Abstract
The most important goal in the treatment of patients with diabetes is to prevent the risk of cardiovascular disease (CVD), the first cause of mortality in these subjects. Thiazolidinediones (TZDs), a class of antidiabetic drugs, act as insulin sensitizers increasing insulin-dependent glucose disposal and reducing hepatic glucose output. TZDs including pioglitazone, rosiglitazone and troglitazone, by activating PPAR-γ have shown pleiotropic effects in reducing vascular risk factors and atherosclerosis. However, troglitazone was removed from the market due to its hepatoxicity, and rosiglitazone and pioglitazone both have particular warnings due to being associated with heart diseases. Specific genetic variations in genes involved in the pathways regulated by TDZs have demonstrated to modify the variability in treatment with these drugs, especially in their side effects. Therefore, pharmacogenomics and pharmacogenetics are an important tool in further understand intersubject variability per se but also to assess the therapeutic potential of such variability in drug individualization and therapeutic optimization.
Keywords: cardiovascular disease, diabetes, pharmacogenetics, pharmacogenomics, single nucleotide polymorphisms, thiazolidinediones, vascular risk factors
Rosiglitazone, pioglitazone & troglitazone
Diabetes is a pandemic disease particularly in the industrialized countries. The major cause of death in these patients (>65%) are related to cardiovascular disease (CVD). Therefore, given the significance of morbidity and mortality associated with diabetes, and its complications, identification and optimization of an appropriate treatment of the pathological condition is crucial. Over the last few decades, there have been a significant number of new agents developed for the treatment of Type 2 diabetes (T2D) with the final goal to regulate the blood level of glucose and therefore to prevent the risk for CVD. These drugs include sulfonylureas, biguanides, α-glucosidase inhibitors and dipeptidyl peptidase IV inhibitors (DPP-IV) [1] . Among those, thiazolidinediones (TZDs) are a class of antidiabetic drugs that act as insulin sensitizers increasing insulin-dependent glucose disposal, and reducing hepatic glucose output. Importantly, TZDs have been demonstrated to reduce insulin resistance not only in a state of T2D but also in nondiabetic conditions such as obesity, which is another important risk factor for CVD [2,3] . Further, it has been demonstrated that TZDs have a capacity to lower glycated hemoglobin (A1c) by approximately 0.5–1.4% [4] . Since their ability in decreasing hyperglycemia and their pleiotropic effects in preventing vascular diseases is substantial, the TZDs have received particular attention of the scientific community and are discussed below in the present review.
To prepare the present manuscript, three investigators sequentially selected the articles and evaluated study quality independently. Selected articles were searched on the PubMed, and Cochrane electronic databases. The search was limited to studies published in English. The principal key words used for the research were: pharmacogenetics, pharmacogenomics, genetics, single nucleotide polymorphisms, genetics, diabetes, metabolic syndrome, cardiovascular disease, thiazolidinediones (rosiglitazone, pioglitazone and troglitazone), side effects, vascular risk factors, atherosclerosis, human studies, animal studies and obesity, in combination between them. We excluded abstracts presented to conferences, case reports and case series articles.
As seen with the majority of other drugs, interindividual variability in response to the treatment with TZDs has been reported to be pronounced. The observed variability defines the importance of investigating the impact of individual genetic makeup on the pharmacological response to these drugs, especially to their side effects [5] . The three drugs in this hypoglycemic class introduced in the market are rosiglitazone, pioglitazone and troglitazone. Troglitazone was removed from the US market in 2000 due to its hepatoxicity. However, besides troglitazone also rosiglitazone and pioglitazone both have particular warnings due to being associated with congestive heart failure [6] . Recently, rosiglitazone is being withdrawn in Europe and in New Zealand due to the increase of myocardial infarction [7] and bone fractures incidence [8] , while it still used in US together with metformin. However, according to the American Heart Association/American Diabetes Association consensus statement on TZDs use, their beneficial effects on vascular risk factors support their prescription to diabetic subjects at high risk for CVD [9] .
Unfortunately, it remains unclear whether side effects such as hepatotoxicity, myocardial infarct and heart failure are related to the characteristic of these drugs as the unique tocopherol side chain of troglitazone, or are related with other unknown mechanisms [10] . Therefore, the argument has been debated in scientific community. Several clinical trials such as the PROactive Study [11] , and further meta-analyses [12] clearly established that therapy with TZDs reduces mortality for cardiovascular events, especially myocardial infarction and stroke. However, although these studies highlighted the positive effects of TZDs against both hyperglycemia and CVD, other recent studies raised concerns on possible augmented incidence of important diseases such as bladder tumors after long use of these drugs, especially in certain individuals [13] . For this reason, the field of pharmacogenetics and pharmacogenomics may improve the understanding in the beneficial properties of TZDs, and the management of CVD risk prevention in diabetic patients.
Mechanism of action: role of PPARs & PPAR-γ
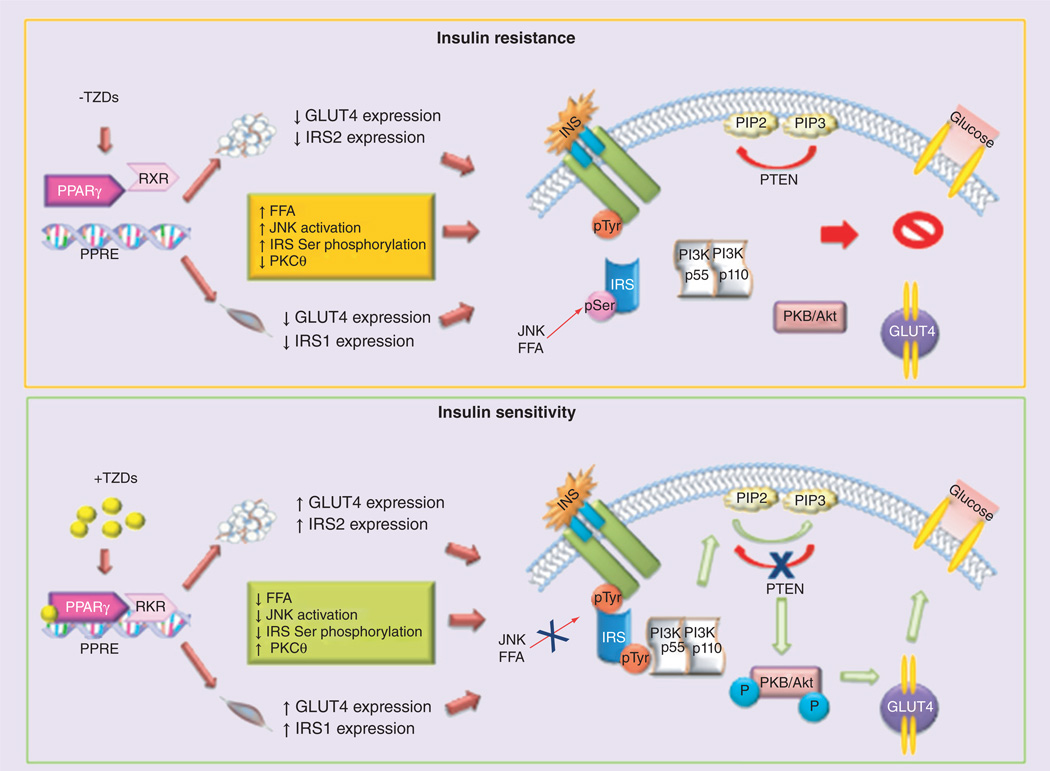
TZDs act mainly through the PPAR-γ [3,14]. The PPA R- γ is a member of the PPAR nuclear receptor superfamily of ligand-inducible transcription factors. In mammals, there are three PPARs: PPARα, PPARβ/δ and PPAR-γ [15]. By binding to PPAR responsive regulatory elements of a target gene (PPRE, a direct repeat of ‘AGGTCA’ gapped by a nucleotide) as obligate heterodimers with RXR, activated by the endogenous agonist 9-cis retinoic acid, the PPARs control the expression of genetic networks involved in adipogenesis, lipid metabolism, inflammation, and maintenance of metabolic homeostasis [15]. Ligand binding as TZDs induces a conformational change in the receptor that allows for differential recruitment of cofactors and subsequent modulation of PPAR-γ activity [15]. In absence of a ligand, the PPAR-γ:RXR complex can recruit corepressor complexes and bind to PPRE, suppressing the transcription of target genes [15]. Adipose tissue is the major mediator of PPAR-γ action on insulin sensitivity. PPAR-γ activation in mature adipocytes induces the expression of a number of genes involved in the insulin signaling cascade such as GLUT4 and CAP, thereby improving insulin sensitivity in patients with T2D [16]. Although no complete PPRE has been found in the GLUT4 promoter, PPAR-γ and its heterodimers partner RXR have been found to bind and repress the promoter activity of GLUT4. The repression is augmented in the presence of the natural ligand, 15D–prostaglandin J2, but completely alleviated by rosiglitazone [17]. This may be a novel mechanism by which a PPAR-γ ligand exerts antidiabetic effect by detaching the PPAR-γ transcription complex from the promoter, thereby increasing the expression of target genes. PPAR-γ agonists potentiate insulin signaling and improve insulin sensitivity at various cellular steps [18], mainly by activation of PI3K, PIP3, and serine/ threonine kinases, including Akt (Akt1 and 2) pathway [18]. In Figure 1 is schematically reported the mechanism by which TZDs induce insulin sensitivity. Treatment with troglitazone increases insulin-stimulated IRS-1-associated PI3K and Akt activity in skeletal muscle biopsies from T2D patients, and enhanced Akt phosphorylation in skeletal muscle from glucose-tolerant, insulin-resistant, first-degree relatives of T2D patients [19]. Moreover, PPAR-γ activation controls the expression of numerous factors secreted from adipose tissue that greatly influence insulin sensitivity: levels of circulating adipocytokines or adipokines, TNF-α, leptin, resistin, 11 beta-hydroxysteroid dehydrogenase 1 and adiponectin [2,19].
Figure 1. Schematic representation of thiazolidinediones mechanism of action.
TZDs acting mainly through PPAR-γ by binding to PPRE as obligate heterodimers with RXR, control the expression of several genetic networks. In particular, TZDs increase insulin-stimulated IRS-1/2-associated PI3K and Akt activity in skeletal muscle and adipose tissue, enhancing Akt phosphorylation and subsequently GLUT4 translocation.
FFA: Free fatty acids; Ins: Insulin; pTyr: phospho tyrosine; TZD: Thiazolidinedione.
