Abstract
Background and Purpose
Post-operative ileus (POI) is induced by intestinal inflammation. Here, we aimed to clarify the effects of 5-HT3 receptor antagonists against POI.
Experimental Approach
We administered three 5-HT3 receptor antagonists, ondansetron, tropisetron and palonosetron, to a mouse model of POI induced by surgical intestinal manipulation (IM). Immunohistochemistry, intestinal transit, inflammatory mediator mRNA expression and 5-HT content were measured. In some experiments, 5-HT3A receptor null mice were used.
Key Results
Three 5-HT3 receptor antagonists reduced IM-induced infiltration of inflammatory CD68-positive macrophages and myeloperoxidase-stained neutrophils. Ondansetron exhibited no anti-inflammatory actions in 5-HT3A receptor null mice. Ondansetron inhibited expression of the chemokine CCL2, IL-1β, IL-6, TNF-α and iNOS mRNAs up-regulated by IM, and also ameliorated the delayed gastrointestinal transit. Peritoneal macrophages, but not most infiltrating monocyte-derived macrophages, expressed 5-HT3 receptors. IM stimulation increased the 5-HT content of peritoneal lavage fluid, which up-regulated mRNA expression of proinflammatory cytokines in peritoneal macrophages. Immunohistochemical localization of 5-HT3 receptors suggests that ondansetron suppressed expression of these mRNAs in activated peritoneal macrophages, adhering to the serosal region of the inflamed intestinal wall.
Conclusion and Implications
5-HT3 receptor antagonists were anti-inflammatory, mainly targeting peritoneal macrophages expressing these receptors. They also restored the delayed gastrointestinal transit by IM. 5-HT3 receptor antagonists should be therapeutically useful agents against POI.
Tables of Links
| TARGETS |
|---|
| Ligand-gated ion channelsa |
| 5-HT3 receptor |
| α7nAChR |
| Enzymeb |
| TPH, tryptophan hydroxylase |
| LIGANDS |
|---|
| Ondansetron |
| Palonosetron |
| Tropisetron |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b).
Introduction
5-Hydroxytryptamine (5-HT) is synthesized from its precursor L-tryptophan via tryptophan hydroxylase (TPH). Enterochromaffin cells of the gastrointestinal mucosal layer comprise the main sites of 5-HT synthesis and localization. Platelets, macrophages and the CNS also contain 5-HT (Gershon and Tack, 2007). A wide range of physiological activities is exhibited by 5-HT in the CNS and peripheral nervous systems, the gastrointestinal tract and the CVS (Kim and Camilleri, 2000). Seven major types of 5-HT receptors (5-HT1 to 5-HT7) have been identified and some have been subclassified into several subgroups (Hannon and Hoyer, 2008).
The 5-HT3 receptor is a ligand-gated cation channel that is widely expressed in the gastrointestinal tract as well as in the CNS and spinal cord (Maricq et al., 1991). Activation of 5-HT3 receptors can stimulate intestinal secretion, gastrointestinal motility and sensory nerves to induce an emetic response and pain (Jackson and Yakel, 1995). Emesis, nausea (Candiotti et al., 2007; Panteleev et al., 2013) and irritable bowel syndrome (Spiller, 2004) are currently treated using 5-HT3 receptor antagonists.
In addition to mediating physiological functions, 5-HT can also induce inflammation (El-Salhy et al., 1997; Linden et al., 2003; Ghia et al., 2009). The severity of clinical disease and histological damage because of dextran sulphate sodium salt (DSS) or dinitrobenzene sulphonic acid-mediated colitis is reduced in mice lacking TPH (TPH null mice) (Ghia et al., 2009). Changes in 5-HT content are associated with both Crohn's disease and ulcerative colitis (Oshima et al., 1999; Heimes et al., 2009). Although the 5-HT receptors regulating immune and inflammatory response in the gut remain somewhat obscure, activated 5-HT3 receptors that are expressed in immune cells such as monocytes, dendritic cells and T-cells, promote the secretion of IL-6 and IL-1β, which in turn accelerates molecular and cellular inflammatory responses (Durk et al., 2005). Recent findings also support the notion that the activation of 5-HT3 receptors plays an important role in the induction of inflammation. Administration of 5-HT3 receptor antagonists ameliorates intestinal mucositis induced by 5-fluorouracil (Yasuda et al., 2013), haemorrhagic shock (Oshima et al., 1999) and DSS-induced colitis (Oshima et al., 1999), but the mechanisms of anti-inflammation via 5-HT3 receptors are not understood in detail.
Post-operative ileus (POI) is a complication of abdominal surgery characterized by gastrointestinal dysmotility with vomiting and abdominal pain (Prasad and Matthews, 1999). Local inflammation of the gastrointestinal wall is generally considered to prolong and complicate POI (Kalff et al., 2000; Bauer and Boeckxstaens, 2004; Wehner et al., 2007). Resident and infiltrating muscularis macrophages as well as neutrophils and mast cells play key roles in inducing local inflammation of the gastrointestinal wall (Mattei and Rombeau, 2006). Inflammatory stimuli and mechanical manipulation can activate these sources of inflammation, which is followed by the production of PGE2, inflammatory cytokines, chemokines and NO that consequently induce motility disorders (Schwarz et al., 2001; Turler et al., 2006). We found that PGE2 released from muscularis resident macrophages can activate muscularis macrophages to produce NO via EP2 and EP4 receptors in an autocrine and/or paracrine manner, followed by decreased intestinal motility (Tajima et al., 2012).
The present study aimed to clarify the therapeutic effects of three 5-HT3 receptor antagonists, ondansetron, tropisetron and palonosetron, against POI, and to clarify their anti-inflammatory effects. The results indicated that these 5-HT3 receptor antagonists reduced intestinal muscularis inflammation induced by intestinal manipulation (IM) in a mouse model of POI, which in turn ameliorated gastrointestinal dysmotility, suggesting that 5-HT3 receptor antagonists might be useful as therapeutic agents against POI. Thus, the data indicate that 5-HT3 receptor antagonists can target peritoneal macrophages expressing 5-HT3 receptors.
Methods
Animals
All animal care and experimental procedures complied with the Guide for Animal Use and Care published by the University of Tokyo and were approved by the Institutional Review Board of the University of Tokyo (approval code P10-482). Studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 172 animals were used in the experiments described here.
