Abstract
Background and Purpose
Tricyclic antidepressants are used clinically as first-line treatments for neuropathic pain. Opioid receptors participate in this pain-relieving action, and preclinical studies in receptor-deficient mice have highlighted a critical role for δ-, but not μ-opioid receptors. In this study, we investigated whether κ-opioid (KOP) receptors have a role in the antiallodynic action of tricyclic antidepressants.
Experimental Approach
We used a model of neuropathic pain induced by unilateral sciatic nerve cuffing. In this model, the mechanical allodynia was evaluated using von Frey filaments. Experiments were conducted in C57BL/6J mice, and in KOP receptor-deficient mice and their wild-type littermates. The tricyclic antidepressant nortriptyline (5 mg·kg−1) was delivered twice a day for over 2 weeks. Agonists and antagonists of opioid receptors were used to test the selectivity of the KOP receptor antagonist norbinaltorphimine (nor-BNI) in mice with neuropathic pain.
Key Results
After 12 days of treatment, nortriptyline relieved neuropathic allodynia in both wild-type and KOP receptor-deficient mice. Surprisingly, acute nor-BNI reversed the effect of nortriptyline in both wild-type and KOP receptor-deficient mice. Further experiments showed that nor-BNI action was selective for KOP receptors at a late time-point after its administration (8 h), but not at an early time-point, when it may also interact with δ-opioid (DOP) receptors.
Conclusions and Implications
KOP receptors are not necessary for the effect of a tricyclic antidepressant against neuropathic allodynia. These findings together with previous data indicate that the DOP receptor is the only opioid receptor that is necessary for the antiallodynic action of antidepressants.
Tables of Links
| TARGETS | |
|---|---|
| Opioid receptors | κ receptor (KOP receptor) |
| δ receptor (DOP receptor) | μ receptor (MOP receptor) |
| LIGANDS | |
|---|---|
| Dynorphin | Norbinaltorphimine |
| Morphine | Nortriptyline |
| Naloxone | SNC80 |
| Naltrexone | U-50,488H |
| Naltrindole |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Neuropathic pain is defined as a consequence of a lesion or a disease affecting the somatosensory system (Jensen et al., 2011). It is generally a chronic condition resistant to classical analgesic drugs (Attal et al., 2010). The recommended pharmacotherapy for neuropathic pain includes the use of monoamine re-uptake inhibitors, such as tricyclic antidepressant (TCA) drugs or 5-HT and noradrenaline re-uptake inhibitors (Dworkin et al., 2007; Attal et al., 2010). Preclinically, studies on TCA action in neuropathic pain models highlighted the involvement of opioid receptors (Valverde et al., 1994; Gray et al., 1998; Marchand et al., 2003; Anjaneyulu and Chopra, 2006; Mico et al., 2006; Nozaki and Kamei, 2006; Benbouzid et al., 2008a,b,; Bohren et al., 2010; Wattiez et al., 2011). However, these pharmacological studies showed some discrepancies concerning the roles of the different opioid receptors.
Some studies have shown a preferential involvement of the δ-opioid (DOP) receptor in the antinociceptive effect of antidepressants (Gray et al., 1998; Schreiber et al., 1999; Benbouzid et al., 2008a), while others have demonstrated the involvement of both μ-opioid (MOP) and DOP receptors (Schreiber et al., 2000; Marchand et al., 2003; Nozaki and Kamei, 2006). The conflicting results observed in the literature may be related to differences between the neuropathic pain models that were used or the symptoms that were studied, but also to the limited in vivo selectivity of opioid receptor antagonists. Selectivity problems may be solved in part by using genetic approaches with different opioid receptor knockout mice. Indeed, in a murine model of neuropathic pain that is sensitive to long-term, but not acute antidepressant treatment (Benbouzid et al., 2008a,b,; Yalcin et al., 2009; 2014,), we recently showed that the antiallodynic effect of chronic nortriptyline treatment was lost in DOP receptor-deficient mice (Benbouzid et al., 2008b). Conversely, this effect was maintained in MOP receptor-deficient mice (Bohren et al., 2010), demonstrating the critical role of DOP, but not of MOP receptors, in the antiallodynic action of a TCA. However, the role of κ-opioid (KOP) receptors in the treatment of neuropathic pain remains unclear.
The endogenous κ-opioid system is involved in a variety of physiological processes including analgesia, addiction, antipruritic activity, diuresis, feeding, respiratory and cardiovascular functions (Butelman et al., 2012; Feng et al., 2012). KOP receptors are widely expressed throughout the brain, spinal cord and dorsal root ganglia (Minami and Satoh, 1995; Sim and Childers, 1997), and they can display antinociceptive activity in a variety of animal pain models, especially visceral pain (Simonin et al., 1998; Riviere, 2004). Moreover, mice lacking prodynorphin display increased tail-flick responses (Wang et al., 2001), while an up-regulation of dynorphin, an endogenous KOP receptor ligand, occurs in the dorsal horn of the spinal cord following persistent inflammatory pain (Parra et al., 2002). This suggests that KOP endogenous pathway modulates pain responses. In neuropathic pain models, intraplantar injection of a KOP receptor agonist produces a significant antinociceptive effect, reversed by the co-administration of the KOP receptor antagonist norbinaltorphimine (nor-BNI) (Keita et al., 1995). Partial sciatic nerve ligation induces a sustained release of endogenous prodynorphin-derived opioid peptides and the increased KOP receptor activation in the spinal dorsal horn produces antinociceptive effects (Xu et al., 2004). Moreover, KOP receptor-deficient mice have enhanced thermal hyperalgesic responses, similar to nor–BNI-treated mice following sciatic nerve ligation (Xu et al., 2004). While the κ-opioid system can be altered in neuropathic conditions, its involvement in the therapeutic effect of antidepressant drugs has yet to be elucidated. A pharmacological study in neuropathic mice treated with the TCA nortriptyline showed that nor-BNI could acutely reverse the antiallodynic action of the chronic TCA treatment (Benbouzid et al., 2008a). However, this effect was only present with 5 mg·kg−1, but not 2 mg·kg−1, of nor-BNI and has not been confirmed using KOP receptor-deficient mice.
In the present study, we use genetic and pharmacological approaches to determine whether the KOP receptor participates to the action of nortriptyline in neuropathic pain. Our data provide evidence that KOP receptors are not necessary for the antiallodynic action of nortriptyline. We also showed that the KOP receptor antagonist nor-BNI displays a time-dependent selectivity. In particular, nor-BNI still acts in KOP receptor knockout mice at early time-points after s.c. administration, demonstrating a lack of acute selectivity.
Methods
The nomenclature for drugs and their molecular targets conforms to the British Journal of Pharmacology Guide to Receptors and Channels (Alexander et al., 2013).
