Abstract
Background
Almost half of children with an inhibited temperament will develop social anxiety disorder by late adolescence. Importantly, this means that half of children with an inhibited temperament will not develop social anxiety disorder. Studying adults with an inhibited temperament provides a unique opportunity to identify neural signatures of both risk and resilience to social anxiety disorder.
Methods
Functional MRI was used to measure brain activation during the anticipation of viewing fear faces in 34 young adults (17 inhibited, 17 uninhibited). To identify neural signatures of risk, we tested for group differences in functional activation and connectivity in regions implicated in social anxiety disorder, including the prefrontal cortex, amygdala, and insula. To identify neural signatures of resilience, we tested correlations between brain activation and both emotion regulation and social anxiety scores.
Results
Inhibited subjects had greater activation of a prefrontal network when anticipating viewing fear faces, relative to uninhibited subjects. No group differences were identified in the amygdala. Inhibited subjects had more negative connectivity between the rostral anterior cingulate cortex (ACC) and the bilateral amygdala. Within the inhibited group, those with fewer social anxiety symptoms and better emotion regulation skills had greater ACC activation and greater functional connectivity between the ACC and amygdala.
Conclusions
These finding suggest that engaging regulatory prefrontal regions during anticipation may be a protective factor, or putative neural marker of resilience, in high-risk individuals. Cognitive training targeting prefrontal cortex function may provide protection against anxiety, especially in high-risk individuals, such as those with inhibited temperament.
Keywords: anticipation, fMRI, functional connectivity, inhibited temperament, social anxiety disorder
Introduction
Social anxiety disorder is highly prevalent1, has a chronic course2, and causes substantial impairment2–4. While the etiologies of most psychiatric disorders remain poorly understood, studies show that social anxiety disorder is often preceded by childhood inhibited temperament5–7, and that inhibited children who remain inhibited as adolescents are at significantly higher risk than those who become less inhibited8,9. Individuals with an inhibited temperament are shy, cautious, and avoidant of new situations. Studies comparing inhibited and uninhibited individuals (i.e. extreme groups approach) have established that inhibited temperament is associated with differences in physiology10 and brain function11. There are consistent findings that adolescents and adults who were inhibited as children have heightened and sustained amygdala responses to novel, neutral, and fearful faces12,13,13–17. Amygdala hyperactivity has also been shown in social anxiety disorder18, and may be an underlying neurobiological risk factor; however, much remains to be understood about the neurobiology of risk for social anxiety disorder.
Patients with social anxiety disorder and individuals with an inhibited temperament experience worry, heightened arousal, and increased anxiety about upcoming social situations, and typically avoid or withdraw from those situations. Studying the anticipation of an aversive experience provides a unique perspective into the neurocircuitry alterations that underlie social anxiety disorder, and its biological risk factor, inhibited temperament. Previous studies have shown that anticipatory processing is altered in social anxiety disorder19,20, and differences in anticipatory processing in anxiety disorders may predict treatment outcomes21 and degree of imapirment22. A recent study by our lab15 showed that when fear faces are expected, uninhibited subjects have increased prefrontal cortex activation and decreased amygdala activation, compared to when fear faces are unexpected. These findings are similar to patterns seen in healthy controls23,24, suggesting that the uninhibited subjects adaptively prepared to view fear faces. In contrast, inhibited individuals have increased amygdala and decreased prefrontal cortex activation to expected fear faces, compared to when fear faces are unexpected. Inhibited individuals may have alterations in the ability to engage brain resources to prepare for an upcoming aversive event; however, this hypothesis has yet to be tested. To address this gap in the field, we conducted a study to test for differences in brain activation during anticipation of viewing aversive faces.
Although inhibited temperament confers substantial risk for social anxiety disorder, half of inhibited children do not develop the disorder6; thus studying inhibited temperament provides a unique opportunity to identify the neurobiological basis of resilience in high-risk individuals. One behavioral study of adolescents who were inhibited as children found that lower levels of response monitoring may be protective25. Other resilience factors are likely to exist, but to date remain undiscovered. For example, high-risk children who are able to regulate their anxiety response may be resilient. Patients with social anxiety disorder use emotion regulation less often26, and emotion regulation is taught in cognitive-behavior therapy27, a common treatment for social anxiety disorder. Emotion regulation is thought to be subserved by the prefrontal cortex. During emotion regulation, the anterior cingulate and dorsolateral prefrontal cortex show increased activation in healthy controls28, but less activation in patients with social anxiety disorder29,30. Based on these findings, we propose that inhibited individuals who have not developed social anxiety disorder by young adulthood may be resilient and may have greater activation of prefrontal cortex regions during anticipation of an aversive event.
We believe that studying adults who were inhibited as children provides a novel perspective on risk and resilience associated with the development of social anxiety disorder. Risk and resilience are likely mediated by function of both reactivity brain regions, such as the amygdala, and regulatory brain regions, such as the prefrontal cortex. One way to probe reactivity and regulation brain regions is to examine brain function during anticipation. Both inhibited and socially anxious individuals often experience worry, heightened arousal, and increased anxiety about upcoming social situations; in contrast, healthy individuals or those with few symptoms may effectively prepare for an upcoming event. In order to identify patterns of brain activation associated with risk for social anxiety disorder, we tested for differences in brain activation while subjects anticipated viewing fear faces and neutral faces. To isolate relevant neurocircuits, we also tested for differences in functional connectivity. To identify patterns of brain activation associated with resilience, we correlated brain activation with both social anxiety and emotion regulation scores within the inhibited temperament group. We hypothesized that the inhibited group would show greater activation of amygdala and insula during anticipation of fear faces, relative to the uninhibited group. We also hypothesized that within the inhibited group, greater activation of the prefrontal cortex would predict less social anxiety and better emotion regulation.
Materials and Methods
Subjects
Forty adults, with either an extreme inhibited (n = 20) or uninhibited temperament (n = 20) participated in the study (Table 1). Inhibited and uninhibited subjects did not differ significantly on age, handedness, gender, or ethnicity. The Vanderbilt University Institutional Review Board approved the study and written informed consent was obtained after providing subjects with a complete description of the study.
Table 1.
