Abstract
Proper lymphatic function is necessary for the transport of fluids, macromolecules, antigens and immune cells out of the interstitium. The lymphatic endothelium plays important roles in the modulation of lymphatic contractile activity and lymph transport, but it's role as a barrier between the lymph and interstitial compartments is less well understood. Alterations in lymphatic function have long been associated with edema and inflammation although the integrity of the lymphatic endothelial barrier during inflammation is not well-defined. In this paper we evaluated the integrity of the lymphatic barrier in response to inflammatory stimuli commonly associated with increased blood endothelial permeability. We utilized in vitro assays of lymphatic endothelial cell (LEC) monolayer barrier function after treatment with different inflammatory cytokines and signaling molecules including TNF-α, IL-6, IL-1β, IFN-γ and LPS. Moderate increases in an index of monolayer barrier dysfunction were noted with all treatments (20–60% increase) except IFN-γ which caused a greater than 2.5 fold increase. Cytokine-induced barrier dysfunction was blocked or reduced by the addition of LNAME, except for IL-1β and LPS treatments, suggesting a regulatory role for nitric oxide. The decreased LEC barrier was associated with modulation of both intercellular adhesion and intracellular cytoskeletal activation. Cytokine treatments reduced the expression of VE-cadherin and increased scavenging of β-catenin in the LECs and this was partially reversed by LNAME. Likewise the phosphorylation of myosin light chain 20 at the regulatory serine 19 site, which accompanied the elevated monolayer barrier dysfunction in response to cytokine treatment, was also blunted by LNAME application. This suggests that the lymphatic barrier is regulated during inflammation and that certain inflammatory signals may induce large increases in permeability.
Introduction
Microcirculatory exchange in most tissues classically occurs between 3 interacting compartments, the blood, the interstitial spaces, and the lymphatic compartments. The lymphatic compartment passes its constituents through the lymph nodes en route to emptying lymph into the blood in the great veins of the upper chest. Thus fluid and macromolecular homeostasis depends on the balance of these interactions between the 3 compartments. The endothelium of both the blood and lymphatic vessels play important roles in the regulation of the movement of fluid and solutes from the blood to the interstitial space and from the interstitium to the lymph.
The lymphatic system is derived from a budding of cells from the cardinal vein during development and it functions primarily as a network to return fluid from the interstitial space through the lymph nodes en route to the blood [1,2]. However a growing body of evidence suggests that while the lymphatic system does return fluid to the blood, one of it's primary functions may be that of immune surveillance and support of the adaptive immune response [3–8]. Structurally, lymphatics can de loosely divided into three general types; 1) initial lymphatics/lymphatic capillaries with a thin cytoplasm, an incomplete basal lamina and disjointed cellular junctions followed by 2) transitional pre-collecting vessels and collecting lymphatics, which have a complete basement membrane, continuous junctions and variable smooth muscle investment and 3) the large transport/conduit lymphatics that are primarily postnodal vessels [9,8,10].
During inflammation and angiogenesis the endothelium of the blood vasculature becomes a permissive barrier that allows the flux of cells, macromolecules, and fluids into the interstitial space, which under controlled circumstances is a normal part of the immune system's response to insult. Angiogenesis usually accompanies chronic inflammation and mechanisms that regulate permeability such as nitric oxide (NO) signaling also play an important part in blood vasculature expansion[11,12]. NO has been shown to be an integral part of regulating vascular permeability and proliferation in blood vascular endothelial cells (BECs), additionally NO production in a shear-dependent manner is a major regulator of pumping activity of lymphatic vessels [13–16]. Thus, physiological production of NO in lymphatic vessels may help maintain barrier integrity, promote proliferation, and regulate pumping, while the pathological levels of NO production that often accompanies inflammation may alter endothelial barrier function in addition to its modulation of lymphatic contractile functions [17].
Hyper-permeability of the lymphatic system would seem to be counter-intuitive to its role in fluid and macromolecule homeostasis leading to an accumulation of fluid and potential tissue damage. Interestingly, recent work has demonstrated that inflammation can reduce the clearance of the interstitial space by reduction of pumping activity of the lymphatics via NO dependent mechanisims [18,19]. It would stand to reason that if inflammation can have major effects on lymphatic pumping function it is also possible that it may compromise the barrier function of the lymphatic endothelium. Thus, while it may play an important role for the isolation and protection against pathological infections, chronic inflammation also results in aberrant lymphatic dysfunction. This could be especially detrimental in mucosal associated lymphatic structures such as the mesentery where lymph is highly enriched in lipid content and gut derived antigens and the loss of the compartmentalization of these factors may lead to exacerbated inflammation in that region.
Despite a growing interest in lymphatic permeability, very little is known about what physiological or pathological processes alter it, or what roles it may play in lymphatic and immune functions. These questions are exceptionally pertinent when considering instances of inflammation. Edema is a classic hallmark of inflammation, and if lymphatic permeability is compromised during inflammation it may partially explain the decreased clearance of interstitial fluids by lymphatics as well as the accumulation of inflammatory cells. Conversely increased lymphatic permeability may be a normal response during inflammation in the light of recently described MHCII+ immune cells that are found in close contact with lymphatic vessels where modulation of lymphatic permeability may be important for the trafficking of these cells or to modulate their ability to sample the antigen-rich lymph [8].
Materials and methods
All animal usage was approved by the Texas A&M and Scott and White IACUC and adhered to the regulations provided in the NIH guide to animal care and use. Endothelial cells for these studies were obtained from mesenteric collecting lymphatics of rats through a modification of techniques we have previously published using vessel isolation and eversion (to prevent contamination of lymphatic muscle cells) [20]. Sprague-Dawley rats (200–250g) were anesthetized by intramuscular injections of Diazepam and InnovarVet. Briefly, a Prenodal mesenteric lymphatic was isolated from the mesentery of the small intestine from anesthetized rats. After removal of all adipocytes, the lymphatic was cannulated on its peripheral end and the contents were flushed out with sterile PBS. Then the lymphatic was inverted using suction and removed from the micropipette. The ends of the vessel were ligated with 12–0 opthalmic suture to minimize lymphatic muscle cell contamination and were and placed onto a fibronectin-coated dish in EGM-2 (Lonza). Endothelial cells were allowed to migrate off the vessels for ~ 1 week before the vessel was removed and the cells were allowed to expand out. After the first passage of the cells, they were confirmed as > 95% LECs via the expression of Prox-1 and LYVE-1. The verified rat lymphatic endothelial cells (RLEC) were maintained in culture under 5% CO2 in EGM-2 media and used from passage 6 to passage 11.