Animals
Mice lacking KOP receptors were generated as described previously (Simonin et al., 1998). These mice were under a C57BL/6J background for over 10 generations. Heterozygous mice (KOP+/−) were bred in our animal facilities, genotyped upon weaning, and the experiments were conducted in adult male KOP+/+ and KOP−/− littermate mice weighing 25–30 g. The pharmacological experiments of Figure 6 used adult male C57BL/6J mice provided by the onsite breeding facilities of the Chronobiotron UMS3415, and were between 8 and 12 weeks-old at the time of surgery.
Figure 6.
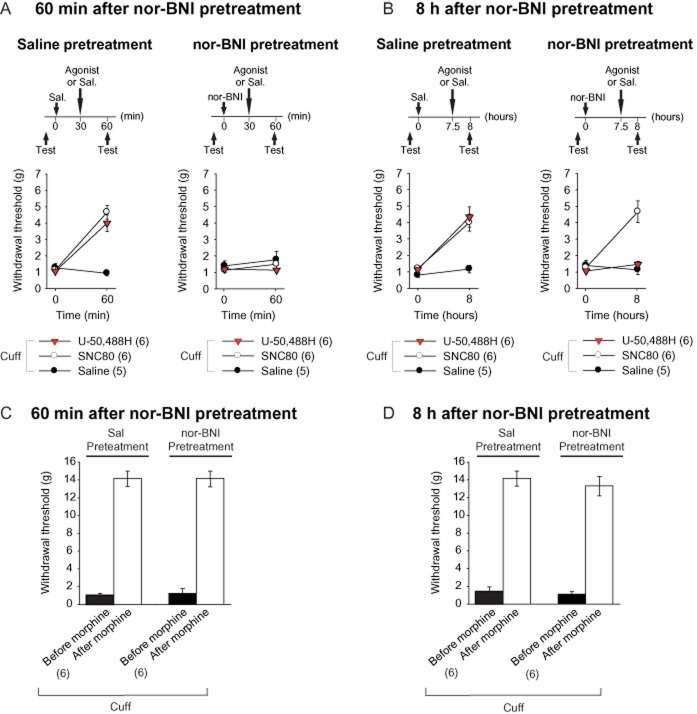
The KOP receptor antagonist nor-BNI has a time-dependent selectivity for opioid receptors. (A) Neuropathic animals (cuff) were pretreated with saline (s.c.) or nor-BNI (5 mg·kg−1, s.c.). The mice then received an acute administration of a DOP receptor agonist (SNC80, 10 mg·kg−1, s.c.), a KOP receptor agonist (U-50,488H, 5 mg·kg−1, s.c.) or of saline 30 min after the nor-BNI injection, and the mechanical threshold was tested before and 60 min after the pretreatment. Nor-BNI prevented the analgesic effect of both agonists. (B) The same procedure was used, except that the DOP and KOP receptor agonists were administered 7 h 30 min after nor-BNI pretreatment, and the hindpaw mechanical threshold was tested before and 8 h after pretreatment. Nor-BNI prevented the antiallodynic effect of the KOP receptor agonist, but not of the DOP receptor agonist. (C, D) The effect of nor-BNI on morphine-induced analgesia (10 mg·kg−1, s.c.) was also evaluated in neuropathic animals (cuff). Nor-BNI did not affect the morphine-induced analgesia, whether morphine was administered 30 min (C) or 7 h 30 (D) after nor-BNI. Data are expressed as mean ± SEM, n (number of animals) are given in parentheses.
Mice were group-housed three to five per cage, maintained under a 12 h light/dark cycle and allowed access to water and food ad libitum. The animal facilities are legally registered for animal experimentation under the Animal House Agreement C67-482-1. All procedures were performed in accordance with the guidelines for animal experimentation of the International Association for the Study of Pain and the European Community Council Directive 86/609. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010), and a total of 192 animals were used in the experiments described here.
Neuropathic pain model
Neuropathic pain was induced by inserting a cuff around the main branch of the right sciatic nerve (Yalcin et al., 2014). Surgical procedures were carried out under ketamine/xylazine anaesthesia (ketamine: 17 mg·mL−1, xylazine: 2.5 mg·mL−1, i.p., 4 mL·kg−1; Centravet, Taden, France). The common branch of the right sciatic nerve was exposed and a 2 mm section of split PE-20 polyethylene tubing (Harvard Apparatus, Les Ulis, France) was placed around it (cuff group). The shaved skin was closed using sutures. Sham-operated mice underwent the same surgical procedure described above but did not have a cuff inserted (sham group).
Nociceptive test
The mechanical threshold of hindpaw withdrawal was determined using von Frey filaments and the results were expressed in g (Bohren et al., 2010; Barrot, 2012; Yalcin et al., 2014). Mice were placed in clear Plexiglas® boxes (7 × 9 × 7 cm; SEPIB, Strasbourg, France) on an elevated mesh screen. Calibrated von Frey filaments (Bioseb, Chaville, France) were applied to the plantar surface of each hindpaw in a series of ascending forces. Each filament was applied until it bent slightly. The g value of the lower filament that gave a positive response, that is, that induced at least three paw responses out of five trials, was considered as the paw withdrawal threshold for this animal. The effect of long-term antidepressant treatment was evaluated at given time-points (at least twice per week), before the morning antidepressant injection (Figure 1). The rationale for this protocol was that a therapeutic treatment must stay effective over time: an animal treated on the previous evening should not be allodynic again on the following morning (Benbouzid et al., 2008a). The effect of acute drug injections was evaluated before (pre-test) and at different time-points (post-tests or time course) following the injection of the drug under consideration.
Figure 1.
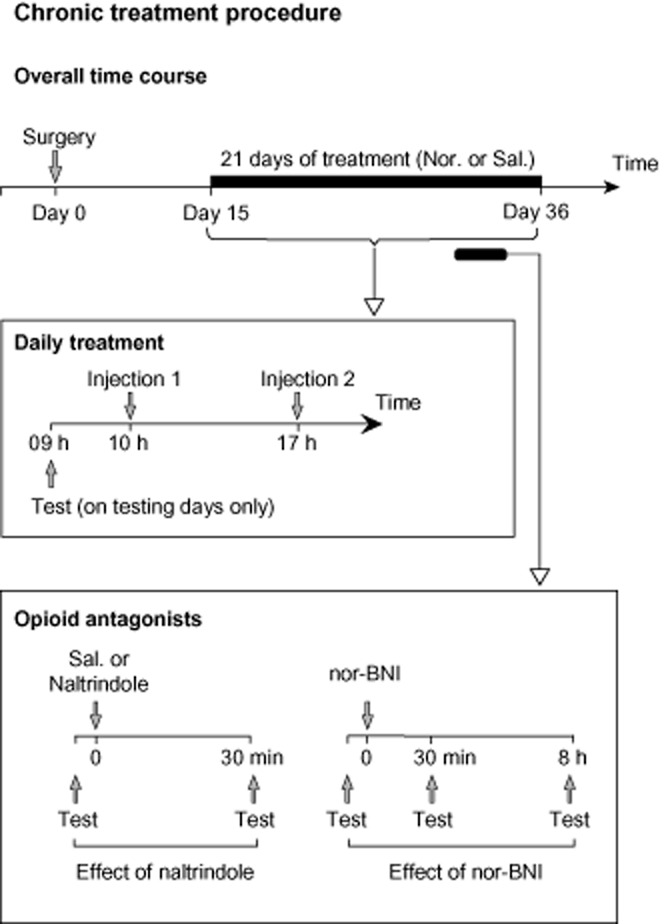
Timeline of the long-term nortriptyline treatment procedures. Nortriptyline treatment began at day 15 post-surgery, with mice receiving two daily injections, morning and afternoon. For the time-course experiment (Figure 3), animals were tested for mechanical allodynia at least twice a week. The mechanical threshold was tested before a morning injection. For the opioid antagonist experiments, after at least 2 weeks of treatment and while the daily saline and nortriptyline treatments were still maintained, the acute effect of the DOP receptor antagonist naltrindole (Figure 4) and of the KOP receptor antagonist nor-BNI (Figure 5) were tested.
