Abstract
In normal cells multiple microRNAs (miRNAs) converge to maintain a proper balance of various processes, including proliferation, differentiation and cell death. miRNA dysregulation can have profound cellular consequences, especially because individual miRNAs can bind to and regulate multiple mRNAs. In cancer, the loss of tumour-suppressive miRNAs enhances the expression of target oncogenes, whereas increased expression of oncogenic miRNAs (known as oncomirs) can repress target tumour suppressor genes. This realization has resulted in a quest to understand the pathways that are regulated by these miRNAs using in vivo model systems, and to comprehend the feasibility of targeting oncogenic miRNAs and restoring tumour-suppressive miRNAs for cancer therapy. Here we discuss progress in using mouse models to understand the roles of miRNAs in cancer and the potential for manipulating miRNAs for cancer therapy as these molecules make their way towards clinical trials.
MicroRNAs (miRNAs) are a class of small, non-coding RNAs that post-transcriptionally control the translation and stability of mRNAs. The first miRNAs were identified through detailed forward genetic screens, which enabled the placing of these miRNAs into defined genetic pathways, thus providing a great deal of information regarding the biological roles of miRNAs in stem cell development1–5. More recent identification of miRNAs has been accomplished through enormous, high-throughput biochemical screens that unveiled a plethora of over 1,000 human miRNAs6. Interestingly, hundreds of these miRNAs map to regions of the human genome that are known to be altered in cancer7, and a similar number are aberrantly expressed in cancerous tissues, and/or bodily fluids or waste products from cancer patients (reviewed in REF. 8). This new wealth of knowledge points to miRNAs as being novel cancer genes and biomarkers. For example, miRNA expression profiles are now used to classify tumours based on the tissue type and stage of disease8–10. Unfortunately, the lack of high-throughput techniques to study miRNA functions has resulted in a pipeline of miRNAs that are ‘cancer related’, without having clearly defined molecular roles. Although hundreds of miRNAs are known to have dysregulated expression in cancer, key studies evaluating their biological and molecular roles, and their potential therapeutic applications, are still rare. Yet understanding the functions of miRNAs is crucial if we hope to uncover the roles of this form of gene regulation in cancer and to harness this knowledge for therapeutic benefit.
In this Review we focus on mouse models in which specific miRNAs are overexpressed or knocked out in order to understand the biological and molecular roles of miRNAs in cancer and metastasis. We also review the recent literature regarding the transition of these master regulators into clinical settings both as direct cancer therapeutics and as tools to sensitize tumours to traditional chemotherapeutics.
Uncovering miRNA functions using mouse models
Although individual miRNAs are dysregulated in various diseases, clear, causal evidence of their role in cancer has only recently come to light. Specifically, several strains of mice lacking or overexpressing cancer-associated miRNAs have been developed and characterized. These include germline transgenic or knockout mice for: miR-155; miR-21; miR-17~92 and its paralogues; miR-15 and miR-16; miR-146; and miR-29. Additional mouse models are the LIN-28-overexpressing strain (which begins to evaluate the in vivo loss of mature let-7) and the multiple conditional DICER knockout models (TABLE 1). Interestingly, most of these mouse models for miRNA dysregulation present with defects in the immune system, and many of these models progress to haematopoietic cancers and, in some cases, solid tumours.
Table 1.
Germline overexpression and knockout models to evaluate in vivo miRNA functions
| Gene product | Expression in the study | Observed phenotypes | Refs |
|---|---|---|---|
| Oncogenes | |||
| miR-155 | Overexpressed in the B cell lineage | Late-onset B cell malignancy | 11 |
| Overexpressed in mature B cells | Increased fraction of germinal centre B cells | 14 | |
| Deleted in B cells | Reduction in the number of germinal centre B cells | 14 | |
| Ubiquitously deleted | Lung airway remodelling; enteric inflammation; impaired protective immunity owing to diminished B and T cell responses and impaired dendritic cell integrity | 13 | |
| Ubiquitously deleted | Loss of antigen- and tissue-specific inflammation | 12 | |
| miR-21 | Overexpressed in nestin-expressing cells | Pre-B-cell lymphoma | 21 |
| Ubiquitously overexpressed | No phenotype alone; potentiated KrasG12D-induced lung tumorigenesis | 19 | |
| Ubiquitously deleted | Attenuated KrasG12D-induced lung tumourigenesis | 19 | |
| Ubiquitously deleted | Reduction in DMBA–TPA-induced skin papillomas | 20 | |
| miR-29‡ | Overexpressed in immature and mature B cells | Indolent B-CLL | 59 |
| miR-17~92 | Overexpressed in lymphocytes | Lymphoproliferative disease and autoimmunity | 24 |
| Ubiquitously deleted | Post-embryonic premature death, lung hypoplasia and ventricular septal defect; inhibited pro-B to pre-B transition; in combination with miR-106b~25 deletion, led to death at midgestation | 23 | |
