SUMMARY
Differentiated cells possess a remarkable genomic plasticity that can be manipulated to reverse or change developmental commitments. Here, we show that the leprosy bacterium hijacks this property to reprogram adult Schwann cells, its preferred host niche, to a stage of progenitor/stem-like cells (pSLC) of mesenchymal traits by downregulating Schwann cell lineage/differentiation-associated genes and upregulating genes mostly of mesoderm development. Reprogramming accompanies epigenetic changes and rendered infected cells highly plastic, migratory and immunomodulatory. We provide evidence that acquisition of these properties by pSLC promotes bacterial spread by two distinct mechanisms: direct differentiation to mesenchymal tissues, including skeletal and smooth muscles, and by forming granuloma-like structures and subsequently release bacteria-laden macrophages. These findings support a model of host cell reprogramming in which a bacterial pathogen uses the plasticity of its cellular niche for promoting dissemination of infection, and provide an unexpected link between cellular reprogramming and host-pathogen interaction.
INTRODUCTION
Differentiated adult cells are natural targets for many intracellular bacterial pathogens. These pathogens often establish infection in their preferred niches by manipulating or subverting differentiated host cell functions (Falkow, 1991). Although it is now recognized that these cells posses unprecedented genomic plasticity and nuclear reprogramming potential (Gurdon and Melton, 2008; Theise and Wilmut, 2003; Takahashi and Yamanaka, 2006) it is not known if bacterial pathogens have co-evolved to leverage such host cell plasticity for their advantage.
Among differentiated cells, Schwann cells, the glial cells of the adult peripheral nervous system (PNS) that are comprised of myelin-forming and non-myelin-forming phenotypes (Jessen and Mirsky, 2005), show remarkable plasticity and contribute to the regeneration capacity of adult PNS even after severe injury (Fawcett and Keynes.,1990). Mycobacterium leprae (ML), which causes human leprosy, establishes infection in adult Schwan cells, a primary non-immune target, and causes subsequent neurological injury leading to sensorimotor loss (Job, 1989; Shetty et al., 1988; Stoner, 1979).
Although ML infection in humans initially presents with inflammation-mediated sensorimotor loss (Job, 1989; Miko et al., 1993; Scollard et al., 2006; Stoner, 1979) the early events of PNS infection in human are unknown. ML is a strictly obligate intracellular pathogen with a severely decayed bacterial genome and is totally dependent on host cell functions for survival (Cole et al., 2001). Recent studies have suggested that ML uses the regeneration properties of the PNS for expansion of bacterial niche within Schwann cells (Rambukkana, 2010; Rambukkana et al., 2002; Tapinos et al., 2006). In patients with advanced leprosy, regeneration of damaged peripheral nerves has been documented despite the bacterial presence (Miko et al., 1993). This may also reflect the bacterial efforts to secure and propagate Schwann cell niche during human infection. Thus, once invaded, ML uses strategies that promote Schwann cell endurance or rejuvenation in order to maintain infected cells in active stage so that essential host factors critical for bacterial survival can be acquired. In addition, Schwann cells also serve as a safe haven for ML, since the PNS blood-nerve barrier protects ML from host immune assault (Job, 1989; Stoner, 1979). Such favorable conditions, which are assisted with nontoxic, non-cytopathic, non-apoptotic and non-tumorigenic nature of ML, permit bacterial residence within host cells for a long period (Lahiri et al., 2010; Tapinos and Rambukkana, 2005).
The bacillary load in Schwann cells is a critical determinant for the subsequent immunopathology that manifest in various tissues following ML dissemination (Miko et al., 1993). After Schwann cell colonization leprosy bacilli need an exit route in order to successfully infect other tissues and transmit infection. In leprosy patients, disseminated ML could be seen in several tissues including skeletal muscles and smooth muscles (Pearson et al. 1970; Job et al., 1994; Kaur et al., 1981; Scollard et al., 2006; Werneck et al., 1999). Also, the involvement of skeletal muscles in human leprosy is considered secondary due to peripheral neuropathy with the obvious peripheral nerve innervations of skeletal muscles (Pearson et al., 1970; Werneck et al., 1999). However, it is unknown how initial colonization of ML in Schwann cells subsequently leads to the spread of infection to other tissues.
In this study, we show that leprosy bacteria trigger reprogramming of adult Schwann cells to a stage of progenitor/stem-like cells with migratory and immunomodulatory properties that promote bacterial dissemination. Reprogrammed cells facilitate bacterial spread by two distinct mechanisms – by direct differentiation to mesenchymal tissues, skeletal muscles and smooth muscles, and by contributing to form granuloma-like structures that subsequently release bacteria-laden macrophages. Our findings present an unexpected link between cellular reprogramming and host-pathogen interaction, and thus direct to potential new therapeutic strategies for combating infections with pathogenic bacteria.
RESULTS
Schwann Cells Derived from Adult Peripheral Nerves Undergo Reprogramming Events in Response to Intracellular M. leprae
We recapitulated the colonization of ML and subsequent early molecular events in adult Schwann cells by infecting primary Schwann cells isolated from adult mouse peripheral nerves. We isolated Schwann cells from adult wild type and Sox2-GFP transgenic mice and purified by FACS sorting using antibody to Schwann cell surface marker p75 NTR or GFP expression under the control of Sox2 promoter respectively (Figures 1A–C; 2A, B; S1, S2-A). Single cell-derived Schwann cells were generated from FACS sorted cells and characterized extensively (Figure S1). All were positive for the markers for late Schwann cell development and myelination-associated genes (Figures 1C, S1). Most of these differentiation markers are not present in neural crest stem cells and do not appear until around the time of birth (Jessen and Mirsky, 2005; Finzsch et al, 2010). Therefore, they represent mature de-differentiated Schwann cells. Moreover, karyotyping of these cells showed intact chromosome numbers and no evidence of translocation, suggesting their genomic stability (Figure S1).
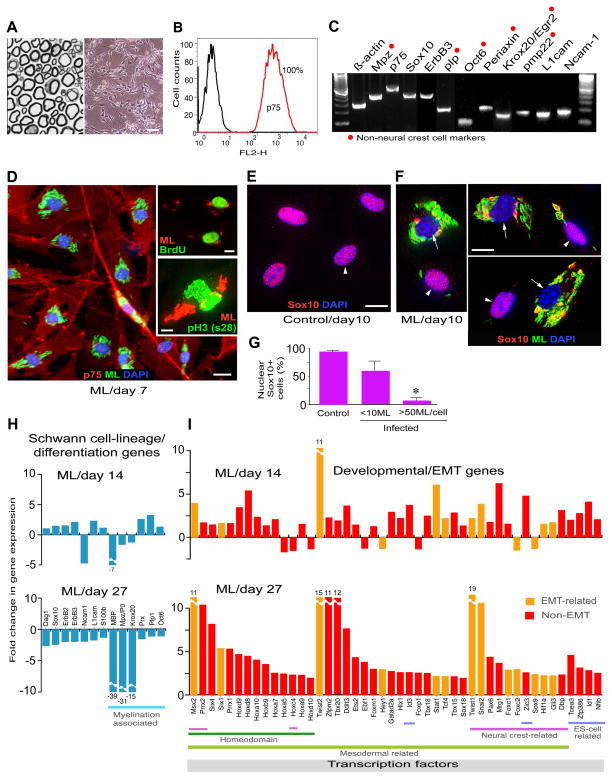
Figure 1. Adult Peripheral Nerve-Derived Schwann Cells Undergo Reprogramming Events in Response to Intracellular M. leprae.
(A and B) Methylin-blue stained semithin section of adult nerves (A, left) from which Schwann cells were isolated (phase image; A, right), and purified by FACS sorting using anti-p75 antibody (B). Scale bar represents 50μm.
(C) RT-PCR of purified p75+ Schwann cells. Highlighted genes (red dots) are late Schwann cell developmental and myelin markers that are not present in neural crest cells. See also Figure S1.
(D) Purified Schwann cells were infected with M. leprae (ML), fixed at day 7 and immunolabeled with anti-p75 (red) and anti-PGL-1 (iML in green) antibodies, counterstained with DAPI (blue). Insets: BrdU uptake (green; top) and antibody to phospho-histone H3 (ser28) (green; bottom) double labeled with PGL-1 antibody (red). Scale bars represent 50μm.
(E–G) Expression of nuclear Sox10 in control (E) and infected (F) Schwann cells at day 10 post-infection; antibodies to Sox10 (red) and PGL-1 (iML; green), counterstained with DAPI (blue) (F). Shown in (F) are selected representative Deltavision images from infected cultures with high (arrows) and low bacterial load (arrowheads). (G) Quantification of nuclear Sox10 in control and infected Schwann cells with iML per cell. Scale bars represent 10μm.
(H and I) Genearray analyses at day 14 and 27 post-infection. Schwann cell lineage/differentiation-associated genes (H) and development and EMT-associated transcription factor (TF) genes (I) are shown as fold change in expression. See also Figure S2 and Table S1.