Subject Characteristics.
| Inhibited Temperament (n = 20) |
Uninhibited Temperament (n = 20) |
||||
|---|---|---|---|---|---|
|
| |||||
| Mean | SD | Mean | SD | p-value | |
| Childhood Temperament | 3.33 | 0.52 | 1.41 | 0.19 | <.001 |
| Adult Temperament | 3.23 | 0.49 | 1.59 | 0.22 | <.001 |
| Social Anxiety Score | 96.5 | 29.7 | 14.4 | 18.6 | <.001 |
| Emotion Regulation Score | 28.4 | 16.6 | 43.1 | 12.7 | .006 |
| Demographics | |||||
| Age (years) | 21.7 | 1.8 | 21.8 | 2.3 | ns |
| Percent | n | Percent | n | p-value | |
|---|---|---|---|---|---|
| Social Anxiety Disorder | 30% | 6 | 0% | 0 | |
| Handedness (% right) | 90% | 18 | 90% | 18 | ns |
| Gender (% female) | 60% | 12 | 60% | 12 | ns |
| Ethnicity | ns | ||||
| % Caucasian | 70% | 14 | 75% | 15 | |
| % African-American | 20% | 4 | 15% | 3 | |
| % Asian | 10% | 2 | 10% | 2 |
Note: SD = standard deviation; ns = non-significant p-value.
Recruitment and Screening
Subjects were recruited by seeking individuals ages 18-25 who were “extremely shy or outgoing”. Consistent with prior studies12–15, individuals with an extreme inhibited or extreme uninhibited temperament were identified using the Adult Self-Report of Inhibition (ASRI)31 and the Retrospective Self-Report of Inhibition (RSRI)31. Subjects were selected for having a stable temperament (i.e. being extremely inhibited or extremely uninhibited as both an adult and a child), defined by scores on both the ASRI and RSRI that were greater than one standard deviation from published means31. Other inclusion criteria included: passing an MRI safety screen, being free of psychoactive medications within the past six months, having no history of brain trauma, and having no psychiatric illness (based on clinical interview), except anxiety disorders in the inhibited temperament group.
Psychiatric diagnosis was assessed by a trained clinical interviewer using the Structured Clinical Interview for DSM-IV (SCID)32. In order to examine individual differences in resilience, we did not exclude inhibited subjects who met criteria for a current or past anxiety disorder. In the inhibited group, six subjects had current social anxiety disorder (two with comorbid specific phobia, one with comorbid specific phobia and obsessive-compulsive disorder), and three subjects had current specific phobias. No subjects in the inhibited group had a history of social anxiety disorder (without current social anxiety disorder). No subjects in the uninhibited group had current or past psychiatric disorders.
Measures
Social anxiety scores were measured using the Social Phobia Anxiety Inventory (SPAI; Table 1)33. Emotion regulation was measured with the Cognitive Emotion Regulation Questionnaire (CERQ)34. The CERQ consists of positive and negative subscales. The positive subscales are: acceptance, refocus on planning, positive refocusing, positive reappraisal, and putting into perspective. The negative subscales are: self-blame, blaming others, rumination, and catastrophizing. Subjects rated how frequently they used each strategy from 1 (almost never) to 5 (almost always). Subscale scores were created by summing the scores across all four items for each subscale. The higher the score for each subscale, the more frequently that strategy was used. Because both more frequent use of positive strategies and less frequent use of negative strategies are associated with better outcomes35–37, we created a single score to represent adaptive emotion regulation, defined as positive emotion regulation - negative emotion regulation..
Experimental Design
fMRI Task
Subjects were trained to associate a cue (square or diamond) with a social stimulus (fear or neutral face). Following the training, subjects verbally confirmed the cue/emotion pairing.
Subjects were told that during the task, they would see the same cues, followed by the same types of emotional faces. The task consisted of four runs of 10 fear and 10 neutral trials per run. Each trial (Figure 1) consisted of: a cue (1s); an anticipation period (5-9s, jittered between each trial); a face (1s); and a blank screen (5-9s, jittered between each trial). The emotional faces (40 females, 40 males) were selected from the Karolinska Directed Emotional Faces database38 and stimuli were presented using Eprime software (Version 2.0, Psychology Software Tools, Sharpsburgh, PA).
Figure 1.
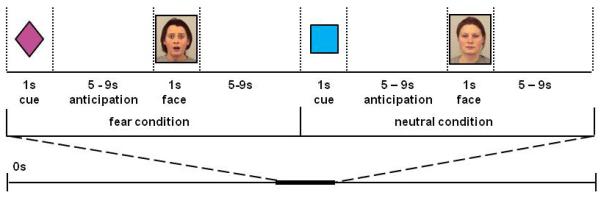
Study Design. The test phase included four runs of 10 fear and 10 neutral trials, for a total of 40 fear trials and 40 neutral trials. Each trial consisted of a cue (1s), an anticipation period (5-9s), and a face (1s). Following each trial, a 5-9s blank screen was shown to allow for return to baseline. Fear and neutral trials were randomized within runs and the duration of the anticipation period was jittered between trials.
Behavioral Data
To ensure attention to the task and provide a measure of reaction time, the task included a button-press. Subjects were told to press a single button with the index finger of their dominant hand as quickly as possible whenever they saw a cue or a face (for both valences). The button-press ensured that subjects maintained attention to the task while not adding an explicit cognitive component to the task, given findings that active tasks can reduce amygdala activation39. Following the functional MRI scan, subjects rated each face in Eprime on a Likert scale of valence (1 = very unpleasant, 7 = very pleasant) and arousal (1 = very excited, 7 = very calm).
fMRI Data Acquisition
Anatomic and echoplanar (EPI) images were collected on a 3T Philips magnet. High-resolution T1-weighted anatomical images were acquired using the following sequence: 256 mm FOV, 170 slices, 1 mm slice thickness, 0 mm gap. EPI images were acquired using a sequence optimized for the amygdala: 2s TR, 22 ms TE, 90° flip angle, 1.8 SENSE factor, 240 mm FOV, 3 × 3 mm in plane resolution. Each EPI volume comprised 40 2.5 mm axial oblique slices with a .25 mm gap between slices, tilted 15 degrees, anterior higher than posterior, relative to the intercommisural plane.
fMRI Data Preprocessing
Functional MRI data were pre-processed using SPM5 (http://www.fil.ion.ucl.ac.uk/) and MATLAB (Version 7.5.0; Mathworks, Inc., Natick, MA). Data were slice-time corrected, corrected for motion (aligned to the first volume), coregistered to the structural image, normalized into standard space (EPI template, SPM5), high pass filtered (128 s), and spatially smoothed (6 mm FWHM). For each subject, motion was assessed and EPI images were visually inspected for artifacts, signal dropout, and coverage. Six subjects were removed for high motion (> 3 mm translation, > 3 degrees of rotation), resulting in a final sample of 17 inhibited and 17 uninhibited subjects.
