The potential copers' vastus lateralis maximum CSA (P = .047), total quadriceps muscle volume (P = .020) and maximum CSA (P = .015), and quadriceps-hamstring ratio volume (P = .021) and maximum CSA (P = .007) demonstrated quadriceps atrophy. However, only the ACL-deficient limb of the older (mean ± SD age, 27.4 ± 11.4 versus 19.9 ± 3.3 years; P = .032) and lower-activity-level (3.3 ± 0.5 versus 3.6 ± 0.5; P = .098) noncoper group demonstrated reduced rectus femoris (P = .057) and lateral hamstring (P = .064) neuromuscular control in comparison to their uninvolved limb.
Conclusion
These findings suggest that quadriceps and hamstring muscle function, rather than muscle size, may be an important factor in the varied response early after ACL injury.
Keywords: ACL, knee, MRI
In the United States, about 250 000 people tear their anterior cruciate ligament (ACL) each year.15,26 In 2006, according to US national data, approximately 125 000 ACL reconstructions were performed.20 Many individuals express the desire to return to their prior level of activity without ACL reconstruction; however, the return rate varies from 19% to 82%,30-33 perhaps because of persistent movement dysfunction.9 Most ACL injuries occur during sports that involve dynamic movements such as jumping, pivoting, and cutting,3,14 so returning to preinjury levels of activity requires high-level neuromuscular control to dynamically stabilize the femur on the tibia.
Within the ACL-injured population, there are high- and low-functioning groups. A screening tool has been developed that may help distinguish individuals within 6 months of injury who have the potential to return to their previous activity level without surgery (potential copers) from those who are recommended for ACL reconstruction (noncopers).13,18 Potential copers represent a select group of high-functioning individuals and must have all of the following: 80% or greater on a timed hop score of the ACL-deficient limb in comparison to the uninvolved limb, a Knee Outcome Survey activities of daily living score of 80% or greater, a global rating of knee function of 60% or greater, and no more than 1 episode of giving way since the injury. Noncopers, on the other hand, are characteristically identified by recurrent episodes of giving way34 and reduced neuromuscular control,35-39 and, unfortunately, represent the majority of individuals with ACL injuries.13,17,27 Potential copers have been characterized as having coordinated muscle contractions during functional tasks, such as walking and jogging,5 and during a timed hop test.18 Ideally, those classified as potential copers go on to be true copers, defined as being 1 year from injury and having returned to sport without episodes of giving way with nonsurgical management.
The difference between potential copers and noncopers is sometimes unclear. In a previous study39 comparing muscle morphology and neuromuscular control between noncopers, true copers, and uninjured controls, the noncopers were found to have quadriceps atrophy and poorer neuromuscular control, whereas few differences existed between true copers and uninjured controls. However, recent evidence suggests that those initially classified as noncopers, despite their initial classification, have a 70% chance of becoming true copers.29 A prior study28 that did not classify coping status raises the question of whether surgery may always be necessary, as individuals randomly assigned to a nonoperative group performed better than a matched surgical group in 2 of 4 hop tests. Peak isometric knee extensor strength does not differentiate potential copers from noncopers,18 but noncopers were found to have significantly greater side-to-side limb asymmetry than potential copers with isokinetic knee extension strength tested at 60°/s at angles less than 40° of knee extension.10 Individual muscles within the quadriceps may be selectively recruited during terminal knee extension while contracting isokinetically, but it is not known if individual muscles within the knee extensors are differentially affected between groups. Furthermore, others have found that ACL injury has a varied effect on neuromuscular control and does not similarly affect all injured individuals.39 There is a need to improve our ability to accurately differentiate those who require surgery from those who are capable of returning to their prior level of function without ACL reconstruction.
The purpose of this study was to describe the knee muscle morphology and voluntary neuromuscular control of ACL-deficient noncopers and potential copers within 6 months of injury to better differentiate these groups. We hypothesized that (1) noncopers would demonstrate quadriceps atrophy and poorer neuromuscular control on their injured side, whereas the potential copers would not; and (2) neither group would demonstrate hamstring atrophy on their injured side.
Methods
Participants
Twenty participants (10 ACL-deficient noncopers and 10 ACL-deficient potential copers) were included in this study. All were regular participants (greater than 50 hours per year) in level 1 or 2 activities that included running, cutting, and jumping (eg, soccer, basketball, football) prior to their injury.7 Each group included 8 males and 2 females. All ACL tears were diagnosed by a sports fellowship–trained orthopaedic surgeon and were confirmed by noncontrast magnetic resonance imaging and a 3-mm or greater side-to-side knee anterior laxity difference7 (KT1000 arthrometer; MEDmetric Corporation, San Diego, CA). The coping status of the participants was determined by an established screening examination that differentiates people who may be able to return to their prior level of function without undergoing ACL reconstructive surgery.13 The Knee Outcome Survey was filled out by all subjects. Exclusion criteria were a previous ACL injury, concomitant ligament injury, fracture, greater than trace knee effusion, pathological gait, and hip or ankle pathology. Participants were only included if the duration of their ACL injury was less than 6 months, to prevent any confounding effects of chronic knee instability (TABLE 1). Parameters included in TABLE 1 were collected at the time the participants completed the previously described screening examination for ACL-deficient coping status, including their activity level prior to injury. Participants were not excluded from the study if there was a contact mechanism of injury. All participants provided written informed consent, in accordance with the University of Delaware Institutional Review Board, which approved the research protocol and the informed-consent form.