Treatments
The nortriptyline (5 mg·kg−1) treatment began 15 days after the surgery, and lasted at least 20 consecutive days without interruption (Figure 1) (Benbouzid et al., 2008a). Both nortriptyline and NaCl solutions were administered i.p. twice a day (morning and evening) in a volume of 5 mL·kg−1 (Figure 1). This dose and the treatment regimen were chosen based on a previous dose–response study (Benbouzid et al., 2008a) in which nortriptyline at this dose displayed an antiallodynic action after chronic treatment, but no acute analgesic action. The s.c. injection of the DOP receptor antagonist naltrindole (5 mg·kg−1), of its control (NaCl 0.9%), or of the KOP receptor antagonist nor-BNI (5 mg·kg−1) was done after at least 2 weeks of nortriptyline treatment (Figure 1).
Pharmacological profile of nor-BNI
The s.c. injection of either morphine, the KOP receptor agonist U-50,488H or the DOP receptor agonist SNC80 was done in the neuropathic condition only. Beforehand, a pretreatment was done with nor-BNI or its control (NaCl 0.9%). In order to test the selectivity of nor-BNI in the early phase after its injection, morphine (10 mg·kg−1), SNC80 (10 mg·kg−1) and U-50,488H (5 mg·kg−1) or saline (NaCl 0.9%) were injected 30 min later. The mice were tested before and 60 min after the pretreatment (experimental design in Figure 6A), that is 30 min after the agonist administration. In order to test the selectivity of nor-BNI in the late phase after its injection, morphine, SNC80 and U-50,488H were injected 7 h 30 min after the pretreatment. In this condition, the mice were tested before and 8 h after the pretreatment (experimental design in Figure 6B), that is 30 min after the agonist administration. The 30 min test delay after the agonist administration was chosen based on the known analgesia time course of these compounds (Sounvoravong et al., 2004; Nozaki et al., 2012). Independent sets of mice were used for each condition.
Statistical analysis
Data are expressed as mean ± SEM. Statistical analyses were performed using multifactor anova. The surgical procedure (sham or cuff) and the treatments (saline vs. drug injections) were taken as between-group factors. When needed, the time of nociceptive testing (either time course or preinjection vs. postinjection data) was taken as a within-subject factor. The Duncan test was used for post hoc comparisons. The significance level was set at P < 0.05.
Chemicals
The following drugs were used: nortriptyline hydrochloride, nor-BNI dihydrochloride, the DOP receptor antagonist naltrindole hydrochloride, and the DOP receptor agonist 4-[(R)-[(2S,5R)-2,5-dimethyl-4-prop-2-enylpiperazin-1-yl]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide (SNC80) were obtained from Sigma-Aldrich (St Quentin Fallavier, France), and the KOP receptor agonist trans-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]-benzeneacetamide (U-50,488H) was obtained from Tocris Biosciences (Bristol, UK). Morphine sulphate was kindly supplied by Francopia (Paris, France). All the drugs were dissolved in 0.9% physiological saline solution (NaCl) that was also used for control injections.
Results
Mechanical sensitivity
KOP−/− mice had the same baseline values for mechanical sensitivity as their wild-type littermates KOP+/+ (Figure 2A and 2B). The sham surgery did not affect the long-term paw withdrawal threshold, although a transitory drop in mechanical sensitivity was observed after the surgical procedure (Figure 2B). Conversely, cuff-implanted mice showed long-lasting ipsilateral mechanical allodynia, which was present in KOP+/+ and in KOP−/− mice (Surgery × Time interaction; KOP+/+ F6,138 = 2.4, P < 0.05; KOP−/− F6,132 = 2.4, P < 0.05; post hoc: cuff < sham in each genotype at P < 0.0001 on post-surgery days 1–15) (Figure 2B). Mechanical allodynia was unaffected by the presence or absence of the KOP receptor (genotype effect; F6,270 = 1.0, P > 0.40).
Figure 2.
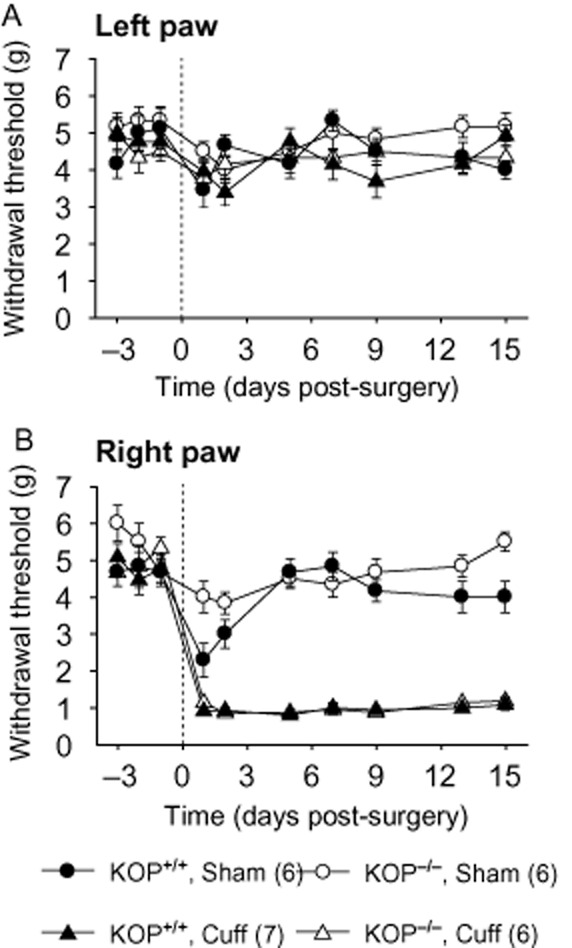
Long-lasting mechanical allodynia after sciatic nerve injury in KOP+/+ and KOP−/− mice. Unilateral cuffing of the main branch of the sciatic nerve induced long-lasting mechanical allodynia, as tested using von Frey filaments. (A) Insertion of the cuff did not affect the mechanical threshold of the contralateral paw (left paw). (B) The cuff induced an ipsilateral (right paw) mechanical allodynia in both KOP+/+ and KOP−/− mice. Data are expressed as mean ± SEM, n (number of animals) are given in parentheses.