Data Analysis
Behavioral Data
To confirm that subjects learned the cue-face association and were attending to the stimuli, we compared mean reaction times by emotion (fear/neutral) and by event type (cue/image) using analysis of variance (ANOVA) in SPSS. Analyses were restricted to hits, defined as a button press during the cue or face stimulus (subjects pushed the button for 97.9% of the stimuli). We tested for group differences in accuracy, reaction time, arousal, and valence ratings using a repeated measures ANOVA with emotion (fear/neutral) as the within-subjects factor.
fMRI Data Modeling
For each subject, a general linear model was estimated in SPM5 with seven different regressors: instructions, fear cue, neutral cue, fear anticipation, neutral anticipation, fear face, and neutral face. Trials in which the subject did not press the button (cue or face) were not explicitly modeled. Contrast images were created for fear – neutral anticipation.
Regions of Interest Analysis
Analyses were restricted to regions of interest (ROIs) previously identified in anticipation studies19,21,22,40–43 in order to reduce the overall number of comparisons. The ROIs were: the anterior cingulate cortex (ACC); dorsolateral prefrontal cortex (dlPFC); dorsomedial prefrontal cortex (dmPFC); insula; and amygdala. The regions were defined using anatomical guidelines specified by the AAL templates44 implemented in the WFU Pick Atlas (Version 2.4)45. Within each a priori region, we tested for significant voxel-wise differences. Family-wise error (FWE) control was provided by cluster based thresholding46. Cluster sizes were determined using AlphaSim (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). Calculations were performed for each mask individually using 3 × 3 × 3mm voxels, 6mm smoothing, a voxel p-value of .05, an FWE rate of .05, and 5,000 iterations. Cluster sizes for each ROI were: ACC–29 voxels (783 mL); dlPFC–51 voxels (1377 mL); dmPFC–48 voxels (1296 mL); insula–28 voxels (756 mL); and amygdala–12 voxels (324 mL).
Functional Connectivity
To identify neural circuits involved in risk and resilience to social anxiety disorder, we compared functional connectivity between groups using a psychophysiological interaction (PPI) analysis47. The seed regions were the significant clusters identified in the between-groups analysis (Table 3). Target regions were restricted to the a priori ROIs and cluster-based thresholding was used to control for Type I errors.
Table 3.
Activation during anticipation of viewing fear faces.
| Peak Voxel | ||||||
|---|---|---|---|---|---|---|
| Brain Region (Hemisphere) | Brodmann Area |
Cluster Size (voxels) |
Z Score | x | y | z |
|
Inhibited Temperament > Uninhibited Temperament
| ||||||
| Region of Interest Analysis | ||||||
| Dorsolateral prefrontal cortex (R) | 9 | 140 | 3.27 | 30 | 36 | 24 |
| Dorsolateral prefrontal cortex (L) | 9 | 80 | 3.45 | −42 | 27 | 33 |
| Dorsal anterior cingulate cortex (B) | 24/32 | 62 | 3.78 | −12 | 33 | 27 |
| Rostral anterior cingulate cortex (B) | 24/32 | 29 | 3.11 | 6 | 33 | −6 |
| Insula (L) | 13/38 | 35 | 2.97 | −36 | 3 | −15 |
|
| ||||||
| Whole Brain Analysis | ||||||
| Thalamus (R) | 37 | 3.64 | 12 | −6 | −6 | |
| Extrastriate visual cortex (L) | 19 | 28 | 3.33 | −15 | −51 | −6 |
|
| ||||||
|
Inhibited Temperament – Social Anxiety Score – Negative Correlation
| ||||||
| Dorsal/rostral anterior cingulate cortex (B) |
32 | 109 | 3.55 | 6 | 42 | 21 |
| Rostral anterior cingulate cortex (B) | 11/32 | 42 | 3.44 | −12 | 51 | 0 |
| Amygdala (R) | 17 | 3.04 | 30 | 0 | −24 | |
|
| ||||||
|
Inhibited Temperament – Emotion Regulation Score – Positive Correlation
| ||||||
| Dorsal anterior cingulate cortex (B) | 32 | 79 | 3.33 | 0 | 45 | 12 |
Note: (L) = left hemisphere; (R) = right hemisphere; (B) = bilateral; coordinates are in Montreal Neurological Institute convention.
Social Anxiety and Emotion Regulation in Inhibited Temperament
To determine whether individual differences in social anxiety or emotion regulation scores predicted differences in brain activation in the inhibited temperament group, we performed a regression analysis with social anxiety or emotion regulation as predictor scores and brain activation and connectivity during anticipation as outcome variables. Analyses were restricted to the a priori ROIs and cluster-based thresholding was used to control for Type I errors. To test whether these findings were specific to the inhibited group, we also tested for a correlation between percent signal change (from the regions identified in the inhibited group) and social anxiety symptoms and emotion regulation scores in both the uninhibited temperament group and across the whole sample. In order to illustrate the magnitude and direction of correlations, we provide scatterplots; the associated correlation values provide an effect size for the original statistically significant findings.
Post Hoc Analysis
To ‘unpack’ the findings from initial group comparisons and regressions, post-hoc analyses were performed. Within each group, paired t-tests were performed to compare activation in the fear vs neutral conditions. Percent signal change was extracted using MarsBar48 and data were analyzed using SPSS (Version 18.0.2; SPSS, Inc.; Chicago, IL). For group differences, paired-sample t-tests were performed with emotion (fear/neutral) as the within-subjects factor.
Whole Brain Analysis
To determine if there were any additional regions that showed group differences during anticipation of fear faces, we performed an exploratory voxel-wise whole brain analysis. For the whole brain analysis, a cluster threshold was calculated using AlphaSim. The calculation was done using the whole brain mask image created by SPM, 3 × 3 × 3mm voxels, 6mm smoothing, a voxel p-value of .005, an FWE rate of .05, and 5,000 iterations. For this analysis, a voxel p < .005 and a cluster size of 24 voxels (648 mL) provided a FWE corrected p < .05.
Results
Behavioral Data
Task Valdiation
Overall, there was a main effect of emotion; reaction times were significantly faster for fear trials than neutral trials (p = .002; Table 2). Reaction times were also significantly faster for faces than for cues (p < .001). As expected, fear faces were rated as significantly more arousing (p < .001) and less pleasant than neutral faces (p < .001).
Table 2.