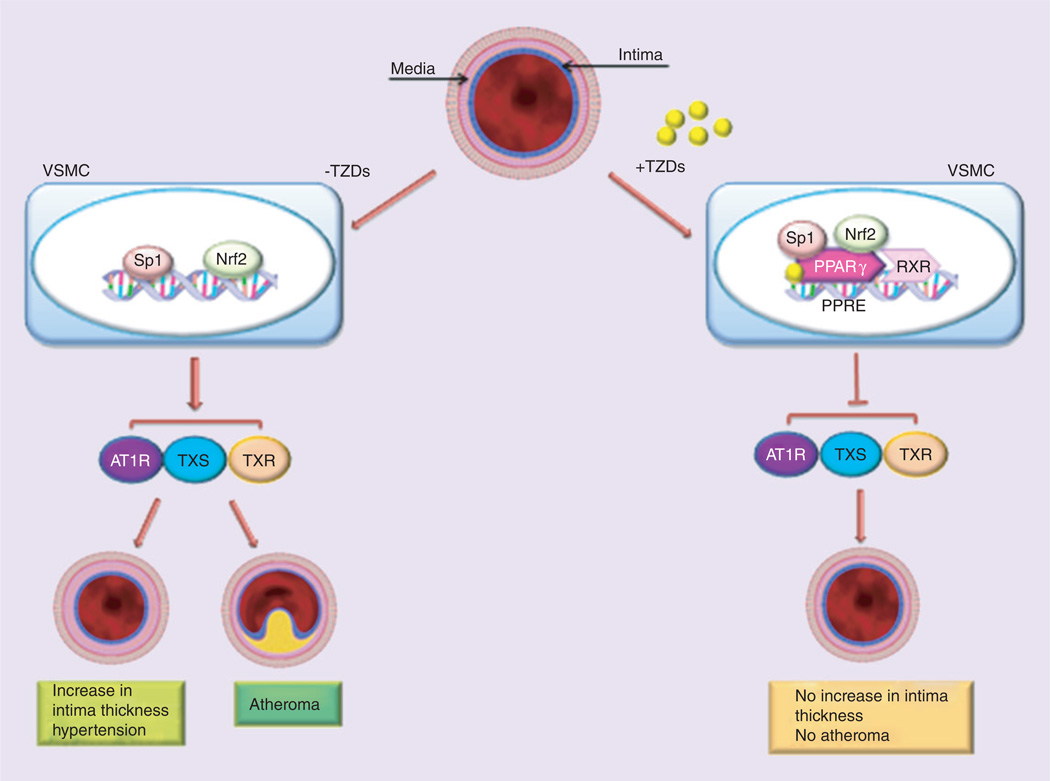
Pleiotropic beneficial effects of TZDs as PPAR-γ agonists have been also demonstrated in the vasculature and against vascular risk factors [15]. PPAR-γ agonists have been shown to lower blood pressure (BP) in animals and humans by suppressing the renin-angiotensin (Ang)-aldosterone system (RAAS) including the inhibition of ATIR expression, Ang-II-mediated signaling pathways and Ang-II-induced adrenal aldosterone synthesis/secretion [20]. PPAR-γ agonists also inhibit the progression of atherosclerosis in animals through a pathway involving the suppression of RAAS and the thromboxane A2 system [20]. Interestingly, TZDs treatment, dose-dependently decrease the expression of AT1R gene [21]. Recently, it was also shown that PPAR-γ agonists exert an inhibitory effect on Ang-II-induced aldosterone synthase expression and aldosterone secretion [22]. Moreover, pharmacological activation of PPAR-γ receptors is noted to suppress the activity of TXS and its receptor TXR expression. These biochemical changes are noted to be involved in the development of atherosclerosis [23,24]. TZDs positively affect the vasculature by decreasing the intimal medial thickness (IMT) and inhibiting the transendothelial migration of monocytes in the vascular smooth muscle cells (VSMC) [25]. Figure 2 represents the protective mechanisms of TZDs against increase of IMT and vascular atherosclerosis.
Figure 2. Thiazolidinediones action on vasculature.
TZDs positively affect the vasculature by decreasing the intimal medial thickness and development of atherosclerosis in the vascular smooth muscle cells. TZDs exert their protective role by inhibiting the gene expression of AT1R, TXS and TXR, involved in mechanism linked with intimal medial thickness and atherosclerosis.
TZD: Thiazolidinedione; VSMC: Vascular smooth muscle cell.
Another mechanism linking TZDs with prevention of atherosclerosis is their ability, as PPAR-γ agonists, to decrease serum levels of oxLDL-cholesterol and triglycerides and increased serum levels of HDL-cholesterol, especially in T2D [26]. Increase in oxLDL has been identified as one of the main risk factor for atherosclerosis and CVD [27]. Specifically, among the TZDs, the most powerful in controlling the blood lipid profile has been shown to be pioglitazone, which compared with rosiglitazone strongly reduce the level of oxLDL [26]. Supporting these findings, an in vitro study conducted in human umbilical vein endothelial cells demonstrated as pioglitazone inhibited inflammatory response which inducing atherosclerosis by the increase of oxLDL [28].
In fact, TZDs prevent atherosclerosis also by decreasing oxidative stress and inflammation [29], which are under control of previously discussed pathways. After an acute insult like ischemia, administration of TZDs significantly increases the production of antioxidant enzymes such as catalase and SOD, increasing free radical scavenging in the periinfarct area [30]. Pretreatment of rats with pioglitazone for 7 days prior to coronary ligation significantly decreased the expression levels of inflammatory markers and the number of infiltrating macrophages in the ischemic region [31].
Animal models have clearly demonstrated that TZD by PPAR-γ activation improve insulin release by preserving pancreatic β-cell function, and reduce vascular risk factors [32]. However, these findings have not been clearly established in humans [2]. Confirming these findings, by using a quantitative real-time polymerase chain reaction (rt-PCR) Wang et al. [33] evaluated gene expression profiling to assess cardiorenal safety in the heart and kidney tissue samples in adult male myocardial infarction-induced heart failure and Zucker diabetic fatty rats treated with rosiglitazone. Although variations in the expression of some genes such as ACE, PPAR-γ, IL-6 and TNF-α. were observed, authors themselves address serious doubts about the transferability of these results obtained in animal models to clinical settings [34].
Pharmacogenomics & pharmacogenetics of rosiglitazone in diabetes
The antidiabetic effect of rosiglitazone ((RS)-5-[4-(2-[methyl (pyridin-2-yl) amino] ethoxy) benzyl] thiazoli-dine-2, 4-dione) is associated with reduction in blood glucose level mainly by its pleiotropic effects in adipose tissue [35]. It is also noted to modulate adipogenesis and affect regulation of the expression of adipocyte factors: TNF-α, IL-6, adiponectin and resistin [36]. Reports on such adipocyte factor genes have revealed that SNPs in such genetic elements are principally responsible for the interindividual and interethnic variability of the hypoglycemic efficacy of rosiglitazone [37]. The relevance of the genetic background is also amply demonstrated by studies in animal models. Using new mouse models of T2D, Pan et al. [38,39], demonstrated that the genetic background can affect adverse hepatic and cardiac response to rosiglitazone. Differential pharmacogenetics responses in the same new experimental model were identified by Leiter et al. [40]. Genetic variation in drug-metabolizing protein (CYP) genes: CYP2C8 (CYP2C8*3) and CYP2C9 (CYP2C9*3), is demonstrated to substantially contribute towards intersubject heterogeneity in terms of the pharmacokinetic behavior of rosiglitazone [41,42]. Clinical data from the Chinese diabetic population has shown that the glucose-lowering effects of rosiglitazone vary in subpopulations representing different ADIPOQ gene polymorphisms [43]. Among T2D patients treated with rosiglitazone, subjects possessing the SNP-11377 CC in the ADIPOQ responded with a greater reduction in fasting plasma glucose levels in comparison to the CG and GG genotypes [43]. Reports have shown that alleles of leptin G-2548A and TNF-α G-308A, important adipocytokines involved in mediating insulin sensitivity and glucose homeostasis, are linked with higher levels of insulin resistance in Chinese and Caucasian subjects suffering from T2D, respectively [44,45]. Liu et al. [37] observed that rosiglitazone showed a markedly increased effect in patients with the AA genotype of leptin G-2548A in terms of fasting serum insulin and postprandial serum insulin compared with the GG+GA genotype. Furthermore, they have shown a reduced efficacy in patients on rosiglitazone with GA+AA genotype of TNF-α. G-308A when compared with its GG in terms of fasting serum insulin. Gene expression background-specific effects on lipid deposition and insulin sensitivity of rosiglitazone administration has also been demonstrated by studies in rodents [46]. Furthermore, Yu et al. [47] have shown that SNPs in KCNQ1 are associated with the therapeutic response of rosiglitazone. Kang et al. [48,49] have shown that genetic variants in adiponectin are intrinsically associated with the modulation of pharmacodynamics of rosiglitazone in diabetic subjects from different populations. Besides evincing their hypoglycemic effects, TDZs also favorably modulate the lipid profile of blood, thus leading to a reduction in the progression of atherosclerosis and associated cardiovascular risk factors among diabetic patients [7,11]. Rosiglitazone treatment in populations representing UCP2–866G/A and ADRB3 Trp64Arg genotypes substantially inhibit triglyceride, LDL-cholesterol and adiponectin levels in the blood [50]. Several other variants have been reported. Genetic variants of adipocytokines modulate the therapeutic efficacy of rosiglitazone in T2D patients [51]. In an earlier study Chen et al. [52] evaluated the glucose lowering effect of rosiglitazone in newly diagnosed Chinese T2D patients. They observed that rosiglitazone treated group with rs6467136 GA+AA variant of PAX4 gene showed greater glucose lowering effects compared with GG homozygotes suggesting that rosiglitazone is more likely to achieve target fasting as well as 2-h glucose levels in GA+AA carriers compared with GG homozygotes. On the other hand another study investigating the effect of rosiglitazone in newly diagnosed T2D, observed that R219K variant of ABCA1 gene had poor rosiglitazone response compared with RR homozygotes [53]. They also reported that neither M883I nor R1587K variant of the ABCA1 gene had any effect of rosiglitazone response. Furthermore, the UCP2 −866G/A genotype is known to regulate rosiglitazone-based control of glucose homeostasis [50], and the G allele of UCP2–866G/A gene is noted to enhance the risk of obesity among diabetic individuals [54]. The ADRB3 Trp64Arg genotype is associated with rosiglitazone-related modulation of blood lipid profile [50]. Zhang et al. [55] observed that Thr394Thr and Gly482Ser SNPs in PGC-1α genes alter the rosiglitazone-induced beneficial effect on plasma lipid profile in T2D subjects. Rosiglitazone also inhibits the pathogenesis of obesity by modulating mitochondrial remodeling [56]. In addition, rosiglitazone markedly ameliorates endothelial dysfunction associated with T2D [57]. However, investigations are required to validate the relevance of existing forms of genetic polymorphisms associated with rosiglitazone-induced glucose control in terms of quantifying its effect on diabetes-associated cardiovascular complications.