Table 1. Demographic Data of Participants.
| Parameter | Noncoper* | Potential Coper* | P Value |
|---|---|---|---|
| Age at injury, y | 27.4 ± 11.4 | 19.9 ± 3.3 | .032 |
| Age at testing, y | 27.6 ± 11.4 | 20.1 ± 3.3 | .031 |
| Duration of injury, mo | 2.4 ± 1.2 | 2.3 ± 0.9 | .393 |
| Height, cm | 172.7 ± 9.7 | 176.3 ± 11.9 | .229 |
| Weight, kg | 72.3 ± 14.3 | 77.8 ± 12.2 | .183 |
| Body mass index, kg/m2 | 24.0 ± 3.6 | 24.9 ± 2.1 | .243 |
| Giving-way episodes, n | 3.2 ± 4.7 | 0.3 ± 0.5 | .035 |
| Knee Outcome Survey, % | 80.7 ± 8.8 | 93.4 ± 3.9 | <.001 |
| Global rating score, % | 77.5 ± 12.5 | 79.5 ± 11.9 | .359 |
| Activity level at testing (1-4) | 3.6 ± 0.5 | 3.3 ± 0.5 | .098 |
| Activity level preinjury (1-4) | 1.4 ± 0.5 | 1.2 ± 0.4 | .178 |
| Knee laxity, mm | 5.3 ± 2.2 | 5.0 ± 3.0 | .394 |
Values are mean ± SD.
Magnetic Resonance Imaging
To obtain muscle morphology data, axial spin-echo, T1-weighted magnetic resonance images were acquired with a 1.5-T Signa LX scanner (GE Healthcare, Waukesha, WI) from the level of the ankle mortise up to the iliac crest, while participants lay supine in the scanner. Imaging for both limbs was acquired simultaneously using the scanner's body coil. The imaging protocol (repetition time, 350 milliseconds; echo time, 9 milliseconds; 256 × 160 matrix; and a field of view that varied with the size of the participant) was repeated 4 times across overlapping sections of the lower leg, knee, thigh, and pelvis. Slices were 10 mm thick, with a 1.5-mm gap between slices in the lower leg, thigh, and pelvis. However, in the knee, the slices were 5 mm thick, with a 1-mm gap between them, to acquire more detailed data.
Image processing was completed in 3 steps. First, the muscle contours were manually traced in each axial slice in which they were present, using IMOD software (The Boulder Laboratory for 3-D Electron Microscopy of Cells, University of Colorado, Boulder, CO)21 and a digital palette (Intuos2; Wacom Technology, Vancouver, WA). A single rater, who was blinded to the group affiliation of the participants and side of injury, performed all digitization. In a preliminary trial of the test-retest reliability and accuracy of a magnetic resonance image phantom of known dimensions, the rater's error was a difference of less than 5% from known dimensions and less than 1% across 4 measurements. This processing method has demonstrated a high degree of reproducibility in a separate test-retest reliability study.39 Muscles were traced as separate objects to differentiate tissue effects. The muscles traced were the semimembranosus, semitendinosus, biceps femoris long head, biceps femoris short head, rectus femoris (RF), vastus medialis (VM), vastus lateralis (VL), and vastus intermedius (VI). Second, the contours of each muscle from each imaging sequence were grouped, and 3-D reconstructions were generated in a custom-written MATLAB program (The MathWorks, Inc, Natick, MA). Third, total muscle volume (cm3) and maximum cross-sectional area (CSA) (cm2) were calculated. In addition, total volume and maximum CSA were calculated for a knee extensor group that combined the RF, VM, VL, and VI, and for a knee flexor group that combined the semimembranosus, semitendinosus, biceps femoris long head, and biceps femoris short head, by summing the values of the individual muscles.
Voluntary Neuromuscular Control Testing
Participants performed a previously developed, seated target-matching protocol to demonstrate the neuromuscular coordination of the muscles about the knee.22-24,35-39 Participants were seated on a cushion placed beneath their ischial tuberosities, so that their thighs were unloaded and free to move (FIGURE 1). Both limbs were tested, and the order of testing was randomized to prevent a test-order effect. Joint angles were standardized to 90° of hip flexion and 70° of knee flexion. The participant's task was to position a circular cursor over a narrow target (FIGURE 2). The cursor and targets were projected with a video projection system onto a wall directly in front of the participant. The targets appeared consecutively, in random order, at 1 of 18 positions located every 20° on the circumference of an invisible circle in the flexion/extension-varus/valgus plane. Participants had to match each target position 4 times, for a total of 72 targets. The cursor moved in response to forces produced against a 6-degrees-of-freedom load cell (F/T model US-150-600; ATI Industrial Automation, Apex, NC) attached to the participants with a 6-cm fiberglass cast and clamp that were located approximately 6 cm superior to the lateral malleolus (FIGURE 1). The cursor moved with 4 degrees of freedom: (1) knee extension/flexion force applications moved the cursor up and down, (2) varus (hip adduction) and valgus (hip abduction) force applications moved the cursor medial and lateral, (3) inferior/superior force applications (along the axis of the shank) made the cursor circumference smaller or larger, and (4) internal/external rotation torque applications of the shank moved the “needle” within the cursor clockwise or counterclockwise.
Figure 1.