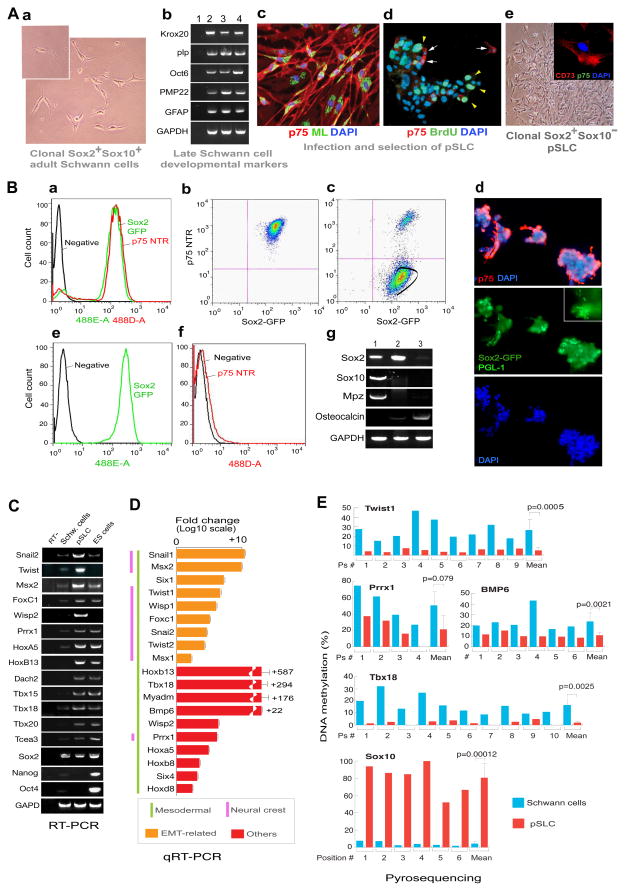
Figure 2. Induction of mesenchymal transition in Clonal Schwann cells by M. leprae generates progenitor/stem-like cells (pSLC).
(A) Phase image of single cell (inset)-derived Schwann cells from wild-type mice (a). (b) RT-PCR of p75+ FACS sorted control Schwann cells from wild type mice (lane 2), clonal Schwann cells from wild type mice (lane 3) and clonal Schwann cells from Sox2-GFP mice (lane 4); Lane 1, negative control (water only). (c) Infected clonal wild type Schwann cells at day 7 (PGL-1+ML, green; p75+ Schwann cells, red). (d) BrdU uptake (green arrowheads) of day 27-infected Schwann cells maintained in mesenchymal stem cell media (Mscm); anti-p75 antibody in red (arrows) and nuclei (DAPI) in blue. (e) Phase image of p75− FACS sorted cells that express CD73 (e-inset). Magnification 20×; Inset (e): 40×.
(B) FACS analysis of GFP+(Sox2+)/p75+ Schwann cells isolated from adult Sox2-GFP mice (a). Clonal GFP+/p75+ cells derived from FACS sorted Schwann cells (b) were infected with ML for 4 weeks (also see Figure S1-M), transferred to Mscm and Sox2+/p75− population was gated for FACS sorting (c). IF analysis of aggregated infected cells maintained in Mscm before FACS sorting; p75+ (red) and DAPI (blue). Inset shows PGL-1+ ML as green rods. (e, f) FACS analysis of sorted cells showing GFP+/Sox2+ (e) and p75− (f) cells, which are referred as pSLC. (g) RT-PCR of control Schwann cells (lane 1), pSLC (lane 2) and pSLC in bone differentiation media (lane 3). Magnification: d: 10×.
(C) RT-PCR of embryonic/developmental marker genes in pSLC as compared to control Schwann cells and mouse ES cells. See also Figure S3.
(D) Quantitative PCR (qPCR) for differential expression of selected embryonic/developmental marker genes in pSLC relative to control Schwann cells. Data are mean +/− SEM from three samples.
(E) Cell fate change of Schwann cells to pSLC accompanies change in DNA methylation statuses of promoter regions of key developmental/EMT and Schwann cell-lineage genes as analysed by pyrosequencing.
As expected, Schwann cells are highly susceptible to ML infection with rapid bacterial engulfment with >90% efficiency (Figures 1D, 2A-c, S1). We performed extensive genome-wide transcriptome profiling using Affymetrix mouse genechips and subsequent RT-PCR and qPCR analyses. Strikingly, infected Schwann cells compared to uninfected/control Schwann cells, which were maintained under identical experimental conditions, showed upregulation of numerous genes of embryonic development, transcription, chromatin remodeling, cell signaling, chemokines and cell division-cycle/DNA replication (Figures 1I, S2). The latter was consistent with a moderate increase of S-phase cells in infected cultures; despite the presence of numerous intracellular ML (iML) infected cells showed BrdU uptake and phospho-Histone H3 (S28) positive nuclei, which represent the S- and mitotic-phases of the cell cycle respectively (Figure 1D-insets). Strikingly, ML infection was followed by the removal or export of Sox10 from Schwann cell nuclei (Figures 1E–G). Whereas infected Schwann cells that carry fewer iML maintained Sox10 exclusively in nuclei similar to uninfected/control cells, high bacterial load invariably caused the removal of nuclear Sox10, which might affect the regulation of its key target Schwann cell genes including myelin gene Mpz (Figures 1E–G; Finzsch et al, 2010; Jessen and Mirsky, 2005; Weider et al., 2012). Indeed, down regulation of myelin genes was found in infected Schwann cells over time (Figures 1H). Sox10 is a master regulator of Schwann cell homeostasis, identity and myelin maintenance as well as differentiation, with nuclear Sox10 being required to recruit chromatin remodeling complexes (Finzsch et al, 2010; Weider et al., 2012). Therefore, bacterial-induced removal of Sox10 from nuclei is likely to perturb normal Sox10-mediated transcriptional events. Together, these findings set the stage for a potential reprogramming or change in Schwann cell fate in response to iML.
Mesenchymal Transition and Induction of EMT-like Program in Adult Schwann Cells
The fate of iML-induced effects on Schwann cells were examined by gene expression analyses over 4 week period. Strikingly, only infected cells gradually ‘turn off’ Schwann cell differentiation/myelination- and lineage-associated genes and ‘turn on’ numerous developmental genes, comprising mostly the mesoderm development including homeodomain/Hox, EMT and neural crest related genes (Figures 1H, I S2); at day 27 post infection, most of the Schwann cell lineage and differentiation/myelination genes, but not negative regulators of myelination like Sox2 and c-Jun (Mirsky and Jessen, 2005), were down regulated (Figures 1H, S2), suggesting that iML gradually shut down the Schwann cell differentiation program. Figure 1I shows the selected key developmental regulated transcription factor (DRTF) genes (with known functions; Table S1).
A major tissue remodeling program that is central to the early mesoderm development during embryogenesis is EMT (Polyak and Weinberg, 2009). Interestingly, master regulators of EMT, Twist-1, -2, Snail2 and Msx2, that are capable of inducing EMT in epithelial cells (Mani et al., 2008) (Table S1), were among the highly upregulated transcription factor (TF) genes in ML infected Schwann cells (Figures. 1I, S2). The observed cell fate changes in Schwann cells are complex as infection involves upregulation of multiple developmental and functional genes (Figures 1H, I, S2; Table 1) and that these events are not associated with the tumor suppressor genes like p53 or Schwann cell tumor associated gene NF1 (Figures S2-A, S3-A).
Our findings also suggest that reprogramming requires high number of intracellular bacteria (>50 iML/cell with >90% infection efficiency) and a long-term incubation; we could not find any significant change in gene expression or the removal of nuclear Sox10 at much lower rate of infection (<10 iML/cell; Figures 1E–G). Shortage or lack of such optimum conditions appear to weaken or fail to induce transcriptional changes. For example, the observed changes in gene expression are highly significant with live ML, as compared to irradiated ML; bacterial components like PGL-1 did not show reprogramming events, despite their capacity to induce rapid demyelination upon their extracellular binding to myelinated Schwann cells (Rambukkana et al., 2002). This appears to be due to high invasion capacity of live ML and thus the long-term exposure of Schwann cells to high number of iML. In addition, incubation of Schwann cells with other mycobacteria like Mycobacterium smegmatis did not lead to reprogramming (data not shown).
