Abstract
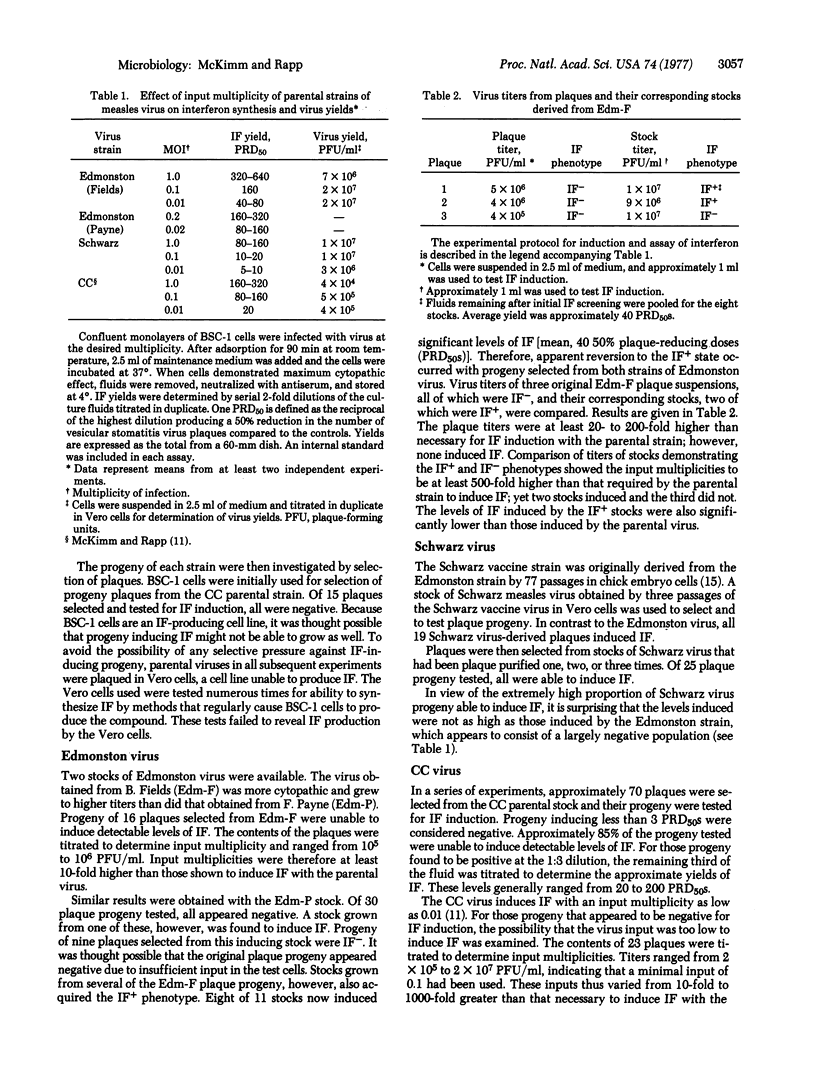
Plaque progeny of three interferon (IF)-inducing strains of measles virus (Edmonston, Schwarz, and CC) were examined for ability to induce IF in BSC-1 cells. Only after passage in Vero cells did any of the Edmonston progeny induce IF. The vast majority of plaque progeny selected from the Schwarz strain induced IF, even though this virus was originally derived from the Edmonston strain. This property was retained even after serial plaque purification of the progeny. However, the Schwarz-derived CC strain consisted of a population generally unable to induce IF. Stocks grown from both Edmonston and CC plaques demonstrating the IF+ phenotype maintained this characteristic as a whole, but it was not a property that was inherited by all progeny in the stocks. Levels of IF induced were approximately the same for all strains, even though the proportion of inducing progeny varied markedly among them. These noninducing variants appeared to be normal, fully infectious measles virions. The results suggest that induction of IF by measles virus is at least partially under the genetic control of the virus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON C. D., ATHERTON J. G. EFFECT OF ACTINOMYCIN D ON MEASLES VIRUS GROWTH AND ON INTERFERON PRODUCTION. Nature. 1964 Aug 8;203:671–671. doi: 10.1038/203671a0. [DOI] [PubMed] [Google Scholar]
- Atkins G. J., Johnston M. D., Westmacott L. M., Burke D. C. Department of Biological Sciences, University of Warwick, Coventry, CV47AL, England. J Gen Virol. 1974 Dec;25(3):381–390. doi: 10.1099/0022-1317-25-3-381. [DOI] [PubMed] [Google Scholar]
- Atkins G. J., Lancashire C. L. The induction of interferon by temperature-sensitive mutants of Sindbis virus: its relationship to double-stranded RNA synthesis and cytopathic effect. J Gen Virol. 1976 Feb;30(2):157–165. doi: 10.1099/0022-1317-30-2-157. [DOI] [PubMed] [Google Scholar]
- Breschkin A. M., Haspel M. V., Rapp F. Neurovirulence and induction of hydrocephalus with parental, mutant, and revertant strains of measles virus. J Virol. 1976 May;18(2):809–811. doi: 10.1128/jvi.18.2.809-811.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE MAEYER E., ENDERS J. F. An interferon appearing in cell cultures infected with measles virus. Proc Soc Exp Biol Med. 1961 Jul;107:573–578. doi: 10.3181/00379727-107-26692. [DOI] [PubMed] [Google Scholar]
- DEMAEYER E., ENDERS J. F. GROWTH CHARACTERISTICS, INTERFERON PRODUCTION AND PLAQUE FORMATION WITH DIFFERENT LINES OF EDMONSTON MEASLES VIRUS. Arch Gesamte Virusforsch. 1965;16:151–160. doi: 10.1007/BF01253804. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Merigan T. C. Current concepts of interferon and interferon induction. Annu Rev Med. 1970;21:17–46. doi: 10.1146/annurev.me.21.020170.000313. [DOI] [PubMed] [Google Scholar]
