Abstract
Bronchiolitis obliterans syndrome (BOS) is a form of chronic graft vs. host disease (cGVHD) and a highly morbid pulmonary complication after allogeneic hematopoietic stem cell transplantation (HSCT). We assessed the prevalence and risk factors for BOS and cGVHD in a cohort of HSCT recipients, including those who received reduced intensity conditioning (RIC) HSCT. Between January 1, 2000 and June 30, 2010, all patients who underwent allogeneic HSCT at our institution (n = 1854) were retrospectively screened for the development of BOS by PFT criteria. We matched the BOS cases with two groups of control patients: (1) patients who had concurrent cGVHD without BOS and (2) those who developed neither cGVHD nor BOS. Comparisons between BOS patients and controls were conducted using t-test or Fisher’s exact tests. Multivariate regression analysis was performed to examine factors associated with BOS diagnosis. All statistical analyses were performed using SAS 9.2. We identified 89 patients (4.8%) meeting diagnostic criteria for BOS at a median time of 491 days (range: 48–2067) after HSCT. Eighty-six (97%) of our BOS cohort had extra-pulmonary cGVHD. In multivariate analysis compared to patients without cGVHD, patients who received busulfan-based conditioning, had unrelated donors, and had female donors were significantly more likely to develop BOS, while ATG administration was associated with a lower risk of BOS. Our novel results suggest that busulfan conditioning, even in RIC transplantation, could be an important risk factor for BOS and cGVHD.
Introduction
Bronchiolitis obliterans syndrome (BOS) is a form of irreversible airflow obstruction and is a late, non-infectious pulmonary complication of allogeneic hematopoietic stem cell transplantation (HSCT) [1]. Depending on the disease definition, the prevalence of BOS ranges from approximately 2–30% [1–8], and is associated with a significant increase in morbidity and mortality within the HSCT population [3,4].
The clinical presentation of BOS is usually insidious and may include a dry cough, shortness of breath, or dyspnea on exertion, but up to 30% of patients are asymptomatic [5,7]. In the absence of routine spirometric screening, reports of disease prevalence likely underestimate the true burden of BOS, since symptomatic patients are typically already suffering from moderate to severe airflow obstruction [5].
The clinical factor most closely associated with the development of BOS is the presence of cGVHD at another site [1,3–5,9–11]. The NIH consensus criteria now consider BOS to be a form of cGVHD of the lungs [12]. Other risk factors reported to be associated with BOS include: acute GVHD, myeloablative busulfan-based conditioning regimens, use of methotrexate for GVHD prophylaxis, respiratory viral infections within the first 100 days after transplant, peripheral blood stem cell source, history of pneumonitis, low immunoglobulin levels, and reduced pretransplant pulmonary function tests [1,2,4–6,9–11,13].
Lower doses of busulfan are increasingly used in combination with fludarabine or melphalan as conditioning in reduced intensity conditioning (RIC) HSCT. The impact of busulfan on the development of BOS and cGVHD after RIC HSCT remains unknown and has important implications for clinical care. We assessed the prevalence and risk factors for BOS and cGVHD in a recent cohort of HSCT recipients, many of whom had undergone RIC HSCT [14].
Methods
Patients
All patients who received an allogeneic HSCT (HSCT) at the Dana Farber Cancer Institute/Brigham and Women’s Hospital (DFCI/BWH) between January 1, 2000 and June 30, 2010 were retrospectively screened for the development of BOS. All pulmonary function tests (PFTs) conducted between January 1, 2000 and June 30, 2010 at the DFCI were screened for the presence of expiratory airflow obstruction (FEV1/FVC ratio ≤ 0.7). PFTs were performed as part of the pretransplant evaluation for all patients. Post-transplant PFTs were performed for respiratory symptoms, re-transplant, enrollment in clinical trials, and at the discretion of the treating physician. Patients indentified as having an FEV1/FVC ratio ≤0.7 after HSCT then underwent a detailed chart review and were excluded if they demonstrated reversible airflow obstruction or had an alternative explanation for their obstructive ventilatory defect such as respiratory infection, or an exacerbation of underlying obstructive lung disease. In addition, the electronic medical record of all patients coded in a central DFCI BMT repository as having cGVHD with lung involvement and/or cGVHD without lung involvement were reviewed for post-transplant PFTs or lung pathology demonstrating BOS. From this cohort, BOS was defined, using modified NIH criteria [12], as (1) new onset airflow obstruction, FEV1/FVC ratio ≤ 0.7 and FEV1 < 80% predicted; (2) irreversible airflow obstruction defined as <12% change and <200 cc absolute change in FEV1 and/or FVC in response to bronchodilator challenge per American Thoracic Society (ATS) criteria [12,15]; (3) if airflow obstruction was noted prior to HSCT, a ≥15% decline in FEV1 from baseline; or (4) BO confirmed by pathology irrespective of meeting the spirometric definition of BOS. Patients with a pretransplant history of pulmonary disease were excluded unless they had post transplant PFTs documenting no response to bronchodilator challenge. Our modifications to the NIH criteria included a more liberal inclusion of an FEV1 < 80% vs. 75%, and we did not include RV/TCL, high resolution CT or bronchoscopy as part of the inclusion criteria.