Bacterially Reprogrammed Schwann Cells Exhibit Properties Similar to Progenitor/Stem-like Cells (pSLC)
To isolate reprogrammed cells from the infected cell population, we transferred clonal infected Schwann cells to mesenchymal stem cell medium (Mscm), which is selective for mesenchymal stem cells (MSC) from bone marrow cells (Li et al., 2009). In Mscm, only infected clonal Schwann cells showed a high proliferation index as demonstrated by BrdU uptake (Figure 2A-d); >90% of these BrdU+ cells were negative for Schwann cell lineage markers p75 and Sox10 as well as other mature phenotypes (Figure 2A). We used this property to select p75− population by FACS sorting. Analyses of these p75− cells by microarrays, RT-PCR, qPCR, immunofluorescence and FACS revealed the loss of all Schwann cell lineage/myelin specific markers and acquisition of various mesodermal and neural crest markers (Figures 2C, D, S3-A, -B). Thus, we concluded that iML converted Sox2+/p75+/Sox10+ Schwann cells to a Sox2+/p75−/Sox10− reprogrammed cells with loss of Schwann cell markers (Figures 2B, S3-A), which we referred as progenitor/stem-like cells (pSLC) because of their stem cell characteristics (see below). Further characterization revealed the expression of CD73, CD44, Sca-1 and Cd29 MSC markers, but the absence of hematopoietic markers CD45, CD34 and c-kit (Figures S3-H). pSLC also acquired highly migratory properties as evident by their migration through connective tissues when administered into injured muscles (Figures S3-F, G).
Reprogramming Removes Schwann cell Lineage/Myelin Regulator Sox10 and Maintains Stem Cell Marker Sox2
Conversion of parent Schwann cells to pSLC led to the loss of Schwann cell master regulator Sox10, but maintained Sox2. To track the Sox2 expression during reprogramming, we generated clonal Schwann cells isolated from adult Sox2-GFP mice (Emilni et al., 2008). Clonal cells in Schwann cell media maintained both Sox10 and other mature Schwann cell markers whereas infected cells lost Sox10 and other Schwann cell markers and subsequently change into pSLC when maintained in Mscm (Figure 2A, B). Infected Schwann cells continued to maintain GFP (Sox2) expression but upon reprogramming, lost expression of p75, Sox10 and other mature markers (Figures 2B-a-d). When pSLC were maintained in Mscm, clusters of infected GFP+ cells with both p75- and p75+ were frequently observed (Figures 2B-d). When p75− reprogrammed cells in Mscm were isolated by FACS sorting they continued to express GFP (Sox2) despite the loss of lineage/mature Schwann cell markers (Figures 2B-c, g). However, GFP/Sox2 expression disappeared when pSLC were subjected to bone differentiation (Pittenger et al., 1999), which in contrast upregulated bone marker osteocalcin (Figures 2B-g, 3C), further underscoring stem cell characteristics of pSLC. Tracking the GFP expression from parent GFP+/Sox2+/p75+/Sox10+ Schwann cells to GFP+/Sox2+/p75−/Sox10− pSLC at clonal level we confirmed that pSLC are derived from Schwann cells, but not from any progenitor cell type within the Schwann cell preparation.
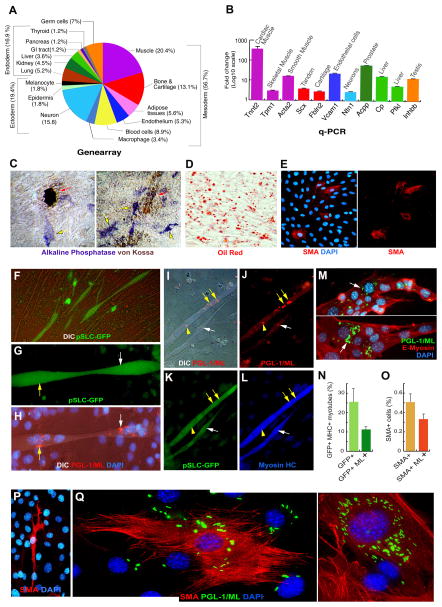
Figure 3. Broader Developmental Potential of Reprogrammed pSLC Promote Bacterial Spread to Mesenchymal Tissues by Direct Differentiation.
(A and B) Expression of tissue-specific gene clusters in pSLC as compared to control Schwann cells as analyzed by genearrays (A). qPCR of some of the selected tissue-specific genes in pSLC. Data are mean +/− SEM from two samples.
(C–F) Differentiation potential of pSLC into various mesenchymal tissues. (C) Bone differentiation as analyzed by von Kossa staining (mineral/calcium deposition in brown; red arrows) and the expression of alkaline phosphatase activity (in blue; yellow arrows) (see also Figure 2B-g). (D) Adipocyte differentiation media produced mature adipocyte-like cells as detected by Oil red staining. (E) pSLC spontaneously differentiate into SMA+ smooth muscle-like cells in culture. (F) Co-culture of GFP+ pSLC with C2C12 myoblasts contributed to GFP+ myotube formation; shown is a live fluorescence/DIC image. Magnifications: 20×.
(G–N) pSLC passively transfer infection to myotubes following differentiation. Infected GFP+ pSLC (green) transfer ML to GFP+ myotubes upon differentiation under the influence of C2C12 myoblasts. Multinucleated GFP+ myotubes (G, H) show the acquisition of ML (white and yellow arrows) as detected by anti-PGL-1 antibody (red) counterstained with DAPI and visualized under fluorescence/DIC (H). These ML+ GFP+ myotubers were also positive for myosin HC (I–L). Antibody to E-myosin detected early stage of multinucleated myotubes that carry numerous iML (M). Quantification of GFP+ myotubes with and without ML (N). Magnifications: G, H, M: 40×; I–L: 20×.
(O–Q) ML infected pSLC spontaneously differentiate into SMA+ smooth muscle-like cells as detected by antibodies to PGL-1 (green) and SMA (red) counterstained with DAPI (blue) (P, Q).
(O) Quantification of SMA+ cells with and without ML. Magnification: P: 40×; Q: 60×.
Reprogramming Schwann cells to pSLC accompanies epigenetic changes
We next examined if this change in cell fate is epigenetically regulated. We analyzed DNA methylation at 5 methylcytosine in cytosine guanine dinucleotide (CpG) in the promoter regions of selected genes that are expressed and repressed in pSLC as compared to parent Schwann cells. Methylation status was assessed by bisulfite pyrosequencing, which allows quantitative determination of the extent of methylation of each cytosine in a given DNA sequence (supplementary data). We found that most CpG sequences in promoter regions of mesodermal/EMT genes, Twist1, Prrx1, Tbx18, and Bmp6, are significantly less methylated in pSLC as compared to Schwann cells (Figure 2E;), suggesting that the epigenetic status of these genes were reprogrammed from a transcriptionally repressed state to an active state in pSLC. In contrast, the extent of methylation of the CpGs in Sox10 is significantly higher in pSLC and was strongly demethylated in Schwann cells. This correlates directly with loss of Sox10 expression in pSLC (Figures 2B-g, S3-A). These findings suggest that reprogramming causes significant epigenetic changes in some of the key regulatory genes.
Differentiation Potential of Reprogrammed Schwann cells to Mesenchymal Tissues
We assessed the ability of pSLC to differentiate into different tissue types in Mscm by analyzing gene arrays (Figure 3A). Of the total tissue-specific genes, 56% of the transcripts accounted for mesoderm-related targets. qPCR of selected such genes confirmed these findings (Figure 3B).
Consistent with these findings, pSLC differentiated into typical mesenchymal tissues under various well-established differentiation protocols. Under bone differentiation conditions, pSLC showed increased alkaline phosphatase activity and mineral deposition (Figures 3C, 2A-g); in adipocyte differentiation media, pSLC produced Oil Red O dye-positive lipid droplets that are characteristic of mature adipocytes (Figure 3D; Pittenger et al., 1999). Based on the muscle specific-gene expression patterns (Figures 3A, B, S3), we also tested the potential of pSLC to differentiate into muscle lineage. Whereas pSLC spontaneously differentiated into SMA+ smooth muscle-like cells (Figure 3E), co-culturing pSLC with C2C12 myoblasts (Shi et al., 2004) contributed to myotube formation (Figure 3F). However, ML-induced reprogramming does not cause Schwann cells to acquire tumor formation capacity, as pSLC did not form teratomas or any kind of tumor formation in SCID mice (data not shown).
Reprogrammed Schwann cells with Stem Cell-like Properties Promote Dissemination of Infection to Skeletal Muscles and Smooth Muscles by Direct Differentiation
We hypothesized that pLSC promote the transmission of ML into mesenchymal tissues by direct differentiation. As skeletal and smooth muscle tissues are known to harbor ML in leprosy patients, we tested this hypothesis directly in vitro and in vivo using mouse models. (Gupta et al., 1975; Kaur et al., 1981; Pearson et al., 1970; Werneck et al., 1999).
Bacterial dissemination by direct differentiation in vitro models
To determine if ML spread to myotubes in a skeletal muscle-like microenvironment in vitro we co-cultured infected pSLC with C2C12 myoblasts, (Shi et al., 2004). For these experiments we re-infected pSLC with ML in order to maintain bacterial load. Also, to track the contribution of pSLC, we introduced Lentivirus based-CopGFP reporter gene into clonal pSLC derived from wild-type Schwann cells. Stably expressed GFP+ cells were isolated by FACS sorting. When infected GFP+pSLC were co-cultured with C2C12 myoblasts pSLC fused with myoblasts and contributed to GFP+ myotube formation (Figure 3F, G). This process accompanied the passive transmission of iML from GFP+pSLC to GFP+myotubes (Figures 3G, H). Immunolabeling with Antibodies to PGL-1 (ML) and myosin heavy chain (Myosin HC) confirmed the transmitted iML in GFP+ multinucleated myotubes (Figures 3G, H) that are also positive for myosin HC (Figures 3I–L). Antibody to E-myosin labeled the early stage of multinucleated myotubes with transmitted iML, and GFP+ myotubes harboring iML clearly showed a passive bacterial transmission from reprogrammed pSLC following differentiation (Figures 3M; 3N).