Monolayer barrier function assays were performed using 0.4μm pore cell culture inserts in 24 well plates (BD falcon) coated with 2% porcine gelatin and seeded at 50% confluence by area with RLECs and allowed to grow to confluence. Assays were performed at least 72 hrs after visual confluence was obtained. This allowed the plated RLECs to establish a stable basement membrane and cell-to-cell contacts, after which the culture media was removed and replaced with experimental medium consisting of phenol-free EBM-2 (with 2% serum and pen/strep mix) with 200μL in the upper chamber and 600μL in the lower chamber. After a 3-hr stabilization period, 100μL of media containing the cytokine treatment was added to the upper chamber (luminal) for 1 hr prior to the addition of 10μL of 10mg/mL of the FITC labeled BSA to the upper chamber. After a 30 min incubation at 37°C, 10μL aliquots of media was removed from the lower chamber and mixed with 90μL Milli-Q water and placed in a 96 well fluorescence plate and read at the excitation and emission pair of 494nm/518nm on a Biotek synergy H1 micro plate reader. All experiments were repeated a minimum of 6 times. Treatments used for all studies included rat TNF-α (10ng/mL) (Peprotec), rat IL-6 (100ng/mL) (Peprotec), rat IL-1β (50ng/mL) (Peprotec), LPS from e. coli (50ng/mL) (Sigma), rat IFN-γ (10ng/mL) (Peprotec) and human VEGFC156s (50ng/mL) (R&D). These doses were selected from the literature [21–26] and after preliminary dose response experiments. Additional experiments were performed using the nitric oxide synthase (NOS) inhibitor LNAME at a concentration of 1μM in the media of the upper and lower chambers to determine the contribution of NOS derived NO had on modulation of LEC monolayer barrier dysfunction. Further experiments were conducted using the NO donor S-Nitroso-N-Acetyl-D,L-Penicillamine (SNAP) at 1, 10 and 100μM to examine the effects of exogenous NO on monolayer permeability. Data was corrected for background and reported as percentage of control. Statistical significance was determined by ANOVA with Dunnet's post test (InStat software).
Griess' assay (Invitrogen) was performed to confirm the production of NO in the LECs resulting from treatment with cytokines. This assay measures nitrite, which is the stable degradation product of NO. We tested the ability of IFN-γ, TNF-α and IL-1β to produce NO at 1 and 24hrs (IFN-γ and IL-1β), in the presence of LNAME at 24hrs (TNF-α) and in a dose dependent manner for IL-1β.
Western blotting was performed on RLECs to determine the state of LEC markers as well as important regulators of endothelial permeability, markers of junctional stability and cellular contraction. RLECs were treated with the same cytokines that were used in the previously described permeability study in addition to SNAP (10μM). The cells were treated in EBM-2 with phenol-containing 2% FBS and penicillin/streptomycin mix to eliminate any influence of growth factors in the growth media. Likewise some of these studies were performed in the presence of LNAME just as the monolayer permeability studies were. Western blots for the junction components VE-cadherin and β-catenin (Santa Cruz) as well as the contractile protein myosin light chain 20 (MLC20; Cell Signaling)/phosphorylated Ser19 myosin light chain 20 (pMLC20;Cell Signaling) were performed on RLECs lysates at 1 hr and 24 hrs post treatment. Western blotting of iNOS (BD Bioscience) expression was also performed after 24 hrs of cytokine treatment to examine induction of iNOS expression in LECs. Cells were lysed in NP-40 lysis buffer containing protease and phosphatase inhibitor cocktails (SIGMA). The lysates were electrophoresed on NuPAGE Bis-Tris gradient (4–20%) gels at 100V for 2 hrs followed by transfer onto nitrocellulose membranes (0.22μm) at 15V overnight followed by 1 hr at 45V. All membranes were blocked in 5% non-fat milk in PBS for 1 hr followed by primary antibody incubation over night at 4°C in 1% non-fat milk and 0.1% fish gelatin followed by incubation with the appropriate HRP-conjugated secondary antibodies in the same buffer at room temperature for 2 hrs. All antibody incubations were followed by 3 washes in tris buffered saline for 10 min at room temperature. Blots were documented on a Fuji LAS4000 imaging system using chemiluminescence The acquired images were converted to TIF format and analyzed using ImageJ software (NIH) to perform densitometry. All quantitative parametric data was analyzed by ANOVA with Dunnett's post test.
Fluorescent microscopy was also used to determine the status of LEC markers and junctional proteins under basal conditions to determine the cells phenotype (collecting or initial LEC) based on junctional morphology. RLECs were grown on coverslips coated with 2% porcine gelatin until confluence at which time the cells were removed from EGM-2 and placed into phenol red-free EGM-2 72 hrs prior to treatment. RLECs were fixed in 4% paraformaldehyde for 10 min at 4°C and washed with cold PBS. RLECs used for staining of internal structures (Prox-1 etc.) were permiabilized in 0.01% Triton-X 100 for 10 min, while those used to stain surface markers were not. RLECs were blocked in 10% normal pre-immune goat serum for 1 hr at room temperature and incubation for all primary antibodies was carried out at 1:200 dilution in antibody dilution buffer (ProHisto) over night at 4°C. Likewise all secondary antibody incubations were done in antibody dilution buffer at 1:200 and all washes were for 15 min in antibody amplifying wash buffer (ProHisto). Cells were mounted using Prolong Gold (Invitrogen) and then were imaged on a Leica AOBS SP2 confocal-multiphoton microscope system using a 40X objective at 0.25 um steps throughout the thickness of the cells. The resulting confocal Z-stacks were merged into average projections using ImageJ software (NIH).
Additional experiments were also performed using DETA-NONOnate as an extended release NO donor to mimic physiological NO production by eNOS. LECs were treated with 100μM DETA-NONOnate for 4 and 8 hrs after which PCNA levels were measured by Western blot as a correlative index of proliferation. Doses and time points were selected due to responses in growth factor receptor expression seen in other work in our lab using this NO donor.
Results
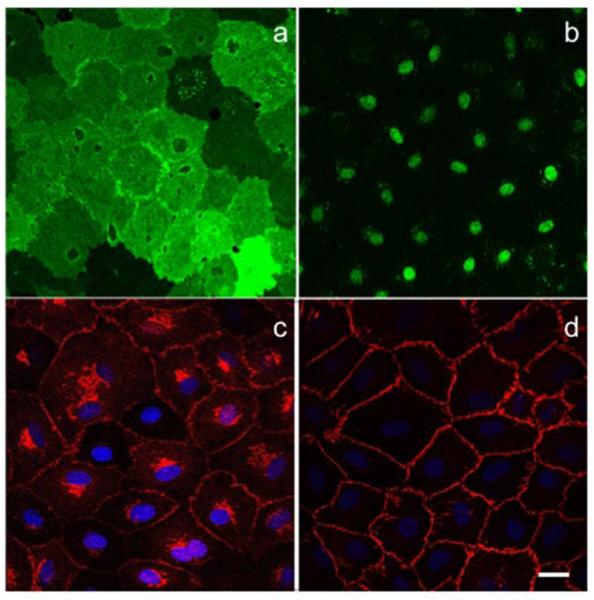
Cells isolated and cultured from the mesenteric collecting lymphatics displayed classic endothelial morphology of small cobblestone like monolayers, which were positive for Prox-1 and LYVE-1 (Fig. 1a,b). Interestingly while LYVE-1 is weakly and variably expressed in muscularized collecting lymphatics, it was significantly expressed in isolated LECs grown originally from these vessels. However, junctional VE-cadherin expression in these cells showed a classic collecting LEC morphology with contiguous belts with little to no apparent gaps at the cell-cell junctions as would be expected in a collecting lymphatic (Fig. 1c,d) [27,28].