| miR-106a~363§ | Ubiquitously deleted | No phenotype | 23 |
| miR-106b~25§ | Ubiquitously deleted | No phenotype alone; death at midgestation when in combination with miR-17~92 deletion | 23 |
| Overexpressed in prostate epithelium (in combination with its host gene, MCM7) | Prostatic intraepithelial neoplasia | 31 | |
| LIN28 | Overexpressed | Enhanced growth and delayed puberty | 60 |
| Tumour suppressors | |||
| miR-15~16 | Point mutation 3' to the stem-loop structure of precursor (pre)-mir-16-1 | Autoimmune and B cell lymphoproliferative disease; B-CLL | 36, 37 |
| Deletion of 13q14 | Indolent B cell-autonomous, clonal lymphoproliferative disorders (monoclonal B cell lymphocytosis, CLL and non-Hodgkin lymphoma); disorders slightly more aggressive than mir-15a- and mir-16-1-null animals | 38 | |
| Deletion of mir-15a~16-1 | Indolent B cell-autonomous, clonal lymphoproliferative disorders (monoclonal B cell lymphocytosis, CLL and non-Hodgkin lymphoma) | 38 | |
| miR-146a‖ | Ubiquitously deleted | Myeloproliferation and haematolymphoid tumours (myeloid sarcomas and lymphomas); autoimmune disorders (splenomegaly, lymphadenopathy, multi-organ inflammation) | 45, 47 |
| DICER | Conditionally deleted in lung | Enhanced lung tumour development in KrasLSL–G12D mice (KrasLSL–G12D;Dicer1+/− and KrasLSL−G12D;Dicer1−/−); reduced survival of KrasLSL–G12D;Dicer1+/− mice when transgenes were induced in the lung | 64,160 |
| Conditionally deleted in lung or muscle | Haploinsufficient tumour suppressor; decreased survival in KrasLSL–G12D; Trp53fl/fl;Dicer1+/− (relative to Dicer1+/+ or Dicer1−/− triple transgenics) | 64 | |
| Conditionally deleted in retinoblasts | Haploinsufficient tumour suppressor in a retinoblastoma-sensitized background | 65 |
Additional data support a role for miR-29a as a tumour suppressor.
Paralogue of miR17~92.
In vitro data support an alternative role for miR-146a as an oncogene. B-CLL, B cell chronic lymphocytic leukaemia; DMBA, 7,12-dimethylbenz(a)anthracene; MCM7, mini-chromosome maintenance protein 7; miRNA, microRNA; TPA, 12-O-tetradecanoylphorbol-13-acetate.
In discussing these mouse models and the supporting cell culture work and human tissue analysis, we have subdivided the following sections according to whether the miRNAs have strong data to support their role as either an oncogene or a tumour suppressor at this time. We follow with discussions of miRNAs for which there is evidence for context-dependent effects, and then we provide an overview of miRNAs that are involved in metastasis.
Oncogenic miRNAs
miR-155
The independent generation of transgenic, mir-155-overexpressing mice and mir-155-knockout mice demonstrated that this gene has a crucial role in the immune system11–14; wild-type levels of miR-155 are essential for preserving normal immune system function, including the maintenance of both major classes of cells (B and T lymphocytes) of the adaptive immune response and dendritic cells, which are involved in the innate immune response13,14. Although mir-155-knockout mice are immunocompromised owing to defects in these cell lineages, overexpression of miR-155 specifically in the B cell lineage results in pre-leukaemic pre-B cell proliferation in the spleen and bone marrow, followed later in life by B cell malignancy11. The delay to malignancy is probably explained by the time that is required to accumulate the necessary secondary mutations, as miR-155 has recently been shown to repress genes encoding DNA damage response proteins15. In this model, miR-155 overexpression represents the ‘first hit’, thus establishing a pre-cancerous environment, which may be pushed towards malignancy by further mutations.
miR-21
One of the first oncogenic miRNAs identified was miR-21. Because of its elevated levels in many different cancers16–18, three groups generated mir-21-knockout or mir-21-overexpressing mice19–21. Forced 15–30-fold inducible overexpression of miR-21 under the control of the nestin promoter resulted in severe pre-B-cell lymphoma21. In concordance, similar if not higher levels of miR-21 are reported in the serum and tumours of patients with cancer16,22. Upon returning miR-21 to endogenous levels the mouse tumours disappeared. Notably, this was the first report indicating the addiction of tumours to a single oncogenic miRNA (termed ‘oncomir addiction’). A miR-21 effect was also observed in a separate series of models19. Ubiquitous expression of miR-21, fourfold to sixfold over endogenous levels, resulted in no obvious phenotypes; however, miR-21 overexpression could potentiate the phenotype of mice with a latent KrasG12D allele (KrasLA2), a constitutively activated version of the KRAS proto-oncoprotein. Doubly transgenic animals had an increased lung tumour burden relative to the KrasLA2 mice, but no increase in the rate of conversion from adenoma to adenocarcinoma. By contrast, the lung tumour burden was decreased in KrasLA2;mir-21−/− animals, relative to the KrasLA2 controls. Unlike the previous study21, here miR-21 is involved in the later stages of tumorigenesis and not in tumour promotion, as it has no effect on tumorigenesis in the absence of oncogenic KRAS.