Antiallodynic effect of the antidepressant drug nortriptyline
Two weeks after the surgery, we started the treatment with either nortriptyline (5 mg·kg−1) or the control saline solution (NaCl 0.9%). The mice received two injections per day and were tested in the morning before drug injection. Previous data showed that this treatment has no acute analgesic effect whereas it relieves neuropathic allodynia after 10–12 days of treatment (Benbouzid et al., 2008a; Bohren et al., 2010). Similarly, the nortriptyline treatment alleviated the cuff-induced allodynia in KOP+/+ mice after 13 days of treatment [Surgery × Treatment × Time interaction; F9,189 = 6.71, P < 0.0001; post hoc: (CuffNor = Sham) > CuffSal at P < 0.01 on post-surgery days 28–35] (Figure 3A). The same antiallodynic effect was also present in KOP−/− mice (F9,180 = 3.7, P < 0.0001; post hoc: (CuffNor = Sham) > CuffSal at P < 0.01 on post-surgery days 26–35] (Figure 3B). In both cases, nortriptyline reversed the cuff-induced allodynia without affecting the mechanical threshold of the mice in the sham group. Thus KOP receptors did not appear to be necessary for the antiallodynic action of nortriptyline.
Figure 3.
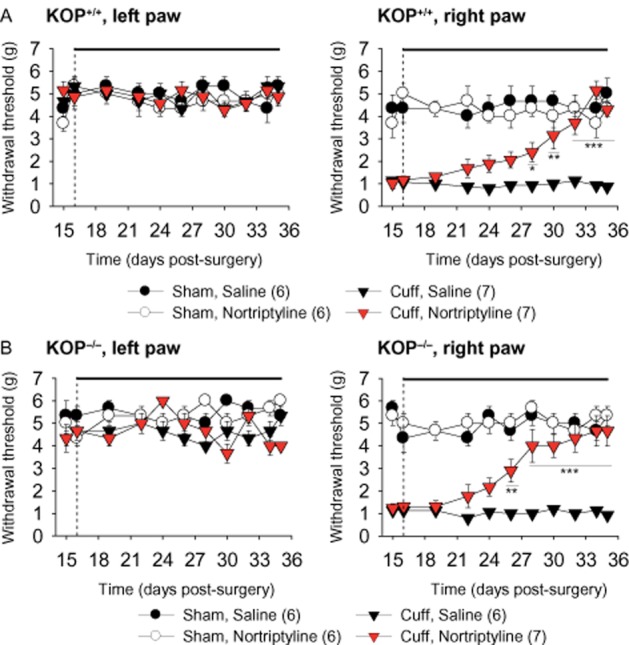
A chronic antidepressant treatment relieves neuropathic allodynia in KOP+/+ and KOP−/− mice. Nortriptyline treatment (5 mg·kg−1, i.p. injection twice a day) or its saline control (NaCl 0.9%) began on post-surgery day 16 and was maintained for at least 20 days (the black line above the graph indicates the treatment period). The mechanical threshold was measured before the morning drug injection to test the effect of chronic treatment. In KOP+/+ (A) and KOP−/− mice (B), the antidepressant treatment did not affect the mechanical threshold of the contralateral paw (left paw), but it reversed the neuropathic allodynia on the ipsilateral paw (right paw). Data are expressed as mean ± SEM, n (number of animals) are given in parentheses. *P < 0.05, **P < 0.01, ***P < 0.001 cuff treated versus cuff saline group.
DOP receptor antagonist effect
Previous data highlighted a critical role of DOP receptors in the antiallodynic action of nortriptyline (Benbouzid et al., 2008a,b,; Choucair-Jaafar et al., 2014). We thus tested the effects of an acute injection of the DOP receptor antagonist naltrindole (5 mg·kg−1) in the KOP+/+ and KOP−/− mice. After at least 2 weeks of treatment with nortriptyline, the injection of naltrindole acutely blocked the antiallodynic effect of nortriptyline (KOP+/+ F1,20 = 10.6, P < 0.01; KOP−/− F1,20 = 9.8, P < 0.01) (Figure 4A and 4B). The injection of naltrindole induced a relapse of allodynia within 30 min after its administration, and this effect was present in both KOP−/− and KOP+/+ mice. We also observed that naltrindole did not induce any change in the mechanical sensitivity of mice with sham surgery or of neuropathic mice treated with saline (Figure 4A and 4B).
Figure 4.
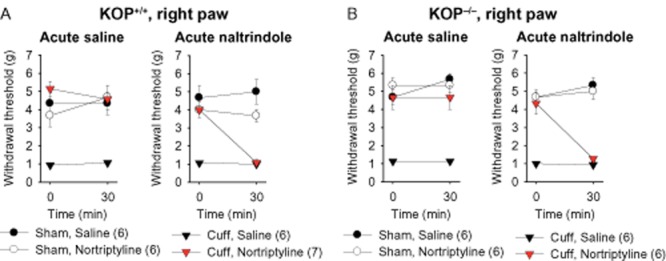
Acute DOP receptor antagonist injection suppressed the antiallodynic effect of nortryptiline treatment in KOP+/+ and KOP−/− mice. After at least 2 weeks of antidepressant or saline treatment, the animals received an injection of the DOP receptor antagonist naltrindole (5 mg·kg−1, s.c.) or its control saline solution. The mechanical threshold was measured before and 30 min after injection. (A) While acute saline injection did not change the paw withdrawal threshold, naltrindole induced a relapse of mechanical allodynia in neuropathic KOP+/+ mice treated with nortriptyline. No effect of naltrindole or saline was seen in sham mice or in saline-treated neuropathic animals. (B) Similar results were obtained in KOP−/− mice. Data are expressed as mean ± SEM, n (number of animals) are given in parentheses.