We next tested the spread of ML to smooth muscle-like cells from infected pSLC. When pSLC were maintained in Mscm for several passages ~0.5% of pSLC spontaneously differentiated into SMA+ smooth muscle-like cells (Figure 3P). This propensity of pSLC allowed direct ML transfer from infected SMA−pSLC to SMA+ cells with clear actin filament network which can easily be distinguished from undifferentiated pSLC (Figures 3P, Q).
Bacterial Dissemination by Direct Differentiation In Vivo
To further validate the in vitro findings, we used a widely used cardiotoxin-induced skeletal muscle injury model, wherein damage muscle fibers are replaced spontaneously by regenerating muscles in the presence of anti-inflammatory microenvironment (Charge and Rudnicki, 2004; Tidball and Villalta, 2010). We used nude mice, since they lack T cells and produce relatively less complex inflammatory microenvironment comprising predominantly macrophages. Our rationale was that pSLC, which express muscle development and differentiation genes similar to muscle progenitors (Figures 3, S3), would contribute to myotube formation under the physiological conditions of injury-induced regenerating microenvironment (Charge and Rudnicki, 2004). Such conditions somewhat mimic the ML infection associated inflammation in muscles, and thus commensurate with the muscle pathology documented in lepromatous form of leprosy patients with high bacterial load (Gupta et al., 1975; Werneck et al., 1999).
We first injected cardiotoxin into the tibialis anterior (AT) muscles of nude mice and the muscle injury, inflammation and the repair process were confirmed by histological studies and immunolabeling (data not shown). GFP+pSLC containing ML was then injected into pre-injured TA muscles and their distribution was examined at different time intervals. Antibody staining to adult myosin clearly showed the intimate interaction and early fusion process of ML-laden GFP+ cells around the muscle fibers at the injury site after day 10 (Figures 4A–D). The engrafted GFP+ pSLC were detected within muscle milieu at day 14 and 21 either as fused to resident regenerating fibers or fully integrated into smaller diameter myofibers that express adult myosin and are demarcated by laminin in the basal lamina (Figures. 4E–H). Anti-PGL-1 antibody staining that specifically identifies ML (Ng et al., 2000) confirmed the bacterial presence within fully integrated GFP+ myofibers (Figure 4I, J). Specific acid-fast mycobacterial staining further confirmed rod-shaped ML within myofibers (Figure 4K, L). In contrast to pSLC, injection of non-reprogrammed infected GFP+ Schwann cells (infection only for 2 days) to injured TA muscles failed to transmit infection to regenerating muscle fibers (data not shown).
Figure 4. Re-differentiation of reprogrammed pSLC contribute to passive bacterial transfer to skeletal muscles and smooth muscles in vivo.
(A–D) Transverse frozen sections of TA muscles injected with ML infected GFP+ pSLC after 10 days. Deltavision images showed GFP+ pSLC (green) either fused with myofibers (A, B) or migrating in between muscle fibers (C, D). Antibodies to adult myosin detected muscle fibers (red in A, B and blue in D) and laminin demarcated individual muscle fibers (red in C). Antibodies to PGL-1 detects ML (in red; arrow) associated closely with muscle fibers (D). Asterisks in all figures denote individual myofibers. Magnifications: 60×.
(E–J) Infected GFP+ pSLC were incorporated into regenerating muscles at 3-week post-injection. GFP+ myofibers are positive for adult myosin (E, F) and are demarcated by laminin (G, H). pSLC incorporation passively transmitted ML to myofibers as detected by PGL-1+ ML (red; arrows) in GFP+ myofibers (I, J). Magnifications (Deltavision images): 40×
(K and L) Bacterial presence within the skeletal muscle fibers (asterisks) was confirmed by acid-fast mycobacterial staining (red). Arrows show clumps of rod-shaped ML in several individual muscle fibers. Magnifications: K, L: 60×
(M–P) Administered infected GFP+ pSLC differentiate into SMA+ smooth muscles in dermal area. Antibody to SMA (red) co-localizes with differentiated GFP+pSLC (green; arrowheads); merged image is shown in (O). Magnifications: M–O: 40×. (P) Higher magnification (100×) showed the PGL+ML (red; arrows) within SMA+ smooth muscles (blue) near dermal vessels.
Similarly, we also found that administered infected GFP+ pSLC can differentiate into SMA+ smooth muscles (or myofibroblasts) and ML can be detected within SMA+ smooth muscles in the dermal area of muscle-skin interphase and associated blood vessels (Figures 4M–P). In contrast, infected GFP+ Schwann cells (non-reprogrammed cells infected for 2 days only) neither differentiated into smooth muscles nor contributed to transferring infection to SMA+ cells (data not shown). Together, these results suggest that under the influence of injury and inflammatory tissue microenvironment, infected pSLC could elaborate their migratory capacity and differentiate into myofibers or smooth muscles and passively transmit ML to these tissues that are favorable for sequestration of infection.
Reprogrammed pSLC Acquire Immunomodulatory Properties
We examined if reprogrammed pSLC also secrete immunomodulatory factors similar to mesenchymal stem cells (MSCs). To test this, we first analyzed proteins released into serum/supplement-free conditioned media (CM) from pSLC using mouse chemokine/cytokine protein arrays. Figure 5A shows the quantitative analysis of differentially expressed soluble proteins released by pSLC. Strikingly, pSLC produced a range of chemokines, cytokines, growth/survival factors, soluble adhesion receptors, and tissue remodeling factors to varying degrees (Figures 5A, S4). These findings also validate the MSC-like functional properties of pSLC because almost all chemokines/cytokines that are known to produce by MSC are also released by pSLC (Figure 5A) (Hoogduijn et al., 2010; Klopp et al., 2011). Although a fewer number of these proteins are also released by control/uninfected Schwann cells the fold increase of their expression is several fold higher in pSLC (Figures 5A, S4), suggesting a greater potential of pSLC to participate in multiple immunomodulatory functions. Interestingly, once reprogrammed, pSLC continued to secrete soluble factors regardless of bacterial presence, as complete removal of ML from pSLC (with no detectable ML genomic DNA) continued to produce almost same soluble proteins (Figures 5A-inset, S4).
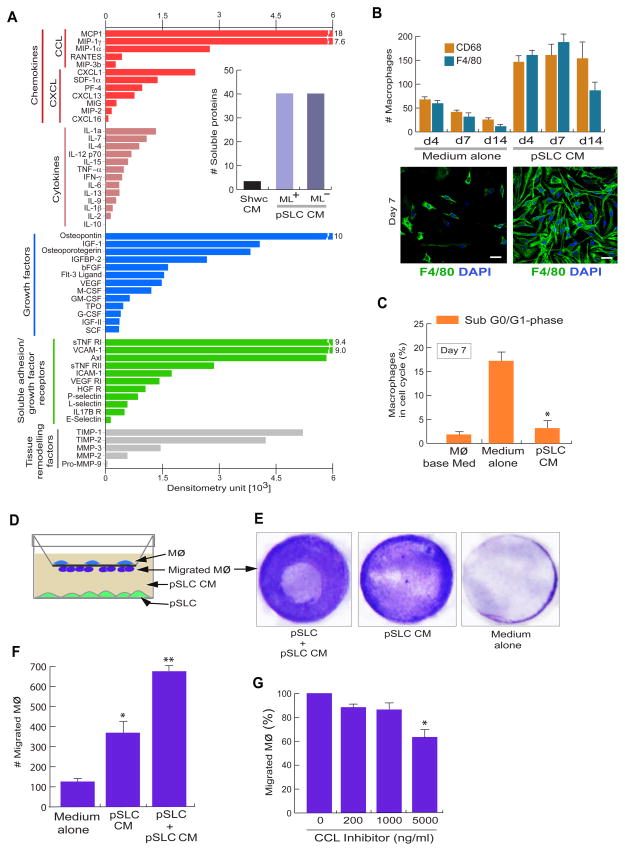
Figure 5. Reprogrammed pSLC secrete immune/growth factors that promote macrophage survival and migration.
(A) Proteinarrays of conditioned media from infected pSLC showing the secretion of indicated classes of soluble factors. Relative levels of released proteins detected by dot blot chemiluminescence and presented as mean values from two arrays. Inset shows the number of detectable soluble factors released from conditioned media from control Schwann cells (Schwc-CM) and pSLC (pSLC-CM) with and without iML (+/−ML). Also see figure S4.