After identifying BOS cases, each case was matched with two sets of controls based on date of HSCT (±90 days). Control Group 1 included patient who developed cGVHD without BOS (“cGVHD”), and Control Group 2 included those who developed neither cGVHD nor BOS (“No cGVHD”). cGVHD was defined clinically by treating physicians. Grading of the severity of cGVHD was not included in this analysis because of the changes in classification schemes during the period that these patients were treated [12]. Patients were not included as controls if: (1) survival was ≤1 year after transplant, (2) there was insufficient clinical follow-up between year 1 and year 2 after transplant, unless autopsy demonstrated no BOS, or (3) there was a decline in FEV1 to less than <80% predicted. If FEV1 was reduced prior to transplantation, patients were excluded from the control groups if they had >10% decline in FEV1 % predicted from baseline. Patients were also excluded from the control groups upon detailed medical record review if they developed chronic cough, shortness of breath, wheeze or unexplained dyspnea on exertion defined as symptoms lasting >30 days, unless PFTs documented no change from baseline. All PFTs were performed according to the ATS guidelines, as part of routine clinical care [15]. Lung function data collected included FEV1, FVC, TLC, RV, DLCO, and RV/TLC, all expressed as a percentage of predicted values. FEV1/FVC was expressed as a ratio [15]. Lung volumes were assessed using helium dilution or plethysmography. All clinical data was collected through the DFCI central database repository. Clinical variables not recorded in the central database were collected from the electronic medical records.
Allogeneic HSCTs were performed under a number of treatment plans and clinical protocols using both myeloablative and reduced intensity conditioning regimens. Myeloablative conditioning (MAC) regimens consisted mostly of cyclophosphamide (3600 mg/m2 or 120 mg/kg) plus total body irradiation (1400 cGy in 7 fractions with lung shielding), or high dose busulfan (12.8 mg/kg intravenously) plus cyclophosphamide (3600 mg/m2). Pharmacokinetic monitoring of busulfan was not performed. Reduced intensity conditioning (RIC) regimens consisted of fludarabine (120 mg/m2) plus intravenous busulfan (3.2–6.4 mg/kg) or fludarabine (125 mg/m2) plus melphalan (140 mg/m2). Selection of MAC vs. RIC regimen for transplantation was based on physician discretion in consultation with the patient. In general, older patients or patients with increased co-morbidities are more likely to get RIC than MAC regimens. Patients received bone marrow, umbilical cord or peripheral blood stem cells from HLA-matched or mismatched, related or unrelated donors. GVHD prophylaxis consisted primarily of a calcineurin inhibitor (cyclosporine or tacrolimus) combined with methotrexate, with or without sirolimus.
This study (Protocol 09-316) was approved by the Institutional Review Board at Dana Farber/Harvard Cancer Center, Boston, MA.
Statistical analysis
We evaluated clinical predictors for an association with development of BOS (see Table I). Each predictor was evaluated for significance between BOS cases and both groups of controls (cGVHD and No cGVHD) and between control groups (cGVHD and No cGHVD). Two-sided Fisher’s exact test was used to compare categorical variables between groups. Two-sided Wilcoxon Rank-Sum test or t-test was used to compare continuous variables as appropriate given the normality of the data. We also performed a multivariate regression analysis predicting the development of BOS and cGVHD. All variables with a P < 0.1 on univariate analysis were eligible for inclusion, using a Forward Selection logistic regression model. Cell source and conditioning regimen were dichotomized for multivariate analysis, peripheral blood stem cell source versus others, and busulfan-based versus others, respectively. All statistical analyses were performed using SAS 9.2.
TABLE I.
Clinical Characteristics of the BOS and Non-BOS Control Cohorts
| BOS, n = 89 | cGVHD, n = 89 | No cGVHD, n = 87 | |
|---|---|---|---|
| Age (y) | 46.1 (12.1) | 44.9 (13) | 42 (12.4)a |
| Male gender | 48 (54%) | 57 (64%) | 49 (56%) |
| White race | 80 (90%) | 85 (96%) | 85 (98%) |
| Active smoking at Tx | 4 (5%) | 5 (6%) | 5 (6%) |
| Pretransplant history of pulmonary diseasec | 20 (22%) | 10 (11%) | 3 (3%)a |
| Respiratory Illness prior to day 100d | 23 (26%) | 11 (13%)a | 9 (10%)a |
| CMV positive | 40 (45%) | 35 (39%) | 28 (33%) |
| Acute GVHDe | 40 (45%) | 34 (38%) | 27 (31%) |
| Pretransplant PFTs | |||
| Pretransplant FEV1% | 89.3 (17) | 101 (14.7)a | 100.2 (14.8)a |
| Pretransplant FVC% | 90.9 (16.8) | 97.9 (12.6)a | 99.4 (14)a |
| Pretransplant FEV1/FVC | 76.1 (8.8) | 79.8 (6.3)a | 79.1 (7)a |
| Pretransplant TLC% | 90.1 (16.8) | 94.0 (11.1) | 94.9 (12.6) |
| Pretransplant RV% | 93.0 (30.8) | 85.2 (24.6) | 87.3 (26.6) |
| Pretransplant RV/TLC% | 102.7 (24.9) | 91.2 (19.4)a | 90.8 (26.0)a |
| Pretransplant DLCO% | 84.4 (22.1) | 90.4 (17.8) | 88.4 (20.7) |
| High-risk diseasef | 64 (72%) | 61 (69%) | 45 (52%)a,b |
| Cell source | |||
| PBSC | 76 (85%) | 73 (82%) | 55 (63%)a,b |
| Bone marrow | 10 (11%) | 13 (15%) | 17 (20%) |
| Cord cells | 3 (3%) | 3 (3%) | 14 (16%)a,b |
| Conditioning regimen | |||
| Busulfan/cyclophosphamidee | 4 (5%) | 2 (2%) | 1 (1%) |
| Cyclophosphamide/TBIg | 37 (42%) | 48 (54%) | 55 (63%)a |
| Busulfan/fludarabineh | 45 (51%) | 34(38%) | 13(15%)a,b |
| Melphalan/fludarabine | 3 (3%) | 3 (3%) | 13 (15%)a,b |
| Other | 0 (0%) | 2 (2%) | 5 (6%)a |
| GVHD prophylaxis | |||
| ATG in conditioning | 6 (7%) | 5 (6%) | 30 (34%)a,b |
| Sirolimus post HSCT | 51 (57%) | 52 (58%) | 37 (43%)b |
| Donor | |||
| Age (y) | 35.3 (11.8) | 39.4 (12.7)a | 33.9 (18.0) |
| Female | 46 (52%) | 32 (36%)a | 28 (31%)a |
| Gender mismatch | 37 (42%) | 38 (43%) | 42 (48%) |
| Unrelated | 65 (73%) | 50 (66%)a | 37 (41%)a,b |
| Mismatched | 14 (16%) | 9 (10%) | 15 (17%) |
| CMV positive | 30 (34%) | 28 (32%) | 25 (29%) |
P <0.05 BOS vs cGVHD and no cGVHD controls
P <0.05 cGVHD vs no cGVHD controls.