Balb/c mice, C57BL/6J mice and 5-HT3A receptor null mice of C57BL/6J background (Jackson Laboratories, Bar Harbor, ME, USA) were housed under controlled conditions (8–12 weeks age, 12 h light–dark cycles). The animals were anaesthetized with pentobarbital sodium (Kyoritsu Seiyaku Co., Tokyo, Japan) to create a mouse model of POI using IM as described (Kalff et al., 1999; 2000,). In this study, the combined procedure of laparotomy and IM constituted the POI model, because laparotomy alone only transiently increased expression of pro-inflammatory cytokines (Kiyosue et al., 2006). In this study, unless otherwise stated, no treatment was used as the control, reference state.
Experimental design
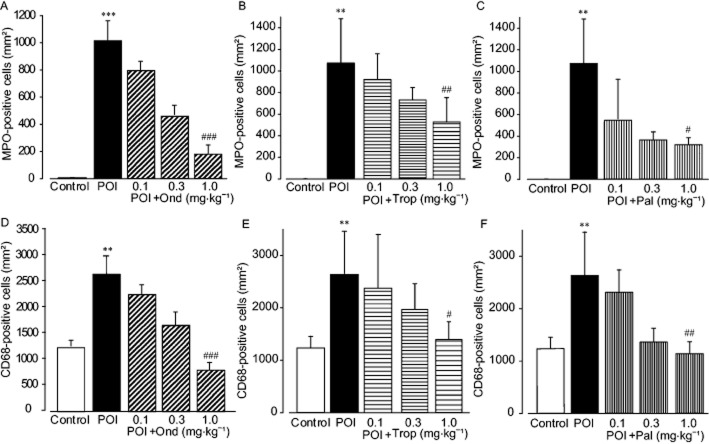
The mice were assigned randomly to the following groups: control, untreated and with fasting; POI, s.c. injected with sterilized physiological saline (1 mg·kg−1); POI + ondansetron (1 mg·kg−1; Sigma-Aldrich, St Louis, MO, USA); POI + tropisetron (1 mg·kg−1; Santa Cruz Biotechnology, Dallas, TX, USA); and POI + palonosetron (1 mg·kg−1; Santa Cruz Biotechnology). Each 5-HT3 receptor antagonist was injected s.c. together with physiological saline into a mouse model of POI before IM. The concentrations of these 5-HT3 receptor antagonists were determined based on the findings of Nagakura et al., (1996) and a dose–response study was also carried out (Fig. 2).
Figure 2.
Effects of ondansetron (Ond), tropisetron (Trop) and palonosetron (Pal) on inflammatory leukocyte infiltration into muscle layer induced by IM. Quantitation of infiltrated MPO-stained neutrophils (A–C) and CD-68-positive macrophages (D–F) at 24 h after IM. Each 5-HT3 receptor antagonist (0.1, 0.3 and 1 mg·kg−1) was s.c. administered 1 h before IM. Data shown are means ± SEM from four independent experiments. **P < 0.01, ***P < 0.001; significantly different from control. #P < 0.05, ##P < 0.01, ###P < 0.001; significantly different from POI.
Myeloperoxidase (MPO) staining
Whole mount ileal muscularis preparations were fixed in 10% paraformaldehyde for 24 h at 4°C, cut into 1 cm squares and washed twice with Tris-buffered saline (TBS) for 30 min, at room temperature. The preparations were stained with physiological salt solution containing 0.1% (w/v) Hanker-Yates reagent (Polyscience, Warrington, PA, USA) and 0.03% (v/v) hydrogen peroxidase (Mitsubishi Gas Chemical Company, Tokyo, Japan) for 5 min, washed for 10 min in PBS and mounted on glass slides. Thereafter, MPO-positive neutrophils in four random selected areas of the myenteric plexus region in each preparation were counted under an ACT-1C for DXM1200C microscope (Nikon, Tokyo, Japan).
Immunohistochemistry
Fixed whole-mount preparations were washed twice with TBS for 30 min and then permeabilized with 0.2% Triton-X-100 and 2% BSA in TBS for 2 h. The permeabilized preparations were rinsed with 2% BSA in TBS for 30 min, incubated with 1:500 diluted rat anti-mouse CD68 antibody (Serotec, Ltd., Oxford, UK) in TBS with 2% BSA at 4°C overnight and then washed for 2 h in TBS. The preparations were labelled with 1:250 diluted goat anti-rat IgG Alexa Fluor 488 secondary antibody (Life Technologies, Carlsbad, CA, USA) for 90 min at room temperature. The number of CD68-positive cells was counted in four randomly selected areas of each preparation and the average number of infiltrating cells was calculated.
Segments of the mouse ileum were removed, fixed by immersion in fresh 0.1 M phosphate buffer containing 4% paraformaldehyde for 2 h at 4°C, and washed three times with PBS. The segments were cryoprotected overnight in 0.1 M phosphate buffer containing 20% sucrose. Tissues were frozen at an optimal cutting temperature (Sakura Finetek, Tokyo, Japan) in mounting medium, and sectioned on a cryostat (Leica Instruments, Nussloch, Germany) at a thickness of 30 μm. The tissue was incubated with 1:75 diluted rabbit anti-mouse 5-HT3R antibody (Calbiochem, Billerica, MA, USA) for about 40 h at room temperature. The preparations were labelled with 1:400 diluted donkey anti-rabbit IgG fluorescein isothiocyanate secondary antibody (Jackson Laboratories) for 4 h at room temperature. Thereafter, the tissues were incubated with 1:300 diluted rat anti-mouse CD68 antibody for about 20 h at room temperature and labelled with 1:400 diluted donkey anti-rat IgG tetramethylrhodamine isothiocyanate (Jackson Laboratories ) for 4 h at room temperature.
Determination of intestinal transit
After a 24 h fast, the mice were randomly assigned to four groups (Control, Control + Ond, POI and POI + Ond). Ond was given s.c. 1 h before IM. The mice received 100 μL of the non-absorbable marker 0.25% (w/v) Phenol Red in 5% (w/v) glucose via a gastric tube at 23 h after IM. After 1 h, the gastrointestinal region was isolated from the abdominal cavity. The intestine and colon were divided into 10 (Sl1-Sl10) and three (Co1-Co3) segments at equal intervals. The stomach and caecum were separated as a single segment (Sto, Cec). Supernatant of each bowel content (1000 μL) was added to 200 μL of trichloroacetic acid solution (20% wt·vol−1) to precipitate the proteins. After centrifugation (10000× g, 20 min), the supernatant (600 μL) was added to 800 μL of NaOH (0.5 N) to develop the maximum intensity of colour. The solutions were read using a spectrophotometer (560 nm wavelength) (Sallam et al., 2007). The volume of Phenol Red in each segment and the geometric centre of distribution were calculated as previously described (Kalff et al., 2000; Schwarz et al., 2001; Sallam et al., 2007).