A third study reported an oncogenic role for miR-21 in skin carcinogenesis20. In this DMBA–TPA model, wild-type animals developed early skin papillomas that progressed into invasive carcinomas. In identically treated mir-21−/− animals, papilloma multiplicity and incidence were reduced. The molecular explanation probably involves increased expression of the pro-apoptotic miR-21 target genes sprouty homologue 1 (SPRY1), PTEN and programmed cell death protein 4 (PDCD4), which negatively regulate the RAF, PI3K and RAL guanine-nucleotide dissociation stimulator (RALGDS) pro-survival signalling pathways, respectively. In accordance, mir-21 loss was associated with enhanced cellular apoptosis and a moderate reduction in proliferation.
These studies, combined with human tissue data and cell culture experiments, confirm that miR-21 is an oncogene and provide a rationale for the therapeutic inhibition of miR-21.
miR-17~92
Often miRNAs are found in large clusters that are expressed polycistronically; in these cases, it is often of value to evaluate the function of the cluster and of the individual miRNAs within the cluster. The mir-17~92 cluster and its paralogues, mir-106b~25 and mir-106a~363, are several such regions that have been extensively examined23,24. The mir-17~92 cluster, contained within a fragile site in the genome7, is amplified in both solid tumours and haematopoietic malignancies25–29. As expected, mice overexpressing the miR-17~92 cluster in lymphocytes develop lymphoproliferative disease and autoimmunity, and they die prematurely24. Note that even in the absence of genomic amplification, miR-17~92 expression can be directly induced through transactivation by MYC or MYCN30. The interplay between MYC and miR-17~92 was demonstrated in a mouse model of B cell lymphoma. Tumour development was accelerated in animals reconstituted with haematopoietic stem cells expressing a truncated version of the cluster, mir-17–19b-1, and Eμ-driven Myc, probably through anti-apoptotic mechanisms27 that might include the downregulation of the miR-17~92 targets PTEN and BCL-2-like protein 11 (BCL2L11; also known as BIM)24. Genetic deletion of mir-17~92 confirmed its importance for B cell development, whereas deletion of the paralogues, mir-106b~25 or mir-106a~363, had no obvious phenotypes23. By contrast, overexpression of the mir-106b~25 cluster cooperated with overexpression of its host gene, mini-chromosome maintenance protein 7 (Mcm7) to induce prostatic intraepithelial neoplasia. This provides an example of overexpression of a single genetic locus contributing to two ‘oncogenic hits’: elevated MCM7 levels and an increased expression of oncogenic miR-106b~25 (REF. 31).
Although the roles for these clusters in oncogenesis have been documented, the causal role for individual miRNAs within the clusters is just beginning to be defined. There are six individual mature miRNAs within the miR-17~92 cluster, subdivided into three distinct families (miR-17, miR-20a and miR-18a; miR-19a and miR-19b; and miR-92a) based on sequence homology within the seed region. Of the three, the miR-19 family has been confirmed by two groups to contain the key oncomirs32,33. Overexpression of the miR-17~92 cluster lacking both miR-19 family members in Eμ-Myc B cell lymphoma cells increased the latency of lymphoma when these cells were injected into a cohort of nude mice, relative to cells with the intact cluster32. In addition, the overexpression of individual miRNAs within the cluster, or inactivating mutations in both mir-19a and mir-19b, confirmed that miR-19 was both sufficient and necessary for promoting MYC-induced lymphomagenesis33. Furthermore, multiple members of the miR-17~92 cluster — miR-19b, miR-20a and miR-92 — are capable of individually promoting NOTCH1-induced T cell acute lymphoblastic leukaemia (T-ALL) in a mouse model34. Contrary to the above experiments that propose miR-19 as the major oncomir in the cluster, miR-20b and miR-92 had similar if not enhanced ability to reduce disease latency. These miRNAs reduce the expression of the tumour suppressors PTEN and BIM, which are frequently downregulated in T-ALL.
Tumour-suppressive miRNAs
miR-15~16
Similarly to the overexpression of miR-17~92, a B cell lymphoproliferative disorder is observed in mice that are deficient for miR-16 and/or miR-15a, which are the first miRNAs that were implicated in cancer35. These two miRNAs constitute a small miRNA cluster that is located in 13q14, a fragile site in the genome that is deleted in >50% of cases of B cell chronic lymphocytic leukaemia (B-CLL). A naturally occurring mouse model, the New Zealand black (NZB) mouse, develops symptoms that are similar to human B-CLL, and a comprehensive genetic study identified a causative point mutation in the 3′ flanking region of mir-16 that reduced miR-16 expression36. Reintroducing miR-15a~16 into cells that were derived from NZB mice restored cell cycle control and increased apoptosis, concurrent with reduced levels of the cell cycle regulator and miR-15 and miR-16 target, cyclin D1 (REF. 37). To further validate that the causal gene within the 13q14 deleted region is the mir-15a~16 cluster, Dalla-Favera and colleagues38 generated two strains of transgenic mice. The first strain lacks the 13q14 minimal deleted region (MDR); this deletion removes the mir-15a~16 cluster and its host gene, deleted in leukaemia 2 (Dleu2). The second strain lacks only the mir-15a~16 cluster. In both strains, a clonal population of B cells became evident as the mice aged. Furthermore, the disease was determined to be B cell autonomous; similar pathologies were obtained whether the deletions were ubiquitous or strictly confined to the B cell lineage. Although mice lacking the MDR had a more aggressive disease course, suggesting that an additional genetic element within the MDR locus contributes to the tumour-suppressive function, restoration of miR-15a~16, but not DLEU2, decreased cellular proliferation in vitro.