KOP receptor antagonist effect
A previous study showed that an acute administration of 5 mg·kg−1 of the KOP receptor antagonist nor-BNI, but not 2 mg·kg−1, attenuated the antidepressant-induced antiallodynic action (Benbouzid et al., 2008a). On account of these discrepancies between pharmacological and knockout results, we then tested the effects of an injection of nor-BNI (5 mg·kg−1) in the KOP+/+ and KOP−/− mice. After at least 2 weeks of treatment with nortriptyline or saline, the injection of nor-BNI suppressed the antiallodynic effect of nortriptyline (Figure 5A). However, this effect was present in both KOP+/+ and KOP−/− animals when we tested the mice 30 min after nor-BNI injection [KOP+/+ F1,20 = 3.4, P < 0.05; KOP−/− F1,20 = 3.4, P < 0.05; post hoc: (CuffNor = CuffSal) < Sham at P < 0.001] (Figure 5A and 5B). The effect of nor-BNI in KOP−/− mice suggests a lack of selectivity of the nor-BNI at the 30 min time-point. Previous studies showed that nor-BNI has long-lasting antagonist activity at KOP receptors (Spanagel et al., 1994; Patkar et al., 2013). Moreover, it was proposed that nor-BNI may act on MOP receptors in the first hour after its administration, whereas the KOP receptor antagonist action gradually increases, reaching its maximum effect a few hours after the injection (Endoh et al., 1992; Wettstein and Grouhel, 1996). Therefore, we tested the mice 8 h after the nor-BNI injection, and at this time-point, nor-BNI no longer had an effect on the mechanical threshold of either KOP+/+ or KOP−/− neuropathic nortriptyline-treated mice (Figure 5A and 5B).
Figure 5.
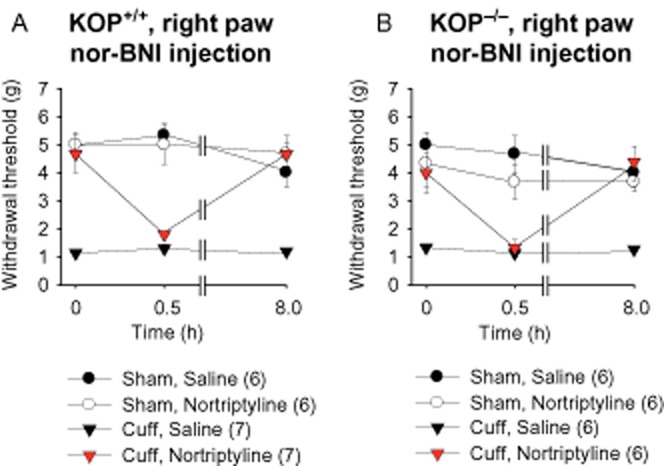
Acute nor-BNI injection suppressed the antiallodynic effect of nortryptiline treatment in KOP+/+ and KOP−/− mice. After at least 2 weeks of antidepressant or saline treatment, the animals received an injection of the KOP receptor antagonist nor-BNI (5 mg·kg−1, s.c.) or its control saline solution. The mechanical threshold was measured before, 30 min and 8 h after nor-BNI injection. (A) nor-BNI induced a transitory relapse of mechanical allodynia in neuropathic KOP+/+ mice treated with nortriptyline. (B) Similar results were obtained in KOP−/− mice. Data are expressed as mean ± SEM, n (number of animals) are given in parentheses.
Time-dependent selectivity of nor-BNI
To further study the selectivity of nor-BNI, we analysed the antiallodynic action of a KOP receptor agonist, U-50,488H (5 mg·kg−1) and a DOP receptor agonist, SNC80 (10 mg·kg−1) in the neuropathic mice (Figure 6). We observed an acute antiallodynic action of the KOP and DOP receptor agonists 60 min after a saline pretreatment [F1,15 = 26.4, P < 0.0001; post hoc (U-50,488H = SNC80) > Sal, P < 0.0001]. However, these antiallodynic effects of U-50,488H and SNC80 were lost 60 min after nor-BNI pretreatment (F1,15 = 0.5, P > 0.6) (Figure 6A). Therefore, at an early time-point, the nor-BNI is able to block the antiallodynic effect of both a KOP and a DOP receptor agonist.
We then did a similar experiment 8 h after pretreatment with saline or nor-BNI. The KOP and DOP receptor agonists displayed an antiallodynic action after the saline pretreatment [F1,15 = 11.00, P < 0.01; post hoc (U-50,488H = SNC80) > Sal, P < 0.0001], but 8 h after the nor-BNI pretreatment, the antinociceptive action of the KOP receptor agonist was blocked, whereas the DOP receptor agonist remained fully effective [F1,15 = 19.6, P < 0.0001; post hoc: (U-50,488H = Sal) < SNC80 at P < 0.001] (Figure 6B). These data illustrate that nor-BNI becomes selective for KOP receptors a few hours after its injection, but may also affect DOP receptors at early time-points.
We also tested whether nor-BNI could block the antiallodynic effect of morphine (10 mg·kg−1), which acts mainly through the MOP receptor to induce its analgesic action (Matthes et al., 1996; Sora et al., 1997). We observed that nor-BNI had no effect on the morphine-induced mechanical analgesia either 60 min (F1,10 = 0.02, P > 0.8) (Figure 6C) or 8 h (F1,10 = 0.8, P > 0.1) (Figure 6D) after nor-BNI pretreatment. These experiments show that nor-BNI becomes selective for KOP receptors several hours after injection, and the non-specific effect observed at the early time-point was mainly due to an action on DOP, but not MOP receptors.
Discussion and conclusions
In this work, we studied the involvement of KOP receptors in the antiallodynic action of the TCA nortriptyline. We show that this antiallodynic action, which can be reversed by a DOP receptor antagonist, does not require KOP receptors. Finally, we provide evidence supporting the poor selectivity of nor-BNI at early time-points after its injection.
The basal mechanical sensitivity was similar in KOP+/+ and KOP−/− mice. This is in agreement with findings from other laboratories (Xu et al., 2004; Schepers et al., 2008), even though in one study a slight reduction in the basal mechanical sensitivity threshold was detected in the KOP−/− mice (Gaveriaux-Ruff et al., 2008), which may depend on the von Frey testing procedure (Barrot, 2012). Following the induction of neuropathy by sciatic nerve cuffing, the intensity of the mechanical allodynia was also similar for both genotypes. However, it should be noted that enhanced allodynia can be observed in another model of neuropathic pain, the partial sciatic nerve ligation (Xu et al., 2004). In certain conditions, KOP receptors may thus have a modulatory action on mechanical sensitivity, but these receptors do not play a critical role in the establishment or the maintenance of neuropathic allodynia.