Real-time RT-PCR
Total RNA was extracted from the ileal muscularis and peritoneal macrophages using Trizol (Molecular Research Center, Inc., Cincinnati, OH, USA) according to the manufacturer's instructions. Total RNA was reverse transcribed using ReverTra Ace in random 9-mer oligonucleotide primers (Takara Bio, Otsu, Japan) at 30°C for 10 min, 42°C for 1 h and 99°C for 5 min. Real-time PCR analysis was performed using SYBR Green (Tajima et al., 2012). The primer sequences and predicted product sizes are listed in Table 2013a. The cDNA were amplified via 4°C for 1 min, denaturation at 95°C for 1 min 45 cycles of 95°C for 15 s, 59°C for 1 min. Relative expression value was shown against mRNA expression of control sample. In some experiments, semi-quantitative RT-PCR was performed to detect expression of the target gene.
Table 1.
Primer sets and the expected sizes for PCR
| Gene | Forward | Reverse | Expected size (bp) |
|---|---|---|---|
| CCL2 | 5′TGTTACCTCAGTTCATCATCCACGG 3′ | 5′CAGAATGGTAATGTGAGCAGGAAG3′ | 316 |
| IL-1β | 5′ TGACGTTCCCATTAGACAGC3′ | 5′ TGGGGAAGGCATTAGAAACA3′ | 497 |
| IL-6 | 5′ TCTCTGGGAAATCGTGGAAA3′ | 5′ GATGGTCTTGGTCCTTAGCC3′ | 397 |
| IL-10 | 5′TGGCCTTGTAGACACCTTGG 3′ | 5′ AGCCGGGAAGACAATAACTG3′ | 362 |
| TNF-α | 5′ AGCCTGTACCCACGTCGTAG 3′ | 5′ GTAGACAAGGTACAACCCATCG 3′ | 324 |
| iNOS | 5′ CAAACCCAAGGTCTACGTTC 3′ | 5′ GAAAAGACTGCACGAAGAT 3′ | 189 |
| 5-HT3A receptor | 5′ CCAGTCCTGACTGGCTGAG 3′ | 5′ AAGTCCTGAGGTCCTCCAAC 3′ | 188 |
| S18rRNA | 5′GACTCAACACGGGAAACCTCAC 3′ | 5′ CACCCACGGAATCGAGAGAAAG3′ | 80 |
| GAPDH | 5′ CAGGGCTGCTTTTAATTCTG 3′ | 5′ AGCACCAGCATCACCCCACT 3′ | 269 |
Isolation of peritoneal macrophage and collection of peritoneal lavage fluids
After exsanguination, the peritoneal cavity was flushed with 5 mL of PBS and peritoneal lavage fluids were centrifuged at 250× g for 5 min at 4°C. The supernatant was aspirated and the pellet was resuspended in PBS. Cells (2 × 106) were incubated in 100 mm dishes at 37°C under a 5% CO2 atmosphere for 2 h, washed with PBS twice, the suspended cells were removed and the adherent cells were collected as peritoneal macrophages. Over 80% of adhering cells were CD68-positive macrophages (Klimetzek and Remold, 1980) (data not shown).
Peritoneal macrophages were fixed by acetone for 5 min at 4°C and washed three times with PBS. Peritoneal macrophages were incubated with 1:75 diluted rabbit anti-mouse 5-HT3 receptor antibody for about 30 h and 1:400 diluted rat anti-mouse F4/80 antibody for 4 h at 4°C. Thereafter, the peritoneal cells were labelled with 1:400 diluted goat anti-rat IgG Alexa Fluor 488 and 1:400 diluted donkey anti-rat IgG Alexa Fluor 594 secondary antibody for 4 h at room temperature.
Peritoneal mast cells were also purified from peritoneal cells as described by Jensen et al., (2006). Briefly, after exsanguination, the peritoneal cavity was flushed with 5 mL of HEPES and peritoneal lavage fluids were centrifuged at 250× g for 90 s at 4°C. The supernatant was removed and the pellet was resuspended in HEPES, and on the 70% Percoll solution, centrifuging at 760× g for 25 min at 20°C. After centrifuging, the supernatant was aspirated and cells resuspended in HEPES and centrifuged at 380× g for 3 min 4°C. The pellet was collected as peritoneal mast cells. We confirmed that these isolated cells were stained by anti-c-kit antibody (Abcam Japan, Tokyo, Japan).
Measurement of 5-HT content of the peritoneal lavage
After exsanguination, the peritoneal cavities of the mice were flushed with 2 mL of PBS containing 5 mM EDTA, the peritoneal lavage fluids were centrifuged at 10 000× g for 10 min at 4°C and then the supernatant was collected. The content of 5-HT in the supernatant of the peritoneal lavage fluid was measured using an elisa (Beckman Coulter, Brea, CA, USA) according to the manufacturer's instructions. Total protein was measured using Lowry protein assays and the results are shown as ng per mg protein (Lowry et al., 1951).
Data analysis
Results are expressed as means ± SEM. Data were analysed using unpaired Student's t-tests for comparisons between two groups and by one-way anova followed by Dunnett's test for comparisons among three groups. P values < 0.05 were considered statistically significant.