These models suggest a crucial function of the DLEU2/mir-15a~16 locus in B cells; however, deletion of this region also occurs in other cancers, such as multiple myeloma and prostate cancer, which indicates its importance in other cellular contexts39,40. For example, miR-15a and miR-16 are often downregulated in the stroma of prostate cancers, implicating a non-cell-autonomous mechanism for the cluster39. Increased miR-15a and miR-16 expression in supporting fibroblasts impaired the expansion of prostate cancer xenografts through direct downregulation of fibroblast growth factor 2 (FGF2) and FGF receptor 1 (FGFR1)39; this pathway promotes cell survival through activation of the RAS–MEK–ERK and PI3K–AKT pathways. (Note that aberrant expression of FGFs and FGFRs are found in multiple cancers, including prostate cancer41.)
These models support a tumour-suppressive role for the mir-15a~16 cluster in vivo. Therefore, miR-15 and miR-16 replacement therapy should be considered for tumours that have lost miR-15a and miR-16 expression or that show elevated expression of cognate target genes.
Context-dependent miRNAs
miR-146
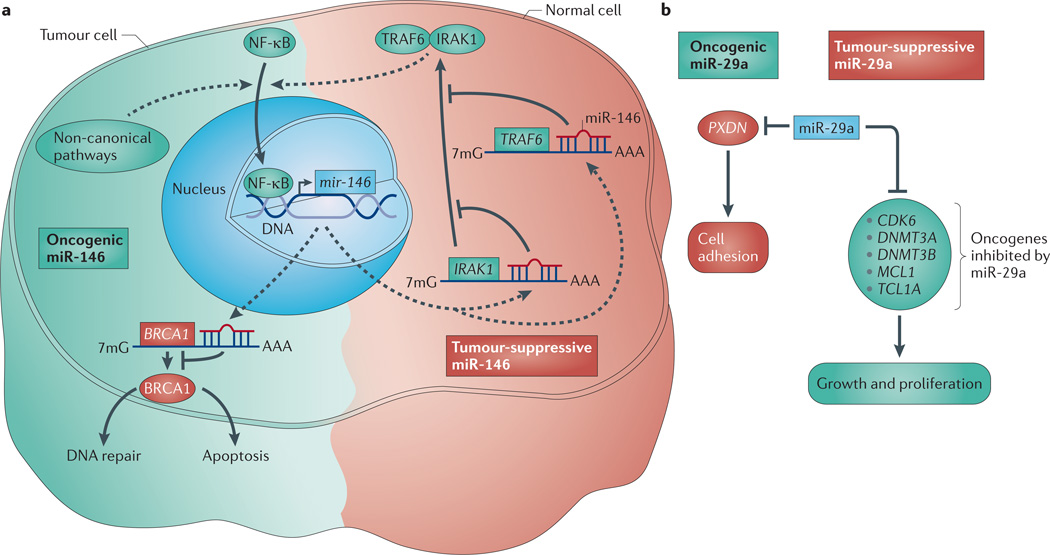
Some miRNAs, such as miR-146, have what seem to be opposing roles in tumorigenesis that depend on the cellular context. Expression levels of miR-146 family members, mir-146a and mir-146b-5p, which are encoded by separate genes, are elevated in breast, prostate, endocrine pancreatic, cervical, thyroid and ovarian carcinomas18,42–44. Whether this elevation drives tumorigenesis or is a cellular safeguard that is put in place to prevent it is not entirely clear. Although most data support a tumour-suppressive function for miR-146 (see below)45–47, a recent report showed that miR-146 can reduce the expression of the DNA repair enzyme BRCA1 (REF. 48) (FIG. 1). Although this remains to be confirmed in miR-146-overexpressing mice, it is suggestive of an oncogenic role in the breast cancer lines in which it was tested.