Pretransplant history of pulmonary disease included the following diagnoses: asthma, chronic obstructive pulmonary disease, emphysema, chronic bronchitis, and bronchiectasis that were not active at the time of diagnosis.
Patients treated for viral or bacterial infection per treating physician prior to day 100.
Acute GVHD was graded by consensus grading criteria [38].
All diseases other than CML CP1, AML/ALL CR1, MDS RA/RS, or AA are considered high-risk in this analysis.
Myeloablative conditioning regimens: cytoxan + total body irradiation 1400 cGY; high dose busulfan cyclophosphamide.
Reduced intensity conditioning regimen: fludarabine + low-dose intravenous busulfan (3.2–6.4 mg/kg).
Results
BOS cohort characteristics
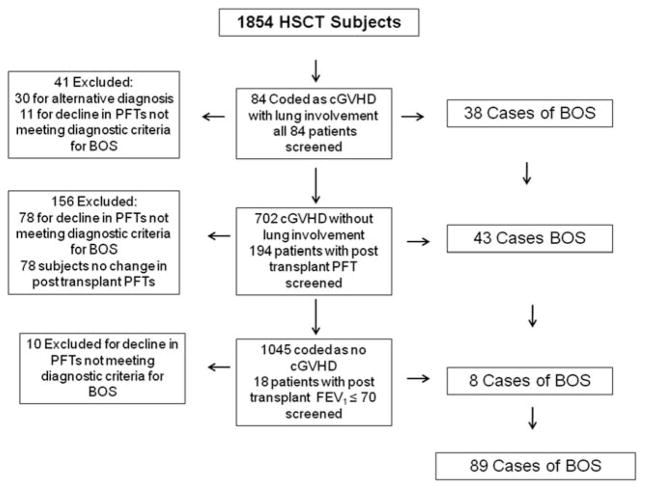
Between January 1, 2000 and June 30, 2010, a total of 1,854 patients underwent 1,967 allogeneic HSCTs at the Dana Farber Cancer Institute/Brigham and Women’s Hospital. BOS patients were identified using three strategies: (1) 84 patients were coded as having cGVHD and lung involvement, 43 of those 84 met BOS inclusion criteria; 41 were excluded for either, cryptogenic organizing pneumonia (COP), interstitial pneumonitis or restrictive lung disease without BOS (n = 30), or for decline in PFTs not meeting inclusion criteria (n = 11). All patients had post-transplant PFT’s available for review. (2) A total of 702 patients were coded as cGVHD without lung involvement, and 194 of them had PFTs that were available for analysis. BOS was confirmed in 38 patients, and 78 were excluded for having a decline in PFTs not meeting diagnostic criteria of BOS. (3) A total of 1068 patients were coded as not having cGVHD. From this list, 18 were identified as having an FEV1/FVC ratio ≤0.7, and from this group, eight cases of BOS were identified (see Fig. 1).
Figure 1.
Flow chart detailing BOS inclusion criteria for analysis.
A total of 89 patients met the diagnostic criteria for BOS resulting in a prevalence of 4.8%. The median time from transplantation to meeting criteria for BOS was 491 days (range: 48–2067). The median time from transplantation to development of symptoms was 430 days (range: 21–2067). Eighty-six (97%) of the BOS patients had evidence of cGVHD affecting other organ systems. Eight patients (9%) had no respiratory symptoms at the time of diagnosis, and the indication for PFTs included: clinical trial enrollment (n = 5), second transplantation (n = 1) follow-up idiopathic Pneumonia Syndrome (n = 1), and bronchiectasis on routine PET scan (n = 1). Ten patients (11%) had biopsy proven disease; of these three (3.4%) did not meet our modified spirometric NIH criteria for the diagnosis of BOS. One patient did not have post-transplant PFTs performed, and the other two had a combined obstructive and restrictive PFT pattern with a normal FEV1/FVC ratio and an elevated RV/TLC. In addition, of the 10 patients with biopsy proven BOS, 6 had lung volumes measured, and 0/6 (0%) had an RV/TLC >120% of predicted at the time of diagnosis. The clinical characteristics of our BOS cohort are summarized in Table I.
The mean FEV1, FVC, TLC, DLCO, and RV/TLC at the time of diagnosis were 52.8% (±15.7), 69.4% (±16.0), 80.0% (±14.9), 65.4% (±18.4), and 126.6% (±27.3) predicted, respectively (Table II). A total of 35/89 (39%) patients had a TLC <80% predicted. A total of 31/35 (89%) had skin manifestations of cGVHD or CT findings that could explain the reduced TLC, and 4 (11%) had no alternative diagnosis that could be established. Of the 35 patients, 18 (51%) demonstrated abnormal CT findings, 11 (31%) had centrilobular nodules, 6 (17%) had pulmonary infiltrates, and 1 (3%) had a pleural effusion.