Behavioural pharmacology studies have shown that the antidepressant-induced analgesia can be inhibited by naloxone, a non-selective opioid receptor antagonist (Biegon and Samuel, 1980; Eschalier et al., 1981; Gray et al., 1998; Wattiez et al., 2011). Moreover, it has also been proposed that chronic antidepressant treatment can change opioid receptor densities and increase the level of opioid peptides in different regions of the nervous system (Antkiewicz-Michaluk et al., 1987; Hamon et al., 1987). While some authors provide pharmacological evidence for the involvement of MOP receptors (Schreiber et al., 2000; Marchand et al., 2003), this is not supported by studies in MOP receptor-deficient mice (Bohren et al., 2010), and the DOP receptor appears to be a major target for the antiallodynic action of antidepressants (Gray et al., 1998; Schreiber et al., 1999; Ozturk et al., 2006; Benbouzid et al., 2008b; Choucair-Jaafar et al., 2014). The link between the opioid system and antidepressant drugs is not limited to their action in a pain context (Lutz and Kieffer, 2013). Indeed, pharmacological blockade of opioid receptors antagonizes the antidepressant effect of antidepressant compounds in a behavioural model of depression (Berrocoso et al., 2004). DOP receptor-deficient mice display anxiety-like and depressive-like behaviours compared with the wild-type animals (Filliol et al., 2000), and recruitment of DOP receptors by a selective agonist has antidepressant-like effects in rodents (Pradhan et al., 2011). Although the opioidergic system appears to be a common target for the treatment of depression and pain, the precise downstream mechanism involved remains to be elucidated.
The role of the KOP receptor in the antidepressant treatment of neuropathic pain remains controversial, only a few studies have suggested the involvement of the KOP receptor (Schreiber et al., 1999; 2002,; Benbouzid et al., 2008a). Using a genetic approach, we observe that the therapeutic benefit of a chronic antidepressant treatment remains present in KOP−/− mice. These results suggest that, as for the MOP receptor (Bohren et al., 2010), the KOP receptor is not necessary for the antiallodynic effect of nortriptyline. However, it has previously been shown that the acute administration of the KOP receptor antagonist nor-BNI acutely blocked the antiallodynic effect of chronic nortriptyline treatment (Benbouzid et al., 2008a). In the present study we confirmed this acute action of nor-BNI, but we observed it in both wild-type and KOP−/− mice.
Our data question the in vivo selectivity of nor-BNI. Nor-BNI is a dimeric naltrexone derivative (Munro et al., 2012) that has a high in vitro binding selectivity for KOP versus MOP and DOP receptors, with Ki values of 0.28, 47.2 and 42.9 nM for KOP, MOP and DOP receptors respectively (Takemori et al., 1988). Although nor-BNI has a high KOP receptor antagonist activity in vitro (Takemori et al., 1988) and a long-lasting KOP receptor antagonist activity in vivo (Endoh et al., 1992; Horan et al., 1992; Broadbear et al., 1994), the temporal component seems to be a critical factor for its in vivo selectivity (Endoh et al., 1992; Spanagel et al., 1994; Munro et al., 2012). For example, in the tail pinch test, the antagonistic action of nor-BNI at KOP receptors gradually increased to reach a maximum effect at 2 h and was maintained over 4 days (Endoh et al., 1992). Moreover, while over 80% of plasma nor-BNI was eliminated within 2 h (Munro et al., 2012; Patkar et al., 2013), nor-BNI (after 10 mg·kg−1 injection) was still present at a low concentration in brain homogenates up to 21 days after a single i.p. administration, and was still able to block KOP receptor agonist-induced analgesia up to 7 days after nor-BNI pretreatment (Patkar et al., 2013). This long-lasting action of nor-BNI in vivo (Horan et al., 1992; Butelman et al., 1993; Broadbear et al., 1994) was present despite non-covalent binding in vitro (Bruchas et al., 2007). It should be noted that previous pharmacokinetic studies (Munro et al., 2012; Kishioka et al., 2013; Patkar et al., 2013) have only detected the nor-BNI molecule itself, which cannot exclude the possibility that nor-BNI may also be biotransformed in vivo into long-lasting metabolites that bind covalently to KOP receptors (Bruchas et al., 2007). However, the selectivity of a drug is usually decreased after biotransformation (except for prodrugs). It has also been proposed that the long-lasting action of nor-BNI could be related to a long-lasting JNK1-mediated desensitization of KOP receptors (Bruchas et al., 2007; Munro et al., 2012).
The non-selective early action of nor-BNI might be partly due to peripheral opioid receptors. Indeed nor-BNI is mostly distributed in plasma, after its systemic administration, with levels peaking at 30 min and declining within 2 h (Munro et al., 2012). Nor-BNI has been reported to antagonize morphine-induced, but not U-50,488H-induced analgesia in the first 30 min after its administration (Endoh et al., 1992). In fact, nor-BNI suppressed morphine-induced analgesia at a very high dose (30 mg·kg−1), but not at lower doses (3 and 10 mg·kg−1) (Wettstein and Grouhel, 1996), which is in agreement with our results, showing no effect of 5 mg·kg−1 nor-BNI on morphine-induced analgesia. At a dose often used for in vivo studies (Butelman et al., 1993; Menendez et al., 1993), our study establishes an interaction of nor-BNI with DOP receptor-related mechanisms at early time-points after its administration, but confirms the selectivity of nor-BNI for KOP receptors 8 h after its administration. Lastly, it should be noted that our experiments were all conducted on the antiallodynic action in a model of neuropathic pain. Most of the studies that showed a time-dependent selectivity of nor-BNI on the KOP receptor have been done in naïve animals (Endoh et al., 1992; Horan et al., 1992; Butelman et al., 1993). However, there is evidence of neuropathy-induced plasticity of the endogenous κ-opioid system (Stevens et al., 1991; Xu et al., 2004). Therefore, we cannot rule out the possibility that a yet unknown mechanism induces off-target actions of nor-BNI in a neuropathic pain state.
In conclusion, KOP receptors are not necessary for the antiallodynic action of the TCA nortriptyline. Together with previous studies on opioid receptor-deficient mice, the present findings support the idea that the DOP receptor is the only opioid receptor that is critical for the relief of neuropathic mechanical allodynia following TCA treatment. Furthermore, caution should be taken when using nor-BNI as a KOP receptor antagonist for behavioural studies. In particular, its time-dependent selectivity should be taken into account.
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique ( (UPR3212), the University of Strasbourg and the Neurex Network (Program Interreg IV Upper Rhine). We thank Rhian Alice Ceredig for her comments on the paper.
Glossary
- DOP receptor
δ-opioid receptor
- KOP receptor
κ-opioid receptor
- MOP receptor
μ-opioid receptor
- nor-BNI
norbinaltorphimine
- SNC80
4-[(R)-[(2S,5R)-2,5-dimethyl-4-prop-2-enylpiperazin-1-yl]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide
- TCA
tricyclic antidepressant
- U-50,488H
trans-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]-benzeneacetamide
Author contributions
M. B., S. M., Y. B. and I. Y. designed the experiments. S. M. and Y. B. performed and analysed the experiments. I. Y. performed the surgeries. B. L. K. and C. G. supplied the transgenic mouse breeders. S. D. managed the transgenic mouse colonies. M. B., S. M., M-J. F-M., I. Y., C. G-R. and B. L. K. wrote the paper.