(B) Soluble factors secreted by pSLC promote macrophage survival. Quantification of adherent F4/80+ and CD68+ macrophages maintained in pSLC-CM and media alone for up to 14 days (B, top panel). Immunofluorescence of adherent F4/80+ macrophages in the presence of pSLC-CM (right) and media alone (left) at day 7 (B, bottom panel). Magnifications: 20×.
(C) Macrophages in indicated culture conditions in the Sub G0/G1 cell cycle phase that corresponds to apoptotic cells, as analysed using Propidium Iodide. Data are presented as mean+/− SEM from three experiments; *: p<0.001.
(D–G) pSLC secretory factors promote macrophage migration. (D) Schematic for the macrophage transwell migration assay in response to conditioned media or media containing viable pSLC. (E) Crystal violet dye staining of migrated macrophages through the membrane under the influence of indicated conditions. (F) Quantification of migrated macrophages. (G) Effect of CC-chemokine inhibitor on macrophage migration in response to pSLC conditioned media. The data shown in F and G are presented as mean ± SEM from 3 experiments; *: p<0.01, **: p<0.001.
pSLC-Derived Soluble Factors Mediate Macrophage Survival and Migration
We next examined the functional effects of pSLC-derived immunomodulatory factors on tissue macrophages. As a source, we used freshly isolated mouse peritoneal macrophages which comprised >85% F4/80+ ~100% CD68+ macrophages. When incubated with pSLC-CM alone, both F4/80+ and CD68+ macrophages were maintained >14 days whereas the majority of macrophages in medium alone did not survive or lifted off early in cultures (Figure 5B). Macrophages maintained in pSLC-CM showed a firm attachment and elongated morphology with strong F4/80 expression, suggesting possible maturation of macrophages in pSLC-CM. pSLC-CM also influenced increased survival of macrophages with minimum apoptosis (Figure 5C). However, pSLC-CM did not increase S phase cells significantly (data not shown).
Consistent with the chemokine/cytokine production pSLC-CM attracted a significant number of macrophages when cell migration assay was performed using Trans-well system (Figures 5D–F). Migration capacity was further increased in the presence of live pSLC, implying that freshly secreted pSLC factors attracted higher number of macrophages (Figures 5E, F). Although pSLC-CM-induced macrophage migration was slightly decreased (<10%) in the presence of standard dose (200–1000ng) of broader CC-chemokine inhibitor, CCI (recombinant viral CCI-Fc chimera), which has shown to be effective in other studies (Buatois et al, 2010), high dose of non-toxic concentrations of CCI (5000ng) was required to produce a significant inhibition of migration (Figure 5G). Since pSLC produce an array of soluble proteins (Figures 5A, S4), our data suggest that collective effect of immune factors, but not CC-chemokine alone, are required to exert effective macrophage chemotactic ability.
Reprogrammed pSLC Promote Bacterial Dissemination via Macrophages in vivo
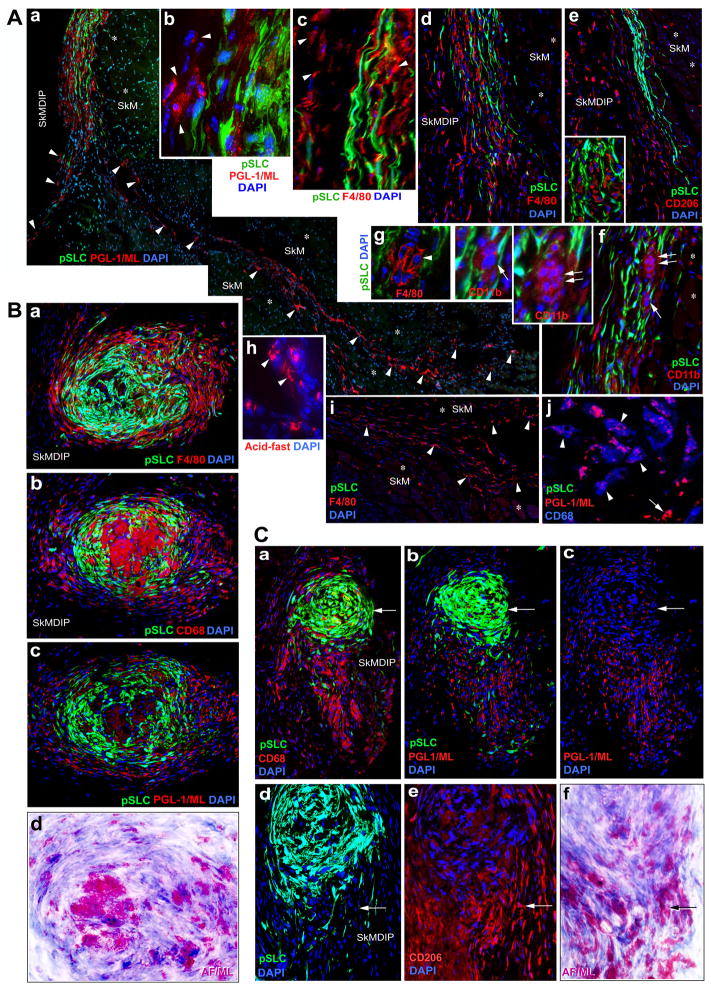
We reasoned that a nude mouse muscle injury model with a predominantly macrophage-containing inflammatory microenvironment would be useful to directly test whether pSLC promote bacterial dissemination via macrophages. To ensure that all injected pSLC express GFP and all bacteria are inside the cells, strongly positive GFP+ pSLC were FACS sorted and reinfected with ML 2 days before cell injection to the pre-injured TA muscles. Analyzing the fate of pSLC and iML at week 1, 2 and 3 post-injection revealed that almost all GFP+ cells migrated further away from the site of injection after one week and continue to spread overtime along the interstitial connective tissues in the perimysium and to the skeletal muscle-dermal inter phase (SkMDIP) at week 3 post-injection (Figures 6A, S5, S6). Strikingly, iML were no longer confined to pSLC as most of iML were detected within recipient non-GFP cells (Figure 6A-a, b, h, j). These data indicate that iML were efficiently transferred from donor GFP+pSLC to GFP− recipient tissue cells in vivo. Both anti-PGL-1 antibody and acid-fast mycobacterial staining confirmed these findings (Figures 6A-j; S5). These ML+ GFP− cells were identified as endogenous macrophages as >90% of them were positive for macrophage markers F4/80, CD11b, CD68, and CD206 (Figures 6A-c-j; S5).
Figure 6. Reprogrammed pSLC Promote Bacterial Dissemination via Macrophages and Granuloma Formation in vivo.
(A) pSLC intimately associated with infiltrating macrophages and transfer infection effectively to recipient tissue cells in TA muscles. ML infected GFP-pSLC were administered into pre-injured TA muscles of athymic nude mice and transverse sections at day 14 and 21 post-injection were labeled with antibodies to PGL1 (ML in red; a, b) and various macrophage markers, F4/80 (c, g, i), CD206 (e), CD11b (f), CD68 (j). In (j), ML (red) were co-localize with CD68 (blue; arrow heads). Endogenous non-GFP tissue cells that have taken up ML are shown in arrowheads (a) and are predominantly comprised of infiltrated macrophages of M1 and M2 phenotypes (e, i, j). ML localization is confirmed by acid-fast staining (red) in parallel sections (h). Insets in e, f, and in g (arrows and arrowheads) show the formation of early smaller macrophage granuloma-like structures. SkM: skeletal muscles (asterisks); SkMDIP: skeletal muscle-dermal interphase. Magnifications: a, d, e, f and I: 10×; b, c, g, h: 40×.
(B) pSLC contribute to the formation of typical macrophage granuloma-like structures (GLS) in SkMDIP. Migrated pSLC formed organized GLS with M2 and M1 macrophages that show distinct distribution and morphological features, as analyzed by antibodies to F4/80 (a) or CD206 (see also Figure S7)) and CD68 (b) respectively. The distribution of ML (red) within GLS was localized in parallel sections with antibody to PGL-1 (c), and by Fite’s staining (in purple) which detects mycobacteria in infected tissues (d; also see Figure S7). Note the strongly F4/80+ cells in the periphery and fused and multinucleated CD68+ cells in the core of GLS. Magnifications are 20×. (C) Disintegration and release of bacterial-laden macrophages from granulomas. Sections distant from the granulomas (as in B) within the same tissues show disintegration of both CD68 (a) and CD206 (e) macrophages from tapered GFP+pSLC aggregates (a-c), and the emigrating macrophages carrying large number of ML as detected by anti-PGL-1 (red; b, c) and Fite’s staining (f). Arrows in the top and bottom panels show the pSLC with much fewer iML and high content of acid-fast+ ML in migrating macrophages respectively. Magnifications: a-c: 10×; d-f: 40×. See also Figure S5, S6 and S7.