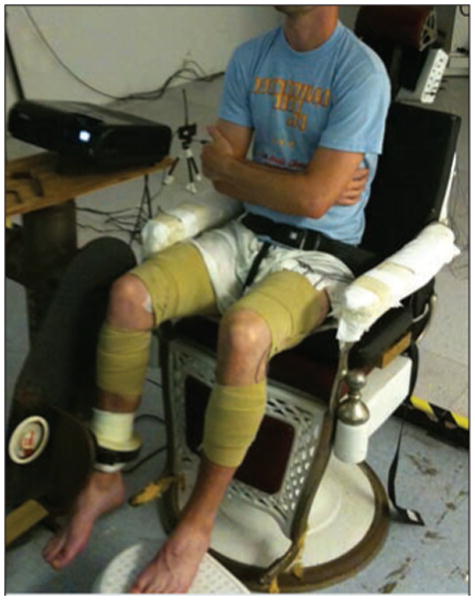
Experimental setup for the voluntary neuromuscular control protocol.
Figure 2.
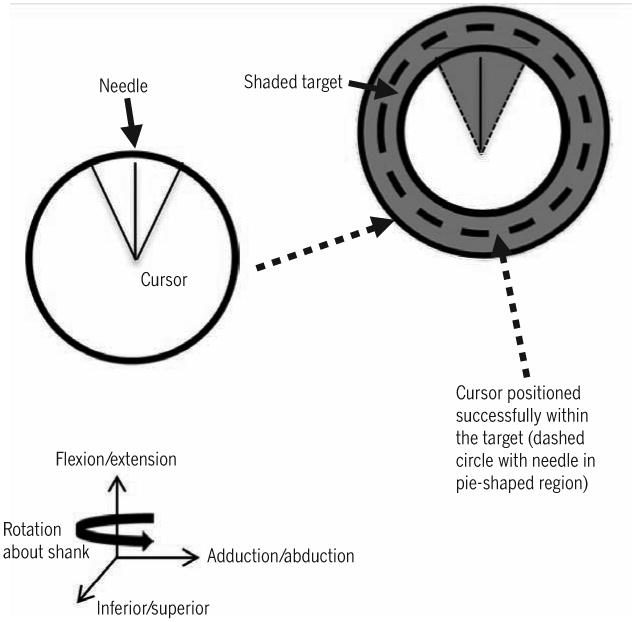
Target-matching task visual feedback used to measure voluntary neuromuscular control. Adapted from Williams et al.39
Participants performed maximum voluntary isometric contractions to determine the peak forces and moments that they could produce in each plane and around each axis. A load of 30% of the magnitude of the smaller of the varus/ valgus-directed forces recorded during the maximum voluntary isometric contractions was required to successfully position the cursor within the target and to standardize the loads between participants. Criteria required for success were that the cursor was larger than the inner target circle and smaller than the larger target circle. In addition, the “needle” was required to remain in a neutral position. Participants were required to hold the cursor within the target area for 0.5 seconds before the trial was considered successful. The electromyography (EMG) and force data during the 0.5 seconds were recorded for analysis. Prior to collecting data from each limb, participants performed 18 random targets to familiarize themselves with the task and to reduce the effects of task novelty and learning. The participants had no time limit for the target-matching task and were encouraged to rest whenever they felt the need. The individual administering the target-matching task was not blinded to the group affiliation of the participants or side of injury.
Electromyography
Electrodes, using a standard location, were placed on the following muscles: RF, VM, VL, and medial hamstrings (MH) and lateral hamstrings (LH).16 Prior to application, all hair was shaved from the electrode placement sites and the skin in the region was cleaned to facilitate optimal electrode contact and to reduce the impedance at the electrode-skin interface. Adhesive Ag/AgCl surface electrodes (Norotrode 120; Myotronics-Noromed, Inc, Kent, WA) were applied at a 2-cm interelectrode distance, then connected to a differential preamplifier with a gain of 20 and a 2-pole (20-2000 Hz) band-pass filter. Surface electrodes and preamplifiers were further secured with tape (HYPAFIX; Smith & Nephew, London, UK), then wrapped with 10-cm-wide elastic bands (SuperWrap; Fabrifoam Products, Exton, PA). The analog signal was anti-aliased at 500 Hz through a backpack unit (MA300-28; Motion Lab Systems, Inc, Baton Rouge, LA) and sampled at 1000 Hz. A resting trial was recorded prior to the experimental protocol, so that baseline noise could be removed during EMG data reduction. The EMG signal of each muscle was full-wave rectified and normalized to the peak EMG magnitudes recorded prior to testing during maximum voluntary isometric contractions at 70° of knee flexion.
Data Reduction of Muscle Activity
Muscle activity was evaluated using a specificity index that has been well described in the literature,8,25,35,36,39 to quantify how specific the muscle activation pattern of a single muscle was to a single force direction, or, in this case, the target. The specificity index was calculated using circular statistics due to the polar coordinate system of the force directions.1,12 The formula for the sum of the vectors divided by the scalar magnitude was as follows: Σ(EMGi×λ)/Σ|EMGi|, where EMGi is a vector describing the EMG magnitude in each target direction and λ is the unit vector in the EMG direction. A specificity index of 1.0 indicated that a muscle was only active when 1 of the 18 targets was present and was therefore specific to that direction, whereas a specificity index of 0.0 indicated that the muscle was equally active at each of the 18 targets and thus not specific to any force direction.