TABLE II.
Baseline PFTs and PFTs at the Time of Diagnosis of BOS
| Pre-BOS | Post-BOS | |
|---|---|---|
| FEV1 | 89.3 (17) | 52.8 (15.7) |
| FVC | 90.9 (16.8) | 69.4 (16.0) |
| FEV1/FVC | 76.1 (8.8) | 57.9 (10.7) |
| TLC | 90.1 (16.8) | 80.0 (14.9) |
| RV | 93.0 (30.8) | 99.8 (29.2) |
| RV/TLC | 102.7 (24.9) | 126.6 (27.3) |
| DLCO | 84.4 (22.1) | 65.4 (18.4) |
FEV1/FVC is expressed as a ratio and all other values are expressed as % predicted. Pre-BOS PFTs were all performed at baseline prior to transplantation. Post-BOS PFTs were the PFTs performed when the subject meet our modified NIH spirometric criteria.
Control cohort characteristics
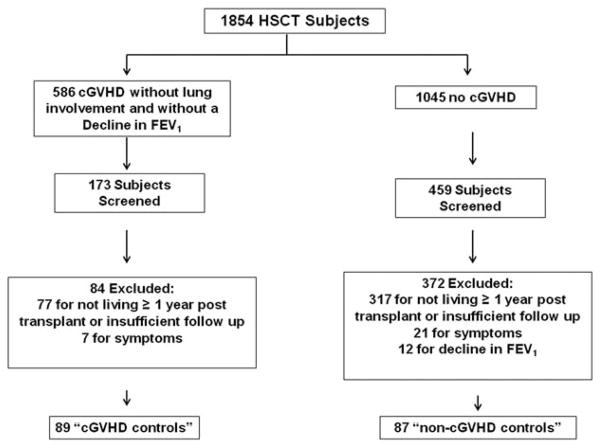
Control populations were identified from the remaining non-BOS patients and categorized as cGVHD without lung involvement (“cGVHD”) and those without cGVHD (“no cGVHD”). The control patients meeting the pre-defined criteria closest to the index BOS case (±90 days) were chosen. There were 586 patients who were coded in the data repository as cGVHD without lung involvement. One hundred and seventy three charts were reviewed to identify 89 controls, 59 (66%) had post-transplant PFTs available. Eighty four were excluded: 77 for not living >1 year after transplantation or insufficient clinical follow-up and 7 for symptoms of chronic cough, shortness of breath, wheeze, or unexplained dyspnea on exertion (Fig. 2).
Figure 2.
Flow chart detailing control subject inclusion criteria for analysis.
There were 1045 patients coded in the BMT repository as not having cGVHD. From these we screened 459 patients to identify 87 controls, 26 (30%) had post-transplant PFTs available. We could not identify patients meeting control criteria that fell within ±90 days for HSCT for two of the BOS cases. Three hundred and seventy two patients were excluded: 317 for not living >1 year after transplantation or insufficient clinical follow-up, 21 for symptoms of chronic cough, shortness of breath, wheeze, or unexplained dyspnea on exertion, 12 for decline in post-transplant PFTs not meeting BOS criteria, and 22 for evidence of cGVHD not recorded in the database at the time the data was extracted (Fig. 2).
Risk factors for BOS
Comparison with “no cGVHD” cohort
As shown in Table I, univariate analysis of patients with BOS compared with patients without cGVHD or BOS demonstrated a number of factors as being significantly associated with BOS. We observed a strong association with BOS and busulfan-based conditioning, even in reduced intensity transplantation where the busulfan dose was ≤6.4 mg/kg. We also observed that the use of ATG as part of the pretransplant conditioning regimen was associated with a lower risk for the development of BOS. We additionally confirmed a number of previously reported risk factors associated with the development of BOS including: older patient age, pretransplant history of pulmonary disease, a history of respiratory illness within the first 100 days after transplant, lower baseline FEV1 % predicted, lower baseline FVC % predicted, lower baseline FEV1/FVC, higher baseline RV/TLC% predicted, high-risk underlying disease, peripheral blood stem cells, female donor, and unrelated donor. Conversely, cyclophosphamide/TBI conditioning, melphalan/fludarabine conditioning and the use of cord cell transplant were associated with a lower prevalence of BOS.
Multivariate analysis demonstrated that busulfan-based conditioning, unrelated donor, female donor, lower baseline FEV1 % predicted, patient CMV sera-positive at time of transplant, acute GVHD, pre-transplant history of pulmonary disease, and high-risk disease were independent factors associated with the development of BOS. The use of ATG in the conditioning regimen was associated with a reduced risk for the development of BOS (Table III).
TABLE III.
Multivariate Predictors of BOS vs No cGVHD Without BOS
| Risk factor | OR | 95% CI | P value |
|---|---|---|---|
| Busulfan | 6.37 | [2.37,17.13] | <0.001 |
| ATG | 0.08 | [0.02, 0.27] | <0.001 |
| Unrelated donor | 4.01 | [1.55,10.42] | 0.004 |
| Female donor | 4.20 | [1.63, 10.86] | 0.003 |
| Reduced pretransplant FEV1% | 1.04 | [1.01, 1.07] | <0.01 |
| CMV positive | 3.44 | [1.34, 8.87] | 0.01 |
| Acute GVHD | 3.34 | [1.29, 8.67] | 0.01 |
| Pretransplant history of lung disease | 9.99 | [1.66, 59.80] | 0.01 |
| High-risk disease | 2.76 | [1.02, 7.45] | <0.05 |
Comparison with cGVHD patients without BOS
Univariate analysis of patients with BOS compared to patients with cGVHD but no pulmonary involvement demonstrated that BOS was significantly associated with a history of respiratory illness within the first 100 days after transplantation, lower baseline FEV1 % predicted, lower baseline FVC % predicted, lower baseline FEV1/FVC, higher baseline RV/TLC% predicted, lower donor age, female donor, unrelated donor. There was a trend toward busulfan-based conditioning P = 0.07. Multivariate analysis revealed that lower baseline FEV1 % predicted, unrelated donor, female donor, and a history of respiratory illness within the first 100 days after transplantation were independent factors associated with the development of BOS (Table IV).