Fig 1.
Immunofluorecent images of LYVE1 (green) (a) and Prox-1 (green) (b) stained with Alexafluor 488 secondary antibodies in RLECs, note the cell-to-cell variability in LYVE1 expression. Images of VE-cadherin (red) (c) and β-catenin (red) (d) co-stained with DAPI (blue) in RLECs stained with an Alexafluor 647 secondary antibody, the cells show a continuous belt of junction similar to cells of a collecting vessel type. All images were taken at 40X with 2X zoom magnification using a LEICA confocal microscope, images are average projections of stacks with a 0.5μm step size. Scale is 10μm
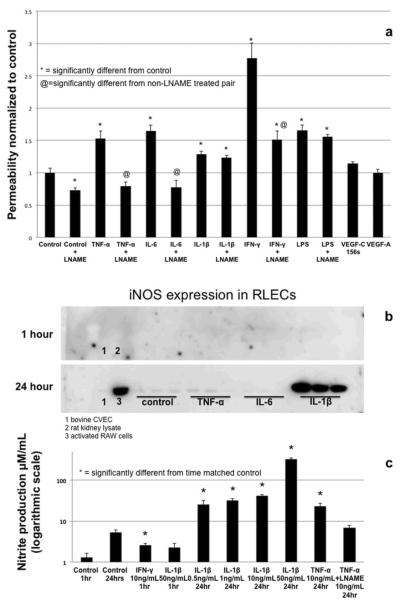
The primary goal of this study was to determine the permeability potential of lymphatic endothelium to cytokines and bacterial products known to elicit increased permeability in BECs. RLECs responded to a number of inflammatory mediators with increased permeability, though generally not as great as the magnitude seen in BEC. Both TNFα and IL-1β (at 10 and 50ng/mL respectively) significantly albeit modestly increased permeability by ~ 20% at 1 hr, while IL-6 and LPS (at 100ng/mL and 50ng/mL respectively) induced a significant but increase in monolayer barrier dysfunction of ~60% (Fig. 2a). Interestingly IFN-γ treatment (10ng/mL) induced a strong, ~2.5 fold increase in permeability. This was a much greater increase than the other cytokines tested and suggests these cells have a great dynamic range of permeability (Fig. 2a).
Fig 2.
Permeability of RLEC monolayers to FITC-labled bovine serum albumin in response to inflammatory cytokines TNF-α (10ng/mL), Il-6 (100ng/mL), Il-1β (50ng/mL), LPS (50ng/mL) IFN-γ (10ng/mL). These treatments were carried out alone and in the presence of 1μM LNAME to test NO dependency of permeability (a). Western blot of TNF-α, IL-6 and IL-1β treated cells at 24 hrs showing that IL-1β alone out of all tested cytokines induces an up regulation of iNOS (b). Specific cytokines (IFN-γ, IL-1β and TNF-α) were selected for further testing of NO production by Griess assay. TNF-α was used to test the efficacy of LNAME on NO production (after 24 hr to allow accumulation of nitrite), while IFN-γ and IL-1β represented the cytokines that had the greatest NO-dependent and NO-independent permeability effects respectively (c). Permeability data is representative from 6 experiments, n=3–6. Greiss assay data is representative of 3 experiments with n=3. * denotes significant departure from control (p≤0.05) as determined by ANOVA with Dunnett's post test.
Pretreatment with the NOS-inhibitor LNAME completely blocked the increases in monolayer barrier dysfunction caused by IL-6 and TNF-α and significantly reduced but did not completely inhibit the permeability increase due to IFN-γ. However, LNAME had no effect on the increase in barrier dysfunction induced by IL-1β nor LPS (Fig. 2a). Treatment with VEGF-C156s (50ng/mL) as a positive control induced a 14% increase in monolayer barrier dysfunction but did not reach significance. Interestingly VEGF-A (50ng/mL) had no effect at all on RLEC barrier function. iNOS levels in RLECs under control conditions were very low and were not apparently changed after 1 hr of cytokines treatment.. Treatment of RLEC with TNF-α, IL-6, LPS or IFN-γ did not alter iNOS expression at 24 hrs (data not shown for LPS and IFN-γ). However, treatment with IL-1β consistently induced a substantial and significant increase in the level of iNOS after 24 hrs of treatment (Fig. 2b). Data from the Griess assay suggests that LECs in culture produce a small amount of NO basally and that this level can be increased significantly by cytokine treatment, even at 1hr (Fig. 2c). We tested the ability of IFN-γ vs IL-1β to increase NO production at 1hr due to the relative impact of these cytokines on barrier function and the NO-dependency of IFN-γ, and NO-independent effects of IL-1β on monolayer barrier dysfunction. We found that IFN-γ treatment at 1hr significantly increased NO production but IL-1β treatment at 1hr did not. However, at 24hrs, IL-1β induced a very large increase in NO, which we propose is a direct effect of the upregulated iNOS expression. TNF-α increased NO production in an LNAME-sensitive manner that also corresponds to the prevention of monolayer barrier dysfunction with LNAME application prior to TNF-α exposure.
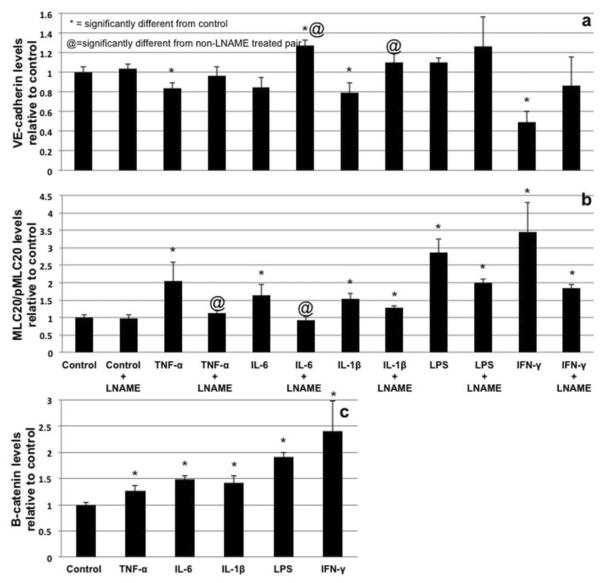
Western blots of RLEC after treatment with TNF-α, IL-6, IL-1β and INF-γ (1hr) revealed a significant reduction of the protein levels of VE-cadherin. INF-γ had the most profound effect with a reduction of VE-cadherin to less than 50% of control levels. Interestingly, LPS did not decrease VE-cadherin levels. LNAME-pretreatment inhibited the loss of VE-cadherin levels and in some cases (IL-6, IL-1β and LPS) it appeared to increase VE-cadherin levels (Fig. 3a). All cytokines caused an apparent increase in β-catenin (1hr of treatment), with IFN-γ causing the greatest increase (Fig. 3c).