Data Analysis
To compare between groups, muscle morphology data were converted to an index by using a limb-symmetry index, calculated by dividing the ACL-deficient limb muscle volume or maximum CSA by the respective value of the uninvolved limb. Demographic data were compiled and compared between groups using t tests. Comparisons of the muscle morphology (volume and maximum CSA) and specificity indices for side-to-side differences and between groups were made using t tests. The level of significance (alpha) was set at .05. Parameters for a priori power estimates (G*Power Version 3.0.10)11 were as follows: α = .05, 1 − β = 0.80. Effect size (d) was determined by group means and standard deviations using a built-in algorithm in the software based on the literature and pilot studies. The calculated sample size from the a priori tests of statistical power of 0.80 was between 10 and 20 subjects in a group for the muscles studied. Cohen d was calculated to present effect size, or the standardized estimate of the magnitude of the differences between means.6 Cohen6 described an effect size of 0.2 as small, an effect size of 0.5 as medium, and an effect size of 0.8 as large.
Results
The participants in the potential coper group were younger (P = .032), had fewer giving-way episodes of the knee (P = .035), a higher Knee Outcome Survey score (P = .0003), and a higher activity level at the time of screening (P = .098) when compared to the noncopers (TABLE 1). There were no significant differences for the other demographic variables.
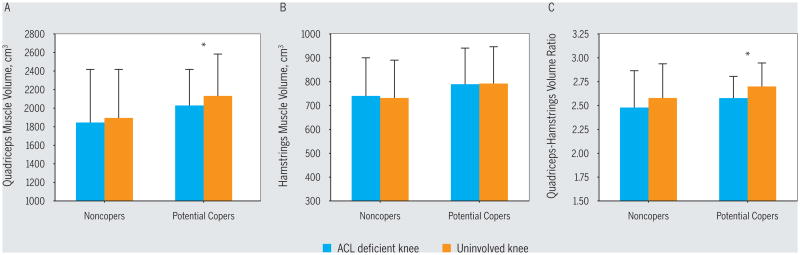
The knee extensor group of the involved side of the potential copers demonstrated significant atrophy when compared to the uninvolved side (FIGURE 3, TABLE 2); in contrast, the noncopers had atrophy in only 2 of the vasti (VM and VI) and not the knee extensor group as a whole. There were no significant differences for the normalized limb volumes or maximum CSAs of the quadriceps muscles between the potential coper and noncoper groups (TABLE 2). There were no significant side-to-side asymmetries or between-group differences for the hamstring muscle morphology (FIGURE 3, TABLE 2).
Figure 3.
Limb comparisons for (A) quadriceps, (B) hamstrings, and (C) quadriceps-hamstring ratio muscle volume data across the 2 groups. Values are mean ± SD.
*Significant difference between the ACL-deficient and the uninvolved side (P<.05). Abbreviation: ACL, anterior cruciate ligament.
Table 2. Muscle Volume and Maximum Cross-sectional Area for the 2 Groups.
| Potential Copers | Noncopers | Between-Groups P Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Muscle | ACL Deficient* | Uninvolved* | P Value | Effect Size | ACL Deficient* | Uninvolved* | P Value | Effect Size | |
| Volume, cm3 | |||||||||
| Vastus lateralis | 794.5 ± 131.2 | 807.8 ± 143.4 | .17 | 0.1 | 783.7 ± 225.3 | 802.4 ± 235.1 | .20 | 0.08 | .29 |
| Vastus intermedius | 419.3 ± 79.1 | 423.1 ± 71.6 | .42 | 0.1 | 349.9 ± 107.8 | 398.7 ± 129.5† | .01 | 0.41 | .18 |
| Vastus medialis | 435.4 ± 71.1 | 444.9 ± 72.5 | .19 | 0.1 | 412.8 ± 146.7 | 432.8 ± 141.3† | .02 | 0.14 | .44 |
| Rectus femoris | 297.8 ± 53.0 | 300.6 ± 68.3 | .38 | 0.0 | 280.0 ± 77.5 | 278.6 ± 71.5 | .32 | 0.02 | .23 |