TABLE IV.
Multivariate Predictors of BOS vs cGVHD Without BOS
| Risk factor | OR | 95% CI | P value |
|---|---|---|---|
| Reduced pretransplant FEV1% | 1.05 | [1.03, 1.07] | <0.001 |
| Unrelated donor | 2.67 | [1.28, 5.59] | <0.01 |
| Female donor | 2.22 | [1.11, 4.45] | 0.03 |
| Respiratory illness Prior to day 100 | 2.58 | [1.05, 6.32] | 0.04 |
Comparison of cGVHD patients without BOS and No cGVHD
Univariate analysis of patients with cGVHD without BOS compared with patients without cGVHD demonstrated that the development of cGVHD was significantly associated with high-risk disease, PBSC, busulfan-based conditioning, sirolimus GVHD prophylaxis, and unrelated donors. Multivariate analysis that revealed that RIC busulfan-based conditioning, and unrelated donors were independent factors associated with the development of cGVHD and ATG was protective (Table V).
TABLE V.
Multivariate Predictors cGVHD Without BOS vs No cGVHD
| Risk factor | OR | 95% CI | P value |
|---|---|---|---|
| Busulfan | 2.86 | [1.32, 6.22] | <0.01 |
| ATG | 0.09 | [0.03, 0.28] | <.0001 |
| Unrelated donor | 2.24 | [1.12, 4.48] | 0.02 |
Discussion
With the increasing use of reduced intensity conditioning regimens, and better supportive care, early transplant mortality after HSCT is on the decline, and late complications, including chronic GVHD and BOS are emerging as major causes of morbidity and mortality for transplant survivors. BOS and chronic GVHD after HSCT are intricately linked, and based on the current NIH consensus criteria for cGVHD diagnosis, BOS is a diagnostic/distinct manifestation of cGVHD in the lungs [12]. Risk factors associated with BOS/cGVHD in the era of RIC transplantation have not been well described. In this large single center retrospective study, we have identified that the use of busulfan, even in RIC transplantation, appears to be a risk factor associated with the development of cGVHD and BOS.
To date there have been multiple retrospective studies identifying full dose busulfan-based regimens as risk factors for the development of BOS and cGVHD [6,11,16,17]. The strongest evidence implicating such an association was a randomized clinical trial of 167 patients with leukemia treated with myeloablative busulfan or total body irradiation (TBI) in addition to cyclophosphamide. Patients in this study were observed for a period of 5–9 years. The authors found that the incidence of cGVHD and BOS were significantly greater in the busulfan-treated patients compared with the TBI patients (59% versus 47%) and (26% versus 5%), respectively [13]. However, to our knowledge, there have been no studies that have examined the association of busulfan with BOS and cGVHD development in RIC transplantation, where low-dose busulfan is commonly used in combination with fludarabine.
The mechanisms of busulfan-induced lung injury are currently unknown, but may include direct toxicity to the epithelial lining cells of the lungs as well as the liver, with the latter being associated with the development of veno-occlusive disease [6,13,16,18,19]. The direct toxicity to the pulmonary epithelial cells may expose antigens and result in donor allo-reactivity contributing to the development of BOS [20].
Another notable finding of our analysis is that the use of ATG as part of the conditioning regimen appears to confer a protective effect against the development of cGVHD and BOS. Our findings confirm previously reported studies demonstrating that ATG conditioning is associated with lower incidence of BOS and cGVHD [21–27].
The overall prevalence for BOS in our patient population was 4.8%, which is similar to a recent study conducted by Au et al., demonstrating a prevalence of 5.5% using NIH consensus criteria [1]. The rate of decline in FEV1 in BOS has also been shown to be variable, from rapid progression to reversible airflow obstruction [5], which is similar to other forms of cGVHD that have demonstrated variable grades of severity and progression of disease [28,29]. One could speculate that a decline in FEV1 after transplantation that does not meet the diagnostic criteria for BOS may represent milder forms of the disease. This may explain why our study was able to replicate many of the previously reported risk factors associated with BOS. Our case–control study was designed specifically to exclude patients who may have mild forms of BOS or unrecognized symptomatic disease.
One of the limitations of our retrospective cohort was the lack of a standardized screening protocol for BOS. We suspect that this most likely resulted in an underestimation of the true prevalence of BOS in our population, given that 9% of our BOS cohort was asymptomatic at the time of diagnosis, and only 28% of our patients with identified cGVHD had PFTs performed after transplantation. Utilizing a strict screening protocol of PFTs every 3 months for 3 years after transplant, Kuzmina et al. found a prevalence of BOS to be 27% at three years, suggesting we may be vastly underestimating the true prevalence of BOS [7]. In addition, patients who present with a combined obstructive/restrictive pulmonary deficit and resulting FEV1/FVC >0.7 may have BOS not meeting the NIH diagnostic criteria, suggesting the current criteria may lack sensitivity for the diagnosis of BOS. We identified three patients with biopsy proven disease who did not meet NIH spirometric criteria for the diagnosis of BOS. One patient did not have PFTs performed and the other two patients demonstrated a combined obstructive/restrictive deficit with a preserved FEV1/FVC ratio. Bergeron et al. recently published on a cohort of “atypical” BOS patients with a reduced FEV1 and FVC with a preserved ratio and found up to 31% of their cohort met these criteria [30]. In addition, within our cohort 35 (39%) of patients had a TLC <80% predicted consistent with a combined obstructive/restrictive deficit. These data suggest BOS, when combined with a restrictive deficit, may be under recognized, and more work is needed to identify and validate more sensitive and specific non-invasive means of diagnosis such as Quantitative Computed Tomography [31–33].