Inflammatory conditions in cardiotoxin-induced TA muscles are known to bring a sequential appearance of macrophage subpopulations, initially with CD68+ M1 subtype peaks at week 2 followed by high CD163/CD206 M2 population that overlaps within M1 population at week 2–3 post-injury (Tidball and Villalta, 2010). These established conditions allowed us to correlate GFP+pSLC distribution with infiltrated macrophage subtypes in injured muscles in a timely manner. Indeed, our findings with serial tissue sections from infected GFP+pSLC engrafted mice at week 3 post-infection showed that CD68+, CD163+ CD206+ or F4/80+ cells in the interstitial tissues were co-distributed with GFP+pSLC. Double immunolabeling with Aan antibody to PGL-1 and macrophage markers confirmed iML within these macrophages (Figures 6A–j: S5-K, L). Once ML transferred from pSLC to macrophages, they appeared to transmit infection to uninfected macrophages and thus facilitate ML spread via these tissue macrophages along the connective tissues in between muscle fibers and SkMDIP. Figures 6A-a and S5, S6 clearly show examples illustrating the presence of iML within numerous non-GFP recipient cells along the perimysium/connective tissues and SkMDIP. Analysis of serial tissue sections strongly suggestive of ML transfer from pSLC to both M1 and M2 macrophages (Figures 6, S5).
pSLC Contribute to Granuloma-like Formation in vivo: Role in Bacterial Dissemination
Strikingly, infected pSLC that have migrated to the loosely associated connective tissue areas within skeletal muscle-dermal inter phase (SkMDIP) formed organized aggregates together with macrophages (Figures 6B, S6). These organized aggregates resemble typical granulomas seen in mycobacterial infections, both in murine models and humans (Flynn and Chen, 2001; Bold and Ernst, 2009; Modlin, and Rea, 1988). Analyzing the cellular distribution of these granuloma-like structures (GLS) revealed that pSLC contributed to a major cellular mass highly organized into a spiral form of GFP+ cells and interposed between infiltrated macrophages in both core area and the periphery of GLS (Figure 6B). Interestingly, a distinct distribution of macrophage subpopulation was observed within these GLS: the exterior of the GLS are strongly positive for F4/80 (F4/80+high) and CD206 populations whereas the core areas were weakly positive for F4/80 (F4/80+low) but strongly positive for CD68+ cells with tightly packed multinucleated macrophages (Figures 6B-a, S8). This phenotypic distribution of macrophages with F4/80+high/CD206+high and F4/80+low/CD68+high corresponds to M1 and M2 subtypes respectively (Figures 6B, S7, S8) (Mosser and Edwards, 2008). Anti-PGL-1 and Fite’s staining (modified acid-fast mycobacterial staining) in serial sections clearly showed that macrophages in both core and periphery of GLS harbor large number of iML (Figures 6B, S6, S7). In contrast, we were unable to detect such GLS when infected GFP+Schwann cells were administered under similar experimental conditions.
Release of ML-laden Macrophages from the granulomas
We found a significant shift in distribution of macrophages when analyzed across granuloma-containing tissues within SkMDIP. Figures 6B and 6C illustrate the distribution of pSLC and macrophages and their bacterial contents within the GLS in serial sections taken from 250–300μm apart tissue areas. In one end of the GLS bearing tissues, CD68+ cells with high number of iML were organized into the core area surrounded by GFP+pSLC and bacterial laden F4/80+high/CD206+high macrophages in the exterior whereas in the other end, both CD68+ and CD206+ macrophages were found migrating out of the tapered aggregates of pSLC carrying iML with them (Figures 6B, 6C, S6). These data collectively suggest not only the transfer of ML from pSLC to macrophages but also the migration of these bacterial-laden macrophages from the organized GLS.
In vitro Granuloma-like formation by pSLC and Macrophages
Next we developed an in vitro model of GLS. We found that pSLC tend to form aggregates at high density and no leaked bacteria were found outside the aggregates (Figure 7A). Real-time microscopy revealed that uninfected macrophages migrated into infected GFP+pSLC aggregates when peritoneal macrophages were added (Figure 7B). Most of these macrophages reached the core area of the GLS as early as 6h and 18h and acquired ML from pSLC; bacterial transfer from some infected pSLC to macrophages was also observed adjacent to the GLS (Figures 7B). Some macrophages were also found to contain GFP+pSLC cell debris with the bacteria in them, suggesting the phagocytosis of dead or apoptotic GFP+pSLC by macrophages (Figures 7B,). However, only ~0.04% TUNEL positive cells were detected in these cell aggregates. Overtime, an increasing number of macrophages continued to migrate and form larger GLS and at this point most of the ML were transferred to macrophages (Figures 7C ). These organized GLS then gradually started to disintegrate and subsequently release ML-laden macrophages (Figures 7D). Quantification of bacterial transfer from pSLC to macrophages showed an effective transmission of infection to macrophages overtime (Figures 7E). These findings suggest that chemoattractants released from pSLC aggregates may have provided the right signals for macrophage migration and GLS formation. Further studies on dissection of regulatory signaling pathways involved may establish new links between host cell reprogramming and innate phase immune responses during infection.
Figure 7. In vitro Granuloma-like Formation with pSLC recapitulates Bacterial Spread via Macrophages.
(A) Infected GFP+pSLC form aggregates in culture (top panel) and ML confined strictly within the aggregates (bottom left) and form bacterial clusters (red; bottom insets), as detected by antibody to PGL-1 (red; bottom left), counterstained with DAPI (blue).
(B–E) Macrophages were added to infected pSLC aggregates and incubated for 6h, 18h (B), 72h (C) and 96h (D) and labeled with antibodies to PGL-1 and macrophage markers, F4/80, CD11b. Shown are confocal images after 3-D reconstructions (B); arrows (top panel) indicate phagocytosed ML infected pSLC (green; ML in red) by macrophages (blue) within GLS. At 18h, high number of ML (green) was found in macrophages (blue) that penetrated into GLS (B, bottom left panels). Occasional TUNEL+ cells (red; 0.04%) were also found in these GLS (nuclei are shown in yellow). Bacterial transfer from GFP+pSLC to F4/80+ macrophages (red) was also seen outside GLS (B, bottom right). (C) At 72h, more macrophages (blue) were incorporated into GLS and ML were taken up by these macrophages (arrows); note that less PGL+ML in pSLC (left and middle panels) and departure of some macrophages carrying ML with them; yellow arrows depict macrophages carrying both pSLC debries and ML (right panels). (D) Disintegration of GLS and the release of ML-laden macrophages (arrows). (E) Correlation coefficients of bacterial transfer from pSLC to macrophages overtime based on bacterial presence or absence (+/−ML) in pSCL (green) and in macrophages (blue). Scale Bars, (A) 5μm; (B–D) 20μm.
(F) The proposed model: Schwann cells in the adult peripheral nervous system (PNS) infected with ML undergo a reprogramming process that convert Schwann cells to pSLC that promote bacterial dissemination. BNB: blood nerve barrier.
DISCUSSION
In this study, we provide evidence that leprosy bacteria perturb dynamic mechanisms that normally preserve the lineage commitment of adult Schwann cells, and hence change the Schwann cell fate to pSLC with mesenchymal characteristics that promote bacterial dissemination. The findings describe an unexpected but natural exploitation of adult Schwann cell plasticity by ML during infection. Once invaded, ML gradually ‘turn off’ Schwann cell lineage/differentiation-associated genes/TFs and ‘turn on’ numerous embryonic/developmental genes and transcription factors of mesoderm and neural crest. Such alterations in gene expression are likely to disrupt the stoichiometry of transcriptional regulators, leading to reprogramming of infected cell nuclei.
Of particular interest is the induction of transcription factors of the homeodomain/Hox family and EMT in Schwann cells in response to ML. It is known that the fate of somatic cells can be altered by forced expressions of both Hox and EMT genes (Schneuwly et al., 1987; Mani et al., 2009). ML-infected cells upregulated several Hox and key EMT-associated transcription factors including the master regulators of EMT, Twist and Snail (Polak and Weinberg, 2009; Mani et al., 2009). Demethylation of the promoter region of Twist1 in reprogrammed cells further suggests the change in cell fate accompanies the change in epigenetic status. Reprogramming that follows the silencing of Sox10, the master regulator of Schwann cell lineage and differentiation/myelination (Finzsch et al, 2010; Jessen and Mirsky, 2005), directly correlate with significant DNA methylation of Sox10 in pSLC. On the other hand, no change in DNA methylation in the Sox2 promoter region (data not shown) suggests the continuous expression of Sox2 in both parent Schwann cells and pSLC. Thus, the silencing Sox10 and maintaining or induction of Sox2 may be critical for Schwann cell reprogramming during infection.