Fig 3.
Quantification of Western blotting of VE-cadherin (a) (normalized to β-actin) and MLC20/pMLC20 ratios (b) after 1hr of treatment with TNF-α, IL-6, IL-1β, LPS and IFN-γ alone and in the presence of LNAME. All data is compared to its corresponding control. Quantification of β-catenin levels (normalized to β-actin) after cytokine treatment showing apparent salvaging of β-catenin (c). * denotes significant departure from control (p≤0.05) as determined by ANOVA with Dunnett's post test, @ denotes significant difference from non-LNAME treated pair as determined by Student's two tailed t-test.
Treatment with TNF-α, IL-6, IL-1β, LPS and IFN-γ (1hr) each stimulated the phosphorylation of the contractile protein myosin light chain 20 at the regulatory serine 19 site with IFN-γ inducing the greatest phosphorylation increase (~3.4 fold) (Fig. 3b). Elevated MLC20 phosphorylation induced by some cytokines was partially blocked by LNAME except in the cases of IL-1β, LPS and IFN-γ treatment, (Fig. 3b).
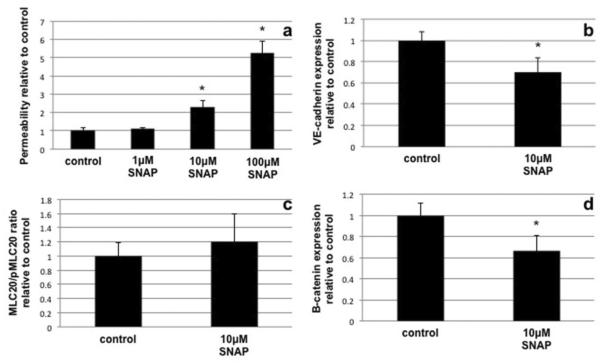
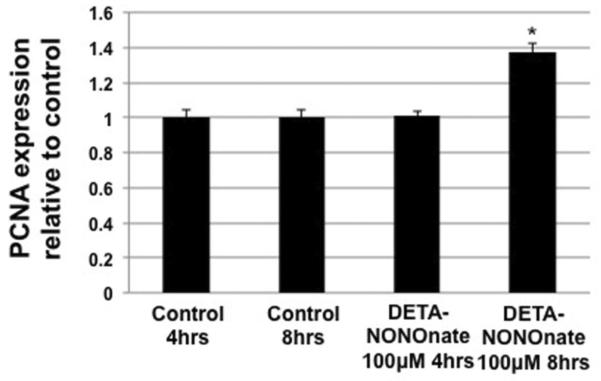
To determine the direct acute effects of NO on barrier function, LECs were treated with the rapid release NO donor SNAP. SNAP treatment at 1μM had no significant effects on LEC monolayer barrier function while treatment at 10μM yielded a increase in FITC-BSA leak across the monolayer similar to what was seen in IFN-γ treated cells (~2.3 fold increase) (Fig. 4a). Treatment of LECs with 100μM SNAP induced a massive increase in barrier dysfunction and was due to the loss of cell integrity in the monolayer. Likewise SNAP treatment (10μM at 1hr) significantly decreased the level of VE-cadherin (Fig. 4b). The phosphorylation of MLC20 was not significantly changed by SNAP (10μM) (Fig. 4c). Interestingly SNAP treatment did not increase β-catenin levels as seen in the cytokine treatments (Fig. 4d). DETA-NONOnate, which mimics a slower and sustained physiological NO release, induced a significant elevation of the marker of proliferation, PCNA (Fig. 5), at 8hrs post treatment however there was no elevation of this protein at 4hrs of treatment.
Fig 4.
Effects of SNAP the NO donor on permeability (a), junctional components VE-cadherin (b) and β-catenin (d) and the MLC20/pMLC20 levels (c). SNAP treatment (10μM) induced an increase in permeability roughly equivalent to IFN-γ while SNAP (1μM) had no significant effect and 100μM SNAP caused cells to lift from the monolayer. SNAP treatment at 10μM caused a significant reduction in the levels of VE-cadherin after 1 hr of treatment however the salvaging phenomenon of β-catenin seen with cytokine treatment was not observed. MLC20 phosphorylation was not increased by SNAP treatment (10μM) which was unexpected. Data is representative of 3 experiments with n=3 and significance was determined by ANOVA with Dunnett's post test in the case of permeability experiments and Student's t-test in all other cases. * denotes significant departure from control (p≤0.05)
Fig 5.
Effects of DETA-NONOnate on RLEC proliferation marker PCNA. Treatment with 100μM DETA-NONOnate caused no change in PCNA at 4 hrs (vs. 4 hrs control) while there was significant elevation of PCNA levels at 8 hrs (vs. 8hrs control), suggesting that NO can increase indices of proliferation in RLECs
Discussion and conclusions
We determined the effects of 5 classic inflammatory cytokines TNF-α, IL-6, IL-1β, INF-γ and LPS, as well as the lymphangiogenic factor VEGF-C156s on lymphatic endothelial barrier function. Each cytokine variably increased LEC monolayer permeability to FITC-albumin (increases from ~25% to 250%). The cytokine-induced monolayer barrier dysfunction was sensitive to NO blockade by LNAME with the exception of IL-1β and LPS (which is similar to their effects in blood vascular endothelial cells) with corresponding alterations in junctional and cytoskeletal rearrangements [29–31]. These findings may provide the basis for a new outlook of lymphatic function and dysfunction during inflammation. Additionally these changes may affect the whole body, not just the local site of inflammation, by decompartmentalizing the lymph via the immune system.
While much attention has been recently focused on lymphangiogenesis and lymphatic contractile function, still very little data exists about the regulation of permeability in the lymphatic endothelium. This paper supports earlier findings that documented that the lymphatic endothelium is an actively-regulated macromolecular barrier, in which the lymphatic endothelium could both positively and negatively alter permeability. We believe, the data in this report ties lymphatic function to immune function through the impact on lymphatic permeability of traditional inflammatory mediators released during immune responses [16,2,32,33].
We have recently reported that there are several different immune cell types in close proximity and in some cases attached to lymphatic vessels [34], which complicates the interpretation of altered lymphatic vessel leakage. Because of this we decided to utilize in vitro measures of barrier function in cultured RLECs to eliminate the confounding factors of the immune cells. While this may not completely recapitulate events in vivo, it does provide direct evidence how the lymphatic endothelium may responds to inflammation [23,35,26].