The lack of a standard BOS screening protocol should bias toward the null for our cGVHD control cohort, as many cGVHD patients did not have post-transplant screening PFTs and were included as controls on the basis of not developing pulmonary symptoms. As such, it is possible that some patients in our cGVHD control cohort could have had undiagnosed asymptomatic BOS. This may explain, at least in part, the weaker univariate association (P = 0.07) for busulfan when our BOS cohort was compared to the cGVHD control cohort. However, unrecognized BOS is unlikely to be as significant an issue for the no-cGVHD control cohort since it is uncommon to have BOS without cGVHD affecting other organ systems. Within our cohort, 97% of the patients diagnosed with BOS had evidence of cGVHD affecting other organ systems. Prior reports suggest the incidence of cGVHD affecting other organ systems in patients diagnosed with BOS ranges from 69% to 100% with an average of 89% [1,2,5,6,9,10,20,22,30,34]. Our data suggests that busulfan even at RIC doses increases the overall risk of cGVHD and BOS.
Our multivariate model revealed that a lower baseline FEV1 % predicted, unrelated donor, female donor, and a history of respiratory illness within the first 100 days after transplantation were independent factors associated with the development of BOS, compared to patients with cGVHD and no BOS. Our results confirm the previously reported association between respiratory infections early after transplantation and the development of BOS [3,35,36]. Thus, strict adherence to routine post-transplant guidelines for preventing respiratory infections early after HSCT would appear to be an essential secondary strategy for the prevention of BOS [37].
In summary, our findings provide new information with regard to risk factors associated with BOS and cGVHD, demonstrating that busulfan, even when used at lower doses in RIC HSCT, confers an increased risk for BOS and cGVHD, while the use of ATG as part of the pretransplant conditioning regimen appears to be protective. These observations could have important clinical implications for patients being considered for RIC HSCT. There may be some utility in the selection of non-busulfan-based RIC regimens, such as those using fludarabine-melphalan or fludarabine plus low-dose TBI, in patients who have other risk factors for BOS, such has prior underlying pulmonary disease, and/or abnormal pretransplant PFTs. Larger registry based analyses and/or prospective clinical trials are needed to confirm these results that might impact the selection of conditioning regimens for HSCT.
Acknowledgments
Contract grant sponsor: National Institutes of Health; Contract grant numbers: 5T32HL007118-35, 5T32HL007680-20, and 5T32HL007633-27 (Dr. Gazourian), K23HL089353-05, HL107246-03 and HL116473-01 (Dr. Washko), and HL091957 (Dr. Baron);
Contract grant sponsor: Ted and Eileen Pasquarello Research Fund (The DFCI BMT data repository);
Contract grant sponsor: NIH; Contract grant number: CA142106 and PO1HL070149-02 (The DFCI BMT data repository);
Contract grant sponsor: the Jock and Bunny Adams Education and Research Fund (The DFCI BMT data repository). Contract grant sponsor: Parker B. Francis Foundation (Dr. Rogers).
The authors would like to thank Diana C. Pierce-Tremblay and Qiheng Yang for their contributions to this study.
Footnotes
Conflict of interest: Nothing to report.
Author Contributions
Lee Gazourian, M.D: Guarantor of the manuscript, contributed to the creation and final approval.
Ruby Ibanga: contributed to the creation and final approval of the manuscript.
Angela J. Rogers, M.D., Gerald L. Weinhouse, M.D., Victor Pinto-Plata, M.D., Jerome Ritz, M.D., Robert J. Soiffer, M.D., Joseph H. Antin, M.D., George R. Washko, M.D., and Rebecca M. Baron, M.D.: contributed to the creation and final approval of the manuscript.
Vincent T. Ho, M.D.: facilitated data collection and contributed to the creation and final approval of the manuscript.