Results
5-HT3 receptor antagonists prevent the infiltration of macrophages and neutrophils into inflamed myenteric plexus regions induced by IM
Recent findings have shown that 5-HT3 receptor antagonists exert anti-inflammatory effects (Vega Lde et al., 2005; Maleki-Dizaji et al., 2010; Liu et al., 2011; Yasuda et al., 2013). We therefore investigated, using immunohistochemical techniques, whether 5-HT3 receptor antagonists have anti-inflammatory effects in a mouse model of POI, at 24 h after IM (Fig. 1 and Fig. 2). Ramified cells, immunohistochemically identified as CD68-positive macrophages, were detected in the myenteric plexus region of the intestines from control mice, whereas MPO-positive neutrophils were undetectable anywhere in the control mouse intestine. More CD68-positive macrophages and MPO-positive neutrophils were identified in the intestinal muscle layer of POI mice, compared with those of control mice. The 5-HT3 receptor antagonist, ondansetron (1 mg·kg−1 s.c.) inhibited the infiltration of macrophages and neutrophils induced by IM (Fig. 1). As shown in Figure 2, all 5-HT3 receptor antagonists (ondansetron, tropisetron and palonosetron) dose-dependently inhibited inflammation induced by IM, and maximum responses were obtained by administration of 1 mg·kg−1 s.c.
Figure 1.
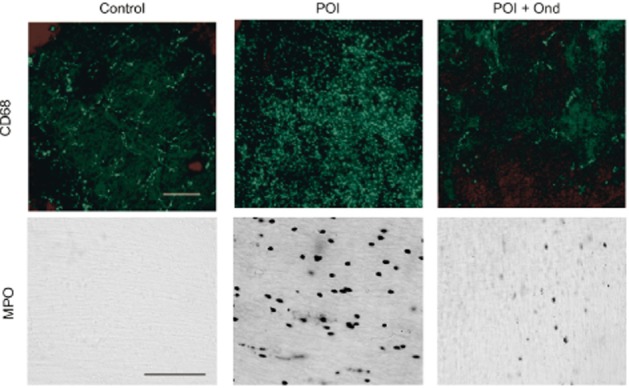
Effects of ondansetron (Ond) on macrophage and neutrophil infiltrations into myenteric plexus region induced by IM. Typical images of CD68-positive macrophages and MPO-positive neutrophils in myenteric plexus region at 24 h after IM are shown from six independent experiments. Ondansetron (1 mg·kg−1) was s.c. administered 1 h before IM. Scale bar shows 100 μm.
We further investigated effects of ondansetron on IM-induced inflammation in 5-HT3A receptor null mice. IM induced macrophages and neutrophils infiltrations into the intestinal muscle layer in 5-HT3A receptor null mice (macrophages: control; 734 ± 60 cells per mm2, IM; 2945 ± 330, neutrophil: control; 9 ± 10, IM; 1076 ± 412). The anti-inflammatory actions of ondansetron (1 mg·kg−1 s.c.) were absent from 5-HT3A receptor null mice with IM treatment (macrophages; IM + ondansetron; 3327 ± 261, neutrophils; IM + ondansetron; 3327 ± 399, n = 4–5).
Ondansetron inhibits the mRNA expression of inflammatory mediators induced by IM
We investigated the effect of ondansetron on the mRNA expression of inflammatory mediators induced 3 h after IM, because expression reached a maximum around 3–6 h after IM, as previously described (Tsuchida et al., 2011). IM up-regulated the mRNA expression of the chemokine CCL2, IL-1β, IL-6 and TNF-α, and tended to increase iNOS mRNA expression (Figure 3). Ondansetron significantly reduced the expression of CCL2, IL-1β, IL-6 and TNF-α, and tended to inhibit iNOS. On the other hand, IM decreased the mRNA for IL-10 and ondansetron tended to reverse this decrease.
Figure 3.

Effects of ondansetron (Ond; 1 mg·kg−1) on mRNA expression of inflammatory mediators in the muscle layer of intestine from POI mice. Messenger RNA levels of CCL2, IL-1β, IL-6, TNF-α, IL-10 and iNOS (A–F). Data shown are means ±SEM from four independent experiments. *P < 0.05, **P < 0.01; significantly different from control. #P < 0.05, ##P < 0.01; significantly different from POI.
Ondansetron ameliorates delayed gastrointestinal transit induced by IM
Intestinal inflammation induced by IM delays gastric emptying and intestinal transit in POI (Kalff et al., 2000; Schwarz et al., 2001). We therefore examined the effect of ondansetron on gastrointestinal dysmotility induced by IM. About 10% of orally administered labelled Phenol Red remained within the stomach of the control group, whereas 90% was transported down the intestine to the distal end of the ileum, peaking at SI5-8 (Fig. 4A). On the other hand, about 30% of Phenol Red remained in the stomach and 70% was transported from SI1 to SI4 in the POI mice, indicating delayed gastrointestinal transit (Fig. 4B). The average calculated geometric centres of distribution are shown in Figure 4D. Ondansetron significantly prevented the delayed intestinal transit caused by IM, in which 10% of Phenol Red remained in the stomach, while 90% of the transported Phenol Red content moved between SI5 and Cec (Fig. 4C). The average geometric centre was also restored to a value similar to that of the control, suggesting that ondansetron normalized the gastrointestinal dysmotility induced by IM in POI mice (Fig. 4D). Ondansetron alone in control mice did not affect gastrointestinal transit (geometric centre, 7.4 ± 0.85; n = 4).
Figure 4.

Ameliorative action of ondansetron (Ond; 1 mg·kg−1) on gastrointestinal transit in a mouse model of POI. Data shown are means ± SEM of ratio (%) of Phenol Red content (A–C). Geometric centre calculated from A to C (D). **P < 0.01; significantly different from control. ##P < 0.01; significantly different from POI. Data shown are means ± SEM from four independent experiments.