Pharmacogenomics & pharmacogenetics of pioglitazone in diabetes
Pioglitazone ((RS)-5-(4-[2-(5-ethylpyridin-2-yl) ethoxy] benzyl) thiazolidine-2, 4-dione) is one of the most widely used drugs for the treatment of T2D, and is the only TZDs available in the market in several countries [58]. However, a critical understanding of the clinical outcome associated with pioglitazone-based treatment of T2D and related cardiovascular complications has revealed that variability among subjects, rooted in environmental and physiological factors, is an important reason for the submaximal success of this drug [59]. Pharmacogenomics promises to delineate the mechanistic basis of the variability in pioglitazone efficacy. The principal biotransformation products of pioglitazone produced in various phases of drug metabolism are M-I, M-II, M-III, M-IV, M-V and M-VI [60]. M-III and M-IV are two metabolites documented to exert a rather sustained hypoglycemic effect and are thus accentuate the overall efficacy of pioglitazone per se [60]. CYP2C8 and CYP3A4 are the principal cytochrome P450 enzymes responsible for the breakdown of pioglitazone in the body into the above-mentioned metabolites [61]. Clinical data have shown that genetic polymorphisms associated with CYP2C8:*1 homozygotes and the *3 variant allele carriers, are responsible for variability in bioavailability of pioglitazone among various treatment populations [62,63]. Kadam et al. [64] have recently reported that oral clearance of pioglitazone is substantially higher in CYP2C8*3 carriers and is one of the main factors for the observed variability in pharmacokinetic efficacy of pioglitazone. Moreover, pharmacological inhibition of CYP2C8 enhances bioavailability of pioglitazone in CYP2C8*3 and CYP2C8*1 carriers, thus underlining the clinical significance of pharmacogenomics in pioglitazone pharmacotherapy of diabetes [62,63].
Polymorphism in PPAR-γ encoding gene PPARG p.Pro12Ala (rsl801282) is an important genetic polymorphism established to play a critical role in mediating glucose metabolism [65]. In vitro experiments demonstrated that P12A mutation in PPAR-γ2 gene is responsible for the variation in pioglitazone-induced attenuation of transcriptional activation of a reporter gene in a construct containing the PPRE [66]. This study also indicated that P12A mutation might also be linked to adipogenesis [66]. Furthermore, various research groups have shown that biological variability linked with the glucose-lowering effect of pioglitazone is also partly ascribed to the polymorphism associated with PPARG gene [67–69]. The PTPRD SNP rs17584499, located in intron 10, has been identified in Chinese populations as another important factor leading to pharmacodynamic variability in the glucose-lowering effect of pioglitazone [69]. A substantial alteration of pioglitazone-induced reduction in HbAlc was observed in ADIPOQ C-11377G genotype and ADIPOR2 G795A genotype in Chinese and Iranian T2D populations [43,70]. Retn expression is induced by adipocyte differentiation and is inhibited by PPAR-γ agonists in isolated 3T3-L1 cell cultures [71]. In vivo studies demonstrated that systemic release of retn from adipocytes is substantially enhanced during diabetes in obese mice. PPAR-γ agonists like TZDs attenuate this increase in serum retn levels in laboratory animals as well as in clinical subjects. Mice overexpressing the retn in the liver have greater serum retn and insulin resistance, whereas retn(−/−) mice have reduced fasting blood glucose [72,73]. Therefore, it can be concluded that elevated serum retn levels cause insulin resistance in rodents. A study in a Japanese population revealed that retn C-420G (rs1862513) polymorphism in the GG genotype, an adipokines known to antagonize insulin, is linked with a stronger glucose-lowering effect of pioglitazone [74].
In addition to the glucose-lowering effect, recent clinical studies report that pioglitazone prevent dyslipidemia-based progression of atherosclerotic macro-vascular changes in the carotid and coronary arteries of diabetic subjects [7,11,75]. Certain animal studies have shown that pioglitazone attenuates myocardial ischemia/reperfusion injury in rodents via endothelial NOS-, COX-2- and ERK-1/2-dependent pathways 43,76–78]. A genetic study by Saitou et al. [79] has shown that the ameliorative potential of pioglitazone on diabetic aggravation of atherosclerosis is independent of the tested genotypes. Polymorphism (S447X) in the LPL gene influences the beneficial effects of pioglitazone on cardiovascular complications of diabetes mellitus [80]. Such SNP mediates pioglitazone-linked increase in the synthesis and secretion of LPL in multiple types of parenchymal cells [81,82]. Studies have further shown that the S447X variant is intrinsically associated with the pathogenesis of dyslipoproteinemia and coronary artery disease and thus markedly reduces the efficacy of pioglitazone in the LPL S447X genotype when compared with subjects with the S447S genotype [82].
Pharmacogenomics & pharmacogenetics of troglitazone in diabetes
Troglitazone ((RS)-5-(4-[(6-hydroxy-2,5,7,8-tetramethylchroman-2-yl) methoxy] benzyl) thiazolidine-2, 4-dione) was introduced for the first time on the pharmaceutical market in March 1997 as a novel therapy for the treatment of T2D [83]. After a short period of surveillance several cases of troglitazone-induced hepatotoxicity were reported [84]. Therefore, in 2000 the drug was abruptly removed from the market [83]. The symptoms observed in patients were related to an idiosyncratic hepatocellular injury-type liver toxicity determined by the specific metabolism of the troglitazone defined as ‘metabolic idiosyncrasy’ [85]. After removal of the drug from the market, a few studies have assessed whether specific genetic variants could predispose to the hepatotoxicity caused by troglitazone. Watanabe et al. [86], in a study performed on 25 Japanese T2D patients with abnormal increase in levels of ALT or AST during troglitazone treatment, analyzed 51 different genes related to drug metabolism, apoptosis, production and elimination of reactive oxygen species, including signal transduction pathways of PPAR-γ2 and insulin. The genotyping analysis demonstrated that the presence of the combined GSTT1 and GSTM1 null genotype [87,88] was a specific risk factor for the enhanced susceptibility to transaminase increase associated with troglitazone. Oniki et al. [89], in a subsequent study performed on 215 participants from the health screening program, reviewed the results of previous study suggesting that the combined GSTM1 and GSTT1 null genotypes might also be a risk factor for alcoholic mild liver dysfunction. Kumashiro et al. [90], in 2003 examined retrospectively the polymorphism of CYP450 CYP2D6 and CYP2C19 in eight T2D patients with, and 31 patients without troglitazone-induced liver injury. The frequency of CYP2D6 SNP was identical for both groups while homozygous or compound heterozygous polymorphisms of CYP2C19 (2*/2*, 2*/3*, 3*/3*: poor metabolizers genotypes) were significantly more frequent in patients with liver injury (p = 0.0401).
In the TRIPOD study [91], performed on 93 Hispanic women with a history of gestational diabetes treated with troglitazone for 3 months, no correlation were found between PPARG common polymorphism P12A (rsl801282) and response to troglitazone, suggesting that PPAR-γ is the main target of the TZDs but its genetic variants may even not modify the response to the therapy. To clarify this issue, Wolford et al. [92], screened by direct Sanger sequencing 40kb corresponding to all exons, introns and flanking regions of PPARG in 93 non diabetic women with previous gestational diabetes who had participated in the TRIPOD study. Among the 131 identified variants, 8 (not including P12A) were associated with the variation to troglitazone monotherapy response (p < 0.05). A screening by Florez et al. [93], performed in a cohort of 3458 subjects from the DPP study did not find any association between P12A and troglitazone therapy, supporting previous findings from TRIPOD study. In addition the authors genotyped five of the eight SNPs reported in the Wolford study [92] without find any significant associations between these genetic loci and troglitazone ‘responders’ and troglitazone ‘non-responders.’ These controversial results have been extensively analyzed and some potential explanations have been found in the differences from the selected study populations especially in term of race-ethnicity, and in the different statistical methodologies employed in these studies. Moreover, it must be considered the different sensitivity among methods used to evaluate insulin levels (TRIPOD – direct measurement, DPP – indirect measurement) [94]. In Table 1 are reported some of the most important studies in this field.
Table 1.
Summary of genetic studies on troglitazone.
| Patients (male/female) |
Controls (male/ female) |
Country | Ethnicity | Gene (*OMIM) |
Gene function | Polymorphism | Comment | Ref. |
|---|---|---|---|---|---|---|---|---|
| 25(7/18) (abnormal increase in ALT levels) |
85 (40/45) | Japan | Asiatic |
GSTT1 (*600436) |
Catalyze the conjugation and the detoxification of glutathione and reactive toxic compounds, chemicals and metabolites |
Wild/null | The combined null genotype of GSTT1 and GSTM1 is a susceptible genetic factor for troglitazone-associated abnormal increases in liver enzyme levels |
[75] |
|
GSTM1 (*138350) |
Wild/null | |||||||
| 8 patients with Type 2 diabetes with troglitazone- induced liver injury |
31 patients unaffected after using troglitazone |
Japan | Asiatic |
CYP2C19 (*124020) |
Enzymatic system responsible for metabolism and elimination of endogenous and exogenous molecules and ingested chemicals |
CYP2C19*1 CYP2C19*2 CYP2C19*3 |
The frequency of CYP2C19 homozygous polymorphism was higher in patients with troglitazone-induced liver injury |
[79] |
|
CYP2D6 (*124030) |
CYP2D6*1 CYP2D6*2 CYP2D6*5 CYP2D6*10 |
The frequency of CYP2D6 polymorphism was identical in both groups |
||||||
| 93 women TRIPOD study |
– | USA | Hispanic |
PPARG (*601487) |
Fat cell differentiation and whole-body insulin sensitivity |
Pro12Ala | Pro12Ala variant did not account for the prevalence of troglitazone nonresponders in the TRIPOD cohort |
[80] |
| 93 women Troglitazone in TRIPOD study |
– | USA | Hispanic | rs13073869 rs880663 rs4135263 rs1152003 rs6806708 rs13065455 rs13088205 rs13088214 |
8 of 131 PPARG variants may underlie response to thiazolidinedione therapy in women at risk for Type 2 diabetes |
[81] | ||
| 3548 subjects enrolled in DPP study |
– | USA | Caucasian African- American Hispanic Asian American American Indian |
Pro12Ala rs880663, rs4135263 rs1152003 rs6806708 rs13065455 |
PPARG P12A and the five variants have little or no effect on the beneficial response to troglitazone |
[82] | ||
| 3356 subjects (1116/2240) enrolled in the DPP study |
– | Pro12Ala | At 1 year of treatment with troglitazone, Ala12 allele carriers tended to gain more weight than Pro12 allele homozygotes |
[84] | ||||
| 3548 subjects enrolled in the DPP study |
– |
CDKN2A/B (*600160– *600431) |
Influence on cell cycle regulatory pathways |
rs10811661 | Improvement in the functionality of the pancreatic β-cell after 1 year of troglitazone treatment |
[85] | ||
| 3007 subjects (1017/1990) enrolled in DPP study |
– |
SLC30A8 (*611145) |
Zinc transporter and provider in insulin-secreting pancreatic β-cells |
rs13266634 | No genotype-treatment interactions at 1 year of troglitazone treatment |
[86] | ||
Franks et al. [95], tested the impact of PPARG Pro-12Ala SNP on modification in the obesity-related traits in 3356 subjects included in the DPP study treated with metformin (n = 989), troglitazone (n = 363) or lifestyle modification (n = 1004) versus placebo (n = 1000). After 1 year of follow-up the results showed that in the metformin and lifestyle arms all individuals lost weight, especially those carrying the Ala12 allele (p = 0.01), while in troglitazone arm subjects had trend to gain weight. In particular, this association between troglitazone and the weight gain was greater in the Ala12 allele carriers rather than Pro12 homozygotes. Moree et al. [96] performed a genetic wide association study in 3548 subjects at high risk of T2D enrolled in the DPP. One of the aims of the project was to evaluate the genetic predisposition in different respond to the troglitazone therapy. The results showed an improvement in the functionality of the pancreatic β-cell for patients carrying the protective genotype at CDKN2A/B SNP rsl0811661 at 1 year of troglitazone treatment (p = 0.01) and lifestyle modification (p = 0.05). Majithia et al. [97], genotyped the missense SNP rsl3266634 of the zinc transporter gene SLC30A8 in 3007 participants from DPP and examined the association with fasting proinsulin and insulin at baseline and at 1 year postintervention. The results showed higher proinsulin levels at baseline for carriers of C allele. After 1 year, proinsulin levels decreased significantly in all groups receiving active intervention (lifestyle, metformin or troglitazone) compared with placebo, but no genotype- active intervention correlations were observed.