All the cytokines as well as LPS increased permeability of the LEC monolayers, although most induced modest increases of 20–60%. However, IFN-γ caused a more than 2.5 fold increase in monolayer barrier dysfunction, which suggests that LECs posses a great dynamic range of barrier regulation. But perhaps only a few inflammatory signals, such as intracellular pathogens, will induce a great change in LEC permeability. To put these changes into perspective TNF-α, IL-1β and LPS all induce a 2.5 fold increase in permeability in HUVECs at the same doses used in this study [36], while TNF-α at doses up to 100ng/mL did not significantly increase the magnitude of permeability of RLECs beyond that seen at 10ng/mL (sup fig. 1). This may be a compensatory mechanism to ensure that the lymphatic endothelial barrier does not extraneously collapse and further impair the critical role that the lymphatics play in fluid homeostasis. As the primary site for products of tissue catabolism and secreted molecules, the regional lymphatics are subject to elevated cytokines loads under physiological and pathophysiological conditions [37,38]. As compared to BEC, the blunted permeability values in the LECs reported here may be indicative of a desensitization process to maintain lymphatic fluid homeostatic function in response to the physiological loads. However, our data suggest that the significant dramatic increase in permeability in response to Th1-associated cytokines such as IL-1β and IFN-γ may be relevant to the notion that lymphatic tissues may be co-opted as a reservoir and a means to spread pathogenic organisms.
To put these changes into perspective of other studied hyperpermeability agents, the lymphangiogenic growth factor VEGF-C156s only increased barrier dysfunction 14% in our study, which is similar to what was found by other investigators using trans-endothelial electrical resistance (TEER) [26]. Unlike the previous work by Breslin et al using VEGF-C156s, we did not find this increase to be statistically significant, presumably due to differences in approach. Interestingly, VEGF-A had no effect on RLEC barrier function in our hands, which was unexpected. When compared to the action of VEGF-A on blood endothelium it seems the VEGF-C interaction with VEGF receptor 3 in RLECs does not induce large changes in permeability [39,40,26]. We also examined the effects of slow, low-level release of NO on RLECs due to our findings with the lymphangiogenic growth factor VEGF-C156s. We found that low levels of NO, while not sufficient to induce changes in permeability can cause LECs to prepare for proliferation as evidenced by an increase in PCNA. Proliferation of endothelial cells is usually preceded by the dissolution of the junctions and may explain why there was a very mild increase in permeability in ours, and others studies in response to VEGF-C. However this does not explain completely why VEGF-C does not behave like VEGF-A. This may be a physiologically sound adaptation given that lymphatics usually grown into areas that require greater removal of tissue debris, fluid and cells and compromising the permeability of the existing vessels in the tissue space would exacerbate the problem driving lymphangiogenesis, not correct it.
The low magnitude disruption of the monolayer barrier induced by most inflammatory cytokines (excepting IFN-γ) may be due to the expression of different isoforms of the suppressor of cytokine signaling (SOCS) than what is normally found in endothelial cells of blood vascular origin Again we speculate that it may represent another adaptive change of the lymphatic endothelium to cope with the cytokine-rich environment these cells exist in. The lymphatic endothelium is intimately exposed to interior mileu of the parenchyma and may have developed tolerances to inflammatory stimuli to maintain normal physiological function,. This may play important functions regulating the recruitment of immune cells from the parenchyma to the lymphatics and subsequently the lymph node while retaining the ability to keep the lymph compartment separate from the interstitial space. We are now pursuing what the loss of lymph compartmentalization may mean physiologically and, possibly more important, immunologically.
Endothelial para-cellular permeability is classically modulated by two types of events; the first is intercellular junctional disruption and the second is intracellular contraction of the actin/myosin cytoskeleton, both of which are thought to play important roles in regulating solute flux through the blood endothelium. The most often studied junctional components in regards to endothelial permeability are VE-cadherin and β-catenin, which are parts of the adherens junction and also play important roles in both WNT-mediated signaling (β-catenin) and as a shear/stretch signaling complex with VEGFR2, and PECAM (VE-cadherin) [44]. For permeability to increase, the normal association between VE-cadherin and β-catenin must be disrupted via phosphorylation of VE-cadherin. This results in the disassociation of the two proteins from each other and the actin cytoskeleton. The disassociation of VE-cadherin and β-catenin often results in the translocation of β-catenin to the nucleus (except in the cases of GSK-3β activation resulting in β-catenin phosphorylation and degradation) [45,30]. The increase in β-catenin signal we observed may be due to a number of reasons including impaired turn over of β-catenin, sequestration in the cytosol or increased β-catenin signaling and reduced degradation. SNAP treatment reduced the level of β-catenin significantly after 1 hr suggesting that in RLECs this signaling pathway is independent of, and may in fact be, inhibited by NO. This result would suggest that the lymphatic endothelial junction is very dynamic under normal conditions with a high turnover of integral components given that the half life of β-catenin averages from 30min to 120min depending on cell type and that LECs may differentially regulate β-catenin turn over [46–48]. Interestingly treatment with 10μM SNAP induced similar monolayer barrier dysfunction and VE-cadherin reduction levels as IFN-γ, which suggests that in RLECs the primary modality of NO is to disrupt junctional components. The second major event involved in increasing endothelial permeability is contraction of the cellular cytoskeleton via phosphorylation of myosin light chain 20 (MLC20) much like similar events in muscle. In the case of the lymphatic endothelium, Rho-associated protein kinase (ROCK) is the major kinase that increases the phosphorylation of MLC20 after activation resulting in retraction of the cellular borders after the degradation of junctional complexes [35,49]. We observed that many of the cytokines tested had profound effects on both junctional and contractile components of permeability. These results suggest that edema associated with inflammation may partially be due to the rapid remodeling of the lymphatic endothelial junctions and loss of lymph compartmentalization.
Previous studies of LEC barrier using similar treatments found surprisingly different effects. Work by Chaitanya et al in large T transformed mouse LECs found that IL-1β actually increased barrier integrity and that in human cells there was little response to most cytokines tested. However the alteration in permeability was at least in the same direction as what we have found [23]. We note that IL-1β had a similar effect to what Chaitanya et. al. described in earlier experiments in cells that had lost contact inhibition (data not shown) because of the transformed nature of the cells they used in their experiments. When comparing our data on VEGF-C induced permeability to that of Breslin et. al. it must be noted that we actually detected a similar increase of permeability (14% vs. 12%) but due to differences in the technique (monolayer permeably vs. TEER) we did not find this to be significant. In fact in the case of the previous two authors, endothelial dysfunction was estimated by TEER and no solute movement was measured.
We found that levels of pMLC20 were elevated in all cytokine treated groups. However, pMLC20 levels and levels of barrier function were not strictly correlated, suggesting that MLC20 is very sensitive to phosphorylation by certain cytokines such as TNF-α in RLECs, but that inter-endothelial junctional stability may be more important in regulating barrier function in RLECs. Given the highly dynamic nature of stresses and strains found in phasing pumping collecting lymphatics, maintainence of stable inter-endothelial junctions might be paramount to regulating permeability. Furthermore we found that NOS inhibition eliminated the effects of inflammatory cytokines on MLC20 phosphorylation, which is consistent with what has been found by other groups in BECs [50]. This suggests that NOS has an important role in the regulation of LEC contractile status.