Most mucosal and infiltrated muscularis macrophages do not express 5-HT3 receptor, whereas many adherent peritoneal macrophages on the serosal layer of the inflamed intestine express these receptors
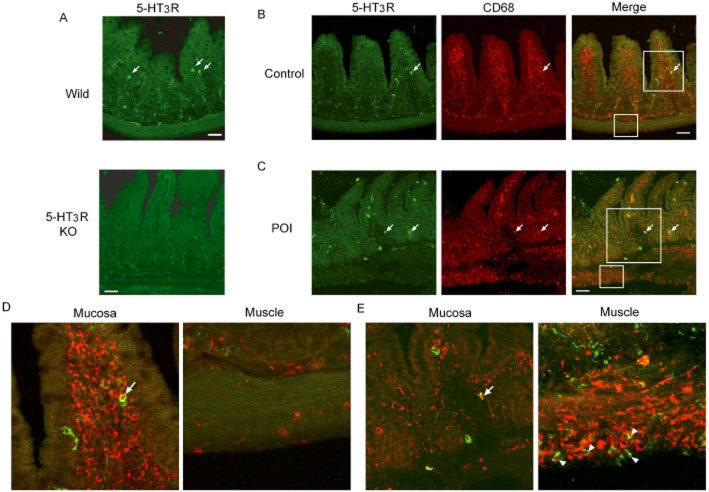
At first, we examined the specificity of the 5-HT3 receptorantibody. Figure 5A showed immunohistochemistry of 5-HT3 receptor-positive cells in the small intestines of wild-type and 5-HT3A receptor null mice. In ileal mucosal layer of wild-type mice, leukocytes, with a rounded shape, immuno-positive for 5-HT3 receptors were randomly detected. These immuno-positive cells were not seen in similar ileal samples from 5-HT3A receptor null mice, suggesting that this antibody specifically detects 5-HT3A receptors expressed in leukocytes. Many CD68-positive resident macrophages were found in the mucosal layer, in contrast to those in the muscle layer of control ileum (Fig. 5B and D). Among CD68-positive macrophages detected in the mucosal layer, a very minor proportion expressed 5-HT3 receptors. The total number of CD68-positive mucosal resident macrophages including those expressing 5-HT3 receptors did not change in the intestinal wall of the POI mice, compared with controls (Fig. 6B and C). As shown in Figure 5C and E, many CD68-positive infiltrating macrophages were detected in the myenteric plexus region at 24 h after IM. Although mRNA for 5-HT3 receptors in the smooth muscle layer of POI mice at 24 h after IM and controls were similar (Fig. 6A), some CD68-positive cells also expressing 5-HT3 receptors, were found around the serosal area of the inflamed muscle layer (Fig. 5E). The summary results also showed an increased proportion of 5-HT3 receptor-positive cells in the total CD68-positive cells, found in the inflamed muscle layer in POI mice (Fig. 6C). In addition, we confirmed that many CD68-positive macrophages seemed to adhere to the serosal surface in horizontal sections of the intestinal wall inflamed by IM. In addition, some CD68-positive macrophages expressed 5-HT3 receptors (Fig. 6D).
Figure 5.
Double-staining for 5-HT3R and CD68-positive macrophages in ileum of mice. (A) Examination of specificity of anti-5-HT3 receptor antibody. Upper panel or lower panel shows immunohistochemistry by anti-5-HT3 receptor antibody in ileum of wild-type mice or 5-HT3A receptor null mice respectively. Each picture shows a typical result from three independent experiments using two mice. (B) Double-staining of 5-HT3 receptor and CD68 in the ileum of control mice and POI mice. Arrow shows double-positive cells. Typical results from four independent experiments are shown. (D and E) Higher magnification pictures in both mucosal and muscle layer of ileum in control mice (D) or POI mice (E). Each picture was magnified from square area of (B) and (C). Arrow head and arrow indicate double-positive cells. Green or red stain indicates 5-HT3 receptors or CD68 respectively. Scale bar shows 50 μm.
Figure 6.
Expression of mRNA for 5-HT3 receptors in muscle layer, quantification of 5-HT3 receptors and CD68 double-positive cells in intestine of control mice or POI mice.and double staining for 5-HT3 receptors and CD68 in the serosal surface of intestinal wall in longitudinal section of ileum in POI mice. (A) Expression of mRNA for 5-HT3 receptors in muscle layer. Data shown are means ± SEM from four independent experiments. (B) Quantification of CD68-positive cells of intestinal mucosal and muscle layer in control mice and POI mice. Cell number was calculated per 0.25 mm2. Data shown are mean ±SEM from four independent experiments. **P < 0.01; significantly different from control. (C) 5-HT3 receptor-positive cells as proportion of CD68-positive cells. Data shown are mean ±SEM from four independent experiments. ***P < 0.001; significantly different from control. (D) Green or red stain indicates 5-HT3 receptor- or CD68-positive macrophages respectively. High-magnification images of serosal surface of intestinal wall in longitudinal section are typical of control ileum and ileum at 24 h after IM. Bar, 20 μm. Arrows show typical merged cells stained for both 5-HT3 receptors and CD68.
Ondansetron inhibits mRNA expression of IM-induced inflammatory mediators in peritoneal macrophages
We investigated which cells found in the peritoneal cavity expressed 5-HT3 receptors. We detected expression of mRNA for 5-HT3 receptors on peritoneal macrophages, but not on peritoneal mast cells (Fig. 7A). We were also able to detect cells immuno-positive for 5-HT3A receptors among the adherent peritoneal cells. Over 80% of F4/80 positive cells were 5-HT3 receptor-positive (Fig. 7B). These data indicated that these macrophages could be targets of inflammation induced through the activation of 5-HT3 receptor in POI mice.
Figure 7.
Expression of mRNA for 5-HT3A receptors in peritoneal mast cells and macrophages, effects of ondansetron (Ond) on expression of mRNA for inflammatory mediators in peritoneal macrophages, and 5-HT content in peritoneal lavage. (A) Expression of 5-HT3A receptor mRNA in peritoneal cells. Peritoneal macrophages and mast cells were separated from peritoneal cells as described. (B) Immunohistochemistry of 5-HT3A receptors and F4/80 of adherent peritoneal cells. Typical pictures are shown out of four independent experiments. (C–H) Effect of ondansetron (Ond) on CCL2, IL-1β, IL-6, TNF-α, IL-10 and iNOS mRNA expressed by peritoneal macrophages isolated from POI mice. Amplification of cDNA derived from RNA in mouse peritoneal cells was performed by real-time PCR. (H) 5-HT content in mouse peritoneal lavage. Each dot shows the data from one mouse. Columns show means ±SEM from four independent experiments. *P < 0.05, **P < 0.01; significantly different from control. #P < 0.05, ##P < 0.01; significantly different from POI. Control; n = 8, POI; n = 11.
We then assessed the effect of ondansetron on the mRNA expression of CCL2, IL-1β, IL-6, iNOS, TNF-α and IL-10 in peritoneal cells from POI mice (Fig. 7C–H). These mRNA were increased at 3 h after IM and this increased expression was inhibited by treatment with ondansetron. By contrast, the mRNA for IL-10 was decreased by POI and reversed by ondansetron treatment (Fig. 7G).