Muraglitazar, a nonthiazolidinedione/nonfibrate PPARα/γ dual agonist, has been shown to present similar side effects of troglitazone. However, even if for these adverse effects this drug has never been introduced in the market, it could be of particular interest for future investigation in the field of pharmacogenomics. Muraglitazar completed different clinical trials where it significantly reduced serum triglycerides, glucose and Alc and increased HDL cholesterol levels [98–100]. In a further clinical trial conducted in 2005, Nissen et al. [101] compared muraglitazar based treatment with placebo or pioglitazone. The former was associated with an excess incidence of the composite end point of death, major adverse cardiovascular events and congestive heart failure. Based on these results, they recommended not approving this drug to treat diabetes until safety was documented trough a trial designed to assess cardiovascular events. Consequently in 2006 Bristol-Myers Squibb announced that it had discontinued further development. In a subsequent pharmacogenomic trial performed in 730 patients affected by T2D was evaluated the interaction between muraglitazar and 213 SNPs from 63 genes [102]. Results showed that several common polymorphism in ren (rs2368564) and edn-1 (rs5370) genes were associated with reduced risk of edema, while the β-1 adrenergic receptor rsl801253 was associated with increased susceptibility to edema [102]. These findings may be indicative of how much pharmacogenomic studies may help in selection of therapeutic drugs potentially prone to cause side effects in certain categories of subjects such as diabetic patients [103].
Pharmacogenomics & pharmacogenetics of TZDs in cardiovascular risk factors
T2D is an established independent risk factor for CVD. As previously reported in the introduction, the American Heart Association/American Diabetes Association consensus statement on the use of TZDs asserts that a therapy based on these drugs should be privileged in patients at high risk for CVD, given that TZDs have antiinflammatory and antiatherogenic properties [9]. Aimed to investigate the genetic impact in the atheroprotective effects of TZDs, Saitou et al. [79] analyzed 99 candidate SNPs and their possible associations with conventional vascular risk factors and the progression or regression of carotid atherosclerosis in diabetic patients treated with pioglitazone or only with diet. Among the analyzed genetic variants, the I/D polymorphism in the ACE gene and the C677T polymorphism in the MTHFR gene showed a significant association with changes in the values of averaged and maximum IMT, a subclinical markers of atherosclerosis, in both group of patients. In particular, the group of patients carrying the ACE D-allele and treated with pioglitazone had lower increase in IMT than the group treated with diet alone (p = 0.01). In addition, pioglitazone exerts a protective effect attenuating the increase in IMT (p = 0.03) in patients carriers the 677T allele in the MTHFR gene which is generally associated to higher homocysteine level and progression of atherosclerosis [79]. These findings strongly suggest as TZDs may have a pleiotropic effects exerting their action through PPAR-γ at different molecular levels such as renin-angiotensin modulation. However, their pleiotropic actions could be also the reason of the side effects characteristic of this class of drug, such as heart failure and edema. The positive response of the treatment is based on the presence mainly of the beneficial effect that can results by a different individual dosage based on EC50 and different individual genetic profiles. In Table 2 are reported the specific intracellular targets coupled with the benefical and side effects associated with TZDs therapy.
Table 2.
Specific intracellular targets coupled with beneficial/side effects associated with thiazolidinediones therapy.
| Thiazolidine diones |
Receptors | Intracellular targets | Benefical effects | Side effects |
|---|---|---|---|---|
| Pioglitazone | PPAR-γ |
|
|
|
| Rosiglitazone |
|
|
|
|
| Troglitazone |
|
|
|
Genetic variances of CYP450 enzymes have been involved in CVD [104,105]. This association is mediated by CYP-catalyzed release of arachidonic acid metabolites with vasodilatation, antiinflammatory and antiatherogenic properties, such as epoxyeicosatrienic acids, and dihydroxyeicosatrienoic acids (DHETs) [106,107]. In vitro, the TZDs are strong inhibitors of CYP2C8, a member of CYP with function in the oxidative system [108]. Moreover, in a pharmacokinetics study, rosiglitazone was found to be a CYP2C8 substrate and its metabolism increased in individuals with CYP2C8*3 allele [42]. Kichheiner et al. [41] analyzed 31 healthy individuals, receiving a daily oral dose of rosiglitazone for two weeks, in order to confirm the impact of CYP2C8 polymorphisms and rosiglitazone effects on urinary excretion of epoxyeicosatrienic acids and DHETs. One of the most interesting data showed that subjects with CYP2C8*3 carriers had greater 14–15 DHET formation rates independently by rosiglitazone treatment, showing in both cases a direct correlation between the amount of produced metabolites and the number of alleles CYP2C8*3 (p < 0.05 and p < 0.01, respectively). On the contrary, carriers of the slow metabolizer haplotype C, had a trend to have a lower DHET excretion. Overall the effect of rosiglitazone in these subjects was a decrease in DHET excretion by approximately 10% (p < 0.02) [41]. Therefore, according to these findings, understanding the physiological role of CYP2C8 genetic variants in arachidonic acid metabolism and their correlation with TZDs could be pivotal to improve our knowledge in their impact on cardiovascular physiopathology.
Although TZDs have beneficial effects on cardiovascular parameters such as lowering BP and blood lipid levels, as previously discussed in this review, the treatment plan with the goal to reduce risk for CVD must be carefully individualized for each patient, since several side effects may accompany the use of these drugs (Table 2). The main side effects that can worsen the preexisting pathological situation in a patient with risk for CVD have been identified in fluid retention, edema and weight gain. For this reason, both pioglitazone and rosiglitazone are strongly not recommended in patients with established heart failure NYHA’s class III or IV (Prescribing information Actos and Avandia [109]). However, it needs to be acknowledged that although both belonging to the same class of drug, their effect on cardiovascular events may be different. Rosiglitazone was associated with a high risk of CVD and all-cause mortality [7,110,111]. Controversially with previous discusses findings, an overall examination of clinical trials adopting pioglitazone did not show a clear advantage in terms of reducing cardiovascular related morbidity [112]. Mainly, this compound has often proven to be significantly protective against plaque progression and micro- and macrovascular events [11,113]. However, a more comprehensive evaluation of TZDs cardiovascular effect and safety could be achieved considering the contribution of SNPs in genes involved in vascular physiological pathways affecting the etiology of TZDs-induced side effects. With this objective, Ruaño et al. [114] performed a retrospective cross sectional study on 87 T2D patients treated with rosiglitazone or pioglitazone. Authors used physiogenomics methodology to test for SNP associations across a PC-Array, consisting of 384 SNPs from 222 candidate genes involved in cardiovascular and metabolic pathways. They reported a significant association between body mass index (BMI) and TZDs therapy with genetic variants in ADORA1 (p < 0.0003), ADIPOR2 (p < 0.007), PKM2 (p < 0.002) and UCP2 (p < 0.008). In contrast, edema induced by TZDs therapy was associated with SNPs in genes controlling vascular permeability such as NPY (p < 0.006) and CCL2 (p < 0.015) [114]. One of the most interesting finding emerging from this study is the strong correlation between risk-associated SNPs and protective SNPs for one or both investigated outcomes, suggesting the importance of monitoring genes involved in different but combinated pathways in order to improve TZDs tolerability and safety.
As we previously mentioned, edema is one of the most common adverse side effects associated to rosiglitazone treatment [115]. Bailey et al. [116], in a genetic study from the multiethnic DREAM trial, tested 32,088 SNPs in 4197 patients treated with rosiglitazone. One SNP, rs6123045 in the NFATC2 was significantly associated with edema (p = 7.68 × 10−3). Specifically, European individuals homozygous for the risk allele (CC) showed higher rate of TZDs-induced edema compared with the individuals heterozygous (CT) or homozygous (TT) for the protective allele. In contrast, the rs6123045 SNP did not show a significant association with edema onset in Latin Americans under similar treatment. However, a haplotype defined by six SNPs in NFATC2 was associated with edema both in Europeans and Latin Americans (p = 2.26 × 10−5 and p = 1.47 × 10−2, respectively) [116]. These data further support a variation in response to therapy and its side effects across race-ethnicities characterized by different genetic patterns.
To better understand mechanisms involved in TDZs therapy related-edema, Chang et al. [117], genotyped 28 tag SNPs from five candidate genes encoding for water channel proteins (AQP2), sodium transport proteins (NHE3, SLC12A1, SCNN1G) and for the TZDs target (PPAR-γ) in 168 T2D patients receiving TZDs treatment (rosiglitazone or pioglitazone). They found association between early edema onset and rs296766 T allele variant of AQP2 (p = 0.0059), and rs12904216 G allele variant of the SLC12A1 (p = 0.049). In regards to PPAR-γ, the Pro12Ala variant, a PPAR-γ SNP associated with induction of fluid retention in T2D Caucasian patients [118], was not evaluated in this study since its low minor allelic frequency in the analysed Asian population. However, other 8 PPAR-γ SNPs were considered for haplotype analysis and statistically significant association was found between PPAR-γ haplotype and edema induced by TZD after adjusting for sex and age [117].
Identifying the interaction between genetic factors controlling fat metabolism and response to TZDs treatment is pivotal in preventing cardiovascular accidents. Weight gain emerged as another common adverse drug reaction after TZDs therapy, especially in patients with already vascular complications [119]. With the aim to investigate association between adipocytokines involved in regulation of insulin activity and rosiglitazone response, Liu and colleagues [37], analysed leptin G-2548A and TNF-α G-308A SNPs in 245 T2D patients before and after 12 weeks of rosiglitazone treatment. Patients carrier of leptin G allelic variant had smaller basal values of BMI (p = 0.038) respect to the one carried AA genotype. After rosiglitazone monotherapy, all T2D patients showed a decrease in fasting and postprandial plasma glucose, in A1c and leptin levels, and a significant increase in HDL levels, suggesting that rosiglitazone can reverse insulin resistance also by decreasing serum leptin level. Particularly, T2D patients’ carrier leptin AA genotypes had better response to treatment, showing higher differential values of fasting and postprandial serum insulin. Moreover, patients with GA+AA TNF-α. genotype had lower value of fasting serum insulin than those carrying the GG genotype, indicating that these particular SNPs may be associated with a better response to rosiglitazone monotherapy [37].