Behavioral results.
| Overall (n = 34) |
Inhibited Temperament (n = 17) |
Uninhibited Temperament (n = 17) |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean | SD | Mean | SD | Mean | SD | |
| Hit Rate (%) | 97.9 | 3.2 | 97.1 | 3.9 | 98.8 | 2.1 |
| Reaction Times (ms) | ||||||
| Fear Cue | 415.9 | 60.1 | 427.5 | 61.0 | 404.3 | 58.7 |
| Neutral Cue | 420.4 | 62.7 | 433.3 | 63.2 | 407.4 | 61.2 |
| Fear Face | 379.5 | 60.7 | 391.5 | 60.3 | 367.4 | 60.3 |
| Neutral Face | 389.6 | 66.7 | 401.8 | 66.5 | 377.5 | 66.7 |
| Arousal Ratings | ||||||
| Fear Face | 3.4 | 0.5 | 3.4 | 0.6 | 3.4 | 0.4 |
| Neutral Face | 4.2 | 0.7 | 4.0 | 0.6 | 4.4 | 0.7 |
| Valence Ratings | ||||||
| Fear Face | 3.0 | 0.6 | 2.9 | 0.7 | 3.1 | 0.5 |
| Neutral Face | 3.9 | 0.5 | 3.7 | 0.5 | 4.1 | 0.4 |
Note: Subjects rated arousal and valence of the faces on a 1-7 Likert scale. Arousal ratings ranged from 1 (very excited) to 7 (very calm). Valence ratings ranged from 1 (very unpleasant) to 7 (very pleasant).
Group Differences
There were no significant differences in accuracy, reaction times, and arousal ratings between groups or interactions of group × emotion (all p > .10; Table 2). Valence ratings differed by group (p = .02); inhibited individuals rated faces (both fear and neutral) as more negative.
fMRI Anticipation Task
To identify neural substrates of risk for social anxiety disorder, we compared brain activation between inhibited and uninhibited subjects. There were group differences in activation in the left insula and multiple prefrontal cortical regions, including the dorsal anterior cingulate (dACC), rostral anterior cingulate cortex (rACC), and bilateral dorsolateral prefrontal cortex (dlPFC) (all p < .05, FWE corrected; Table 3 and Figure 2). No group differences were identified within the dorsomedial prefrontal cortex or amygdala ROIs. There were no regions were the uninhibited group had larger values on the fear > neutral anticipation contrast.
Figure 2.
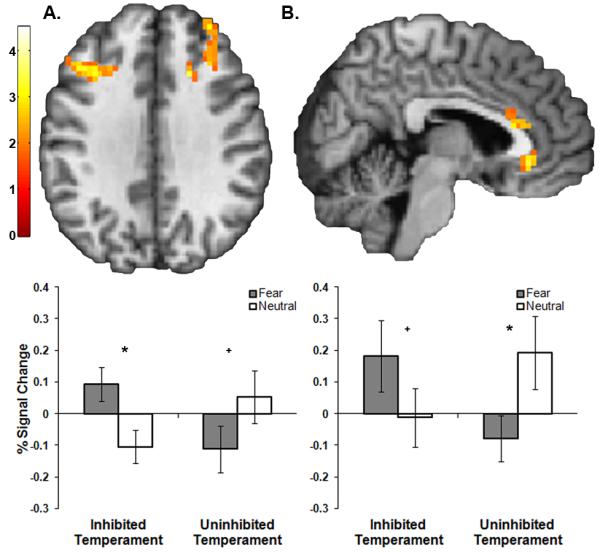
The inhibited group had significantly greater activation across a prefrontal network during anticipation of fear faces, relative to anticipation of neutral faces. During anticipation of fear faces, relative to neutral, the inhibited temperament group had significantly greater activation in (A) the dorsolateral prefrontal cortex and (B) the dorsal and rostral anterior cingulate cortex. Color bar displays t-values. Graphs (left: left dorsolateral prefrontal cortex; right: rostral anterior cingulate cortex) show percent signal change from significant group differences. Error bars are standard error of the mean. Within-group differences in percent signal change for anticipation of fear faces, relative to anticipation of neutral faces are indicated by * (p < .05) or + (trend, p ≤ .10).
To clarify the nature of these findings, we performed post-hoc paired t-tests within each group. In the inhibited group, there was significantly greater activation for fear anticipation, relative to neutral anticipation, in the dACC (p = .001), left dlPFC (p = .009), and right dlPFC (p = .006). Activity in the rACC trended towards significantly greater for fear anticipation (p = .10) and there were no differences in the left insula (p = .22). In the uninhibited temperament group, there was significantly more deactivation for fear anticipation, relative to neutral anticipation, in the rACC (p = .03), left insula (p = .04), left dlPFC (p = .04), and there was a trend towards more deactivation in the right dlPFC (p = .06). Activation in the dACC was similar and close to zero across both conditions (p = .21). Taken together, these results show that the dACC was preferentially engaged during fear anticipation in the inhibited temperament group, but was not differentially engaged during anticipation in the uninhibited temperament group. The insula showed the opposite pattern; the insula showed lower activation during fear anticipation in the uninhibited group and no difference in the inhibited group. The rACC and dlPFC were differentially engaged by both the inhibited and uninhibited temperament groups, but in opposite directions.
Functional Connectivity
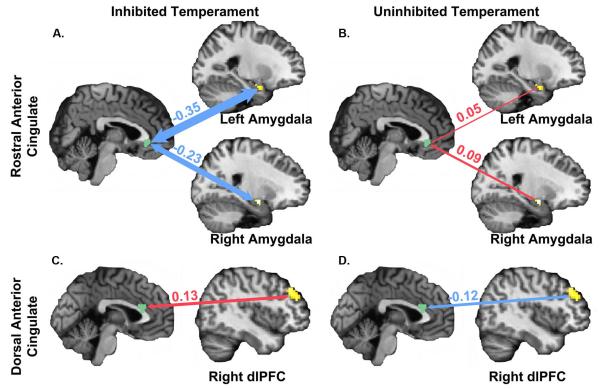
To identify group differences in neurocircuitry activated during anticipation, a functional connectivity analysis was performed. Broadly, the two groups differed in functional connectivity across a neural circuit including the insula, amygdala, and prefrontal cortex (Table 4 and Figure 3). Connectivity between the rACC and the bilateral amygdala differed between groups (left amygdala: p = .023, right amygdala: p = .005). Inhibited subjects had significantly more negative rACC-amygdala connectivity during fear face anticipation, relative to neutral; in contrast, the uninhibited group had similar connectivity across both conditions. The two groups also differed in connectivity between the dorsal ACC (dACC) and the right dlPFC (p = .005) in a region more posterior and lateral than the region of group difference in functional MRI (see Table 3). Inhibited subjects had significantly greater positive connectivity during fear face anticipation, relative to neutral; in contrast, the uninhibited group had negative connectivity across both conditions. Connectivity between the left insula and several brain regions also differed between groups. The inhibited group had negative connectivity between the insula and several regions of the prefrontal cortex including the rACC, dACC and dlPFC; connectivity between these regions was positive in the uninhibited group.