| Semitendinosus | 204.5 ± 59.7 | 209.0 ± 61.0 | .17 | 0.1 | 211.1 ± 49.2 | 213.2 ± 48.9 | .36 | 0.04 | .47 |
| Semimembranosus | 249.2 ± 33.5 | 251.9 ± 38.6 | .28 | 0.1 | 222.1 ± 46.9 | 224.2 ± 50.3 | .35 | 0.04 | .34 |
| Biceps femoris (long) | 213.7 ± 35.7 | 211.6 ± 38.2 | .24 | 0.1 | 199.8 ± 40.9 | 201.7 ± 48.1 | .37 | 0.04 | .33 |
| Biceps femoris (short) | 91.8 ± 16.9 | 89.5 ± 14.8 | .23 | 0.1 | 99.9 ± 32.6 | 98.0 ± 33.9 | .21 | 0.06 | .49 |
| Hamstrings | 789.5 ± 150.3 | 790.8 ± 154.8 | .45 | 0.0 | 739.4 ± 160.3 | 730.7 ± 156.6 | .23 | 0.05 | .32 |
| Quadriceps group | 2027.2 ± 407.5 | 2130.2 ± 444.2† | .02 | 0.2 | 1845.0 ± 570.3 | 1893.8 ± 523.8 | .16 | 0.09 | .24 |
| Quadriceps-hamstring ratio | 2.6 ± 0.2 | 2.7 ± 0.2† | .02 | 0.5 | 2.5 ± 0.4 | 2.6 ± 0.3 | .16 | 0.29 | .45 |
| Cross-sectional area, cm2 | |||||||||
| Vastus lateralis | 32.1 ± 5.22† | 34.0 ± 6.1† | .05 | 0.3 | 33.8 ± 7.9 | 34.1 ± 8.6 | .40 | 0.04 | .18 |
| Vastus intermedius | 24.2 ± 3.9 | 24.5 ± 3.8 | .40 | 0.1 | 20.7 ± 5.3 | 22.7 ± 6.6† | .03 | 0.33 | .24 |
| Vastus medialis | 24.2 ± 4.0 | 24.9 ± 4.3 | .16 | 0.2 | 23.4 ± 6.6 | 24.5 ± 6.8† | .02 | 0.17 | .40 |
| Rectus femoris | 15.6 ± 2.9 | 15.8 ± 3.6 | .40 | 0.1 | 14.2 ± 2.7 | 14.2 ± 2.9 | .38 | 0.03 | .17 |
| Semitendinosus | 11.0 ± 3.5 | 11.1 ± 3.2 | .37 | 0.0 | 10.5 ± 2.1 | 10.8 ± 2.0 | .13 | 0.14 | .30 |
| Semimembranosus | 14.9 ± 2.0 | 15.0 ± 2.5 | .45 | 0.0 | 13.3 ± 2.6 | 13.0 ± 2.0 | .33 | 0.09 | .15 |
| Biceps femoris (long) | 14.4 ± 2.5 | 14.4 ± 2.1 | .45 | 0.0 | 13.4 ± 2.5 | 13.6 ± 2.7 | .18 | 0.09 | .26 |
| Biceps femoris (short) | 7.2 ± 1.1 | 7.1 ± 1.1 | .35 | 0.1 | 74 ± 2.0 | 7.4 ± 2.2 | .41 | 0.02 | .21 |
| Hamstrings | 48.4 ± 8.0 | 48.5 ± 7.9 | .46 | 0.0 | 44.8 ± 7.8 | 44.6 ± 7.2 | .37 | 0.03 | .14 |
| Quadriceps group | 100.1 ± 19.4† | 105.5 ± 19.7† | .01 | 0.3 | 93.1 ± 23.1 | 94.4 ± 21.7 | .30 | 0.06 | .41 |
| Quadriceps-hamstring ratio | 2.1 ± 0.18† | 2.2 ± 0.2† | .01 | 0.5 | 2.1 ± 0.4 | 2.1 ± 0.3 | .30 | 0.10 | .15 |
Abbreviation: ACL, anterior cruciate ligament.
Values are mean ± SD.
Significant asymmetry between ACL-deficient and uninvolved limbs (P<.05).
In the noncoper group, the VM muscle volume (P = .021) and maximum CSA (P = .024) and VI muscle volume (P = .001) and maximum CSA (P = .031) of the involved side were significantly smaller compared to the uninvolved side (TABLE 2). In the potential coper group, the VL muscle maximum CSA (P = .047), total quadriceps muscle volume (P = .020) and maximum CSA (P = .015), and quadriceps-hamstring ratio volume (P = .021) and maximum CSA (P = .007) of the involved side were all significantly smaller compared to the uninvolved side.
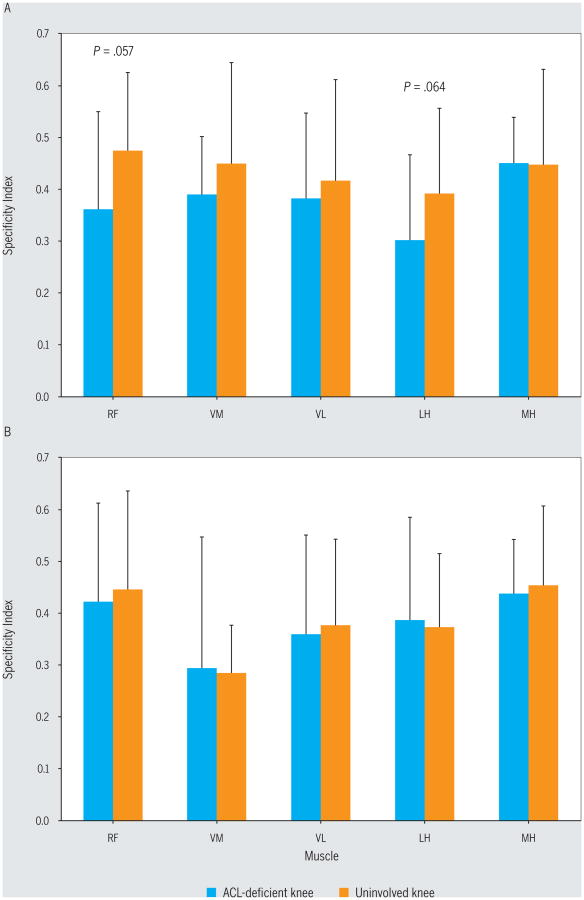
For the noncopers, the specificity index for the RF (mean ± SD, 0.36 ± 0.19) and LH (0.30 ± 0.16) on the involved side was lower than that on the uninvolved side (RF, 0.47 ± 0.15 and LH, 0.39 ± 0.17), suggesting less-refined neuromuscular control of the RF (P = .057, Cohen d = 0.66) and LH (P = .064, Cohen d = 0.55) muscles of the involved limb (FIGURE 4). Based on the specificity index, there were no significant neuromuscular control differences found between limbs for the potential copers (P>.22, Cohen d<0.12) or between the injured limbs of the potential copers and the noncopers (P>.14, Cohen d<0.50).