Moreover, one of major risk factors for CVD and stroke is the excess in adiposity localized in the visceral compartment [120]. PPAR-γ Pro12Ala SNP, since its role in adipocyte differentiation and its significant association with BMI, has been one of the most studied genetic variant in association with risk for obesity and CVD risk [121,122]. Franks et al. [95], tested the possible association between the PPAR-γ Pro12Ala variant and the risk for weight gain and change in adipose distribution in 3356 T2D patients participating to the DPP (Diabetes Prevention Program), in which the effects of metformin, troglitazone treatment and/or intensive lifestyle intervention in diabetes were investigated. At baseline, patients carrying Ala12 allele had larger waist circumference (WC) (p < 0.001). After treatment with troglitazone, the Ala12 allele was associated with a trend in development greater weight gain respect to the PPAR-γ Pro12 variant [95]. By using the same database, same authors further demonstrated as adjusting for age, sex and race-ethnicity, the minor A allele at FTO rs9939609 SNP showed a significant association with heavier weight (p = 0.03) and higher BMI (p = 0.003) at baseline, while after treatment it appeared to be associated to a greater increase in subcutaneous adipose tissue in the placebo group with respect to metformin and intensive lifestyle intervention after 1 year of treatment [123]. Interaction with troglitazone treatment was analysed only for the allelic variant of INSIG2 rs7566605 SNP but no significant association was found with variation in increase of adipose tissue in T2D patients [123]. In Table 3 are reported some of the most important studies in this field.
Table 3.
Summary of genetic studies on thiazolidinediones.
| Patients (male/ female) |
Healthy controls (male/ female) |
Country | Race/ethnicity | Gene (*OMIM) |
Gene function | Polymorphisms | Comment | Ref. |
|---|---|---|---|---|---|---|---|---|
| 87 (57/30) | nd | USA | African- American Caucasian Hispanian |
ADORA1 (*102775) |
Adipocyte energy homeostasis | rs903361 | Results from these comparative physiogenomic analysis associations suggest mechanistic links between adenosine signaling and BMI, and between vascular permeability and drug-induced edema |
[32] |
| PKM2 (*179050) | Intracellular glucose regulation | rs2856929 | ||||||
| ADIPOR2 (*607946) | Mediate increased AMPK and PPAR-α ligand activities Mediate fatty acid oxidation and glucose uptake | rs7975375 | ||||||
|
UCP2 (*601693) |
Mitochondrial protein involved in the control of the metabolism of fatty acids |
rs660339 | ||||||
|
APOH (*138700) |
Lipoprotein metabolism | rs8178847 | ||||||
|
NPY (*162640) |
Long-lasting constrictor agent Enhancer of endothelial permeability Participates in pulmonary and larynx edema |
rs1468271 | ||||||
|
GYS1 (*138570) |
Glycogen molecule synthesis | rs2287754 | ||||||
|
CCL2 (+158105) |
Increases the permeability of the blood-brain barrier Participates in vasogenic brain edema |
rs3760396 | ||||||
|
OLR1 (*602601) |
Participates in capillary formation |
rs2742115 | ||||||
|
GHRH (*139190) |
Stimulates GH secretion | rs6032470 | ||||||
| 4197 | Rosiglitazone (1750) Ramipril Placebo (1761) |
North America South America Europe India Australia |
Europeans Latin Americans South Asians African- American Native North American East Asian Other |
NFATC2 (*600490) |
Activated T-cell transcription factor |
rs6123045 | A significant interaction was detected between the SNPrs6123045 of the (NFATC2) gene and edema among Europeans receiving rosiglitazone treatment |
[33] |
| 268 (126/142) |
Asia | Chinese |
AQP2 (*107777) |
Renal AQP2 water channel | rs296766 | A model based on combination of genetic scores from AQP2 and SLC12A1 and nongenetic risk factors was developed in order to clinically predict TZD-related edema |
[96] | |
|
SLC12A1 (*600839) |
Transport of Na+, K+ and CI− across the plasma membrane |
rs12904216 | ||||||
| 3356 (1116/2240) |
Placebo (29.8%) Metformin (29.5%) Lifestyle intervention (29.9%) Troglitazone (10.8%) |
USA | European African- American American Hispanic Asian/Pacific Islander American Indian |
PPARG (*601487) |
Binding of chemicals that induce proliferation of peroxisome, organelles that contribute to the oxidation of fatty acids |
Pro12Ala | Weight gain, induced by troglitazone treatment, tended to be significantly greater in PPARG Ala12 allelic carriers |
[84] |
|
FTO (*610966) |
Regulation of food intake | rs9939609 | A nominal association exist between common variants in FTO and INSIG2 and obesity- related traits, by interacting with metformin or lifestyle intervention, but not with troglitazone |
[105] | ||||
| INSIG2 (*608660) | Control of cholesterol and fatty acid metabolism via regulation of SREBFs |
rs7566605 | ||||||
| nd | 31 (15/16) | nd | nd |
CYP2C8 (+601129) |
Drug-metabolizing CYP450 isoform |
rs2275622 rs7909236 rs1058930 rs1113129 rs11572080 |
Genetic variants of CYP2C8 gene may affect cardiovascular functions modulating epoxyeicosatrienoic acid- formation |
[68] |
| 222 (144/78) |
160–diet only 62– pioglitazone |
Japan | Japanese |
MTHFR (*607093) |
Catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, a cosubstrate for homocysteine remethylation to methionine |
rs1801133 (C677T) | Pioglitazone exerts an antiatherosclerotic effect on Type 2 diabetic patients carrying the ACE-D allele and the MTHFR 677T allele |
[98] |
|
ACE (+106180) |
Blood pressure regulation and electrolyte balance by hydrolyzing angiotensin I into angiotensin II |
rs1799752 (l/D) | ||||||
| 245 (132/113) |
122 (68/54) | China | Chinese |
Leptin (*164160) |
Regulation of body weight by inhibiting food intake and stimulating energy expenditure Regulation of insulin secretion and serum glucose levels |
G2548A |
Leptin G- 2548A and TNF-α G-308A polymorphisms are associated with therapeutic efficacy of rosiglitazone treatment of Type 2 diabetes patients |
[99] |
|
TNF-α (*191160) |
Multifunctional proinflammatory cytokine Inhibition of lipogenesis and stimulation of lipolysis Impairment of insulin signal pathway |
G-308A | ||||||
nd: Not determined
Conclusion & future perspective
Combining the predictive power of SNPs of genes affecting the etiology of TZDs-induced side effects could be important in order to improve the rational clinical application of these drugs. According to results from previous discussed studies, a model incorporating higher weighted genetic score based on these genetic variants, combined with specific nongenetic risk factors, such as female sex and older age, could help to improving the clinical prediction of TZD-related side effects in diabetic subjects with high risk for CVD.
The potential of TZDs as the basis of therapeutic strategies in treating cardiovascular complications during diabetes has been affirmed by preclinical data, which seem to hold promise and therefore should be used as an adjunct therapy to provide maximal relief to the cardiovascular system. However, an exhaustive pharmacogenomic assessment of TZDs has highlighted the significance of studying the importance of population-specific genetic variability and the manner in which this variability influences the therapeutic effect of TZDs. This observation attains a heightened significance when assessed in the context of life-threatening acute cardiovascular complications during diabetes, as even a minor genetic polymorphism-based variation may snowball into a significant cardiovascular problem. Therefore, pharmacogenomics is an important tool in not only validating the clinical relevance of intersubject variability per se but also to assess the therapeutic potential of such variability in drug individualization and therapeutic optimization. Furthermore, among the wide arrays of genetic variations proposed in the literature and discussed above, it is also important to define first, the bigger picture of interrelationship between the genetic variations and therapeutic efficacy of these drugs; second, the comparisons between the relative quantitative significance of various forms of genetic polymorphism in mediating the pharmacological effect of TZDs on the pathogenesis of diabetes-related cardiovascular complications; and third, the identification of the principal sources of therapeutically relevant fluctuations in drug effect that may be effectively regulated by appropriate methods.
Executive summary.
Introduction: rosiglitazone, pioglitazone & troglitazone
Thiazolidinediones (TZDs) are a class of antidiabetic drugs that act as insulin sensitizers increasing insulin-dependent glucose disposal and reducing hepatic glucose output.
The three drugs in this hypoglycemic class introduced in the market are rosiglitazone, pioglitazone and troglitazone.
TZDs have shown pleiotropic effects against vascular risk factors and atherosclerosis.
Troglitazone was removed from the market due to its hepatoxicity, and rosiglitazone and pioglitazone both have particular warnings due to being associated with congestive heart failure.
Mechanism of action: role of PPARs & PPAR-γ
TZDs act mainly through the PPAR-γ.
In absence of a ligand, the PPAR-γ: RXR complex can recruit corepressor complexes and bind to PPRE, suppressing the transcription of target genes.
Among these genes, PPAR-γ activation induces the expression of genes involved in the insulin signaling cascade such as GLUT4 and CAP, thereby improving insulin sensitivity in patients with diabetes.
Pharmacogenomics & pharmacogenetics of rosiglitazone in diabetes
Genetic variation in drug-metabolizing protein (CYP450) genes: CYP2C8 (CYP2C8*3) and CYP2C9 (CYP2C9*3) contribute towards intersubject heterogeneity in pharmacokinetic behavior of rosiglitazone.
Glucose-lowering effects of rosiglitazone vary in subpopulations representing different ADIPOQ gene polymorphisms.
Rosiglitazone shows a markedly increased effect in patients with the AA genotype of leptin G-2548A.
Pharmacogenomics & pharmacogenetics of pioglitazone in diabetes
SNPs associated with CYP2C8:*1 homozygotes and the *3 variant allele carriers are responsible for variability in bioavailability of pioglitazone among various study populations.
Glucose-lowering effect of pioglitazone is partly ascribed to the polymorphisms associated with PPARG gene.
retn C-420G (rs1862513) polymorphism in the GG genotype is linked with a stronger glucose-lowering effect of pioglitazone.
Pharmacogenomics & pharmacogenetics of troglitazone in diabetes
Presence of the combined GSTT1 and GSTM1 null genotype is a specific risk factor for the enhanced susceptibility to hepatoxicity associated with troglitazone.
TRIPOD study found no correlation between PPARG common polymorphism P12A and response to troglitazone.
Improvement in the pancreatic β-cell functionality for patients carrying the protective genotype at CDKN2AIB SNP rs10811661 at 1 year of troglitazone treatment.
Pharmacogenomics & pharmacogenetics of TZDs in cardiovascular risk factors
Patients carrying the ACE-D allele or MTHFR Sill allele and treated with pioglitazone have lower risk for atherosclerosis than the group treated with diet alone.
CYP2C8 genetic variants in arachidonic acid metabolism and their correlation with TZDs are pivotal in the variability for risk in cardiovascular disease.
Edema induced by TZDs is associated with SNPs in genes controlling vascular permeability such as A/PVand CCL2.
Leptin G-2548A and TNF-α G-308A SNPs are associated with variability in vascular risk.
Acknowledgments
AK Rehni and KR Dave are supported by NIH grant NS073779.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Meerza D, Naseem I, Ahmed J. Pharmacology of signaling pathways: in Type 2 diabetes. Diab. Metabol. Syndr. 2013;7(3):180–185. doi: 10.1016/j.dsx.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 2. Yki-Jarvinen H. Thiazolidinediones. N. Engl. J. Med. 2004;351(11):1106–1118. doi: 10.1056/NEJMra041001. •A very remarkable review describing the mechanisms, indications and limitations of thiazolidinedione therapy.