The loss of barrier dysfunction of the LEC monolayers in response to inflammatory cytokines in this study was primarily an NO-dependent process with the exception of IL-1β, and LPS. IL-1β, and LPS induced acute increases in monolayer barrier dysfunction. However these two cytokines did not work through NO, suggesting that IL-1β and LPS may play a unique role in the regulation of acute lymphatic permeability. This increase in monolayer barrier dysfunction is in contrast to earlier findings using transformed mouse LECs in which IL-1β had no effect, which may be explained by species differences, analysis differences or isolation variances [23]. It is interesting to note that despite partially rescuing changes in VE-cadherin and pMLC levels, LNAME had no effect on changes in permeability induced by IL-1β or LPS. The inability of LNAME to inhibit IL-1β monolayer barrier dysfunction was not surprising because it is well established that IL-1 receptor 2 signals through pathways that bypass NOS signaling [24,30]. Additionally IL-1β was the only factor that increased expression of iNOS in the LECs suggesting that chronic IL-1β may have a unique role in inflammation in the lymphatic endothelium. The inability of LNAME to inhibit LPS induced monolayer barrier dysfunction was surprising as it appears that LPS induced permeability in BECs is NO dependent [41]. However this can possibly be explained by the fact that LPS had a very profound effect on MLC20 phosphorylation that was only partially ablated by LNAME, though it did not affect VE-cadherin levels. This may suggest that cytoskeletal contraction in these cells, if of a high enough magnitude, can induce changes in permeability. LECs have been previously demonstrated to express functional TLRs, including TLR4, and mesenteric lymph can contain a significant endotoxin load since it drains the gut and is the sole route of transport of chylomicrons that actively promote the absorption and dissemination of LPS [42,43]. The impact of cytokine release by LPS stimulation on permeability and the post-prandial mesenteric lymphatic permeability may be of particular interest especially under conditions of chronic inflammation such as IBD.
For many decades the basic understanding of the permeability of lymphatic vessels suggested that the permeability of each lymphatic was essentially fixed with variations in permeability dependent on the type of lymphatic (i.e. initial vs. collecting lymphatic) and the structure of the cellular junctions [49,1,28]. The fixed permeability of the collecting vessels was assumed to passively retain any molecule above ~2,300 dalton in the lumen and that active vesicular transport was the only means of regulating trans-endothelial movement of any particle above that size in collecting vessels [49,1,51]. Additionally in discussions of lymphatic permeability, one must also think about the direction that the permeant molecule is moving, i.e. into or out of the lymphatic and how that affects overall lymph transport. Therefore the most important factors in regulating permeability were the size of macromolecules and the type of lymphatic vessel [28,49,51,2]. Previously it was suggested that collecting lymphatics generally had permeabilities that were very low and relatively invariant [49]. Recently careful studies have shown that the basal permeability of collecting lymphatics to molecules such as albumin is very similar to the permeability of post capillary venules [52]. Furthermore, an increased focus has been placed on the permeability potential of lymphatics and in short order a small group of mediators such as adrenomedullin, VEGF-C, natriuretic peptide as well as LYVE-1 internalization, shear stress and their mechanisms (Rho/ROCK, cAMP, etc.) have been shown to have profound effects on barrier function and permeability of lymphatics [26,53,54,2,35,55]. These data were gathered using a variety of techniques to evaluate lymphatic permeability/barrier function, including isolated vessel measures, in situ measurements and trans-endothelial electrical resistance, yet all of the studies concluded that lymphatic vessels and more correctly the lymphatic endothelium can regulate its permeability in fashions akin to what is seen in the blood vessels.
This study found that much like blood vascular endothelial cells, lymphatic endothelial cells increase their permeability in response to several inflammatory cytokines. However the LECs seem to have unique mechanisms in place to regulate their permeability and respond in a manner somewhat different than what is expected of blood vascular endothelial cells. This is not surprising due to the vastly different environment that lymphatic endothelial cells exist in as compared to blood endothelial cells as well as the significantly different transport roles these endothelial cells serve in their respective vessels. This difference in permeability may be responsible for maintaining the convective transport of fluid that occurs in the interstitial space during inflammation. Additionally the relatively modest permeability responses of RLECs to most inflammatory cytokines may serve to maintain convective transport during modest inflammation but allow macromolecular signals to influence immune surveillance and responses to infection. This strongly supports the growing concept that despite some similarities, lymphatic and blood endothelium should be treated as a unique and independent cell types that need further study to understand the differences and similarities in their respective functions.
Supplementary Material
Supplementary Fig 1 Dose response curves of cytokines tested on RLEC monolayer permeability. Each graph is a single experiment with n=3. These data along with the literature was used to determine which doses to use for further experiments.
Supplementary Fig 2 Western blots representative data for β-catenin, VE-cadherin and pMLC20/MLC20 ratios from TNF-α, IL-6, IL-1β, LPS and IFN-γ treated RLECs at 1 hour.
Acknowledgments
Supported by: Supported by NIH grants HL070308, DK099221, HL094269, CA140732 and the Scott & White Wigley award.
References
- 1.Casley-Smith JR. How the lymphatic system works. Lymphology. 1968;1(3):77–80. [PubMed] [Google Scholar]
- 2.Miteva DO, Rutkowski JM, Dixon JB, Kilarski W, Shields JD, Swartz MA. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ Res. 2010;106(5):920–931. doi: 10.1161/CIRCRESAHA.109.207274. doi:10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24(2):203–215. doi: 10.1016/j.immuni.2006.01.003. doi:10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Angeli V, Randolph GJ. Inflammation, lymphatic function, and dendritic cell migration. Lymphat Res Biol. 2006;4(4):217–228. doi: 10.1089/lrb.2006.4406. doi:10.1089/lrb.2006.4406. [DOI] [PubMed] [Google Scholar]