These results raised the issue of whether IM increased the 5-HT content in the peritoneal cavity. We therefore measured the 5-HT content in peritoneal lavage fluid using an elisa (Fig. 7I) and found that it was increased well above the control level of <5 ng·mg−1, at 24 h after IM.
Discussion
A number of 5-HT3 receptor antagonists are marketed as antiemetic drugs (Celio et al., 2012; Miura et al., 2013). However, recent studies have shown that 5-HT3 receptor antagonists have anti-inflammatory effects against trinitrobenzenesulfonic acid (TNBS)-induced colitis (Linden et al., 2003), haemorrhagic shock (Liu et al., 2011) and peritonitis. Local inflammation of the intestinal wall is important to induce POI (Engel et al., 2010; Snoek et al., 2012) and its amelioration improves intestinal dysmotility and results in the prevention of POI. We found here that the 5-HT3 receptor antagonist ondansetron inhibited the infiltration of inflammatory cells as well as the mRNA expression of inflammatory mediators and normalized the gastrointestinal dysmotility induced by IM in a mouse model of POI. Thus, we suggest that 5-HT3 receptor antagonists could be potent drugs for the treatment or prevention of POI.
5-HT is an important endogenous mediator of gastrointestinal inflammatory diseases, because inflammatory responses in a model of colitis are reduced in TPH1-deficient mice that have reduced 5-HT levels in the gastrointestinal tract (Ghia et al., 2009). Immune cells including monocytes, dendritic cells and T-cells (Fiebich et al., 2004) as well as the CNS and peripheral nerve system (Maricq et al., 1991) express 5-HT3 receptors. However, which of the immune reactive cells are targets of 5-HT3 receptor antagonists in the amelioration of POI has remained obscure. The expression of 5-HT3 receptor mRNA in the muscle layer of the intestine did not change between control and our model of POI. A minor population of CD68 and 5-HT3 receptor double-positive mucosal macrophages might be target cells that could ameliorate intestinal mucositis induced by 5-fluorouracil (Yasuda et al., 2013). Our immunohistochemical analysis at high magnification also detected minor populations of 5-HT3 receptor-positive mucosal resident macrophages in the intestine of control mice. However, the populations of 5-HT3 receptor-positive mucosal macrophages did not change at 24 h after IM. In addition, the main inflammatory response in POI is induced in the muscle, rather than in the mucosal layer (Fig. 5). Therefore, the small proportion of mucosal macrophages expressing 5-HT3 receptors are unlikely to be the major targets of 5-HT3 receptor antagonists in the amelioration of POI. In the muscle layer in contrast to mucosal layer, many CD68-positive macrophages infiltrated into the muscle layer (Fig. 5), but only a minor proportion of these CD68-positive macrophages around the serosal area expressed 5-HT3 receptors.
Surgical manipulation injured the peritoneal cavity without infection. We therefore postulated that peritoneal cells such as peritoneal macrophages and mast cells might also be involved in the induction of inflammation after IM. We found that peritoneal macrophages expressed 5-HT3 receptor mRNA whereas mast cells did not, and that peritoneal CD68-positive macrophages adhered to the serosal region of the inflamed intestinal wall in our model of POI, as shown in Fig. 6D. In addition, ondansetron reduced the mRNA for IL-1β, CCL2, IL-6, iNOS, TNF-α, induced by IM in peritoneal cells. These findings indicate that peritoneal macrophages play a pivotal role in inducing inflammation in POI, in addition to macrophages expressing 5-HT3 receptors that infiltrated the muscle layer. We found that 5-HT3 receptor antagonists inhibited the inflammatory actions of these target cells, which in turn ameliorated POI. This finding raised the issue of whether the 5-HT content was increased by IM in the peritoneal cavity. The elevated 5-HT content was sustained in the peritoneal cavity for at least until 24 h after IM. Peritoneal macrophages and mast cells are likely sources of 5-HT secretion after IM, because these cells have been reported to synthesize and release 5-HT (Kim, 2012).
Interestingly, in the present study, mRNA for IL-10 was decreased in POI mice and ondansetron tended to restore these levels, suggesting that changes in the anti-inflammatory cytokines such as IL-10 could also contribute to the anti-inflammatory effects of 5-HT3 receptor antagonists. However, the changes in IL-10 mRNA were much smaller than those for the pro-inflammatory cytokines and further investigation will be required to clarify the point.
It has been reported that the myenteric neurons expressing 5-HT3 receptors comprise both motor and sensory neurons (Gershon et al., 1965). The activation of sensory nerves can induces anti-inflammatory effects via the afferent vagal pathway (Bonaz et al., 2013; Sun et al., 2013). Stimulation of the afferent vagal nerve activates the hypothalamic–pituitary–adrenal axis, which in turn increases the amount of glucocorticoid hormone released from the adrenal gland to inhibit inflammation. Thus, stimulation of vagal afferent nerves should ameliorate POI (Tracey, 2009). However, the 5-HT3 receptor antagonists inhibit sensory neurons (Panteleev et al., 2013), indicating that submucosal neural cells that express 5-HT3 receptors are not the target cells of the anti-inflammatory action of 5-HT3 receptor antagonists.
The selective 5-HT3 receptor antagonist tropisetron is a partial agonist at α7nAChRs (Hibbs et al., 2009; Shi et al., 2013). Stimulation of α7nAChRs on leukocytes induces anti-inflammatory action in animal models (Pena et al., 2010; Costa et al., 2012) including POI (Tsuchida et al., 2011). Thus, whether anti-inflammatory actions induced by 5-HT3 receptor antagonists could be mediated through 5-HT3 receptors and/or α7nAChRs remains unclear. In the present study, the anti-inflammatory actions of ondansetron were absent in the POI model using 5-HT3A receptor null mice, indicating that, at least ondansetron, exerted its anti-inflammatory actions via 5-HT3A receptors. Further detailed investigation is needed to assess the contribution of activation of α7nAChRs to the anti-inflammatory actions of tropisetron or palonosetron.
Cell signalling mechanisms mediating the anti-inflammatory actions of 5-HT3 receptor antagonists have recently been described. Ondansetron induces p-38MAPK phosphorylation, which leads to reduced mRNA expression of IL-6 and TNF-α (Liu et al., 2011). Tropisetron inhibits T-cell activation through the calcineurin pathway (Vega Lde et al., 2005) but whether this effect is due to α7nAChR activation or to 5-HT3 receptor antagonism remains to be determined by further studies.