- ENDERS J. F. Measles virus. Historical review, isolation, and behavior in various systems. Am J Dis Child. 1962 Mar;103:282–287. [PubMed] [Google Scholar]
- HO M., ENDERS J. F. Further studies on an inhibitor of viral activity appearing in infected cell cultures and its role in chronic viral infections. Virology. 1959 Nov;9:446–477. doi: 10.1016/0042-6822(59)90135-7. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Duff R., Rapp F. Isolation and preliminary characterization of temperature-sensitive mutants of measles virus. J Virol. 1975 Oct;16(4):1000–1009. doi: 10.1128/jvi.16.4.1000-1009.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Knight P. R., Duff R. G., Rapp F. Activation of a latent measles virus infection in hamster cells. J Virol. 1973 Oct;12(4):690–695. doi: 10.1128/jvi.12.4.690-695.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M., Armstrong J. A. Interferon. Annu Rev Microbiol. 1975;29:131–161. doi: 10.1146/annurev.mi.29.100175.001023. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt W. J. Biochemistry of interferon and its inducers. Annu Rev Biochem. 1972;41(10):517–542. doi: 10.1146/annurev.bi.41.070172.002505. [DOI] [PubMed] [Google Scholar]
- Lai M. H., Joklik W. K. The induction of interferon by temperature-sensitive mutants of reovirus, UV-irradiated reovirus, and subviral reovirus particles. Virology. 1973 Jan;51(1):191–204. doi: 10.1016/0042-6822(73)90379-6. [DOI] [PubMed] [Google Scholar]
- Lockart R. Z., Jr, Bayliss N. L., Toy S. T., Yin F. H. Viral events necessary for the induction of interferon in chick embryo cells. J Virol. 1968 Oct;2(10):962–965. doi: 10.1128/jvi.2.10.962-965.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B., Burke D. C. Interferon production by temperature-sensitive mutants of Semliki Forest virus. J Gen Virol. 1970 Jul;8(1):55–68. doi: 10.1099/0022-1317-8-1-55. [DOI] [PubMed] [Google Scholar]
- McKimm J., Rapp F. Inability of measles virus temperature-sensitive mutants to induce interferon. Virology. 1977 Jan;76(1):409–415. doi: 10.1016/0042-6822(77)90312-9. [DOI] [PubMed] [Google Scholar]
- Mirchamsy H., Rapp F. Role of interferon in replication of virulent and attenuated strains of measles virus. J Gen Virol. 1969 Jun;4(4):513–522. doi: 10.1099/0022-1317-4-4-513. [DOI] [PubMed] [Google Scholar]
- SCHWARZ A. J. Preliminary tests of a highly attenuated measles vaccine. Am J Dis Child. 1962 Mar;103:386–389. doi: 10.1001/archpedi.1962.02080020398042. [DOI] [PubMed] [Google Scholar]
- Thacore H., Youngner J. S. Cells persistently infected with Newcastle disease virus. II. Ribonucleic acid and protein synthesis in cells infected with mutants isolated from persistently infected L cells. J Virol. 1970 Jul;6(1):42–48. doi: 10.1128/jvi.6.1.42-48.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