Figure 4.
Specificity indices of the thigh muscles for the 2 groups: (A) noncopers and (B) potential copers. Values are mean ± SD. All P values for potential copers were greater than .22. With the exception of the RF (P = .057) and LH (P = .064), all other P values for noncopers were greater than .24. Abbreviations: LH, lateral hamstrings; MH, medial hamstrings; RF, rectus femoris; VL, vastus lateralis; VM, vastus medialis.
Discussion
The purpose of this study was to compare the morphological and voluntary neuromuscular control qualities of the ACL-deficient knee of individuals considered potential copers versus those considered noncopers. In support of our hypothesis, when compared to the noninvolved side, the ACL-deficient knee of the noncopers had VM and VI atrophy. Contrary to our hypothesis, when compared to the noninvolved side, the ACL-deficient knee of potential copers had significant atrophy of the total quadriceps muscle group and a lower quadriceps-hamstring ratio. In partial support of our initial hypothesis, the noncopers had poorer voluntary neuromuscular control of the RF (mean ± SD, 0.36 ± 0.19) and LH (0.30 ± 0.16) muscles of the ACL-deficient side in comparison to their uninvolved limb (0.47 ± 0.15, P = .057 and 0.39 ± 0.17, P = .064, respectively). Side-to-side differences in voluntary neuromuscular control for the potential coper group were all of much smaller magnitude. There were no significant differences in quadriceps or hamstring muscle morphology between groups (P>.14).
The varied response to ACL injury has been suggested to be related to the neuromuscular function of the muscles about the knee.34,36,39 It has been found that noncopers fire their quadriceps muscles during knee flexion tasks, whereas healthy controls do not.35 The giving-way episodes that in part distinguished noncopers in this study (TABLE 1) may actually be caused by this counterproductive quadriceps function, as others have suggested.37 This study did not find potential copers to have large side-to-side differences in neuromuscular control, as measured with the specificity index (all differences less than 0.02, P>.22). In contrast, the noncopers were found to have lesser control on the affected side for both the RF (difference in specificity index, 0.11; P = .057) and LH (difference in specificity index, 0.09; P = .064) (FIGURE 4). Others have suggested that noncopers use a quadriceps muscle–stiffening strategy during gait, in an effort to avoid giving way and to stabilize the knee.34 Further, previous work has established that noncopers may attempt to stabilize the tibiofemoral joint by asynchronously firing their medial and lateral hamstring muscles4 to avoid anterior tibial translation.19 In contrast, potential copers were able to maintain more normal tibial positioning, perhaps with greater medial quadriceps muscle activation.4 The potential findings of less-refined LH activation in this study are supported by data from Beard et al,2 who found that slower hamstring activation was correlated with episodes of giving way. Determining how potential copers stabilize their knee within the first 6 months of injury may allow clinicians to help a greater number of noncopers avoid surgery if they so desire. Further, these findings improve our understanding of what makes potential copers different from noncopers within 6 months of injury, so that screening tools used to classify groups may be refined.
Compared to the uninvolved side, the ACL-deficient side of potential copers had a smaller total knee extensor group and quadriceps-hamstring ratio, in contrast to that of the noncoper group, in which only the VM and VI were smaller. Noncopers were found to have significantly smaller VL, VI, total quadriceps muscle volumes and maximum CSAs, and quadriceps-hamstring ratio volume and maximum CSAs on the involved side in a previous experiment.39 Findings from the present study are in agreement with these previous findings for VI; however, the means and standard deviations overlapped for the other muscles. Therefore, the 2 studies are not in disagreement with regard to the muscle morphology of the noncopers.
There were a number of limitations in this study. The sample size was small; however, a priori power analysis participant numbers were achieved. The cross-sectional design of the study could be improved by following the participants longitudinally to determine if the voluntary neuromuscular control task was predictive of long-term coping status (if all participants did not undergo reconstructive surgery). Finally, the potential coper group was younger and at a higher activity level at the time of screening than the noncoper group. Future studies should match groups for age and activity level to overcome this limitation.
In the current study, potential copers were characterized by global knee extensor group atrophy rather than individual muscle atrophy, whereas 2 of 4 individual muscles of the noncopers' quadriceps were atrophic. Further, the potential copers had significant asymmetry in their quadriceps-hamstring ratio on their ACL-deficient side due to knee extensor atrophy with concomitant hamstrings symmetry. An explanation of these findings may be that the VL is the largest contributor to total volume and maximum CSA of the quadriceps muscle group, accounting for approximately 35% to 45% of the total, whereas the VM and VI each comprise approximately 20% to 25%, and the RF contributes the least, about 15%.37 Therefore, findings within the current study of atrophy only within the VM and VI of the noncopers may explain why noncopers did not demonstrate total quadriceps muscle atrophy.
Conclusion
Findings from this study indicate that individuals with an ACL-deficient knee classified within 6 months of injury as noncopers have significant vasti (VM and VI) atrophy, whereas potential copers have significant knee extensor atrophy and reduced quadriceps-hamstring ratio compared to their uninvolved side. The older and lower-functioning noncoper group was also found to have less-refined neuromuscular control of the RF (P = .057) and LH (P = .064) in comparison to their uninvolved limb, whereas there were no significant differences in the potential coper group. Perhaps better neuromuscular function, rather than muscle size, allows potential copers to have higher levels of function.