References
- 1.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biology of blood and marrow transplantation. Journal of the American Society for Blood and Marrow Transplantation. 2011;17:1072–1078. doi: 10.1016/j.bbmt.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudek A. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biology of Blood and Marrow Transplantation. 2003;9:657–666. doi: 10.1016/s1083-8791(03)00242-8. [DOI] [PubMed] [Google Scholar]
- 3.Chien JW, Martin PJ, Gooley TA, Flowers ME, Heckbert SR, Nichols WG, Clark JG. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. American Journal of Respiratory and Critical Care Medicine. 2003;168:208–214. doi: 10.1164/rccm.200212-1468OC. [DOI] [PubMed] [Google Scholar]
- 4.Clark JG, Schwartz DA, Flournoy N, Sullivan KM, Crawford SW, Thomas ED. Risk factors for airflow obstruction in recipients of bone marrow transplants. Annals of Internal Medicine. 1987;107:648–656. doi: 10.7326/0003-4819-107-5-648. [DOI] [PubMed] [Google Scholar]
- 5.Clark JG, Crawford SW, Madtes DK, Sullivan KM. Obstructive lung disease after allogeneic marrow transplantation. Clinical presentation and course. Annals of Internal Medicine. 1989;111:368–376. doi: 10.7326/0003-4819-111-5-368. [DOI] [PubMed] [Google Scholar]
- 6.Santo Tomas LH, Loberiza FR, Jr, Klein JP, Layde PM, Lipchik RJ, Rizzo JD, Bredeson CN, Horowitz MM. Risk factors for bronchiolitis obliterans in allogeneic hematopoietic stem-cell transplantation for leukemia. Chest. 2005;128:153–161. doi: 10.1378/chest.128.1.153. [DOI] [PubMed] [Google Scholar]
- 7.Kuzmina Z, Krenn K, Petkov V, Kormoczi U, Weigl R, Rottal A, Kalhs P, Mitterbauer M, Ponhold L, Dekan G, Greinix HT, Pickl WF. CD19+ CD21low B cells and patients at risk for NIH-defined chronic graft-versus-host disease with bronchiolitis obliterans syndrome. Blood. 2013;121:1886–1895. doi: 10.1182/blood-2012-06-435008. [DOI] [PubMed] [Google Scholar]
- 8.Diab KJ, Yu Z, Wood KL, Shmalo JA, Sheski FD, Farber MO, Wilkes DS, Nelson RP., Jr Comparison of pulmonary complications after nonmyeloablative and conventional allogeneic hematopoietic cell transplant. Biology of blood and marrow transplantation. Journal of the American Society for Blood and Marrow Transplantation. 2012;18:1827–1834. doi: 10.1016/j.bbmt.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Holland HK, Wingard JR, Beschorner WE, Saral R, Santos GW. Bronchiolitis obliterans in bone marrow transplantation and its relationship to chronic graft-v-host disease and low serum IgG. Blood. 1988;72:621–627. [PubMed] [Google Scholar]
- 10.Nakaseko C, Ozawa S, Sakaida E, Sakai M, Kanda Y, Oshima K, Kurokawa M, Takahashi S, Ooi J, Shimizu T, Yokota A, Yoshiba F, Fujimaki K, Kanamori H, Sakai R, Saitoh T, Sakura T, Maruta A, Sakamaki H, Okamoto S. Incidence, risk factors and outcomes of bronchiolitis obliterans after allogeneic stem cell transplantation. International Journal of Hematology. 2011;93:375–382. doi: 10.1007/s12185-011-0809-8. [DOI] [PubMed] [Google Scholar]
- 11.Nishio N, Yagasaki H, Takahashi Y, Muramatsu H, Hama A, Tanaka M, Yoshida N, Watanabe N, Kudo K, Yoshimi A, Kojima S. Late-onset non-infectious pulmonary complications following allogeneic hematopoietic stem cell transplantation in children. Bone Marrow Transplantation. 2009;44:303–308. doi: 10.1038/bmt.2009.33. [DOI] [PubMed] [Google Scholar]
- 12.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner M, Vogelsang G, Flowers ME. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation. Journal of the American Society for Blood and Marrow Transplantation. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Ringden O, Remberger M, Ruutu T, Nikoskelainen J, Volin L, Vindelov L, Parkkali T, Lenhoff S, Sallerfors B, Mellander L, Ljungman P, Jacobsen N. Increased risk of chronic graft-versus-host disease, obstructive bronchiolitis, and alopecia with busulfan versus total body irradiation: long-term results of a randomized trial in allogeneic marrow recipients with leukemia. Nordic Bone Marrow Transplantation Group. Blood. 1999;93:2196–2201. [PubMed] [Google Scholar]
- 14.Gratwohl A, Baldomero H, Passweg J, Urbano-Ispizua A. Increasing use of reduced intensity conditioning transplants: report of the 2001 EBMT activity survey. Bone Marrow Transplant. 2002;30:813–831. doi: 10.1038/sj.bmt.1703819. [DOI] [PubMed] [Google Scholar]
- 15.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 16.Marras TK, Chan CK, Lipton JH, Messner HA, Szalai JP, Laupacis A. Long-term pulmonary function abnormalities and survival after allogeneic marrow transplantation. Bone Marrow Transplant. 2004;33:509–517. doi: 10.1038/sj.bmt.1704377. [DOI] [PubMed] [Google Scholar]
- 17.Nakasone H, Kanda J, Yano S, Atsuta Y, Ago H, Fukuda T, Kakihana K, Adachi T, Yujiri T, Taniguchi S, Taguchi J, Morishima Y, Nagamura T, Sakamaki H, Mori T, Murata M. A case–control study of bronchiolitis obliterans syndrome following allogeneic hematopoietic stem cell transplantation. Transplant International: Official Journal of the European Society for Organ Transplantation. 2013 doi: 10.1111/tri.12093. [DOI] [PubMed] [Google Scholar]
- 18.Jochelson M, Tarbell NJ, Freedman AS, Rabinowe SN, Takvorian T, Soiffer R, Anderson K, Ritz J, Nadler LM. Acute and chronic pulmonary complications following autologous bone marrow transplantation in non-Hodgkin’s lymphoma. Bone Marrow Transplant. 1990;6:329–331. [PubMed] [Google Scholar]
- 19.Vergnon JM, Boucheron S, Riffat J, Guy C, Blanc P, Emonot A. Interstitial pneumopathies caused by busulfan. Histologic, developmental and bronchoalveolar lavage analysis of 3 cases. La Revue de medecine interne/fondee par la Societe nationale francaise de medecine interne. 1988;9:377–383. doi: 10.1016/s0248-8663(88)80137-1. [DOI] [PubMed] [Google Scholar]