- 3.Willson TM, Lambert MH, Kliewer SA. Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu. Rev. Biochem. 2001;70:341–367. doi: 10.1146/annurev.biochem.70.1.341. [DOI] [PubMed] [Google Scholar]
- 4.Martens FM, Visseren FL, Lemay J, De Koning EJ, Rabelink TJ. Metabolic and additional vascular effects of thiazolidinediones. Drugs. 2002;62(10):1463–1480. doi: 10.2165/00003495-200262100-00004. [DOI] [PubMed] [Google Scholar]
- 5.Aquilante CL. Pharmacogenetics of thiazolidinedione therapy. Pharmacogenomics. 2007;8(8):917–931. doi: 10.2217/14622416.8.8.917. [DOI] [PubMed] [Google Scholar]
- 6.Filion KB, Joseph L, Boivin JF, Suissa S, Brophy JM. Thiazolidinediones and the risk of incident congestive heart failure among patients with Type 2 diabetes mellitus. Pharmacoepidemiol. Drug Saf. 2011;20(8):785–796. doi: 10.1002/pds.2165. [DOI] [PubMed] [Google Scholar]
- 7. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. ••A major study on the effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes.
- 8.Kumar S, Hoffman SJ, Samadfam R, et al. The effect of rosiglitazone on bone mass and fragility is reversible and can be attenuated with alendronate. J Bone Miner Res. 2013;28(7):1653–1665. doi: 10.1002/jbmr.1918. [DOI] [PubMed] [Google Scholar]
- 9.Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in Type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29(8):1963–1972. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 10.Tolman KG. Thiazolidinedione hepatotoxicity: a class effect? Int. J. Clin. Pract. Suppl. 2000;(113):29–34. [PubMed] [Google Scholar]
- 11. Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with Type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. ••An important clinical study that demonstrated that the pioglitazone reduces the composite of all-cause mortality, nonfatal myocardial infarction and stroke in patients with Type 2 diabetes who have a high risk of macrovascular events.
- 12.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with Type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298(10):1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 13.Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, Allemand H. Pioglitazone and risk of bladder cancer: clarification of the design of the French study. Diahetologia. 2013;56(1):228–229. doi: 10.1007/s00125-012-2769-9. [DOI] [PubMed] [Google Scholar]
- 14.Saltiel AR, Olefsky JM. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes. 1996;45(12):1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- 15.Berger J, Moller DE. The mechanisms of action of PPARs. Annu. Rev. Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 16.Furuta M, Yano Y, Gabazza EC, et al. Troglitazone improves GLUT4 expression in adipose tissue in an animal model of obese Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2002;56(3):159–171. doi: 10.1016/s0168-8227(01)00373-4. [DOI] [PubMed] [Google Scholar]
- 17.Armoni M, Kritz N, Harel C, et al. Peroxisome proliferator-activated receptor-gamma represses GLUT4 promoter activity in primary adipocytes, and rosiglitazone alleviates this effect. J Biol. Chem. 2003;278(33):30614–30623. doi: 10.1074/jbc.M304654200. [DOI] [PubMed] [Google Scholar]
- 18.Jiang G, Dallas-Yang Q, Li Z, et al. Potentiation of insulin signaling in tissues of Zucker obese rats after acute and long-term treatment with PPARgamma agonists. Diabetes. 2002;51(8):2412–2419. doi: 10.2337/diabetes.51.8.2412. [DOI] [PubMed] [Google Scholar]
- 19.Leonardini A, Laviola L, Perrini S, Natalicchio A, Giorgino F. Cross-Talk between PPARgamma and Insulin Signaling and Modulation of Insulin Sensitivity. PPAR Res. 2009;2009:818945. doi: 10.1155/2009/818945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugawara A, Uruno A, Kudo M, Matsuda K, Yang CW, Ito S. PPARgamma agonist beyond glucose lowering effect. Korean J. Intern. Med. 2011;26(1):19–24. doi: 10.3904/kjim.2011.26.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugawara A, Takeuchi K, Uruno A, et al. Differential effects among thiazolidinediones on the transcription of thromboxane receptor and angiotensin II type 1 receptor genes. Hypertens. Res. 2001;24(3):229–233. doi: 10.1291/hypres.24.229. [DOI] [PubMed] [Google Scholar]
- 22.Uruno A, Matsuda K, Noguchi N, et al. Peroxisome proliferator-activated receptor-{gamma} suppresses CYP11B2 expression and aldosterone production. J Mol. Endocrinol. 2011;46(1):37–49. doi: 10.1677/JME-10-0088. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda Y, Sugawara A, Taniyama Y, et al. Suppression of rat thromboxane synthase gene transcription by peroxisome proliferator-activated receptor gamma in macrophages via an interaction with NRF2. J Biol. Chem. 2000;275(42):33142–33150. doi: 10.1074/jbc.M002319200. [DOI] [PubMed] [Google Scholar]
- 24.Sugawara A, Uruno A, Kudo M, et al. Transcription suppression of thromboxane receptor gene by peroxisome proliferator-activated receptor-gamma via an interaction with Sp1 in vascular smooth muscle cells. J Biol. Chem. 2002;277(12):9676–9683. doi: 10.1074/jbc.M104560200. [DOI] [PubMed] [Google Scholar]
- 25.Kudzma DJ. Effects of thiazolidinediones for early treatment of Type 2 diabetes mellitus. Am. J. Manag. Care. 2002;8(16 Suppl.):S472–S482. [PubMed] [Google Scholar]
- 26.Olansky L, Marchetti A, Lau H. Multicenter retrospective assessment of thiazolidinedione monotherapy and combination therapy in patients with Type 2 diabetes: comparative subgroup analyses of glycemic control and blood lipid levels. Clin. Ther. 2003;25(Suppl. B):B64–B80. doi: 10.1016/s0149-2918(03)80243-6. [DOI] [PubMed] [Google Scholar]
- 27.Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013;2013:152786. doi: 10.1155/2013/152786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang DQ, Chu LX, Liu ZY, Zhang SB. Pioglitazone decreased CD40/CD40L expression on human umbilical vein endothelial cells induced by oxidized low-density lipoprotein. Clin. Chim. Acta. 2006;370(1–2):94–99. doi: 10.1016/j.cca.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 29.Kapadia R, Yi JH, Vemuganti R. Mechanisms of antiinflammatory and neuroprotective actions of PPAR-gamma agonists. Front. Biosci. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tureyen K, Kapadia R, Bowen KK, et al. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J NeuroChem. 2007;101(1):41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- 31.Ito H, Nakano A, Kinoshita M, Matsumori A. Pioglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates myocardial ischemia/reperfusion injury in a rat model. Lab. Invest. 2003;83(12):1715–1721. doi: 10.1097/01.lab.0000106724.29121.da. [DOI] [PubMed] [Google Scholar]
- 32.Finegood DT, Mcarthur MD, Kojwang D, et al. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes. 2001;50(5):1021–1029. doi: 10.2337/diabetes.50.5.1021. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Liu X, Zhan Y, et al. Pharmacogenomic, physiological, and biochemical investigations on safety and efficacy biomarkers associated with the peroxisome proliferator-activated receptor-gamma activator rosiglitazone in rodents: a translational medicine investigation. J Pharmacol. Exp. Ther. 2010;334(3):820–829. doi: 10.1124/jpet.110.167635. [DOI] [PubMed] [Google Scholar]
- 34.Wang X. Assessment of cardiac safety for PPARgamma agonists in rodent models of heart failure: a translational medicine perspective. Curr. Mol. Pharmacol. 2012;5(2):264–271. doi: 10.2174/1874467211205020264. [DOI] [PubMed] [Google Scholar]
- 35.Hammarstedt A, Andersson CX, Rotter Sopasakis V, Smith U. The effect of PPARgamma ligands on the adipose tissue in insulin resistance. Prostaglandins Leukot. Essent. Fatty Acids. 2005;73(1):65–75. doi: 10.1016/j.plefa.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol. Sci. 2005;26(5):244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Liu HL, Lin YG, Wu J, et al. Impact of genetic polymorphisms of leptin and TNF-alpha on rosiglitazone response in Chinese patients with Type 2 diabetes. Eur. J. Clin. Pharmacol. 2008;64(7):663–671. doi: 10.1007/s00228-008-0483-9. [DOI] [PubMed] [Google Scholar]
- 38.Pan HJ, Reifsnyder P, Vance DE, Xiao Q, Leiter EH. Pharmacogenetic analysis of rosiglitazone-induced hepatosteatosis in new mouse models of Type 2 diabetes. Diabetes. 2005;54(6):1854–1862. doi: 10.2337/diabetes.54.6.1854. [DOI] [PubMed] [Google Scholar]
- 39.Pan HJ, Lin Y, Chen YE, Vance DE, Leiter EH. Adverse hepatic and cardiac responses to rosiglitazone in a new mouse model of Type 2 diabetes: relation to dysregulated phosphatidylcholine metabolism. Vase. Pharmacol. 2006;45(1):65–71. doi: 10.1016/j.vph.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Leiter EH, Reifsnyder PC, Zhang W, Pan HJ, Xiao Q, Mistry J. Differential endocrine responses to rosiglitazone therapy in new mouse models of Type 2 diabetes. Endocrinology. 2006;147(2):919–926. doi: 10.1210/en.2005-0839. [DOI] [PubMed] [Google Scholar]