Figure 1. Opposing roles of miRNAs in cancer.
a | Oncogenic (shown in green) versus tumour-suppressive (shown in red) functions of miR-146 can be explained based on upstream nuclear factor κB (NF-κB) signals. In cells that are dependent on interleukin 1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6) for NF-κB signalling, miR-146 expression prevents NF-κB activation, resulting in a tumour-suppressive phenotype. However, if an alternative mechanism for NF-κB activation is present (other than through IRAK1 and TRAF6), activated NF-κB would transactivate miR-146. In addition to targeting IRAK1 and TRAF6, miR-146 also targets BRCA1, thus preventing the pro-apoptotic effects of BRCA1 and resulting in a pro-survival response. b | Tumour-suppressive miR-29a targets multiple oncogenes, such as cyclin-dependent kinase 6 (CDK6), DNA (cytosine-5)-methyltransferase 3A (DNMT3A), DNMT3B, myeloid cell leukaemia sequence 1 (MCL1) and T-cell leukaemia/lymphoma 1A (TCL1A). Targeting inhibits growth and proliferation and, in the case of B cell chronic lymphocytic leukaemia (B-CLL), aggressive disease. Oncogenic miR-29a prevents cell adhesion through repressing peroxidasin homologue (PXDN). 7mG, 7-methylguanine; miRNA, microRNA.
Albeit not a germline transgenic, overexpression of miR-146a in transplanted bone marrow cells provides evidence in support of a tumour-suppressive role46. miR-146 overexpression reduced the survival and engraftment of haematopoietic stem cells in recipient mice, as shown by decreased erythropoiesis and impaired lymphopoiesis46. Likewise, studies from mir-146-knockout mice further imply that miR-146 functions as a tumour suppressor in cells of haematopoietic origin. mir-146a-knockout mice develop massive myeloproliferation followed by tumours of haematolymphoid origin, including myeloid sarcomas and lymphomas45,47. The myeloproliferative phenotype correlates with enhanced nuclear factor κB (NF-κB) signalling, which is most pronounced in the spleen and bone marrow47. Indeed, miR-146a suppresses the NF-κB activators interleukin 1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6)45,49 (FIG. 1).
Based on the reported literature, miR-146 function may depend on the cellular context. Note that NF-κB signalling upregulates miR-146, which indirectly represses NF-κB49 (FIG. 1). Thus, in cells in which the primary driver of NF-κB is IRAK1 and TRAF6, miR-146 will probably have tumour-suppressive effects through the negative regulation of NF-κB (and hence negative autoregulation). By contrast, NF-κB can still be activated in IRAK1- or TRAF6-deficient cells50,51 through other pathways; in these cells negative feedback through IRAK1 or TRAF6 will not have dramatic effects. Therefore, because NF-κB induces mir-146 transcription, the potential downstream oncogenic effects of this miRNA may be brought to the fore in these contexts. Therefore, perhaps the difference between the tumour-suppressive and oncogenic roles of miR-146 in haematopoietic cancers and solid tumours is the degree to which NF-κB is regulated by IRAK1 or TRAF6.
Additionally, the complexity involved in miRNA regulation cannot be ruled out. Some factors that fluctuate greatly between cell types include miRNA expression, processing and nuclear export (FIG. 2) and the relative expression and subset of target genes (FIG. 3). Additionally, alternative polyadenylation, splicing and single nucleotide polymorphisms in either the mRNA or miRNA (BOX 1) can amend the miRNA binding sites (FIG. 3). Many of these anomalies are common in oncogenic transcripts in cancer cells, rendering them less responsive to miRNA-dependent regulation. Regardless, more studies are needed to evaluate miR-146 and other miRNAs whose roles in tumorigenesis are ambiguous.
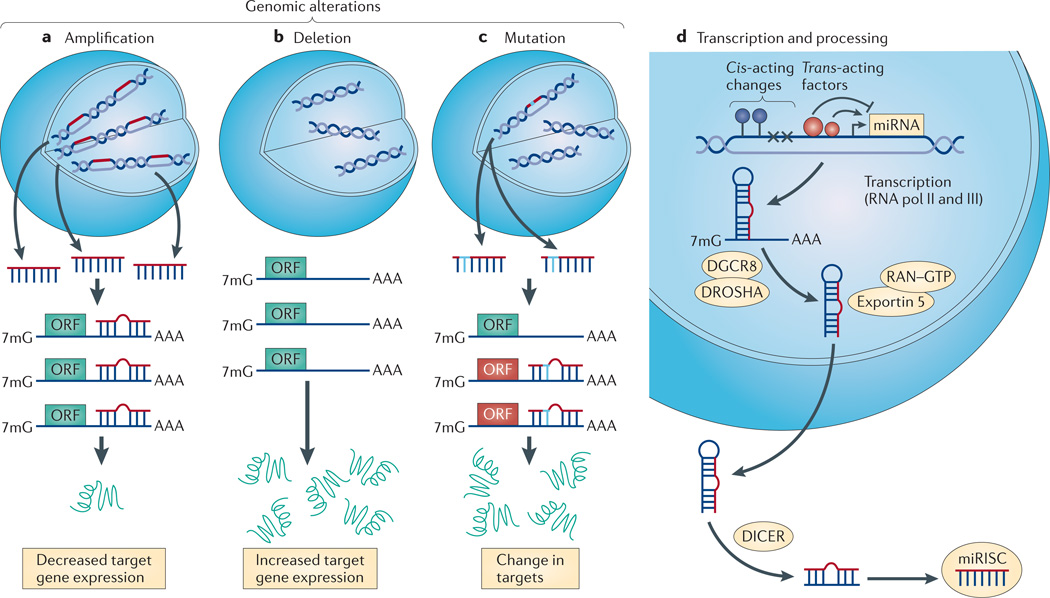
Figure 2. RNAs are subject to genomic, transcriptional and post-transcriptional modes of regulation.