Key Points.
Findings
Individuals with an ACL-deficient knee classified as noncopers may have poorer neuromuscular control than potential copers. While potential copers had knee extensor group atrophy and reduced quadriceps-hamstring ratio, noncopers had atrophy of the vasti (VI and VM).
Implications
These findings suggest that the ability to cope within the first 6 months following ACL injury may, in part, be related to neuromuscular control.
Caution
The static testing protocol used in this study was not as functional as walking or running, so care must be taken when generalizing to more demanding tasks. Further, the noncoper group was older and at a lower activity level at the time of injury than the potential coper group.
Acknowledgments
This study was funded by the National Institutes of Health grant R01 AR46386 (principal investigator, Thomas S. Buchanan). The protocol for this study was approved by the Institutional Review Board of the University of Delaware.
Footnotes
The authors certify that they have no affiliations with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in the article.
References
- 1.Batschelet E. Circular Statistics in Biology. London, UK: Academic Press; 1981. [Google Scholar]
- 2.Beard DJ, Kyberd PJ, O'Connor JJ, Fergusson CM, Dodd CA. Reflex hamstring contraction latency in anterior cruciate ligament deficiency. J Orthop Res. 1994;12:219–228. doi: 10.1002/jor.1100120211. http://dx.doi.org/10.1002/jor.1100120211. [DOI] [PubMed] [Google Scholar]
- 3.Boden BP, Griffin LY, Garrett WE., Jr Etiology and prevention of noncontact ACL injury. Phys Sportsmed. 2000;28:53–60. doi: 10.3810/psm.2000.04.841. http://dx.doi.org/10.3810/psm.2000.04.841. [DOI] [PubMed] [Google Scholar]
- 4.Chmielewski TL, Hurd WJ, Snyder-Mackler L. Elucidation of a potentially destabilizing control strategy in ACL deficient non-copers. J Electromyogr Kinesiol. 2005;15:83–92. doi: 10.1016/j.jelekin.2004.07.003. http://dx.doi.org/10.1016/j.jelekin.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Chmielewski TL, Rudolph KS, Fitzgerald GK, Axe MJ, Snyder-Mackler L. Biomechanical evidence supporting a differential response to acute ACL injury. Clin Biomech (Bristol, Avon) 2001;16:586–591. doi: 10.1016/s0268-0033(01)00050-x. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 7.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22:632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 8.Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118(pt 2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 9.Eastlack ME, Axe MJ, Snyder-Mackler L. Laxity, instability, and functional outcome after ACL injury: copers versus noncopers. Med Sci Sports Exerc. 1999;31:210–215. doi: 10.1097/00005768-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Eitzen I, Eitzen TJ, Holm I, Snyder-Mackler L, Risberg MA. Anterior cruciate ligament-deficient potential copers and noncopers reveal different isokinetic quadriceps strength profiles in the early stage after injury. Am J Sports Med. 2010;38:586–593. doi: 10.1177/0363546509349492. http://dx.doi.org/10.1177/0363546509349492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. http://dx.doi.org/10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 12.Fisher NI. Statistical Analysis of Circular Data. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]
- 13.Fitzgerald GK, Axe MJ, Snyder-Mackler L. A decision-making scheme for returning patients to high-level activity with nonoperative treatment after anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2000;8:76–82. doi: 10.1007/s001670050190. http://dx.doi.org/10.1007/s001670050190. [DOI] [PubMed] [Google Scholar]
- 14.Griffin LY, Agel J, Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141–150. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Griffin LY, Albohm MJ, Arendt EA, et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006;34:1512–1532. doi: 10.1177/0363546506286866. http://dx.doi.org/10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 16.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10:361–374. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 17.Hurd WJ, Axe MJ, Snyder-Mackler L. A 10-year prospective trial of a patient management algorithm and screening examination for highly active individuals with anterior cruciate ligament injury: part 1, outcomes. Am J Sports Med. 2008;36:40–47. doi: 10.1177/0363546507308190. http://dx.doi.org/10.1177/0363546507308190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurd WJ, Axe MJ, Snyder-Mackler L. A 10-year prospective trial of a patient management algorithm and screening examination for highly active individuals with anterior cruciate ligament injury: part 2, determinants of dynamic knee stability. Am J Sports Med. 2008;36:48–56. doi: 10.1177/0363546507308191. http://dx.doi.org/10.1177/0363546507308191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kålund S, Sinkjær T, Arendt-Nielsen L, Simonsen O. Altered timing of hamstring muscle action in anterior cruciate ligament deficient patients. Am J Sports Med. 1990;18:245–248. doi: 10.1177/036354659001800304. http://dx.doi.org/10.1177/036354659001800304. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93:994–1000. doi: 10.2106/JBJS.I.01618. http://dx.doi.org/10.2106/JBJS.I.01618. [DOI] [PubMed] [Google Scholar]