Table 4.
Temperament differences in functional connectivity during anticipation.
| Peak Voxel | ||||||||
|---|---|---|---|---|---|---|---|---|
| Seed Region |
Target | Cluster Size |
x | y | z | Inhibited Temperament (mean ± SD) |
Uninhibited Temperament (mean ± SD) |
p-value |
|
| ||||||||
| rACC | amygdala (L) | 15 | −27 | 0 | −24 | −.35 ± .60 | .05 ± .37 | .023 |
| amygdala (R) | 13 | 24 | −6 | −15 | −.23 ± .34 | .09 ± .28 | .005 | |
|
| ||||||||
| dACC | dlPFC (R) | 59 | 45 | 42 | 27 | .13 ± .22 | −.12 ± .26 | .005 |
| insula (L) | 42 | −30 | 27 | 9 | −.19 ± .31 | .06 ± .39 | .007 | |
|
| ||||||||
| dlPFC (R) | anterior dlPFC (L) | 55 | −27 | 42 | 33 | −.11 ± .38 | .24 ± .30 | .005 |
| posterior dlPFC (L) | 90 | −30 | 21 | 39 | −.08 ± .27 | .33 ± .34 | .001 | |
|
| ||||||||
| insula (L) | rACC | 33 | 3 | 24 | −9 | −.11 ± .32 | .24 ± .39 | .007 |
| dACC | 29 | 3 | 15 | −9 | −.25 ± .35 | .15 ± .47 | .007 | |
| dlPFC (L) | 78 | −18 | 42 | 24 | −.29 ± .31 | .06 ± .39 | .006 | |
Note: connectivity values are represented by beta-values; SD = standard deviation; (L) = left hemisphere; (R) = right hemisphere; (B) = bilateral; rACC = rostral anterior cingulate cortex; dlPFC = dorsolateral prefrontal cortex; dACC = dorsal anterior cingulate cortex; coordinates are in Montreal Neurological Institute convention.
Figure 3.
Group differences in functional connectivity with the anterior cingulate cortex during anticipation of viewing fear faces, relative to anticipation of viewing neutral faces. (A) The inhibited temperament group had significantly more negative connectivity between the rostral anterior cingulate cortex and the bilateral amygdala during anticipation of viewing fear faces, relative to anticipation of viewing neutral faces; (B) the uninhibited group had no significant differences in connectivity during anticipation. (C) The inhibited temperament group had significantly more positive connectivity between the dorsal anterior cingulate cortex (dACC) and the right dorsolateral prefrontal cortex (dlPFC) during anticipation of fear faces, relative to anticipation of neutral faces; (D) the uninhibited temperament group showed a decrease in connectivity. Figures display mean difference between fear anticipation and neutral anticipation conditions in beta-values (mean difference + SEM; IT: rACC-left amygdala = −.35 ± .14, rACC-right amygdala = −.23 ± .08, dACC-right dlPFC = .13 ± .05; UT: rACC-left amygdala = .05 ± .09, rACC-right amygdala = .09 ± .07, dACC-right dlPFC = −.12 ± .06). Significant temperament group differences were calculated using an independent samples t-test (left amygdala: t(32) = −2.39, p = 0.023; right amygdala: t(32) = −3.23, p = 0.005; dACC-right dlPFC: t(32) = 3.03, p = .005).
Correlations with Social Anxiety
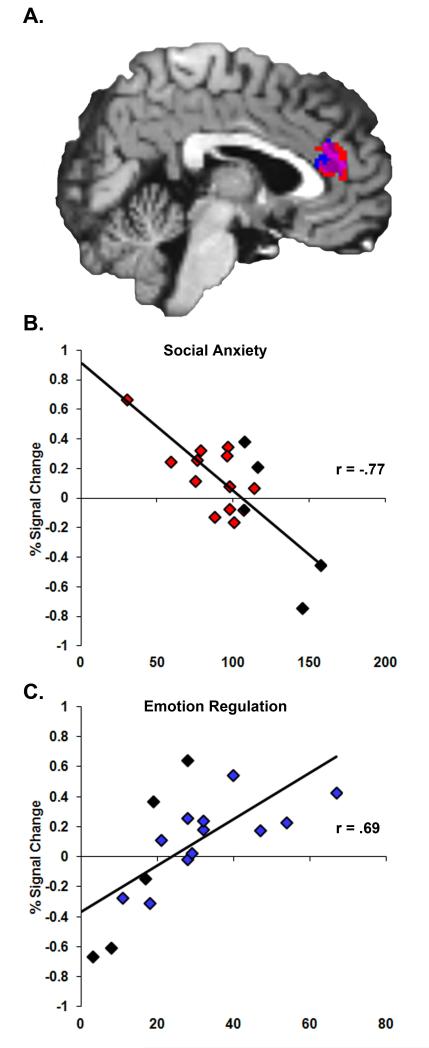
To determine whether individual differences in brain activation were associated with differences in social anxiety symptoms in the inhibited group, we conducted a regression analysis. Individuals with lower social anxiety scores had significantly greater activation of the dACC (r = −.77, p < .001), rACC (r = −.64, p = .005), and right amygdala (trend, r = −.44, p = .08) during anticipation of viewing fear faces, relative to neutral faces (Figure 4A and 4B). These correlations were confirmed using non-parametric testing (Spearman’s rho, all p < .05), which minimizes potential effects of outliers. To determine the degree to which the subjects with social anxiety disorder drove these correlations, we performed the correlation analysis without those subjects. Social anxiety symptoms were still correlated with activation in the dACC (r = −.70, p = .01) with a trend in the rACC (r = −.51, p = .09), but were no longer correlated with activation in the right amygdala (r = −.31, p = .32). Social anxiety was not associated with individual differences in functional connectivity.
Figure 4.