In contrast to Sox2+/Sox10− pSLC, nerve injury-induced de-differentiated Schwann cells maintain Sox2+/Sox10+ and other Schwann cell markers (Le et al., 2005). Safeguarding the Schwann cell lineage markers, particularly Sox10 is critical for an effective differentiation towards myelination during the peripheral nerve regeneration process (Le et al., 2005; Finzsch et al, 2010). This phenotypic difference together with the acquisition of numerous mesoderm developmental genes distinguishes pSLC from nerve injury-induced de-differentiated Schwann cells. On the other hand, our previous studies have shown that the initial interaction of extracellular ML with myelinated Schwann cells induced demyelination, which in turn generated similar de-differentiated cells (Rambukkana et al., 2002; Tapinos et al., 2006). ML appear to use this demyelination strategy to generate de-differentiated Schwann cells as they are highly susceptible to invasion and favorable for bacterial colonization (Rambukkana, 2010). In the present study, we mimicked such conditions by directly isolating de-differentiated Schwann cells from adult mouse peripheral nerves and showed that once invaded, iML reprogrammed these Schwann cells to pSLC. It remains to be determined which mechanisms or signaling is involved in this bacterial-driven cell reprogramming.
The important role of Erk1/2 MAPK signaling in inducing demyelination without immune responses or lesions was first demonstrated using leprosy bacteria as a model (Tapinos et al., 2006). This finding was recently confirmed by using inducible-Raf-kinase transgenic mice in which Erk1/2 activation in myelinated Schwann cells was shown to induce demyelination and inflammatory responses (Napoli et el., 2012). Interestingly, the latter activates p75 whereas reprogramming Schwann cells to pSLC results in the loss of p75 (Figure 2). Also, unlike Erk1/2-induced demyelination where transiently appearing inflammatory cells promote peripheral nerve regeneration, pSLC acquired sustainable secretion of numerous macrophage chemoattractants even after complete removal of ML. This capacity may facilitate continuous attraction of macrophages to pSLC in many tissues during early dissemination process. Although ML use Erk1/2 signaling for Schwann cell manipulation (Tapinos and Rambukkana, 2005; Tapinos et al., 2006), Erk1/2 alone does not appear to contribute to reprogramming as the pharmacological inhibition of Erk1/2 did not abrogate the reprogramming events (data not shown). This further suggests that the activation of Erk1/2 alone does not cause Schwann cell reprogramming.
Although the lineage through which infected Schwann cells are converted to pSLC is not known, this change may have important benefits for a bacterium like ML that depends totally on host cell functions for survival (Cole et al., 2001). Conversion of Schwann cells to pSLC with mesenchymal characteristics sets the stage for ML to use the reprogrammed cells as a vehicle to spread the infection to distal tissues such as skeletal and smooth muscle. We propose two major mechanisms by which ML may use reprogrammed cells to promote bacterial dissemination (Figure 7H). First, ML take advantage of mesenchymal stem cell-like properties of pSLC to migrate and spontaneously differentiate into skeletal and smooth muscles under inflammatory conditions, and thus passively transmit infection to these tissues. Secondly, using immunomodulatory properties of pSLC ML may use reprogrammed cells to create a secondary niche by recruiting macrophages for further bacterial expansion and dissemination.
Importantly, our findings in vivo showed that pSLC contribute to macrophage granuloma formation, one of the pathologic hallmarks of mycobacterial infections in murine models and in patients with both leprosy and tuberculosis (Modlin and Rea, 1988; Flynn and Chen, 2001). Although mycobacterial granulomas are considered to be essential for containment of infection, recent studies in zebrafish have suggested that macrophage granulomas may also promote mycobacterial dissemination during early infection (Davis and Ramakrishnan, 2009). However, unlike other pathogenic mycobacteria, ML use adult Schwann cells as primary non-immune tissue cells for initial colonization (Stoner, 1979). Once colonized, ML take full advantage of Schwann cell plasticity to convert infected cells to pSLC with the capacity to produce chemoattractants and trophic factors, which in turn promote macrophage recruitment, bacterial transfer and survival of infected macrophages (Figures 5, 6, 7H). Interestingly, some of the immune factors/chemokines released from pSLC are also known to foster granuloma formation (Qiu et al., 2001; Chie et al., 2004). Collectively, these events may facilitate pSLC to recruit macrophages and contribute to form granuloma-like structures. Our in vivo and in vitro analyses provide further evidence that ML-laden M1 and M2 macrophages in the granulomas contributed to spread the infection. Recent work showing that the viruses used for reprogramming skin fibroblasts to pluripotent stem cells (Takahashi and Yamanaka, 2006) can promote the efficiency of nuclear reprogramming via activation of innate immune responses (Lee et al., 2012) conceptually support our study. Finally, our findings may have far-reaching implications not only for host-pathogen interactions but also for understanding the basic biology of adult tissue cell plasticity, reprogramming and tissue regeneration.
EXPERIMENTAL PROCEDURES
Primary adult Schwann cell cultures
Detailed experimental protocols for isolation and characterization of mouse primary Schwann cells are described in detail in Supplemental Data.
Infection with M. leprae
Schwann cells were infected with ML according to our previous protocol (Ng et al., 2000; Suppl data). In vivo grown ML derived from nude mouse footpads were prepared as described previously (Truman and Krahenbuhl, 2001; Lahiri et al., 2010), and both viable and non-viable ML were provided by the Laboratory Research Branch of NHDP, Baton Rouge, LA
Mice
These studies employed 4–6 weeks of age young adult CD-1 (ICR) (strain code: 022) mice (Charles-River Laboratory), GFP-mice that constitutively express eGFP [strain: C57BL/6-Tg (ACTB-EGFP) 1Osb/J, stock: 003291; Jackson lab (Bar Harbor, ME)], 6–8 weeks of age CD-1/nude mice (Charles-River), and 4–6 weeks of age NOD-SCID mice (Jackson Lab). Sox-2/GFP mice (Emilni et al., 2008) were kindly provided by R. Jaenisch (Whitehead Institute, MIT, MA). Animals were maintained at the Rockefeller University and University of Edinburgh animal facilities according to institutionally approved protocols.
Flow Cytometry, Immunolabeling, Antibodies and Microscopy
Flow cytometry, primary Schwann cell sorting, generation of clonal cells, immunolabeling with, primary antibodies, imaging (Confocal, Deltavision and Time-laps live cell imaging) are described in Supplementary Data. Antibodies to Sox10, M. leprae PGL-1 and Oct6 were gift from M. Wagner, A. Kolk and D. Meijer respectively.
Schwann cell infection with M. leprae and Reprogramming Protocol
FACS sorted p75+ Schwann cells or clonal Schwann cells generated from wild type mice or Sox2-GFP mice were infected with M. leprae as described (Ng et al., 2000; Tapinos and Rambukkana, 2005). For the isolation of reprogrammed cells, infected cells were transferred to mesenchymal stem cell media that allowed the selection of progenitor/stem-like cell population based on the cell fate change from p75+/Sox10+ cells to p75−/ Sox10−. These cells were FACS sorted using p75 antibody and subjected to detailed characterization (Supplementary Data). pSLC were also transduced with CopGFP-CDH-MSCV-cG reporter vector (System Biosciences, CA) to obtain stable CopGFP expressing pSLC (referred to as GFP+pSLC).
Gene and Protein Expression Analyses
Microarray, proteinarray and q-PCR analyses details are described in the Supplemental Data
pSLC differentiation to mesenchymal tissues
We employed established protocols for bone and adipocyte differentiation from mesenchymal stem cells (Pittenger et al., 1999). Myogenic Differentiation using C2C12 myoblasts was performed as described (Shi et al., 2004). Detailed descriptions are available in the Supplementary Data.
In vivo GFP+ pSLC Transplantation to Skeletal Muscles and Dissemination of infections
CD-1/nude mice (6–8 weeks, Charles-River) were pre-injured with cardiotoxin-1 24h prior to the administration of ML infected GFP+ pSLC or GFP+ Schwann cells. Each indicated experiment was performed using 5–6 mice per experimental group by repeating at least 3–4 times. Experimental details are in the in Supplementary Data.
Macrophage-pSLC Co-Cultures, Migration Assays and in vitro Granuloma Formation
Detailed descriptions of experimental procedures of macrophage isolation and co-cultures and in vitro granuloma formation are described in Supplementary Data.
Supplementary Material
Article Highlights.
Leprosy bacteria reprogram adult Schwann cells by altering host gene expression
Bacterially reprogrammed cells resemble progenitor/stem-like cells (pSLC) of mesenchymal trait
pSLC promote bacterial spread to mesenchymal tissues by re-differentiation
pSLC secrete immune factors, transfer bacteria to macrophages to form granulomas
Acknowledgments
We thank Ian Wilmut and Emil Gotschlich for critical reading of the manuscript. We are grateful to Emil Gotschlich, Paul Nurse, Michael Young, Thomas Sakmar (Rockefeller University), John Savill, Jonathan Sackl, Nick Hastie (University of Edinburgh), and James Krahenbuhl (National Hansen’s Disease Programs; NHDP) for their support during the initial and final phases of this study. Our thanks to Richard Truman (NHDP) for providing Fite’s staining of infected mouse tissue sections; members of the core facilities at the Rockefeller University and the University of Edinburgh for their invaluable assistance; Joe Dybaas, Jennifer Smith, Paola Basilico and Maxmilien Grandclaudon (Rambukkana laboratory) for their participation, and members of Genome Exploration and EpigenDx for technical assistance and data analysis. Mycobacterium leprae were provided by The Laboratory Research Branch of NHDP, Baton Rouge, LA with funding from The American Leprosy Missions and the Hospitaler Order of St. Lazarus of Jerusalem. This work was funded in part by grants from NINDS, NIAID, The Order of MALTALEP Foundation, The Rockefeller University and the University of Edinburgh.