- 5.Jakubzick C, Bogunovic M, Bonito AJ, Kuan EL, Merad M, Randolph GJ. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J Exp Med. 2008;205(12):2839–2850. doi: 10.1084/jem.20081430. doi:10.1084/jem.20081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D'Amore PA, Stein-Streilein J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115(9):2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol. 1999;66(5):698–704. doi: 10.1002/jlb.66.5.698. [DOI] [PubMed] [Google Scholar]
- 8.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5(8):617–628. doi: 10.1038/nri1670. doi:10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 9.Skobe M, Detmar M. Structure, function, and molecular control of the skin lymphatic system. J Investig Dermatol Symp Proc. 2000;5(1):14–19. doi: 10.1046/j.1087-0024.2000.00001.x. doi:10.1046/j.1087-0024.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 10.Zawieja SD, Wang W, Wu X, Nepiyushchikh ZV, Zawieja DC, Muthuchamy M. Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302(3):H643–653. doi: 10.1152/ajpheart.00606.2011. doi:10.1152/ajpheart.00606.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sessa WC. Molecular control of blood flow and angiogenesis: role of nitric oxide. J Thromb Haemost. 2009;7(Suppl 1):35–37. doi: 10.1111/j.1538-7836.2009.03424.x. [DOI] [PubMed] [Google Scholar]
- 12.Spyridopoulos I, Luedemann C, Chen D, Kearney M, Chen D, Murohara T, Principe N, Isner JM, Losordo DW. Divergence of angiogenic and vascular permeability signaling by VEGF: inhibition of protein kinase C suppresses VEGF-induced angiogenesis, but promotes VEGF-induced, NO-dependent vascular permeability. Arterioscler Thromb Vasc Biol. 2002;22(6):901–906. doi: 10.1161/01.atv.0000020006.89055.11. [DOI] [PubMed] [Google Scholar]
- 13.Moncada S, Higgs EA. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Invest. 1991;21(4):361–374. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 14.Davenpeck KL, Gauthier TW, Lefer AM. Inhibition of endothelial-derived nitric oxide promotes P-selectin expression and actions in the rat microcirculation. Gastroenterology. 1994;107(4):1050–1058. doi: 10.1016/0016-5085(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 15.Krieglstein CF, Anthoni C, Cerwinka WH, Stokes KY, Russell J, Grisham MB, Granger DN. Role of blood- and tissue-associated inducible nitric-oxide synthase in colonic inflammation. Am J Pathol. 2007;170(2):490–496. doi: 10.2353/ajpath.2007.060594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol. 2006;575(Pt 3):821–832. doi: 10.1113/jphysiol.2006.115212. doi:10.1113/jphysiol.2006.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmid-Schonbein GW. Nitric oxide (NO) side of lymphatic flow and immune surveillance. Proc Natl Acad Sci U S A. 2012;109(1):3–4. doi: 10.1073/pnas.1117710109. doi:10.1073/pnas.1117710109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu TF, Carati CJ, Macnaughton WK, von der Weid PY. Contractile activity of lymphatic vessels is altered in the TNBS model of guinea pig ileitis. Am J Physiol Gastrointest Liver Physiol. 2006;291(4):G566–574. doi: 10.1152/ajpgi.00058.2006. doi:10.1152/ajpgi.00058.2006. [DOI] [PubMed] [Google Scholar]
- 19.von der Weid PY, Muthuchamy M. Regulatory mechanisms in lymphatic vessel contraction under normal and inflammatory conditions. Pathophysiology. 2009 doi: 10.1016/j.pathophys.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Hayes H, Kossmann E, Wilson E, Meininger C, Zawieja D. Development and characterization of endothelial cells from rat microlymphatics. Lymphat Res Biol. 2003;1(2):101–119. doi: 10.1089/153968503321642606. [DOI] [PubMed] [Google Scholar]
- 21.Chakravortty D, Koide N, Kato Y, Sugiyama T, Kawai M, Fukada M, Yoshida T, Yokochi T. Cytoskeletal alterations in lipopolysaccharide-induced bovine vascular endothelial cell injury and its prevention by sodium arsenite. Clin Diagn Lab Immunol. 2000;7(2):218–225. doi: 10.1128/cdli.7.2.218-225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dudek SM, Munoz NM, Desai A, Osan CM, Meliton AY, Leff AR. Group V phospholipase A2 mediates barrier disruption of human pulmonary endothelial cells caused by LPS in vitro. Am J Respir Cell Mol Biol. 2011;44(3):361–368. doi: 10.1165/rcmb.2009-0446OC. doi:10.1165/rcmb.2009-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaitanya GV, Franks SE, Cromer W, Wells SR, Bienkowska M, Jennings MH, Ruddell A, Ando T, Wang Y, Gu Y, Sapp M, Mathis JM, Jordan PA, Minagar A, Alexander JS. Differential cytokine responses in human and mouse lymphatic endothelial cells to cytokines in vitro. Lymphat Res Biol. 2010;8(3):155–164. doi: 10.1089/lrb.2010.0004. doi:10.1089/lrb.2010.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puhlmann M, Weinreich DM, Farma JM, Carroll NM, Turner EM, Alexander HR., Jr. Interleukin-1beta induced vascular permeability is dependent on induction of endothelial tissue factor (TF) activity. J Transl Med. 2005;3:37. doi: 10.1186/1479-5876-3-37. doi:10.1186/1479-5876-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bove K, Neumann P, Gertzberg N, Johnson A. Role of ecNOS-derived NO in mediating TNF-induced endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2001;280(5):L914–922. doi: 10.1152/ajplung.2001.280.5.L914. [DOI] [PubMed] [Google Scholar]
- 26.Breslin JW, Yuan SY, Wu MH. VEGF-C alters barrier function of cultured lymphatic endothelial cells through a VEGFR-3-dependent mechanism. Lymphat Res Biol. 2007;5(2):105–113. doi: 10.1089/lrb.2007.1004. doi:10.1089/lrb.2007.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204(10):2349–2362. doi: 10.1084/jem.20062596. doi:10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leak LV, Burke JF. Electron microscopic study of lymphatic capillaries in the removal of connective tissue fluids and particulate substances. Lymphology. 1968;1(2):39–52. [PubMed] [Google Scholar]
- 29.Chaitanya GV, Cromer W, Wells S, Jennings M, Mathis JM, Minagar A, Alexander JS. Metabolic modulation of cytokine-induced brain endothelial adhesion molecule expression. Microcirculation. 2012;19(2):155–165. doi: 10.1111/j.1549-8719.2011.00141.x. doi:10.1111/j.1549-8719.2011.00141.x. [DOI] [PubMed] [Google Scholar]
- 30.Barbieri SS, Weksler BB. Tobacco smoke cooperates with interleukin-1beta to alter beta-catenin trafficking in vascular endothelium resulting in increased permeability and induction of cyclooxygenase-2 expression in vitro and in vivo. Faseb J. 2007;21(8):1831–1843. doi: 10.1096/fj.06-7557com. doi:10.1096/fj.06-7557com. [DOI] [PubMed] [Google Scholar]
- 31.Sola-Villa D, Camacho M, Sola R, Soler M, Diaz JM, Vila L. IL-1beta induces VEGF, independently of PGE2 induction, mainly through the PI3-K/mTOR pathway in renal mesangial cells. Kidney Int. 2006;70(11):1935–1941. doi: 10.1038/sj.ki.5001948. [DOI] [PubMed] [Google Scholar]
- 32.Alexander JS, Chaitanya GV, Grisham MB, Boktor M. Emerging roles of lymphatics in inflammatory bowel disease. Ann N Y Acad Sci. 2010;1207(Suppl 1):E75–85. doi: 10.1111/j.1749-6632.2010.05757.x. doi:10.1111/j.1749-6632.2010.05757.x. [DOI] [PubMed] [Google Scholar]