Within the inhibited temperament group, social anxiety scores and emotion regulation scores were correlated with rostral and dorsal anterior cingulate activation during anticipation of viewing fear faces. (A) Shown in red is the region where social anxiety scores were negatively correlated with activation and shown in blue is the region where emotion regulation scores were positively correlated with activation. Shown in purple is the overlap between the two regions. In order to illustrate the magnitude and direction of correlations, we show scatter plots of the data. The associated correlation values provide an effect size for the original statistically significant findings. (B) Social anxiety scores were significantly negatively correlated with activation during anticipation of viewing fear faces in two clusters within the rostral (r = −.64) and dorsal anterior cingulate cortex (r = −.77). (C) Emotion regulation scores were significantly positively correlated with activation in one cluster including the rostral and dorsal anterior cingulate cortex (r = .80). Subjects with social anxiety disorder are indicated with black diamonds. Removing subjects with social anxiety disorder from the analysis had no effect on the significance of the dorsal anterior cingulate region correlations with social anxiety scores (r = −.70, p = .01) or with emotion regulation scores (r = .73, p = .007), but reduced the rostral anterior cingulate correlation with social anxiety to trend level (r = −.51, p = .09).
To determine the specificity of these findings, we also performed a regression analysis within the uninhibited group and across the whole sample. Within the uninhibited temperament group, correlations between percent signal change and social anxiety symptoms were not significant (dorsal anterior cingulate: r = .11, p = .66; rostral anterior cingulate: r = .012, p = .95; right amygdala: r = .24, p = .36). Across all subjects, correlations between percent signal change and social anxiety score were also not significant (rostral anterior cingulate: r = −.19, p = .27; dorsal anterior cingulate: r = −.10, p = .57; right amygdala: r = .05, p = .77).
Correlations with Emotion Regulation
Next we examined the association between emotion regulation—a putative resilience factor—and brain function. Within the inhibited group, individuals with higher emotion regulation scores had significantly greater activity in the dACC (Figure 4C, r = .80, p < .001). This correlation was confirmed using non-parametric testing (Spearman’s rho = .69, p = .002). Interestingly, this region overlapped with the dACC region that was associated with lower social anxiety (Figure 4A). To determine the degree to which the subjects with social anxiety disorder drove these correlations, we performed the correlation analysis without those subjects. Emotion regulation was still correlated with activation in the dACC (r = .73, p = .007). Emotion regulation scores were also correlated with functional connectivity in the inhibited group. Individuals with higher emotion regulation scores had significantly greater connectivity between the rACC and the right amygdala (r = .57, p = .02).
To test the specificity of this relationship, we also performed regression analyses in the uninhibited group and across the whole sample. Within the uninhibited temperament group, the correlation between percent signal change and emotion regulation scores were not significant (r =.40, p = .11). The correlation between rACC-right amygdala connectivity was also not significant (r = .14, p = .59). Across the entire sample, the correlation between percent signal change and emotion regulation score was also not significant (dorsal anterior cingulate: r = .109, p = .54). The correlation between rACC-right amygdala connectivity was significant (r = .54; p = .001); however, this finding was driven by the inhibited temperament group, as it was not significant in the uninhibited temperament group.
Whole Brain Analysis
To identify brain regions associated with group differences in anticipation outside our ROIs, we also performed a voxel-wise whole brain analysis. The inhibited group had greater activation during anticipation of fear compared to neutral faces in the left extrastriate visual cortex and the right thalamus (Table 3). The uninhibited group did not have significantly greater activation in any brain regions.
To clarify the nature of these findings, we performed post-hoc paired t-tests within each group. In the inhibited group, there was significantly greater activation for fear anticipation, relative to neutral anticipation in both the left extrastriate visual cortex (p < .001) and right thalamus (p = .001). In the uninhibited temperament group, there was greater activation for neutral anticipation, relative to fear anticipation in the left extrastriate visual cortex (p = .03) and right thalamus (trend, p = .06).
Discussion
The goals of this study were to identify neural substrates of risk and resilience to social anxiety disorder. There were two major study findings. First, during anticipation of viewing fear faces, the inhibited and uninhibited groups had different neural responses. The uninhibited group had decreased activation in the insula and dorsolateral prefrontal cortex, relative to anticipation of viewing neutral faces. In contrast, the inhibited, high-risk group had increased activation in a prefrontal network, including the bilateral dorsolateral prefrontal cortex and anterior cingulate cortex. This prefrontal network is consistent with the brain regions typically activated in healthy controls during anticipation of viewing aversive images24,40–43. Second, we identified that resilience was associated with activation of the anterior cingulate cortex (ACC) within the high-risk, inhibited group. Greater activation of the ACC was associated with both lower social anxiety and better emotion regulation skills, suggesting that the ability to engage cognitive control regions during anticipation of negative social stimuli may protect against social anxiety disorder in high-risk individuals.
During anticipation of viewing fear faces, relative to neutral faces, the inhibited subjects had greater activation in both the dorsolateral prefrontal cortex (dlPFC) and the dorsal ACC (dACC; also known as anterior mid-cingulate). These prefrontal regions are commonly activated when healthy adults anticipate viewing strongly aversive stimuli40–43, such as mutilated bodies and snakes. Our findings show that inhibited subjects activate the same core anticipatory processing regions during anticipation of more mild social stimuli, such as fear faces. We propose that in inhibited subjects, these faces engage the same anticipatory processes as highly aversive images engage in healthy controls, and that inhibited individuals are more sensitive to mildly negative social stimuli. In contrast, uninhibited subjects had relative deactivation during fear anticipation in multiple regions including the insula, the rostral ACC, and the dlPFC. Decreased activation to aversive, relative to positive faces, has been previously shown in uninhibited temperament49. Deactivation of the insula40,50 and dACC50 has also been shown in healthy controls during anticipation of aversive stimuli; however, this finding is not consistent (see 41,42). Also, these findings are consistent with a recent study51 showing deactivation of the insula and ACC during anticipation of thermal pain in expert meditators, suggesting that anticipatory activity can be down-regulated. Typically, inhibited subjects have increased amygdala responses to social stimuli12–14,16,52; however, in this task, there were no differences in amygdala activation. In the inhibited subjects, it is possible that amygdala activation was inhibited by the prefrontal cortex, consistent with the functional connectivity finding of negative connectivity between the rostral ACC and both the left and right amygdala. Together these findings show that the inhibited subjects engage anticipatory processing to mild social stimuli, and that this anticipatory processing may have effectively inhibited amygdala response.
Studying inhibited young adults provides an opportunity to identify patterns of brain activation associated resilience. Within the inhibited group, greater activation of the rACC and dACC was correlated with fewer social anxiety symptoms and higher emotion regulation scores. Also, greater positive connectivity between the rACC and amygdala was correlated with higher emotion regulation scores. A recent study of adults who were inhibited as children, but were relatively healthy as adults, reported increased rACC activity when engaging emotional control during viewing of fear faces49, providing further support for rACC activity as a protective or resilience factor. Consistent with the notion that the dACC activity mediates resilience, patients with social anxiety disorder show reduced dACC-amygdala connectivity both during face viewing and in the absence of a task53. These findings suggest that in inhibited individuals, greater rACC and dACC activation may compensate for increased amygdala reactivity and that ACC-amygdala connectivity may be critical in the pathogenesis of social anxiety. Being ability to engage the prefrontal cortex and the rACC and dACC, particularly during anticipation of emotional stimuli, may be critical to resilience and protective against developing social anxiety disorder.