a | Allelic amplification (which is typical of oncogenic microRNAs (miRNAs)) results in decreased expression of target genes, including those targets with less miRNA affinity that may not normally be repressed. b | Genomic deletion (which is typical of tumour-suppressive miRNAs) enhances target gene expression. c | Single nucleotide polymorphisms (SNPs) in the miRNA can either create or destroy miRNA binding sites (BOX 1). This alteration in the miRNA changes the ability of the miRNA to bind to and repress target genes. In cancer, SNPs can alter the miRNA such that it now targets tumour-suppressive genes while losing its ability to target oncogenes. d | At the transcriptional level, cis-acting changes to the promoter, including epigenetic regulation (such as promoter methylation, which is depicted by the blue circles) or genomic mutation (which is depicted as ‘×’) and the availability of trans-acting factors change the expression profile of miRNAs in a cell. Finally, miRNA processing defects can change the population of mature miRNAs in a cell. These processing steps include: primary miRNA (pri-miRNA) to precursor miRNA (pre-miRNA) cleavage by the DROSHA–DGCR8 complex; nuclear export by the RAN GTPase and exportin 5; a final cleavage event by DICER; and loading of the mature miRNA into the RNA-induced silencing complex (RISC). 7mG, 7-methylguanine; ORF, open reading frame.
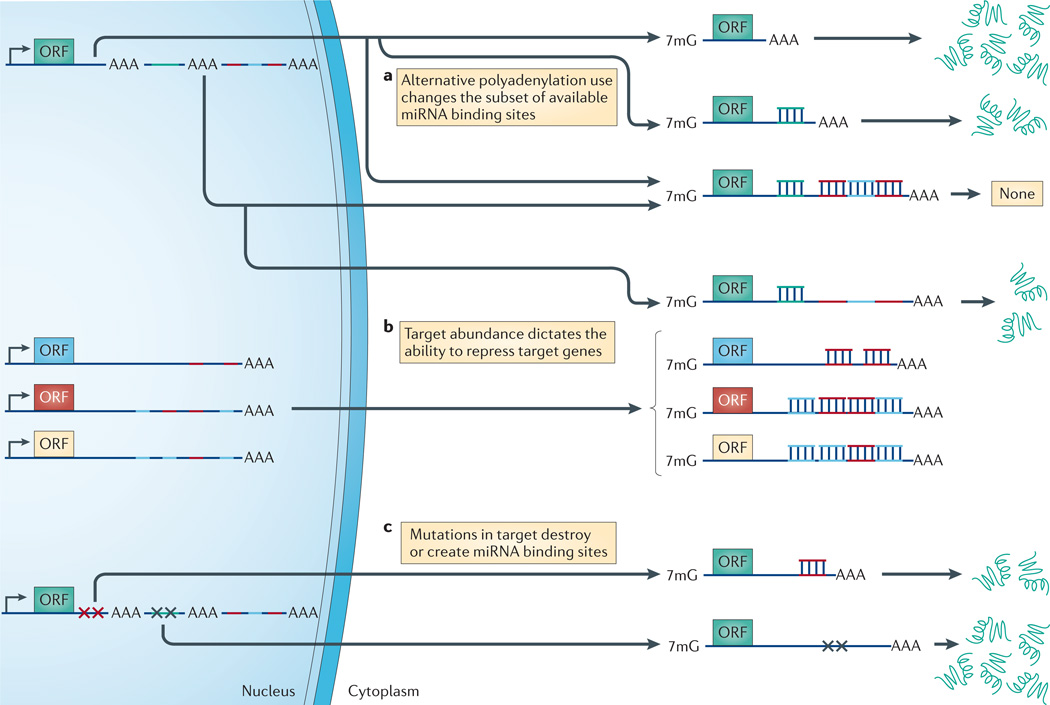
Figure 3. RNA expression patterns dictate miRNA repressibility in cells.
a | Alternative polyadenylation signals give an mRNA transcript the ability to evade regulation by microRNAs (miRNAs). Longer 3′ untranslated regions (UTRs) usually contain more miRNA binding sites and are therefore more sensitive to repression by miRNAs. b | An increased abundance of additional target genes can ’soak-up’ the miRNA pools, leading to target gene derepression. c | Mutations in the target gene can create or destroy miRNA binding sites. 7mG, 7-methylguanine; ORF, open reading frame.
Box 1 | SNPs can destroy and/or create miRNA binding sites.