- 20.Chien JW, Duncan S, Williams KM, Pavletic SZ. Bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation—an increasingly recognized manifestation of chronic graft-versus-host disease. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2010;16:S106–S114. doi: 10.1016/j.bbmt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, Oneto R, Bruno B, Sacchi N, van Lint MT, Bosi A. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2006;12:560–565. doi: 10.1016/j.bbmt.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 22.Duque-Afonso J, Ihorst G, Wasch R, Bertz H, Muller-Quernheim J, Finke J, Prasse A, Marks R. Identification of risk factors for bronchiolitis obliterans syndrome after reduced toxicity conditioning before hematopoietic cell transplantation. Bone Marrow Transplant. 2013 doi: 10.1038/bmt.2013.3. [DOI] [PubMed] [Google Scholar]
- 23.Ditschkowski M, Elmaagacli AH, Trenschel R, Peceny R, Koldehoff M, Schulte C, Beelen DW. T-cell depletion prevents from bronchiolitis obliterans and bronchiolitis obliterans with organizing pneumonia after allogeneic hematopoietic stem cell transplantation with related donors. Haematologica. 2007;92:558–561. doi: 10.3324/haematol.10710. [DOI] [PubMed] [Google Scholar]
- 24.Basara N, Baurmann H, Kolbe K, Yaman A, Labopin M, Burchardt A, Huber C, Fauser AA, Schwerdtfeger R. Antithymocyte globulin for the prevention of graft-versus-host disease after unrelated hematopoietic stem cell transplantation for acute myeloid leukemia: results from the multicenter German cooperative study group. Bone Marrow Transplant. 2005;35:1011–1018. doi: 10.1038/sj.bmt.1704957. [DOI] [PubMed] [Google Scholar]
- 25.Bonifazi F, Bandini G, Stanzani M, Palandri F, Giannini B, Arpinati M, Rosti G, Baccarani M. In vivo T-cell depletion with low-dose ATG is effective in reducing cGVHD after peripheral blood stem cell myeloablative sibling transplants in CML: results from a prospective phase II study. Bone Marrow Transplant. 2005;35:1025–1026. doi: 10.1038/sj.bmt.1704940. [DOI] [PubMed] [Google Scholar]
- 26.Russell JA, Turner AR, Larratt L, Chaudhry A, Morris D, Brown C, Quinlan D, Stewart D. Adult recipients of matched related donor blood cell transplants given myeloablative regimens including pretransplant antithymocyte globulin have lower mortality related to graft-versus-host disease: a matched pair analysis. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2007;13:299–306. doi: 10.1016/j.bbmt.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Socie G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, Volin L, Ruutu T, Heim DA, Schwerdtfeger R, Kolbe K, Mayer J, Maertens JA, Linkesch W, Holler E, Koza V, Bornhauser M, Einsele H, Kolb HJ, Bertz H, Egger M, Grishina O, Finke J. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117:6375–6382. doi: 10.1182/blood-2011-01-329821. [DOI] [PubMed] [Google Scholar]
- 28.Akpek G, Lee SJ, Flowers ME, Pavletic SZ, Arora M, Lee S, Piantadosi S, Guthrie KA, Lynch JC, Takatu A, Horowitz MM, Antin JH, Weisdorf DJ, Martin PJ, Vogelsang GB. Performance of a new clinical grading system for chronic graft-versus-host disease: a multicenter study. Blood. 2003;102:802–809. doi: 10.1182/blood-2002-10-3141. [DOI] [PubMed] [Google Scholar]
- 29.Greinix HT, Loddenkemper C, Pavletic SZ, Holler E, Socie G, Lawitschka A, Halter J, Wolff D. Diagnosis and staging of chronic graft-versus-host disease in the clinical practice. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2011;17:167–175. doi: 10.1016/j.bbmt.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Bergeron A, Godet C, Chevret S, Lorillon G, Peffault de Latour R, de Revel T, Robin M, Ribaud P, Socie G, Tazi A. Bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: phenotypes and prognosis. Bone Marrow Transplant. 2012 doi: 10.1038/bmt.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gazourian L, Coronata AM, Rogers AJ, Weinhouse GL, Soiffer RJ, Antin JH, Ritz J, Ho VT, Baron RM, Washko GR. Airway dilation in bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. Respir Med. 2013;107:276–283. doi: 10.1016/j.rmed.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knollmann FD, Kapell S, Lehmkuhl H, Schulz B, Bottcher H, Hetzer R, Felix R. Dynamic high-resolution electron-beam CT scanning for the diagnosis of bronchiolitis obliterans syndrome after lung transplantation. Chest. 2004;126:447–456. doi: 10.1378/chest.126.2.447. [DOI] [PubMed] [Google Scholar]
- 33.Knollmann FD, Ewert R, Wundrich T, Hetzer R, Felix R. Bronchiolitis obliterans syndrome in lung transplant recipients: use of spirometrically gated CT. Radiology. 2002;225:655–662. doi: 10.1148/radiol.2253011384. [DOI] [PubMed] [Google Scholar]
- 34.Ditschkowski M, Elmaagacli AH, Koldehoff M, Gromke T, Trenschel R, Beelen DW. Bronchiolitis obliterans after allogeneic hematopoietic SCT: further insight-new perspectives? Bone Marrow Transplant. 2013 doi: 10.1038/bmt.2013.17. [DOI] [PubMed] [Google Scholar]
- 35.Magnusson J, Westin J, Andersson L-M, Brittain-Long R, Riise GC. The impact of viral respiratory tract infections on long-term morbidity and mortality following lung transplantation. Transplant J. 2013;95:383–388. doi: 10.1097/tp.0b013e318271d7f0. [DOI] [PubMed] [Google Scholar]
- 36.Versluys AB, Rossen JW, van Ewijk B, Schuurman R, Bierings MB, Boelens JJ. Strong association between respiratory viral infection early after hematopoietic stem cell transplantation and the development of life-threatening acute and chronic alloimmune lung syndromes. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2010;16:782–791. doi: 10.1016/j.bbmt.2009.12.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, Wingard JR, Young JA, Boeckh MJ. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Preface Bone Marrow Transplant. 2009;44:453–455. doi: 10.1038/bmt.2009.254. [DOI] [PubMed] [Google Scholar]