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al., editors. The concise guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjaneyulu M, Chopra K. Possible involvement of cholinergic and opioid receptor mechanisms in fluoxetine mediated antinociception response in streptozotocin-induced diabetic mice. Eur J Pharmacol. 2006;538:80–84. doi: 10.1016/j.ejphar.2006.03.067. [DOI] [PubMed] [Google Scholar]
- Antkiewicz-Michaluk L, Michaluk J, Rokosz-Pelc A, Marona-Lewicka D, Vetulani J. The effect of chronic imipramine and electroconvulsive shock treatment on [3H]DADLE binding to cortical membranes of rats pretreated with chronic reserpine or 6-hydroxydopamine. Pharmacol Biochem Behav. 1987;26:203–206. doi: 10.1016/0091-3057(87)90105-5. [DOI] [PubMed] [Google Scholar]
- Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P, Jensen TS, et al., editors. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–e1188. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- Barrot M. Tests and models of nociception and pain in rodents. Neuroscience. 2012;211:39–50. doi: 10.1016/j.neuroscience.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Benbouzid M, Choucair-Jaafar N, Yalcin I, Waltisperger E, Muller A, Freund-Mercier MJ, et al., editors. Chronic, but not acute, tricyclic antidepressant treatment alleviates neuropathic allodynia after sciatic nerve cuffing in mice. Eur J Pain. 2008a;12:1008–1017. doi: 10.1016/j.ejpain.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Benbouzid M, Gaveriaux-Ruff C, Yalcin I, Waltisperger E, Tessier LH, Muller A, et al., editors. Delta-opioid receptors are critical for tricyclic antidepressant treatment of neuropathic allodynia. Biol Psychiatry. 2008b;63:633–636. doi: 10.1016/j.biopsych.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Berrocoso E, Rojas-Corrales MO, Mico JA. Non-selective opioid receptor antagonism of the antidepressant-like effect of venlafaxine in the forced swimming test in mice. Neurosci Lett. 2004;363:25–28. doi: 10.1016/j.neulet.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Biegon A, Samuel D. Interaction of tricyclic antidepressants with opiate receptors. Biochem Pharmacol. 1980;29:460–462. doi: 10.1016/0006-2952(80)90531-6. [DOI] [PubMed] [Google Scholar]
- Bohren Y, Karavelic D, Tessier LH, Yalcin I, Gaveriaux-Ruff C, Kieffer BL, et al., editors. Mu-opioid receptors are not necessary for nortriptyline treatment of neuropathic allodynia. Eur J Pain. 2010;14:700–704. doi: 10.1016/j.ejpain.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH. Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl) 1994;115:311–319. doi: 10.1007/BF02245071. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, et al., editors. Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem. 2007;282:29803–29811. doi: 10.1074/jbc.M705540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH. Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther. 1993;267:1269–1276. [PubMed] [Google Scholar]
- Butelman ER, Yuferov V, Kreek MJ. Kappa-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci. 2012;35:587–596. doi: 10.1016/j.tins.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choucair-Jaafar N, Salvat E, Freund-Mercier MJ, Barrot M. The antiallodynic action of nortriptyline and terbutaline is mediated by beta(2) adrenoceptors and delta opioid receptors in the ob/ob model of diabetic polyneuropathy. Brain Res. 2014;1546:18–26. doi: 10.1016/j.brainres.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al., editors. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tanaka C, Nagase H. Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn Ther. 1992;316:30–42. [PubMed] [Google Scholar]
- Eschalier A, Montastruc JL, Devoize JL, Rigal F, Gaillard-Plaza G, Pechadre JC. Influence of naloxone and methysergide on the analgesic effect of clomipramine in rats. Eur J Pharmacol. 1981;74:1–7. doi: 10.1016/0014-2999(81)90316-2. [DOI] [PubMed] [Google Scholar]
- Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y. Current research on opioid receptor function. Curr Drug Targets. 2012;13:230–246. doi: 10.2174/138945012799201612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, et al., editors. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Karchewski LA, Hever X, Matifas A, Kieffer BL. Inflammatory pain is enhanced in delta opioid receptor-knockout mice. Eur J Neurosci. 2008;27:2558–2567. doi: 10.1111/j.1460-9568.2008.06223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray AM, Spencer PS, Sewell RD. The involvement of the opioidergic system in the antinociceptive mechanism of action of antidepressant compounds. Br J Pharmacol. 1998;124:669–674. doi: 10.1038/sj.bjp.0701882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon M, Gozlan H, Bourgoin S, Benoliel JJ, Mauborgne A, Taquet H, et al. Opioid receptors and neuropeptides in the CNS in rats treated chronically with amoxapine or amitriptyline. Neuropharmacology. 1987;26:531–539. doi: 10.1016/0028-3908(87)90144-4. [DOI] [PubMed] [Google Scholar]
- Horan P, Taylor J, Yamamura HI, Porreca F. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Exp Ther. 1992;260:1237–1243. [PubMed] [Google Scholar]
- Jensen TS, Baron R, Haanpaa M, Kalso E, Loeser JD, Rice AS, editors. A new definition of neuropathic pain. Pain. 2011;152:2204–2205. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Keita H, Kayser V, Guilbaud G. Antinociceptive effect of a kappa-opioid receptor agonist that minimally crosses the blood–brain barrier (ICI 204448) in a rat model of mononeuropathy. Eur J Pharmacol. 1995;277:275–280. doi: 10.1016/0014-2999(95)00122-2. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishioka S, Kiguchi N, Kobayashi Y, Yamamoto C, Saika F, Wakida N, et al., editors. Pharmacokinetic evidence for the long-lasting effect of nor-binaltorphimine, a potent kappa opioid receptor antagonist, in mice. Neurosci Lett. 2013;552:98–102. doi: 10.1016/j.neulet.2013.07.040. [DOI] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36:195–206. doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand F, Ardid D, Chapuy E, Alloui A, Jourdan D, Eschalier A. Evidence for an involvement of supraspinal delta- and spinal mu-opioid receptors in the antihyperalgesic effect of chronically administered clomipramine in mononeuropathic rats. J Pharmacol Exp Ther. 2003;307:268–274. doi: 10.1124/jpet.103.052613. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, et al., editors. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez L, Andres-Trelles F, Hidalgo A, Baamonde A. Involvement of spinal kappa opioid receptors in a type of footshock induced analgesia in mice. Brain Res. 1993;611:264–271. doi: 10.1016/0006-8993(93)90512-l. [DOI] [PubMed] [Google Scholar]
- Mico JA, Ardid D, Berrocoso E, Eschalier A. Antidepressants and pain. Trends Pharmacol Sci. 2006;27:348–354. doi: 10.1016/j.tips.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Minami M, Satoh M. Molecular biology of the opioid receptors: structures, functions and distributions. Neurosci Res. 1995;23:121–145. doi: 10.1016/0168-0102(95)00933-k. [DOI] [PubMed] [Google Scholar]