Single nucleotide polymorphisms (SNPs) in either the mRNA156 or the microRNA (miRNA) can alter the ability of a miRNA to regulate its target genes. The first mRNA SNP that was identified as affecting a potential miRNA binding site was in a let-7 complementary site in KRAS (REF. 157). SNPs in oncogenes often destroy miRNA binding sites, thus allowing the gene to be expressed at higher levels. Conversely, SNPs in tumour suppressor genes generate novel binding sites, ultimately resulting in decreased expression of the tumour suppressor. As is the case for miRNAs, a single SNP can destroy and/or create a target site. In the case of cancer this would result in the destruction of oncogene repression and the creation of novel tumour suppressor gene repression. SNPs in miRNA promoters or precursors can also lead to expression158 or processing159 defects that alter mature miRNA levels (for a detailed review see REF. 8).
miR-29
The miR-29 family has several seemingly opposing functions in tumorigenesis. miR-29a and miR-29b, which are downregulated in mantle cell lymphoma, are suggested to be tumour suppressors that target multiple cell cycle regulators and oncogenes52–55 (FIG. 1). Consistent with a tumour-suppressive role, overexpression of miR-29 induces apoptosis and suppresses lung cancer and acute myeloid leukaemia (AML) cell tumorigenicity in xenografts52,56. Furthermore, in indolent B-CLL, which has an average survival in humans of 25 years, miR-29 is proposed to target oncogenic TCL1A, potentially preventing fully malignant disease progression54 (reviewed in REF. 57).
By contrast, the observation of miR-29 overexpression in AML and B-CLL, and in vivo miR-29 overexpression studies, imply that mir-29 is an oncogene. In one experiment, lethally irradiated mice were transplanted with miR-29-transduced haematopoietic stem and progenitor cells, resulting in symptoms that were consistent with myeloproliferative disease and a latent progression to AML58. A second system specifically evaluated the contribution of overexpressed miR-29a to B-CLL. When miR-29a was expressed in immature and mature B cells, there was an expansion of CD5+ B cells, mice presented with enlarged spleens, and roughly 20% of animals developed overt leukaemia and died late in life, suggesting that in this case miR-29a can predispose cells to a cancerous state59. The molecular mechanism probably involved direct translational silencing of the tumour-suppressive cell-adhesion molecule peroxidasin homologue (PXDN) by miR-29a (FIG. 1).
These data suggest that miR-29a functions as a tumour suppressor or oncogene, depending on the cellular context.
miRNA processing machinery
LIN28
Models in which the miRNA processing machinery is perturbed give insight into the function of particular miRNA families, as observed in the LIN28-overexpressing strain. LIN28 is an RNA-binding protein that suppresses the maturation of let-7 family miRNA precursors. In this way, a mouse model overexpressing Lin28 overcomes the technical challenges associated with knocking out all 14 let-7 family members60,61. Because let-7 is a bona fide tumour suppressor62, loss of let-7 at the organismal level is predicted to predispose animals to cancer. These studies, which focused on the role of LIN28 in development60 and glucose metabolism61, did not report any cancerous phenotypes. Whether overexpressing LIN28 at later timepoints can induce tumorigenesis or potentiate tumour-prone mice, such as those that overexpress activated Kras or Myc or that lack Trp53, remains to be determined.
DICER
In cancer, miRNA expression is frequently decreased63; defects in DICER or other miRNA processing machinery could elicit such an effect (FIG. 2). Indeed, deletion of Dicer1 reduces the overall levels of mature miRNAs64. Perhaps surprisingly, animals with a single copy of Dicer1 were tumour-prone and succumbed to death earlier than homozygous deleted animals64. Moreover, tumours harvested from Dicer1fl/fl mice, which in the presence of the CRE recombinase should lead to loss of both Dicer1 alleles, retained one intact Dicer1 allele suggesting that DICER functions as a haploinsufficient tumour suppressor64,65. Overexpression of miRNAs or reductions in transcription factors that specifically regulate DICER (discussed below)66,67 recapitulates the phenotypes of Dicer1 heterozygote mice, suggesting that the regulation of DICER in cancer is a multifactorial process. Importantly, human tumours also frequently present with hemizygous deletion of DICER1 and overexpression of miRNAs that target DICER expression66.
Interestingly, a phenotype similar to that of Dicer1fl/fl mice was observed in TAp63−/− mice67. Multiple different isoforms exist for p63: the p63ΔN isoform lacks the transactivation domain and acts in a dominant-negative fashion against the tumour suppressor p53. By contrast, the full-length isoform, TAp63, which contains the transactivation domain, acts in concordance with p53. Recent data suggest that TAp63 functions as a haploin-sufficient tumour suppressor, similar to that of DICER67. In TAp63−/− animals, DICER was expressed at very low levels suggesting a genetic link between them; indeed, TAp63 binds to the Dicer1 promoter and transactivates Dicer1 expression. Accordingly, restoration of DICER expression in TAp63+/− cells reduced their invasive potential.
miRNAs involved in metastasis
miRNAs have been identified as key players in several stages of metastasis (FIG. 4). For this Review we focus on miR-200, the LIN28–let-7 interaction and DICER — which are all involved in primary tumour development and early metastatic disease — and miR-31 and miR-10b, which have roles that are specifically constrained to metastatic progression without affecting the primary tumour. Because this was recently reviewed68 we focus here on recent developments.