- 33.Breslin JW, Gaudreault N, Watson KD, Reynoso R, Yuan SY, Wu MH. Vascular endothelial growth factor-C stimulates the lymphatic pump by a VEGF receptor-3-dependent mechanism. Am J Physiol Heart Circ Physiol. 2007;293(1):H709–718. doi: 10.1152/ajpheart.00102.2007. doi:10.1152/ajpheart.00102.2007. [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee V, Gashev AA. Aging-associated shifts in functional status of mast cells located by adult and aged mesenteric lymphatic vessels. Am J Physiol Heart Circ Physiol. 2012;303(6):H693–702. doi: 10.1152/ajpheart.00378.2012. doi:10.1152/ajpheart.00378.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breslin JW. ROCK and cAMP promote lymphatic endothelial cell barrier integrity and modulate histamine and thrombin-induced barrier dysfunction. Lymphat Res Biol. 2011;9(1):3–11. doi: 10.1089/lrb.2010.0016. doi:10.1089/lrb.2010.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nooteboom A, Van Der Linden CJ, Hendriks T. Tumor necrosis factor-alpha and interleukin-1beta mediate endothelial permeability induced by lipopolysaccharide-stimulated whole blood. Crit Care Med. 2002;30(9):2063–2068. doi: 10.1097/00003246-200209000-00019. doi:10.1097/01.CCM.0000021522.67956.E6. [DOI] [PubMed] [Google Scholar]
- 37.Hunziker T, Brand CU, Kapp A, Waelti ER, Braathen LR. Increased levels of inflammatory cytokines in human skin lymph derived from sodium lauryl sulphate-induced contact dermatitis. Br J Dermatol. 1992;127(3):254–257. doi: 10.1111/j.1365-2133.1992.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 38.Olszewski WL, Pazdur J, Kubasiewicz E, Zaleska M, Cooke CJ, Miller NE. Lymph draining from foot joints in rheumatoid arthritis provides insight into local cytokine and chemokine production and transport to lymph nodes. Arthritis Rheum. 2001;44(3):541–549. doi: 10.1002/1529-0131(200103)44:3<541::AID-ANR102>3.0.CO;2-6. doi:10.1002/1529-0131(200103)44:3<541::AIDANR102>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 39.Glass CA, Harper SJ, Bates DO. The anti-angiogenic VEGF isoform VEGF165b transiently increases hydraulic conductivity, probably through VEGF receptor 1 in vivo. J Physiol. 2006;572(Pt 1):243–257. doi: 10.1113/jphysiol.2005.103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cromer W, Jennings MH, Odaka Y, Mathis JM, Alexander JS. Murine rVEGF164b, an inhibitory VEGF reduces VEGF-A-dependent endothelial proliferation and barrier dysfunction. Microcirculation. 17(7):536–547. doi: 10.1111/j.1549-8719.2010.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu F, Han M, Wilson JX. Tripterine prevents endothelial barrier dysfunction by inhibiting endogenous peroxynitrite formation. Br J Pharmacol. 2009;157(6):1014–1023. doi: 10.1111/j.1476-5381.2009.00292.x. doi:10.1111/j.1476-5381.2009.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawa Y, Ueki T, Hata M, Iwasawa K, Tsuruga E, Kojima H, Ishikawa H, Yoshida S. LPS-induced IL-6, IL-8, VCAM-1, and ICAM-1 expression in human lymphatic endothelium. J Histochem Cytochem. 2008;56(2):97–109. doi: 10.1369/jhc.7A7299.2007. doi:10.1369/jhc.7A7299.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50(1):90–97. doi: 10.1194/jlr.M800156-JLR200. doi:10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–431. doi: 10.1038/nature03952. doi:10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 45.Tharakan B, Hellman J, Sawant DA, Tinsley JH, Parrish AR, Hunter FA, Smythe WR, Childs EW. beta-Catenin Dynamics in the Regulation of Microvascular Endothelial Cell Hyperpermeability. Shock. 2011 doi: 10.1097/SHK.0b013e318240b564. doi:10.1097/SHK.0b013e318240b564. [DOI] [PubMed] [Google Scholar]
- 46.Ding H, Keller KC, Martinez IK, Geransar RM, zur Nieden KO, Nishikawa SG, Rancourt DE, zur Nieden NI. NO-beta-catenin crosstalk modulates primitive streak formation prior to embryonic stem cell osteogenic differentiation. J Cell Sci. 2012;125(Pt 22):5564–5577. doi: 10.1242/jcs.081703. doi:10.1242/jcs.081703. [DOI] [PubMed] [Google Scholar]
- 47.Kang DE, Soriano S, Frosch MP, Collins T, Naruse S, Sisodia SS, Leibowitz G, Levine F, Koo EH. Presenilin 1 facilitates the constitutive turnover of beta-catenin: differential activity of Alzheimer's disease-linked PS1 mutants in the beta-catenin-signaling pathway. J Neurosci. 1999;19(11):4229–4237. doi: 10.1523/JNEUROSCI.19-11-04229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soriano S, Kang DE, Fu M, Pestell R, Chevallier N, Zheng H, Koo EH. Presenilin 1 negatively regulates beta-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of beta-amyloid precursor protein and notch processing. J Cell Biol. 2001;152(4):785–794. doi: 10.1083/jcb.152.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strawitz JG, Eto K, Mitsuoka H, Olney C, Pairent FW, Howard JM. Molecular weight dependence of lymphatic permeability: The concept of regional cancer chemotheraphy by lymphatic perfusion. Microvasc Res. 1968;1(1):58–67. [Google Scholar]
- 50.Rigor RR, Shen Q, Pivetti CD, Wu MH, Yuan SY. Myosin Light Chain Kinase Signaling in Endothelial Barrier Dysfunction. Med Res Rev. 2012 doi: 10.1002/med.21270. doi:10.1002/med.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshikawa H, Takada K, Muranishi S. Molecular weight dependence of permselectivity to rat small intestinal blood-lymph barrier for exogenous macromolecules absorbed from lumen. J Pharmacobiodyn. 1984;7(1):1–6. doi: 10.1248/bpb1978.7.1. [DOI] [PubMed] [Google Scholar]
- 52.Scallan JP, Huxley VH. In vivo determination of collecting lymphatic vessel permeability to albumin: a role for lymphatics in exchange. J Physiol. 2010;588(Pt 1):243–254. doi: 10.1113/jphysiol.2009.179622. doi:10.1113/jphysiol.2009.179622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunworth WP, Fritz-Six KL, Caron KM. Adrenomedullin stabilizes the lymphatic endothelial barrier in vitro and in vivo. Peptides. 2008;29(12):2243–2249. doi: 10.1016/j.peptides.2008.09.009. doi:10.1016/j.peptides.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Price GM, Chrobak KM, Tien J. Effect of cyclic AMP on barrier function of human lymphatic microvascular tubes. Microvasc Res. 2008;76(1):46–51. doi: 10.1016/j.mvr.2008.02.003. doi:10.1016/j.mvr.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hou WH, Liu IH, Tsai CC, Johnson FE, Huang SS, Huang JS. CRSBP-1/LYVE-1 ligands disrupt lymphatic intercellular adhesion by inducing tyrosine phosphorylation and internalization of VE-cadherin. J Cell Sci. 2011;124(Pt 8):1231–1244. doi: 10.1242/jcs.078154. doi:10.1242/jcs.078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig 1 Dose response curves of cytokines tested on RLEC monolayer permeability. Each graph is a single experiment with n=3. These data along with the literature was used to determine which doses to use for further experiments.
Supplementary Fig 2 Western blots representative data for β-catenin, VE-cadherin and pMLC20/MLC20 ratios from TNF-α, IL-6, IL-1β, LPS and IFN-γ treated RLECs at 1 hour.