The study had some limitations, which may be addressed in future research. First, in order to maximize group differences, we compared two extreme groups. This approach is consistent with an extreme discordant phenotypes approach54 and previous studies of inhibited temperament by our group and others10,12–15,17,52,55,56. One important limitation to this approach is that neither group is necessarily “typical”, although in a previous study, uninhibited individuals showed similar findings to healthy control groups13. Future studies should examine anticipatory processing in an average temperament group to determine the generalizability of these findings. Second, we cannot rule out the possibility that the inhibited subjects that we described as “resilient” to social anxiety disorder may still develop social anxiety disorder later in life. However, we propose that this is unlikely given that: 1) the majority of individuals who will develop social anxiety disorder have the disorder by age 151 and 2) in samples of inhibited children, a meta-analysis showed that 40% of inhibited children developed social anxiety disorder by adolescence, which is consistent with estimates of social anxiety disorder in inhibited adults57–59. Third, this study focused on anticipation of social stimuli. It remains unknown whether this pattern of brain responses is specific to social stimuli or reflects a more general response to all stimuli. One research group has examined anticipation of non-social stimuli in patient with social anxiety disorder and found anticipatory activation in some, but not all, of the regions found in this study19,20. Fourth, we tested for differences in five a priori regions of interest, which were selected based on prior studies of anticipatory processing networks. While this approach can increase statistical power by reducing the number of multiple comparisons, a limitation is that findings are limited to the brain regions examined. To address this limitation, we also conducted an exploratory whole brain analysis. Finally, we did not provide specific instructions during the anticipation period; therefore any emotion regulation was implicit, or automatic. Further studies with online anxiety ratings are needed to test the hypothesis that inhibited subjects are regulating their responses during anticipation of social stimuli.
Conclusion
Inhibited adults had greater activation of a prefrontal network when anticipating viewing fear faces. Greater activation of the rACC was associated with fewer social anxiety symptoms and more frequent use of emotion regulation. We propose that greater activation of the ACC mediates resilience to social anxiety disorder. These findings are consistent with theories of cognitive behavior therapy, and findings that use of cognitive control strategies can increase prefrontal cortex activity in patients with anxiety20,60. Given the rapid development of the prefrontal cortex during childhood and adolescence, strategies that increase prefrontal cortex activation and strengthen prefronto-amygdalar connectivity hold promise for increasing resilience in high-risk, inhibited children.
Acknowledgements
Data from this manuscript were presented at the Anxiety Disorders Association of America on April 13th, 2012 in Arlington, Virginia, at the Society of Biological Psychiatry on May 4th, 2012 in Philadelphia, PA, and at the American College of Neuropsychopharmacology on December 4th, 2012 in Hollywood, FL. This work was supported by funding from the National Institute of Mental Health (K01-MH083052, JUB; F30-MH097344, JAC; T32-MH018921), the National Institute on Drug Abuse (K12-DA000357, MMB; R21-DA031441, RLC; R21-DA033341, RLC), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R21-HD053766, RLC), the Vanderbilt Institute for Clinical and Translational Research (NCRR; UL1-RR024975; now at the National Center for Advancing Translational Sciences; 2-UL1-TR000445-06), the Vanderbilt Medical Scholars Program (NIH; TL1-RR024978), the Vanderbilt Medical Scientist Training Program (NIGMS; T32-GM07347), and the Vanderbilt University Institute of Imaging Science. The authors thank Dr. Ellen Leibenluft, Dr. April Seay, and the students of the Developmental Psychopathology seminar for helpful critiques and feedback on the manuscript. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Within the past 3 years, Dr. Cowan has received publication royalties from Lippincott Williams and Wilkins, consultant income from the Southwest Michigan First Life Science Fund and the University of West Alabama, and research and salary support from Shire Pharmaceuticals and Novo Nordisk for projects not overlapping with this report.
Footnotes
All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Wittchen H-U, Beloch E. The impact of social phobia on quality of life. Int Clin Psychopharmacol. 1996;11:15–23. doi: 10.1097/00004850-199606003-00004. [DOI] [PubMed] [Google Scholar]
- 3.Stein MB, Kean YM. Disability and quality of life in social phobia: epidemiologic findings. Am J Psychiat. 2000;157:1606–1613. doi: 10.1176/appi.ajp.157.10.1606. [DOI] [PubMed] [Google Scholar]
- 4.Stein MB, Torgrud LJ, Walker JR. Social phobia symptoms, subtypes, and severity: findings from a community survey. Arch Gen Psychiatry. 2000;57:1046–1052. doi: 10.1001/archpsyc.57.11.1046. [DOI] [PubMed] [Google Scholar]
- 5.Biederman J, et al. Further evidence of association between behavioral inhibition and social anxiety in children. Am J Psychiat. 2001;158:1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- 6.Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J Am Acad Child Adolesc Psychiatry. 2012;51:1066–1075. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirshfeld-Becker DR, et al. Behavioral inhibition in preschool children at risk is a specific predictor of middle childhood social anxiety: a five-year follow-up. J Dev Behav Pediatr. 2007;28:225. doi: 10.1097/01.DBP.0000268559.34463.d0. [DOI] [PubMed] [Google Scholar]
- 8.Essex MJ, Klein MH, Slattery MJ, Goldsmith HH, Kalin NH. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. Am J Psychiat. 2010;167:40. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirshfeld DR, et al. Stable behavioral-inhibition and its association with anxiety disorder. J Am Acad Child Adolesc Psychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- 11.Guyer AE, Masten CL, Pine DS, Vasa RA, Roy AK. Pediatric Anxiety Disorders. Springer; New York: 2013. pp. 23–46. [Google Scholar]
- 12.Blackford JU, Avery SN, Shelton RC, Zald DH. Amygdala temporal dynamics: temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neurosci. 2009;10:145. doi: 10.1186/1471-2202-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackford JU, Avery SN, Cowan RL, Shelton RC, Zald DH. Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Soc Cogn Affect Neur. 2011;6:621–9. doi: 10.1093/scan/nsq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blackford JU, Allen AH, Cowan RL, Avery SN. Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Soc Cogn Affect Neur. 2013;8:143–150. doi: 10.1093/scan/nsr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clauss JA, Cowan RL, Blackford JU. Expectation and temperament moderate amygdala and dorsal anterior cingulate cortex responses to fear faces. Cogn Affect Behav Neurosci. 2011;11:13–21. doi: 10.3758/s13415-010-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pérez-Edgar K, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz CE, et al. A phenotype of early infancy predicts reactivity of the amygdala in male adults. Mol Psychiatr. 2012;17:1042–1050. doi: 10.1038/mp.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiat. 2007;164:1476. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brühl AB, et al. Neural correlates of altered general emotion processing in social anxiety disorder. Brain Res. 2011;1378:72–83. doi: 10.1016/j.brainres.2010.12.084. [DOI] [PubMed] [Google Scholar]