Footnotes
Supplemental Data include extended Supplemental Experimental Procedures and References, 7 figures with figure legends and one table with references, which can be found with this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFRENCES
- Bold TD, Ernst JD. Who benefits from granulomas, mycobacteria or host? Cell. 2009;136:17–19. doi: 10.1016/j.cell.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buatois V, Fagète S, Magistrelli G, Chatel L, Fischer N, Kosco-Vilbois MH, Ferlin WG. Pan-CC chemokine neutralization restricts splenocyte egress and reduces inflammation in a model of arthritis. J Immunol. 2010;185:2544–54. doi: 10.4049/jimmunol.1000182. [DOI] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Chiu BC, Freeman CM, Stolberg VR, Hu JS, Komuniecki E, Chensue SW. The innate pulmonary granuloma: characterization and demonstration of dendritic cell recruitment and function. Am J Pathol. 2004;164:1021–1030. doi: 10.1016/S0002-9440(10)63189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honore N, Garnier T, Churcher C, Harris D, et al. Massive gene decay in the leprosy bacillus. Nature. 2001;409:1007–1011. doi: 10.1038/35059006. [DOI] [PubMed] [Google Scholar]
- Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eminli S, Utikal J, Arnold K, Jaenisch R, Hochedlinger K. Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression. Stem Cells. 2008;26:2467–2474. doi: 10.1634/stemcells.2008-0317. [DOI] [PubMed] [Google Scholar]
- Falkow S. Bacterial entry into eukaryotic cells. Cell. 1991;65:1099–1102. doi: 10.1016/0092-8674(91)90003-h. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- Finzsch M, Schreiner S, Kichko T, Reeh P, Tamm ER, Bosl MR, Meijer D, Wegner M. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J Cell Biol. 2010;189:701–712. doi: 10.1083/jcb.200912142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, Watt FM. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–589. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta JC, Jesupadam T, Gupta MC, Gupta DK. A histopathologic study of striated muscle biopsies in leprosy. Int J Lepr Other Mycobact Dis. 1975;43:348–355. [PubMed] [Google Scholar]
- Gurdon JB. Adult frogs derived from the nuclei of single somatic cells. Dev Biol. 1962;4:256–273. doi: 10.1016/0012-1606(62)90043-x. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Melton DA. Nuclear reprogramming in cells. Science. 2008;322:1811–1815. doi: 10.1126/science.1160810. [DOI] [PubMed] [Google Scholar]
- Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- Hoogduijn MJ, Popp F, Verbeek R, Masoodi M, Nicolaou A, Baan C, Dahlke MH. The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int Immunopharmacol. 2010;10:1496–1500. doi: 10.1016/j.intimp.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Job CK. Nerve damage in leprosy. Int J Lepr Other Mycobact Dis. 1989;57:532–539. [PubMed] [Google Scholar]
- Kaur S, Malik AK, Kumar B. Pathologic changes in striated muscles in leprosy. Lepr India. 1981;53:52–56. [PubMed] [Google Scholar]
- Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F., 3rd Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri R, Randhawa B, Krahenbuhl JL. Infection of mouse macrophages with viable Mycobacterium leprae does not induce apoptosis. J Infect Dis. 2010;201:1736–1742. doi: 10.1086/652499. [DOI] [PubMed] [Google Scholar]
- Le N, Nagarajan Rakesh, Wang James YT, Araki Toshiyuki, Schmidt Robert E, Jeffrey Milbrandt. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci U S A. 2006;102:2596–2601. doi: 10.1073/pnas.0407836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski ES, Pera RR, Yakubov E, Cooke JP. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012;151:547–58. doi: 10.1016/j.cell.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen S, Yuan J, Yang Y, Li J, Ma J, Wu X, Freund M, Pollok K, Hanenberg H, et al. Mesenchymal stem/progenitor cells promote the reconstitution of exogenous hematopoietic stem cells in Fancg−/− mice in vivo. Blood. 2009;113:2342–2351. doi: 10.1182/blood-2008-07-168138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miko TL, Le Maitre C, Kinfu Y. Damage and regeneration of peripheral nerves in advanced treated leprosy. Lancet. 1993;342:521–525. doi: 10.1016/0140-6736(93)91647-5. [DOI] [PubMed] [Google Scholar]
- Modlin RL, Rea TH. Immunopathology of leprosy granulomas. Springer Semin Immunopathol. 1988;10:359–374. doi: 10.1007/BF02053846. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, et al. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Ng V, Zanazzi G, Timpl R, Talts JF, Salzer JL, Brennan PJ, Rambukkana A. Role of the cell wall phenolic glycolipid-1 in the peripheral nerve predilection of Mycobacterium leprae. Cell. 2000;103:511–524. doi: 10.1016/s0092-8674(00)00142-2. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Rees RJ, Weddell AG. Mycobacterium leprae in the striated muscle of patients with leprosy. Lepr Rev. 1970;41:155–166. doi: 10.5935/0305-7518.19700023. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- Qiu B, Frait KA, Reich F, Komuniecki E, Chensue SW. Chemokine expression dynamics in mycobacterial (type-1) and schistosomal (type-2) antigen-elicited pulmonary granuloma formation. Am J Pathol. 2001;158:1503–1515. doi: 10.1016/S0002-9440(10)64101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambukkana A. Usage of signaling in neurodegeneration and regeneration of peripheral nerves by leprosy bacteria. Prog Neurobiol. 2010;91:102–107. doi: 10.1016/j.pneurobio.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Rambukkana A, Salzer JL, Yurchenco PD, Tuomanen EI. Neural targeting of Mycobacterium leprae mediated by the G domain of the laminin-alpha2 chain. Cell. 1997;88:811–821. doi: 10.1016/s0092-8674(00)81927-3. [DOI] [PubMed] [Google Scholar]
- Rambukkana A, Yamada H, Zanazzi G, Mathus T, Salzer JL, Yurchenco PD, Campbell KP, Fischetti VA. Role of alpha-dystroglycan as a Schwann cell receptor for Mycobacterium leprae. Science. 1998;282:2076–2079. doi: 10.1126/science.282.5396.2076. [DOI] [PubMed] [Google Scholar]
- Rambukkana A, Zanazzi G, Tapinos N, Salzer JL. Contact-dependent demyelination by Mycobacterium leprae in the absence of immune cells. Science. 2002;296:927–931. doi: 10.1126/science.1067631. [DOI] [PubMed] [Google Scholar]
- Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338–381. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty VP, Antia NH, Jacobs JM. The pathology of early leprous neuropathy. J Neurol Sci. 1988;88:115–131. doi: 10.1016/0022-510x(88)90210-9. [DOI] [PubMed] [Google Scholar]
- Shi D, Reinecke H, Murry CE, Torok-Storb B. Myogenic fusion of human bone marrow stromal cells, but not hematopoietic cells. Blood. 2004;104:290–294. doi: 10.1182/blood-2003-03-0688. [DOI] [PubMed] [Google Scholar]
- Stoner GL. Importance of the neural predilection of Mycobacterium leprae in leprosy. Lancet. 1979;2:994–996. doi: 10.1016/s0140-6736(79)92564-9. [DOI] [PubMed] [Google Scholar]
- Tapinos N, Ohnishi M, Rambukkana A. ErbB2 receptor tyrosine kinase signaling mediates early demyelination induced by leprosy bacilli. Nat Med. 2006;12:961–966. doi: 10.1038/nm1433. [DOI] [PubMed] [Google Scholar]
- Tapinos N, Rambukkana A. Insights into regulation of human Schwann cell proliferation by Erk1/2 via a MEK-independent and p56Lck-dependent pathway from leprosy bacilli. Proc Natl Acad Sci U S A. 2005;102:9188–9193. doi: 10.1073/pnas.0501196102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theise ND, Wilmut I. Cell plasticity: flexible arrangement. Nature. 2003;425:21. doi: 10.1038/425021a. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1173–1187. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman RW, Krahenbuhl JL. Viable M. leprae as a research reagent. Int J Lepr Other Mycobact Dis. 2001;69:1–12. [PubMed] [Google Scholar]
- Weider M, Küspert M, Bischof M, Vogl MR, Hornig J, Loy K, Kosian T, Müller J, Hillgärtner S, Tamm ER, Metzger D, Wegner M. Chromatin-remodeliing factor brg1 is required for Schwann cell differentiation and myelination. Dev Cell. 2012;23:193–201. doi: 10.1016/j.devcel.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Werneck LC, Teive HA, Scola RH. Muscle involvement in leprosy. Study of the anterior tibial muscle in 40 patients. Arq Neuropsiquiatr. 1999;57:723–734. doi: 10.1590/s0004-282x1999000500001. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.