- 21.Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. http://dx.doi.org/10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd DG, Besier TF. An EMG-driven musculoskeletal model to estimate muscle forces and knee joint moments in vivo. J Biomech. 2003;36:765–776. doi: 10.1016/s0021-9290(03)00010-1. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd DG, Buchanan TS. A model of load sharing between muscles and soft tissues at the human knee during static tasks. J Biomech Eng. 1996;118:367–376. doi: 10.1115/1.2796019. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd DG, Buchanan TS. Strategies of muscular support of varus and valgus isometric loads at the human knee. J Biomech. 2001;34:1257–1267. doi: 10.1016/s0021-9290(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 25.MacLeod TD, Manal K, Silbernagel KG, Snyder-Mackler L, Buchanan TS. Characteristics of human knee muscle coordination during isometric contractions in a standing posture: the effect of limb task. J Electromyogr Kinesiol. 2013;23:1398–1405. doi: 10.1016/j.jelekin.2013.05.004. http://dx.doi.org/10.1016/j.jelekin.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyasaka KC, Daniel DM, Stone ML, Hirshman P. The incidence of knee ligament injuries in the general population. Am J Knee Surg. 1991;4:3–8. [Google Scholar]
- 27.Moksnes H, Engebretsen L, Risberg MA. Performance-based functional outcome for children 12 years or younger following anterior cruciate ligament injury: a two to nine-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2008;16:214–223. doi: 10.1007/s00167-007-0469-7. http://dx.doi.org/10.1007/s00167-007-0469-7. [DOI] [PubMed] [Google Scholar]
- 28.Moksnes H, Risberg MA. Performance-based functional evaluation of non-operative and operative treatment after anterior cruciate ligament injury. Scand J Med Sci Sports. 2009;19:345–355. doi: 10.1111/j.1600-0838.2008.00816.x. http://dx.doi.org/10.1111/j.1600-0838.2008.00816.x. [DOI] [PubMed] [Google Scholar]
- 29.Moksnes H, Snyder-Mackler L, Risberg MA. Individuals with an anterior cruciate ligament-deficient knee classified as noncopers may be candidates for nonsurgical rehabilitation. J Orthop Sports Phys Ther. 2008;38:586–595. doi: 10.2519/jospt.2008.2750. http://dx.doi.org/10.2519/jospt.2008.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myklebust G, Bahr R. Return to play guidelines after anterior cruciate ligament surgery. Br J Sports Med. 2005;39:127–131. doi: 10.1136/bjsm.2004.010900. http://dx.doi.org/10.1136/bjsm.2004.010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myklebust G, Engebretsen L, Brækken IH, Skjølberg A, Olsen OE, Bahr R. Prevention of anterior cruciate ligament injuries in female team handball players: a prospective intervention study over three seasons. Clin J Sport Med. 2003;13:71–78. doi: 10.1097/00042752-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Myklebust G, Holm I, Mæhlum S, Engebretsen L, Bahr R. Clinical, functional, and radiologic outcome in team handball players 6 to 11 years after anterior cruciate ligament injury: a follow-up study. Am J Sports Med. 2003;31:981–989. doi: 10.1177/03635465030310063901. [DOI] [PubMed] [Google Scholar]
- 33.Roos H, Ornell M, Gärdsell P, Lohmander LS, Lindstrand A. Soccer after anterior cruciate ligament injury—an incompatible combination? A national survey of incidence and risk factors and a 7-year follow-up of 310 players. Acta Orthop Scand. 1995;66:107–112. doi: 10.3109/17453679508995501. [DOI] [PubMed] [Google Scholar]
- 34.Rudolph KS, Eastlack ME, Axe MJ, Snyder-Mackler L. 1998 Basmajian Student Award paper: movement patterns after anterior cruciate ligament injury: a comparison of patients who compensate well for the injury and those who require operative stabilization. J Electromyogr Kinesiol. 1998;8:349–362. doi: 10.1016/s1050-6411(97)00042-4. [DOI] [PubMed] [Google Scholar]
- 35.Williams GN, Barrance PJ, Snyder-Mackler L, Axe MJ, Buchanan TS. Specificity of muscle action after anterior cruciate ligament injury. J Orthop Res. 2003;21:1131–1137. doi: 10.1016/S0736-0266(03)00106-2. http://dx.doi.org/10.1016/S0736-0266(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 36.Williams GN, Barrance PJ, Snyder-Mackler L, Buchanan TS. Altered quadriceps control in people with anterior cruciate ligament deficiency. Med Sci Sports Exerc. 2004;36:1089–1097. doi: 10.1249/01.mss.0000131959.20666.11. [DOI] [PubMed] [Google Scholar]
- 37.Williams GN, Buchanan TS, Barrance PJ, Axe MJ, Snyder-Mackler L. Quadriceps weakness, atrophy, and activation failure in predicted noncopers after anterior cruciate ligament injury. Am J Sports Med. 2005;33:402–407. doi: 10.1177/0363546504268042. [DOI] [PubMed] [Google Scholar]
- 38.Williams GN, Snyder-Mackler L, Barrance PJ, Axe MJ, Buchanan TS. Neuromuscular function after anterior cruciate ligament reconstruction with autologous semitendinosus-gracilis graft. J Electromyogr Kinesiol. 2005;15:170–180. doi: 10.1016/j.jelekin.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Williams GN, Snyder-Mackler L, Barrance PJ, Buchanan TS. Quadriceps femoris muscle morphology and function after ACL injury: a differential response in copers versus non-copers. J Biomech. 2005;38:685–693. doi: 10.1016/j.jbiomech.2004.04.004. http://dx.doi.org/10.1016/j.jbiomech.2004.04.004. [DOI] [PubMed] [Google Scholar]