- 20.Brühl AB, Herwig U, Delsignore A, Jancke L, Rufer M. General emotion processing in social anxiety disorder: neural issues of cognitive control. Psychiatry Res. 2013;212:108–15. doi: 10.1016/j.pscychresns.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Nitschke JB, et al. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiat. 2009;166:302. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aupperle RL, et al. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Arch Gen Psychiatry. 2012;69:360–371. doi: 10.1001/archgenpsychiatry.2011.1539. [DOI] [PubMed] [Google Scholar]
- 23.Sarinopoulos I, et al. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cerebral Cortex. 2010;20:929–940. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onoda K, et al. Anterior cingulate cortex modulates preparatory activation during certain anticipation of negative picture. Neuropsychologia. 2008;46:102–110. doi: 10.1016/j.neuropsychologia.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 25.McDermott JM, et al. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biol Psychol. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suveg C, Zeman J. Emotion regulation in children with anxiety disorders. J Clin Child Adolesc. 2004;33:750–759. doi: 10.1207/s15374424jccp3304_10. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann SG. Cognitive mediation of treatment change in social phobia. J Consult Clin Psych. 2004;72:392. doi: 10.1037/0022-006X.72.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ochsner K, Gross J. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66:170–80. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biol Psychiatry. 2009;66:1091–1099. doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reznick JS, Hegeman IM, Kaufman ER, Woods SW, Jacobs M. Retrospective and concurrent self-report of behavioral-inhibition and their relation to adult mental-health. Dev Psychopathol. 1992;4:301–321. [Google Scholar]
- 32.Spitzer RL, Williams JBW, Gibbon M, First MB. The structured clinical interview for DSM-III-R (SCID) .1. History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 33.Turner SM, Beidel DC, Dancu CV, Stanley MA. An empirically derived inventory to measure social fears and anxiety: the Social Phobia and Anxiety Inventory. Psychol Assess. 1989;1:35. [Google Scholar]
- 34.Garnefski N, Kraaij V, Spinhoven P. Negative life events, cognitive emotion regulation and emotional problems. Pers Indiv Differ. 2001;30:1311–1327. [Google Scholar]
- 35.Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. Journal of personality and social psychology. 1989;56:267. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychological assessment. 1995;7:524. [Google Scholar]
- 37.Anderson CA, Miller RS, Riger AL, Dill JC, Sedikides C. Behavioral and characterological attributional styles as predictors of depression and loneliness: review, refinement, and test. Journal of Personality and Social Psychology. 1994;66:549–558. doi: 10.1037//0022-3514.66.3.549. [DOI] [PubMed] [Google Scholar]
- 38.Goeleven E, De Raedt R, Leyman L, Verschuere B. The Karolinska Directed Emotional Faces: a validation study. Cogn Emot. 2008;22:1094–1118. [Google Scholar]
- 39.Shulman GL, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 40.Grupe DW, Oathes DJ, Nitschke JB. Dissecting the anticipation of aversion reveals dissociable neural networks. Cereb Cortex. 2013;23:1874–1883. doi: 10.1093/cercor/bhs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. NeuroImage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 42.Simmons A, Matthews SC, Stein MB, Paulus MP. Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport. 2004;15:2261–65. doi: 10.1097/00001756-200410050-00024. [DOI] [PubMed] [Google Scholar]
- 43.Herwig U, Abler B, Walter H, Erk S. Expecting unpleasant stimuli-An fMRI study. Psychiatry Res-Neuroim. 2007;154:1–12. doi: 10.1016/j.pscychresns.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 45.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 46.Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1993;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 47.McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nature Reviews Neuroscience. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- 49.Jarcho JM, et al. Enduring influence of early temperament on neural mechanisms mediating attention-emotion conflict in adults. Depress Anxiety. 2013 doi: 10.1002/da.22140. doi:10.1002/da.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Archives of General Psychiatry. 2008;65:1275. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lutz A, McFarlin DR, Perlman DM, Salomons TV, Davidson RJ. Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. NeuroImage. 2013;64:538–546. doi: 10.1016/j.neuroimage.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz CE. Inhibited and uninhibited infants ‘grown up’: adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- 53.Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. Abberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety. 2013;30:234–241. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nebert DW. Extreme discordant phenotype methodology: an intuitive approach to clinical pharmacogenetics. Eur J Pharmacol. 2000;410:107–120. doi: 10.1016/s0014-2999(00)00809-8. [DOI] [PubMed] [Google Scholar]
- 55.Reeb-Sutherland BC, et al. Attention to novelty in behaviorally inhibited adolescents moderates risk for anxiety. J Child Psychol Psyc. 2009;50:1365–1372. doi: 10.1111/j.1469-7610.2009.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwartz CE, et al. Structural differences in adult orbital and ventromedial prefrontal cortex are predicted by 4-month infant temperament. Arch Gen Psychiatry. 2010;67:78. doi: 10.1001/archgenpsychiatry.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heiser NA, Turner SM, Beidel DC, Roberson-Nay R. Differentiating social phobia from shyness. Journal of Anxiety Disorders. 2009;23:469–476. doi: 10.1016/j.janxdis.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heiser NA, Turner SM, Beidel DC. Shyness: Relationship to social phobia and other psychiatric disorders. Behaviour Research and Therapy. 2003;41:209–221. doi: 10.1016/s0005-7967(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 59.Chavira DA, Stein MB, Malcarne VL. Scrutinizing the relationship between shyness and social phobia. Journal of Anxiety Disorders. 2002;16:585–598. doi: 10.1016/s0887-6185(02)00124-x. [DOI] [PubMed] [Google Scholar]
- 60.Felmingham K, et al. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol Sci. 2007;18:127–129. doi: 10.1111/j.1467-9280.2007.01860.x. [DOI] [PubMed] [Google Scholar]