- 41.Kirchheiner J, Meineke I, Fuhr U, Rodriguez-Antona C, Lebedeva E, Brockmoller J. Impact of genetic polymorphisms in CYP2C8 and rosiglitazone intake on the urinary excretion of dihydroxyeicosatrienoic acids. Pharmacogenomics. 2008;9(3):277–288. doi: 10.2217/14622416.9.3.277. [DOI] [PubMed] [Google Scholar]
- 42.Kirchheiner J, Thomas S, Bauer S, et al. Pharmacokinetics and pharmacodynamics of rosiglitazone in relation to CYP2C8 genotype. Clin. Pharmacol. Ther. 2006;80(6):657–667. doi: 10.1016/j.clpt.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Li Z, Peng X, Wu Y, Xia Y, Liu X, Zhang Q. The influence of adiponectin gene polymorphism on the pioglitazone response in the Chinese with Type 2 diabetes. Diabetes Obes. Metab. 2008;10(9):794–802. doi: 10.1111/j.1463-1326.2008.00905.x. [DOI] [PubMed] [Google Scholar]
- 44.Ren W, Zhang SH, Wu J, Ni YX. Polymorphism of the leptin gene promoter in pedigrees of Type 2 diabetes mellitus in Chongqing, China. Chin. Med. J. (Engl.) 2004;117(4):558–561. [PubMed] [Google Scholar]
- 45.Sookoian SC, Gonzalez C, Pirola CJ. Meta-analysis on the G-308A tumor necrosis factor alpha gene variant and phenotypes associated with the metabolic syndrome. Obes. Res. 2005;13(12):2122–2131. doi: 10.1038/oby.2005.263. [DOI] [PubMed] [Google Scholar]
- 46.Seda O, Sedova L, Oliyarnyk O, et al. Pharmacogenomics of metabolic effects of rosiglitazone. Pharmacogenomics. 2008;9(2):141–155. doi: 10.2217/14622416.9.2.141. [DOI] [PubMed] [Google Scholar]
- 47.Yu W, Hu C, Zhang R, et al. Effects of KCNQl polymorphisms on the therapeutic efficacy of oral antidiabetic drugs in Chinese patients with Type 2 diabetes. Clin. Pharmacol. Ther. 2011;89(3):437–442. doi: 10.1038/clpt.2010.351. [DOI] [PubMed] [Google Scholar]
- 48.Kang ES, Park SY, Kim HJ, et al. The influence of adiponectin gene polymorphism on the rosiglitazone response in patients with Type 2 diabetes. Diabetes Care. 2005;28(5):1139–1144. doi: 10.2337/diacare.28.5.1139. [DOI] [PubMed] [Google Scholar]
- 49.Kang ES, Cha BS, Kim HJ, et al. The 11482G > A polymorphism in the perilipin gene is associated with weight gain with rosiglitazone treatment in Type 2 diabetes. Diabetes Care. 2006;29(6):1320–1324. doi: 10.2337/dc05-2466. [DOI] [PubMed] [Google Scholar]
- 50.Yang M, Huang Q, Wu J, et al. Effects of UCP2 −866 G/A and ADRB3 Trp64Arg on rosiglitazone response in Chinese patients with Type 2 diabetes. Br. J. Clin. Pharmacol. 2009;68(1):14–22. doi: 10.1111/j.1365-2125.2009.03431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jazet IM, Pijl H, Meinders AE. Adipose tissue as an endocrine organ: impact on insulin resistance. Neth.J. Med. 2003;61(6):194–212. [PubMed] [Google Scholar]
- 52.Chen M, Hu C, Zhang R, et al. Association of PAX4 genetic variants with oral antidiabetic drugs efficacy in Chinese Type 2 diabetes patients. Pharmacogenomics J. 2014;14(5):488–492. doi: 10.1038/tpj.2014.18. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Bao YQ, Hu C, et al. Effects of ABCAl variants on rosiglitazone monotherapy in newly diagnosed Type 2 diabetes patients. Acta Pharmacol. Sin. 2008;29(2):252–258. doi: 10.1111/j.1745-7254.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 54.Krempler F, Esterbauer H, Weitgasser R, et al. A functional polymorphism in the promoter of UCP2 enhances obesity risk but reduces Type 2 diabetes risk in obese middle-aged humans. Diabetes. 2002;51(11):3331–3335. doi: 10.2337/diabetes.51.11.3331. [DOI] [PubMed] [Google Scholar]
- 55.Zhang KH, Huang Q, Dai XP, et al. Effects of the peroxisome proliferator activated receptor-gamma coactivator-1alpha (PGC-1alpha) Thr394Thr and Gly482Ser polymorphisms on rosiglitazone response in Chinese patients with Type 2 diabetes mellitus. J Clin. Pharmacol. 2010;50(9):1022–1030. doi: 10.1177/0091270009355159. [DOI] [PubMed] [Google Scholar]
- 56.Wilson-Fritch L, Nicoloro S, Chouinard M, et al. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin. Invest. 2004;114(9):1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pistrosch F, Passauer J, Fischer S, Fuecker K, Hanefeld M, Gross P. In Type 2 diabetes, rosiglitazone therapy for insulin resistance ameliorates endothelial dysfunction independent of glucose control. Diabetes Care. 2004;27(2):484–490. doi: 10.2337/diacare.27.2.484. [DOI] [PubMed] [Google Scholar]
- 58.Kung J, Henry RR. Thiazolidinedione safety. Expert Opin. Drug Saf. 2012;11(4):565–579. doi: 10.1517/14740338.2012.691963. [DOI] [PubMed] [Google Scholar]
- 59.Scheen AJ. Outcomes and lessons from the PROactive study. Diabetes Res. Clin. Pract. 2012;98(2):175–186. doi: 10.1016/j.diabres.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 60.Hanefeld M. Pharmacokinetics and clinical efficacy of pioglitazone. J Clin. Pract. Suppl. 2001;(121):19–25. [PubMed] [Google Scholar]
- 61.Scheen AJ. Pharmacokinetic interactions with thiazolidinediones. Clin. Pharmacokinet. 2007;46(1):1–12. doi: 10.2165/00003088-200746010-00001. [DOI] [PubMed] [Google Scholar]
- 62.Aquilante CL, Kosmiski LA, Bourne DW, et al. Impact of the CYP2C8 *3 polymorphism on the drug-drug interaction between gemfibrozil and pioglitazone. Br. J. Clin. Pharmacol. 2013;75(1):217–226. doi: 10.1111/j.1365-2125.2012.04343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tornio A, Niemi M, Neuvonen PJ, Backman JT. Trimethoprim and the CYP2C8*3 allele have opposite effects on the pharmacokinetics of pioglitazone. Drug Metab. Dispos. 2008;36(1):73–80. doi: 10.1124/dmd.107.018010. [DOI] [PubMed] [Google Scholar]
- 64.Kadam R, Bourne D, Kompella U, Aquilante C. Effect of cytochrome P450 2C8*3 on the population pharmacokinetics of pioglitazone in healthy Caucasian volunteers. Biol. Pharm. Bull. 2013;36(2):245–251. doi: 10.1248/bpb.b12-00657. [DOI] [PubMed] [Google Scholar]
- 65.Auwerx J. PPARgamma, the ultimate thrifty gene. Diabetologia. 1999;42(9):1033–1049. doi: 10.1007/s001250051268. [DOI] [PubMed] [Google Scholar]
- 66.Masugi J, Tamori Y, Mori H, Koike T, Kasuga M. Inhibitory effect of a proline-to-alanine substitution at codon 12 of peroxisome proliferator-activated receptor-gamma 2 on thiazolidinedione-induced adipogenesis. BioChem. Biophys. Res. Commun. 2000;268(1):178–182. doi: 10.1006/bbrc.2000.2096. [DOI] [PubMed] [Google Scholar]
- 67.Ramirez-Salazar M, Perez-Luque E, Fajardo-Araujo M, Garza SM, Malacara JM. Effect of the Pro12Ala polymorphism of the PPAR gamma 2 gene on response to pioglitazone treatment in menopausal women. Menopause. 2008;15(6):1151–1156. doi: 10.1097/gme.0b013e31816d5b2d. [DOI] [PubMed] [Google Scholar]
- 68.Hsieh MC, Lin KD, Tien KJ, et al. Common polymorphisms of the peroxisome proliferator-activated receptor-gamma (Pro12Ala) and peroxisome proliferator-activated receptor-gamma coactivator-1 (Gly482Ser) and the response to pioglitazone in Chinese patients with Type 2 diabetes mellitus. Metabolism. 2010;59(8):1139–1144. doi: 10.1016/j.metabol.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 69.Pei Q, Huang Q, Yang GP, et al. PPAR-gamma2 and PTPRD gene polymorphisms influence Type 2 diabetes patients’ response to pioglitazone in China. Acta Pharmacol. Sin. 2013;34(2):255–261. doi: 10.1038/aps.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Namvaran F, Azarpira N, Rahimi-Moghaddam P, Dabbaghmanesh MH. Polymorphism of peroxisome proliferator-activated receptor gamma (PPARgamma) Pro12Ala in the Iranian population: relation with insulin resistance and response to treatment with pioglitazone in Type 2 diabetes. Eur. J. Pharmacol. 2011;671(1–3):1–6. doi: 10.1016/j.ejphar.2011.09.158. [DOI] [PubMed] [Google Scholar]
- 71. Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi: 10.1038/35053000. •A significant article that demonstrates that circulating resistin levels are decreased by antidiabetic thiazolidinediones. Resistin has been suggested therefore as a candidate adipocyte derived factor that contributes to insulin resistance in vivo
- 72.Banerjee RR, Rangwala SM, Shapiro JS, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303(5661):1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 73.Rangwala SM, Rich AS, Rhoades B, et al. Abnormal glucose homeostasis due to chronic hyperresistinemia. Diabetes. 2004;53(8):1937–1941. doi: 10.2337/diabetes.53.8.1937. [DOI] [PubMed] [Google Scholar]
- 74.Makino H, Shimizu I, Murao S, et al. A pilot study suggests that the G/G genotype of resistin single nucleotide polymorphism at −420 may be an independent predictor of a reduction in fasting plasma glucose and insulin resistance by pioglitazone in Type 2 diabetes. Endocr. J. 2009;56(9):1049–1058. doi: 10.1507/endocrj.k08e-320. [DOI] [PubMed] [Google Scholar]
- 75. Mazzone T, Meyer PM, Feinstein SB, et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in Type 2 diabetes: a randomized trial. JAMA. 2006;296(21):2572–2581. doi: 10.1001/jama.296.21.joc60158. ••A key clinical trial in patients with Type 2 diabetes mellitus. In this study it was shown that pioglitazone slows progression of carotid artery intima media thickness compared with glimepiride.