In conclusion, 5-HT3 receptor stimulation plays an important role in the induction of inflammation in POI. Elevated 5-HT levels in the peritoneal cavity can stimulate peritoneal macrophages, which adhere to the intestinal wall where they become involved in surgical injury-mediated inflammation, resulting in the induction of POI. In addition to anti-emetic drugs, 5-HT3 receptor antagonists might serve as novel agents for treating POI. Peritoneal macrophages expressing 5-HT3 receptor could be the candidate targets of efforts to ameliorate the inflammation associated with POI.
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education (to M. H., no. 24248050 and no. 25660224 and to H. O., no. 25252055).
Glossary
- IM
intestinal manipulation
- iNOS
inducible NOS
- MPO
myeloperoxidase
- POI
post-operative ileus
- TBS
Tris-buffered saline
- α7nAChR
α7nicotinic ACh receptors
Author contributions
M. H. and T. Ma. planned and designed experiments. T. Ma., K. M. and K. H. performed all experiments. T. Ma., K. M. and M. H. wrote the paper. S. H., S. I., T. Mu., H. T., H. O. and M. H. reviewed and discussed the data. M. K. and S. S. provided 5-HT3aR null mice and discussed about specificity of anti-5-HT3aR antibody.
Conflict of interest
The authors have no conflicts of interest.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-Gated Ion Channels. Br J Pharmacol. 2013a;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, McGrath JC, et al. The Concise Guide to PHARMACOLOGY 2013/14: overview. Br J Pharmacol. 2013b;170:1449–1458. [Google Scholar]
- Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterol Motil. 2004;16(Suppl. 2):54–60. doi: 10.1111/j.1743-3150.2004.00558.x. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Picq C, Sinniger V, Mayol JF, Clarencon D. Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol Motil. 2013;25:208–221. doi: 10.1111/nmo.12076. [DOI] [PubMed] [Google Scholar]
- Candiotti KA, Nhuch F, Kamat A, Deepika K, Arheart KL, Birnbach DJ, et al. Granisetron versus ondansetron treatment for breakthrough postoperative nausea and vomiting after prophylactic ondansetron failure: a pilot study. Anesth Analg. 2007;104:1370–1373. doi: 10.1213/01.ane.0000261474.85547.8b. [DOI] [PubMed] [Google Scholar]
- Celio L, Agustoni F, Testa I, Dotti K, de Braud F. Palonosetron: an evidence-based choice in prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Tumori. 2012;98:279–286. doi: 10.1177/030089161209800301. [DOI] [PubMed] [Google Scholar]
- Costa R, Motta EM, Manjavachi MN, Cola M, Calixto JB. Activation of the α7 nicotinic acetylcholine receptor (α7nAChR) reverses referred mechanical hyperalgesia induced by colonic inflammation in mice. Neuropharmacology. 2012;63:798–805. doi: 10.1016/j.neuropharm.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Durk T, Panther E, Muller T, Sorichter S, Ferrari D, Pizzirani C, et al. 5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes. Int Immunol. 2005;17:599–606. doi: 10.1093/intimm/dxh242. [DOI] [PubMed] [Google Scholar]
- El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–419. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- Engel DR, Koscielny A, Wehner S, Maurer J, Schiwon M, Franken L, et al. T helper type 1 memory cells disseminate postoperative ileus over the entire intestinal tract. Nat Med. 2010;16:1407–1413. doi: 10.1038/nm.2255. [DOI] [PubMed] [Google Scholar]
- Fiebich BL, Akundi RS, Seidel M, Geyer V, Haus U, Muller W, et al. Expression of 5-HT3A receptors in cells of the immune system. Scand J Rheumatol. 2004;119:9–11. [PubMed] [Google Scholar]
- Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Drakontides AB, Ross LL. Serotonin: synthesis and release from the myenteric plexus of the mouse intestine. Science. 1965;149:197–199. doi: 10.1126/science.149.3680.197. [DOI] [PubMed] [Google Scholar]
- Ghia JE, Li N, Wang H, Collins M, Deng Y, El-Sharkawy RT, et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–1660. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Heimes K, Feistel B, Verspohl EJ. Impact of the 5-HT3 receptor channel system for insulin secretion and interaction of ginger extracts. Eur J Pharmacol. 2009;624:58–65. doi: 10.1016/j.ejphar.2009.09.049. [DOI] [PubMed] [Google Scholar]
- Hibbs RE, Sulzenbacher G, Shi J, Talley TT, Conrod S, Kem WR, et al. Structural determinants for interaction of partial agonists with acetylcholine binding protein and neuronal α7 nicotinic acetylcholine receptor. EMBO J. 2009;28:3040–3051. doi: 10.1038/emboj.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Yakel JL. The 5-HT3 receptor channel. Annu Rev Physiol. 1995;57:447–468. doi: 10.1146/annurev.ph.57.030195.002311. [DOI] [PubMed] [Google Scholar]
- Jensen BM, Swindle EJ, Iwaki S, Gilfillan AM. Generation, isolation, and maintenance of rodent mast cells and mast cell lines. Curr Protoc Immunol. 2006;74 doi: 10.1002/0471142735.im0323s74. Unit 3.23.1–3.23.13. [DOI] [PubMed] [Google Scholar]
- Kalff JC, Carlos TM, Schraut WH, Billiar TR, Simmons RL, Bauer AJ. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology. 1999;117:378–387. doi: 10.1053/gast.1999.0029900378. [DOI] [PubMed] [Google Scholar]
- Kalff JC, Schraut WH, Billiar TR, Simmons RL, Bauer AJ. Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology. 2000;118:316–327. doi: 10.1016/s0016-5085(00)70214-9. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2698–2709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- Kim K. Neuroimmunological mechanism of pruritus in atopic dermatitis focused on the role of serotonin. Biomol Ther. 2012;20:506–512. doi: 10.4062/biomolther.2012.20.6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyosue M, Fujisawa M, Kinoshita K, Hori M, Ozaki H. Different susceptibilities of spontaneous rhythmicity and myogenic contractility to intestinal muscularis inflammation in the hapten-induced colitis. Neurogastroenterol Motil. 2006;18:1019–1030. doi: 10.1111/j.1365-2982.2006.00841.x. [DOI] [PubMed] [Google Scholar]