- Munro TA, Berry LM, Van't Veer A, Béguin C, Carroll FI, Zhao Z, et al., editors. Long-acting κ opioid antagonists nor-BNI, GNTI and JDTic: pharmacokinetics in mice and lipophilicity. BMC Pharmacol. 2012;12:5. doi: 10.1186/1471-2210-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki C, Kamei J. Possible involvement of opioidergic systems in the antinociceptive effect of the selective serotonin reuptake inhibitors in sciatic nerve-injured mice. Eur J Pharmacol. 2006;552:99–104. doi: 10.1016/j.ejphar.2006.09.029. [DOI] [PubMed] [Google Scholar]
- Nozaki C, Le Bourdonnec B, Reiss D, Windh RT, Little PJ, Dolle RE, et al., editors. δ-opioid mechanisms for ADL5747 and ADL5859 effects in mice: analgesia, locomotion, and receptor internalization. J Pharmacol Exp Ther. 2012;342:799–807. doi: 10.1124/jpet.111.188987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk Y, Aydin S, Beis R, Herekman-Demir T. The involvement of endogenous opioid mechanisms in the antinociceptive effects induced by antidepressant drugs, desipramine and trimipramine. Pharmacol Biochem Behav. 2006;83:592–597. doi: 10.1016/j.pbb.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Parra MC, Nguyen TN, Hurley RW, Hammond DL. Persistent inflammatory nociception increases levels of dynorphin 1–17 in the spinal cord, but not in supraspinal nuclei involved in pain modulation. J Pain. 2002;3:330–336. doi: 10.1054/jpai.2002.125185. [DOI] [PubMed] [Google Scholar]
- Patkar KA, Wu J, Ganno ML, Singh HD, Ross NC, Rasakham K, et al., editors. Physical presence of nor-binaltorphimine in mouse brain over 21 days after a single administration corresponds to its long-lasting antagonistic effect on kappa-opioid receptors. J Pharmacol Exp Ther. 2013;346:545–554. doi: 10.1124/jpet.113.206086. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al., editors. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl. Acids Res. 2014;42:D1098–1106. doi: 10.1093/nar/gkt1143. (Database Issue): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gaveriaux-Ruff C, Kieffer BL. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci. 2011;32:581–590. doi: 10.1016/j.tips.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere PJ. Peripheral kappa-opioid agonists for visceral pain. Br J Pharmacol. 2004;141:1331–1334. doi: 10.1038/sj.bjp.0705763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers RJ, Mahoney JL, Gehrke BJ, Shippenberg TS. Endogenous kappa-opioid receptor systems inhibit hyperalgesia associated with localized peripheral inflammation. Pain. 2008;138:423–439. doi: 10.1016/j.pain.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S, Backer MM, Pick CG. The antinociceptive effect of venlafaxine in mice is mediated through opioid and adrenergic mechanisms. Neurosci Lett. 1999;273:85–88. doi: 10.1016/s0304-3940(99)00627-8. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Backer MM, Herman I, Shamir D, Boniel T, Pick CG. The antinociceptive effect of trazodone in mice is mediated through both mu-opioid and serotonergic mechanisms. Behav Brain Res. 2000;114:51–56. doi: 10.1016/s0166-4328(00)00185-6. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Bleich A, Pick CG. Venlafaxine and mirtazapine: different mechanisms of antidepressant action, common opioid-mediated antinociceptive effects – a possible opioid involvement in severe depression? J Mol Neurosci. 2002;18:143–149. doi: 10.1385/JMN:18:1-2:143. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Childers SR. Anatomical distribution of mu, delta, and kappa opioid- and nociceptin/orphanin FQ-stimulated [35S]guanylyl-5′-O-(gamma-thio)-triphosphate binding in guinea pig brain. J Comp Neurol. 1997;386:562–572. doi: 10.1002/(sici)1096-9861(19971006)386:4<562::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, et al., editors. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, et al., editors. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci U S A. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sounvoravong S, Takahashi M, Nakashima MN, Nakashima K. Disability of development of tolerance to morphine and U-50,488H, a selective κ-opioid receptor agonist, in neuropathic pain model mice. J Pharmacol Sci. 2004;94:305–312. doi: 10.1254/jphs.94.305. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Almeida OF, Shippenberg TS. Evidence that nor-binaltorphimine can function as an antagonist at multiple opioid receptor subtypes. Eur J Pharmacol. 1994;264:157–162. doi: 10.1016/0014-2999(94)00449-8. [DOI] [PubMed] [Google Scholar]
- Stevens CW, Kajander KC, Bennett GJ, Seybold VS. Bilateral and differential changes in spinal mu, delta and kappa opioid binding in rats with a painful, unilateral neuropathy. Pain. 1991;46:315–326. doi: 10.1016/0304-3959(91)90114-D. [DOI] [PubMed] [Google Scholar]
- Takemori AE, Ho BY, Naeseth JS, Portoghese PS. Nor-binaltorphimine, a highly selective kappa-opioid antagonist in analgesic and receptor binding assays. J Pharmacol Exp Ther. 1988;246:255–258. [PubMed] [Google Scholar]
- Valverde O, Mico JA, Maldonado R, Mellado M, Gibert-Rahola J. Participation of opioid and monoaminergic mechanisms on the antinociceptive effect induced by tricyclic antidepressants in two behavioural pain tests in mice. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1073–1092. doi: 10.1016/0278-5846(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, et al. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci. 2001;21:1779–1786. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattiez AS, Libert F, Privat AM, Loiodice S, Fialip J, Eschalier A, et al., editors. Evidence for a differential opioidergic involvement in the analgesic effect of antidepressants: prediction for efficacy in animal models of neuropatic pain? Br J Pharmacol. 2011;163:792–803. doi: 10.1111/j.1476-5381.2011.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein JG, Grouhel A. Opioid antagonist profile of SC nor-binaltorphimine in the formalin paw assay. Pharmacol Biochem Behav. 1996;53:411–416. doi: 10.1016/0091-3057(95)02043-8. [DOI] [PubMed] [Google Scholar]
- Xu M, Petraschka M, McLaughlin JP, Westenbroek RE, Caron MG, Lefkowitz RJ, et al., editors. Neuropathic pain activates the endogenous kappa opioid system in mouse spinal cord and induces opioid receptor tolerance. J Neurosci. 2004;24:4576–4584. doi: 10.1523/JNEUROSCI.5552-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin I, Choucair-Jaafar N, Benbouzid M, Tessier LH, Muller A, Hein L, et al., editors. beta(2)-adrenoceptors are critical for antidepressant treatment of neuropathic pain. Ann Neurol. 2009;65:218–225. doi: 10.1002/ana.21542. [DOI] [PubMed] [Google Scholar]
- Yalcin I, Megat S, Barthas F, Waltisperger E, Kremer M, Salvat E, et al., editors. The sciatic nerve cuffing model of neuropathic pain in mice. J Vis Exp. 2014;89:e51608. doi: 10.3791/51608. [DOI] [PMC free article] [PubMed] [Google Scholar]