- 76.Honda T, Kaikita K, Tsujita K, et al. Pioglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates myocardial ischemia-reperfusion injury in mice with metabolic disorders. J Mol. Cell Cardiol. 2008;44(5):915–926. doi: 10.1016/j.yjmcc.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Ye Y, Lin Y, Manickavasagam S, Perez-Polo JR, Tieu BC, Birnbaum Y. Pioglitazone protects the myocardium against ischemia-reperfusion injury in eNOS and 1NOS knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2008;295(6):H2436–H2446. doi: 10.1152/ajpheart.00690.2008. [DOI] [PubMed] [Google Scholar]
- 78.Wang H, Zhu QW, Ye P, et al. Pioglitazone attenuates myocardial ischemia-reperfusion injury via up-regulation of ERK and COX-2. BioSci. Trends. 2012;6(6):325–332. [PubMed] [Google Scholar]
- 79.Saitou M, Osonoi T, Kawamori R, et al. Genetic risk factors and the anti-atherosclerotic effect of pioglitazone on carotid atherosclerosis of subjects with Type 2 diabetes — a retrospective study. J. Atheroscler. Thromb. 2010;17(4):386–394. doi: 10.5551/jat.2527. [DOI] [PubMed] [Google Scholar]
- 80.Groenemeijer BE, Hallman MD, Reymer PW, et al. Genetic variant showing a positive interaction with beta-blocking agents with a beneficial influence on lipoprotein lipase activity, HDL cholesterol triglyceride levels in coronary artery disease patients. The Ser447-stop substitution in the lipoprotein lipase gene. REGRESS Study Group. Circulation. 1997;95(12):2628–2635. doi: 10.1161/01.cir.95.12.2628. [DOI] [PubMed] [Google Scholar]
- 81.Bogacka I, Xie H, Bray GA, Smith SR. The effect of pioglitazone on peroxisome proliferator-activated receptor-gamma target genes related to lipid storage in vivo . Diabetes Care. 2004;27(7):1660–1667. doi: 10.2337/diacare.27.7.1660. [DOI] [PubMed] [Google Scholar]
- 82.Wang G, Wang X, Zhang Q, Ma Z. Response to pioglitazone treatment is associated with the lipoprotein lipase S447X variant in subjects with Type 2 diabetes mellitus. Int. J. Clin. Pract. 2007;61(4):552–557. doi: 10.1111/j.1742-1241.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 83.Ikeda T. Drug-induced idiosyncratic hepatotoxicity: prevention strategy developed after the troglitazone case. Drug Metab. Pharmacokinet. 2011;26(1):60–70. doi: 10.2133/dmpk.dmpk-10-rv-090. [DOI] [PubMed] [Google Scholar]
- 84.Watkins PB, Whitcomb RW. Hepatic dysfunction associated with troglitazone. N. Engl. J. Med. 1998;338(13):916–917. doi: 10.1056/NEJM199803263381314. [DOI] [PubMed] [Google Scholar]
- 85.He K, Talaat RE, Pool WF, et al. Metabolic activation of troglitazone: identification of a reactive metabolite and mechanisms involved. DrugMetab. Dispos. 2004;32(6):639–646. doi: 10.1124/dmd.32.6.639. [DOI] [PubMed] [Google Scholar]
- 86.Watanabe I, Tomita A, Shimizu M, et al. A study to survey susceptible genetic factors responsible for troglitazone-associated hepatotoxicity in Japanese patients with Type 2 diabetes mellitus. Clin. Pharmacol. Ther. 2003;73(5):435–455. doi: 10.1016/s0009-9236(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 87.Pemble S, Schroeder KR, Spencer SR, et al. Human glutathione S-transferase theta (GSTTl): cDNA cloning and the characterization of a genetic polymorphism. BioChem J. 1994;300(Pt 1):271–276. doi: 10.1042/bj3000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rebbeck TR. Molecular epidemiology of the human glutathione S-transferase genotypes GSTMl and GSTTl in cancer susceptibility. Cancer Epidemiol. Biomarkers Prev. 1997;6(9):733–743. [PubMed] [Google Scholar]
- 89.Oniki K, Ueda K, Hori M, Mihara S, Marubayashi T, Nakagawa K. Glutathione-S-transferase (GST) Ml null genotype and combined GSTM1 and GSTTl null genotypes as a risk factor for alcoholic mild liver dysfunction. Clin. Pharmacol. Ther. 2007;81(5):634–635. doi: 10.1038/sj.clpt.6100123. [DOI] [PubMed] [Google Scholar]
- 90.Kumashiro R, Kubota T, Koga Y, et al. Association of troglitazone-induced liver injury with mutation of the cytochrome P450 2C19 gene. Hepatol. Res. 2003;26(4):337–342. doi: 10.1016/s1386-6346(03)00165-7. [DOI] [PubMed] [Google Scholar]
- 91.Snitker S, Watanabe RM, Ani I, et al. Changes in insulin sensitivity in response to troglitazone do not differ between subjects with and without the common, functional Pro12Ala peroxisome proliferator-activated receptor-gamma2 gene variant: results from the Troglitazone in Prevention of Diabetes (TRIPOD) study. Diabetes Care. 2004;27(6):1365–1368. doi: 10.2337/diacare.27.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wolford JK, Yeatts KA, Dhanjal SK, et al. Sequence variation in PPARG may underlie differential response to troglitazone. Diabetes. 2005;54(11):3319–3325. doi: 10.2337/diabetes.54.11.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Florez JC, Jablonski KA, Sun MW, et al. Effects of the Type 2 diabetes-associated PPARG P12A polymorphism on progression to diabetes and response to troglitazone. J Clin. Endocrinol. Metab. 2007;92(4):1502–1509. doi: 10.1210/jc.2006-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Distefano JK, Watanabe RM. Pharmacogenetics of anti-diabetes drugs. Pharmaceuticals (Basel) 2010;3(8):2610–2646. doi: 10.3390/ph3082610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Franks PW, Jablonski KA, Delahanty L, et al. The Pro12Ala variant at the peroxisome proliferator-activated receptor gamma gene and change in obesity-related traits in the Diabetes Prevention Program. Diabetologia. 2007;50(12):2451–2460. doi: 10.1007/s00125-007-0826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moore AF, Jablonski KA, Mcateer JB, et al. Extension of Type 2 diabetes genome-wide association scan results in the diabetes prevention program. Diabetes. 2008;57(9):2503–2510. doi: 10.2337/db08-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Majithia AR, Jablonski KA, Mcateer JB, et al. Association of the SLC30A8 missense polymorphism R325W with proinsulin levels at baseline and after lifestyle, metformin or troglitazone intervention in the Diabetes Prevention Program. Diabetologia. 2011;54(10):2570–2574. doi: 10.1007/s00125-011-2234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buse JB, Rubin CJ, Frederich R, et al. Muraglitazar, a dual (alpha/gamma) PPAR activator: a randomized, double-blind, placebo-controlled, 24-week monotherapy trial in adult patients with Type 2 diabetes. Clin. Ther. 2005;27(8):1181–1195. doi: 10.1016/j.clinthera.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 99.Cox SL. Muraglitazar: an agent for the treatment of Type 2 diabetes and associated dyslipidemia. Drugs Today. 2005;41(9):579–587. doi: 10.1358/dot.2005.41.9.925347. [DOI] [PubMed] [Google Scholar]
- 100.Kendall DM, Rubin CJ, Mohideen P, et al. Improvement of glycemic control, triglycerides, and HDL cholesterol levels with muraglitazar, a dual (alpha/gamma) peroxisome proliferator-activated receptor activator, in patients with Type 2 diabetes inadequately controlled with metformin monotherapy: a double-blind, randomized, pioglitazone-comparative study. Diabetes Care. 2006;29(5):1016–1023. doi: 10.2337/diacare.2951016. [DOI] [PubMed] [Google Scholar]
- 101.Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with Type 2 diabetes mellitus. JAMA. 2005;294(20):2581–2586. doi: 10.1001/jama.294.20.joc50147. [DOI] [PubMed] [Google Scholar]
- 102.Geese WJ, Achanzar W, Rubin C, et al. Genetic and gene expression studies implicate renin and endothelin-1 in edema caused by peroxisome proliferator-activated receptor gamma agonists. Pharmacogenet. Genomics. 2008;18(10):903–910. doi: 10.1097/FPC.0b013e32830a6ea0. [DOI] [PubMed] [Google Scholar]
- 103.Panicker GK, Karnad DR, Salvi V, Kothari S. Cardiovascular risk of oral antidiabetic drugs: current evidence and regulatory requirements for new drugs. J Assoc. Phys. India. 2012;60:56–61. [PubMed] [Google Scholar]
- 104.Yasar U, Bennet AM, Eliasson E, et al. Allelic variants of cytochromes P450 2C modify the risk for acute myocardial infarction. Pharmacogenetics. 2003;13(12):715–720. doi: 10.1097/00008571-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 105.Visser LE, Van Schaik RH, Jan Danser AH, et al. The risk of myocardial infarction in patients with reduced activity of cytochrome P450 2C9. Pharmacogenet. Genomics. 2007;17(7):473–479. doi: 10.1097/01.fpc.0000236335.57046.c8. [DOI] [PubMed] [Google Scholar]
- 106.Node K, Huo Y, Ruan X, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285(5431):1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Spiecker M, Liao JK. Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch. Biochem. Biophys. 2005;433(2):413–420. doi: 10.1016/j.abb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 108.Sahi J, Black CB, Hamilton GA, et al. Comparative effects of thiazolidinediones on in vitro P450 enzyme induction and inhibition. Drug Metab. Dispos. 2003;31(4):439–446. doi: 10.1124/dmd.31.4.439. [DOI] [PubMed] [Google Scholar]
- 109.Avandia. www.avandia.com. [Google Scholar]
- 110.Richter B, Bandeira-Echtler E, Bergerhoff K, Clar C, Ebrahim SH. Rosiglitazone for Type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2007;(3) doi: 10.1002/14651858.CD006063.pub2. CD006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Graham DJ, Ouellet-Hellstrom R, Macurdy TE, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304(4):411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 112.Richter B, Bandeira-Echtler E, Bergerhoff K, Clar C, Ebrahim SH. Pioglitazone for Type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;(4) doi: 10.1002/14651858.CD006060.pub2. CD006060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mannucci E, Monami M, Lamanna C, Gensini GF, Marchionni N. Pioglitazone cardiovascular risk. A comprehensive meta-analysis of randomized clinical trials. Diabetes Obes. Metab. 2008;10(12):1221–1238. doi: 10.1111/j.1463-1326.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 114.Ruano G, Bernene J, Windemuth A, et al. Physiogenomic comparison of edema and BMI in patients receiving rosiglitazone or pioglitazone. Clin. C’him. Acta. 2009;400(1–2):48–55. doi: 10.1016/j.cca.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 115.Cho S, Atwood JE. Peripheral edema. Am. J. Med. 2002;113(7):580–586. doi: 10.1016/s0002-9343(02)01322-0. [DOI] [PubMed] [Google Scholar]
- 116.Bailey SD, Xie C, Do R, et al. Variation at the NFATC2 locus increases the risk of thiazolidinedione-induced edema in the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) study. Diabetes Care. 2010;33(10):2250–2253. doi: 10.2337/dc10-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chang TJ, Liu PH, Liang YC, et al. Genetic predisposition and nongenetic risk factors of thiazolidinedione-related edema in patients with Type 2 diabetes. Pharmacogenet. Genomics. 2011;21(12):829–836. doi: 10.1097/FPC.0b013e32834bfff1. ••One major study that by evaluating the association between thiazolidinedione, edema and clinical and genetic variables aimed to develop a simple points system to predict risk of thiazolidinedione edema association.
- 118.Hansen L, Ekstrom CT, Tabanera YPR, Anant M, Wassermann K, Reinhardt RR. The Pro12Ala variant of the PPARG gene is a risk factor for peroxisome proliferator-activated receptor-gamma/alpha agonist-induced edema in Type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2006;91(9):3446–3450. doi: 10.1210/jc.2006-0590. [DOI] [PubMed] [Google Scholar]
- 119.Kawaguchi-Suzuki M, Frye RF. Current clinical evidence on pioglitazone pharmacogenomics. Front. Pharmacol. 2013;4:147. doi: 10.3389/fphar.2013.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wilson PW, D’agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch. Intern. Med. 2002;162(16):1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 121.Masud S, Ye S, Group SaS. Effect of the peroxisome proliferator activated receptor-gamma gene Pro12Ala variant on body mass index: a meta-analysis. J Med. Genet. 2003;40(10):773–780. doi: 10.1136/jmg.40.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gurnell M. ‘Striking the right balance’ in targeting PPARgamma in the metabolic syndrome: novel insights from human genetic studies. PPAR Res. 2007;2007:83593. doi: 10.1155/2007/83593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Franks PW, Jablonski KA, Delahanty LM, et al. Assessing gene-treatment interactions at the FTO and INSIG2 loci on obesity-related traits in the Diabetes Prevention Program. Diabetologia. 2008;51(12):2214–2223. doi: 10.1007/s00125-008-1158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]