- Klimetzek V, Remold HG. The murine bone marrow macrophage, a sensitive indicator cell for murine migration inhibitory factor and a new method for their harvest. Cell Immunol. 1980;53:257–266. doi: 10.1016/0008-8749(80)90327-5. [DOI] [PubMed] [Google Scholar]
- Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–G216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- Liu FC, Liu FW, Yu HP. Ondansetron attenuates hepatic injury via p38 MAPK-dependent pathway in a rat haemorrhagic shock model. Resuscitation. 2011;82:335–340. doi: 10.1016/j.resuscitation.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maleki-Dizaji N, Eteraf-Oskouei T, Fakhrjou A, Maljaie SH, Garjani A. The effects of 5HT3 receptor antagonist granisetron on inflammatory parameters and angiogenesis in the air-pouch model of inflammation. Int Immunopharmacol. 2010;10:1010–1016. doi: 10.1016/j.intimp.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- Mattei P, Rombeau JL. Review of the pathophysiology and management of postoperative ileus. World J Surg. 2006;30:1382–1391. doi: 10.1007/s00268-005-0613-9. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, Watanabe S, Sato K, Makino M, Kobayashi O, Miyao H, et al. The efficacy of triplet antiemetic therapy with 0.75 mg of palonosetron for chemotherapy-induced nausea and vomiting in lung cancer patients receiving highly emetogenic chemotherapy. Support Care Cancer. 2013;21:2575–2581. doi: 10.1007/s00520-013-1835-2. [DOI] [PubMed] [Google Scholar]
- Nagakura Y, Naitoh Y, Kamato T, Yamano M, Miyata K. Compounds possessing 5-HT3 receptor antagonistic activity inhibit intestinal propulsion in mice. Eur J Pharmacol. 1996;311:67–72. doi: 10.1016/0014-2999(96)00403-7. [DOI] [PubMed] [Google Scholar]
- Oshima S, Fujimura M, Fukimiya M. Changes in number of serotonin-containing cells and serotonin levels in the intestinal mucosa of rats with colitis induced by dextran sodium sulfate. Histochem Cell Biol. 1999;112:257–263. doi: 10.1007/s004180050445. [DOI] [PubMed] [Google Scholar]
- Panteleev SS, Busygina II, Liubashina OA. The effects of selective 5HT3 receptor blockade on physiological markers of abdominal pain in awake dogs. Ross Fiziol Zh Im I M Sechenova. 2013;99:471–483. [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena G, Cai B, Liu J, van der Zanden EP, Deitch EA, de Jonge WJ, et al. Unphosphorylated STAT3 modulates α7 nicotinic receptor signaling and cytokine production in sepsis. Eur J Immunol. 2010;40:2580–2589. doi: 10.1002/eji.201040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M, Matthews JB. Deflating postoperative ileus. Gastroenterology. 1999;117:489–492. doi: 10.1053/gast.1999.0029900489. [DOI] [PubMed] [Google Scholar]
- Sallam HS, Oliveira HM, Gan HT, Herndon DN, Chen JD. Ghrelin improves burn-induced delayed gastrointestinal transit in rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R253–R257. doi: 10.1152/ajpregu.00100.2006. [DOI] [PubMed] [Google Scholar]
- Schwarz NT, Kalff JC, Turler A, Engel BM, Watkins SC, Billiar TR, et al. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology. 2001;121:1354–1371. doi: 10.1053/gast.2001.29605. [DOI] [PubMed] [Google Scholar]
- Shi C, Yu R, Shao S, Li Y. Partial activation of α7 nicotinic acetylcholine receptors: insights from molecular dynamics simulations. J Mol Model. 2013;19:871–878. doi: 10.1007/s00894-012-1618-6. [DOI] [PubMed] [Google Scholar]
- Snoek SA, Dhawan S, van Bree SH, Cailotto C, van Diest SA, Duarte JM, et al. Mast cells trigger epithelial barrier dysfunction, bacterial translocation and postoperative ileus in a mouse model. Neurogastroenterol Motil. 2012;24:172–184. doi: 10.1111/j.1365-2982.2011.01820.x. [DOI] [PubMed] [Google Scholar]
- Spiller RC. Irritable bowel syndrome. Br Med Bull. 2004;72:15–29. doi: 10.1093/bmb/ldh039. [DOI] [PubMed] [Google Scholar]
- Sun P, Zhou K, Wang S, Li P, Chen S, Lin G, et al. Involvement of MAPK/NF-κB signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS ONE. 2013;8:e69424. doi: 10.1371/journal.pone.0069424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima T, Murata T, Aritake K, Urade Y, Michishita M, Matsuoka T, et al. EP2 and EP4 receptors on muscularis resident macrophages mediate LPS-induced intestinal dysmotility via iNOS upregulation through cAMP/ERK signals. Am J Physiol Gastrointest Liver Physiol. 2012;302:G524–G534. doi: 10.1152/ajpgi.00264.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida Y, Hatao F, Fujisawa M, Murata T, Kaminishi M, Seto Y, et al. Neuronal stimulation with 5-hydroxytryptamine 4 receptor induces anti-inflammatory actions via α7nACh receptors on muscularis macrophages associated with postoperative ileus. Gut. 2011;60:638–647. doi: 10.1136/gut.2010.227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turler A, Kalff JC, Moore BA, Hoffman RA, Billiar TR, Simmons RL, et al. Leukocyte-derived inducible nitric oxide synthase mediates murine postoperative ileus. Ann Surg. 2006;244:220–229. doi: 10.1097/01.sla.0000229963.37544.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega Lde L, Munoz E, Calzado MA, Lieb K, Candelario-Jalil E, Gschaidmeir H, et al. The 5-HT3 receptor antagonist tropisetron inhibits T cell activation by targeting the calcineurin pathway. Biochem Pharmacol. 2005;70:369–380. doi: 10.1016/j.bcp.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Wehner S, Behrendt FF, Lyutenski BN, Lysson M, Bauer AJ, Hirner A, et al. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut. 2007;56:176–185. doi: 10.1136/gut.2005.089615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Kato S, Yamanaka N, Iimori M, Matsumoto K, Utsumi D, et al. 5-HT3 receptor antagonists ameliorate 5-fluorouracil-induced intestinal mucositis by suppression of apoptosis in murine intestinal crypt cells. Br J Pharmacol. 2013;168:1388–1400. doi: 10.1111/bph.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]