Figure 4. miRNAs that contribute to metastasis.
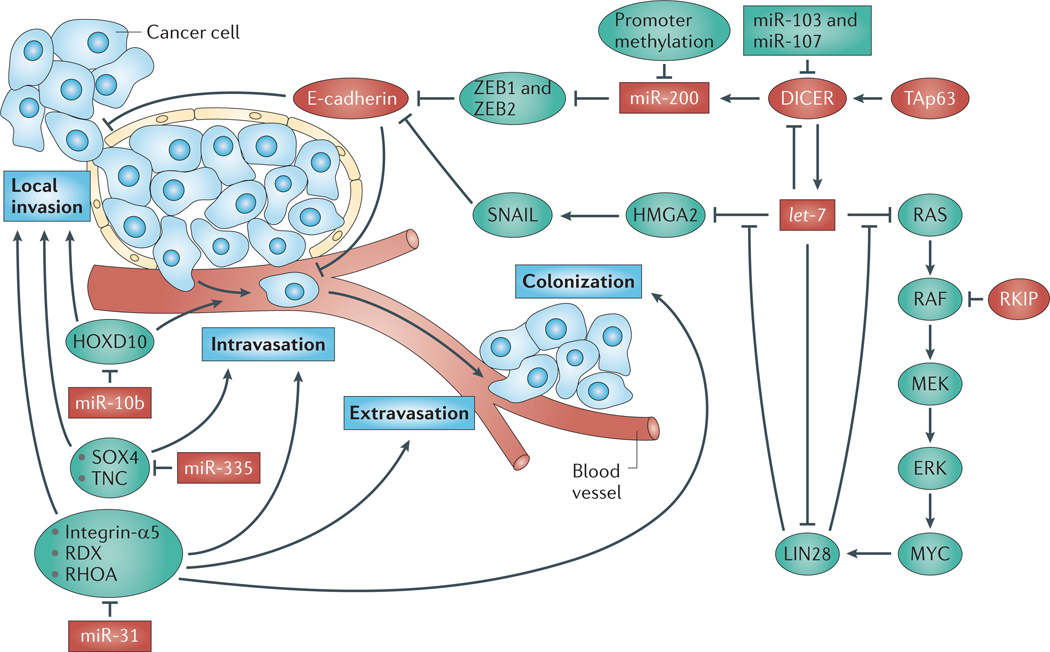
Metastasis occurs through a series of stages: local invasion, intravasation, extravasation and colonization (as indicated by the blue boxes). Protein-coding genes and microRNAs (miRNAs) that promote (shown in green) or prevent (shown in red) metastasis are involved at each step (the figure covers only those pathways that are discussed in the main text). miR-200 directly represses the expression of the mesenchymal markers zinc finger E-box-binding homeobox 1 (ZEB1) and ZEB2 in quiescent cells. As such, during the epithelial to mesenchymal transition (EMT) when miR-200 levels are reduced, the expression of ZEB1 and ZEB2 becomes elevated. ZEB1 and ZEB2 transcriptionally repress E-cadherin, a cell adhesion molecule that is lost in aggressive and metastatic tumours; thus, cells missing miR-200 are more able to disseminate and invade surrounding tissue. Furthermore, the inhibition between miR-200, and ZEB1 and ZEB2 is mutual, leading to a potentially bistable system: either high miR-200 and low ZEB1 and ZEB2 levels, or high ZEB1 and ZEB2 and low miR-200 levels. This latter state would seem to be particularly dangerous and could lead to aggressive tumours. Based on its miRNA-processing ability, DICER is positioned upstream of miR-200. DICER1 itself is positively regulated at the transcriptional level by the full-length isoform of p63 (TAp63), and is negatively regulated by miR-103, miR-107 and let-7. In addition to suppressing DICER expression, let-7 also inhibits the oncogenic RAS–RAF–MEK pathway and HMGA2, an upstream activator of SNAIL. SNAIL inhibits E-cadherin, leading to local invasion and intravasation. Antimetastatic miRNAs that are involved outside of the E-cadherin pathway include miR-10b, which suppresses the expression of HOXD10; miR-335, which impairs SOX4 and tenascin C (TNC) signalling; and miR-31, which prevents all steps of metastasis through inhibiting the expression of integrin-α5, radixin (RDX) and RHOA. RKIP, RAF kinase inhibitory protein.
miR-200
Screens for miRNAs that are downregulated during the epithelial to mesenchymal transition (EMT) have identified five members of the miR-200 family (miR-200a, miR-200b, miR-200c, miR-141 and mir-429)69,70. miR-200 loss is common in aggressive lung, prostate and pancreatic cancers71–73. These studies suggest an association between EMT and the loss of miR-200; however, whether miR-200 loss is capable of inducing metastasis or is just a read-out of disease progression was only recently evaluated. In the highly aggressive KrasLA1;Trp53R172HΔG mouse lung cancer model, in which KrasG12D is activated somatically through a latent allele and wild-type p53 function is perturbed, attenuation of miR-200 was required for metastasis71. Ectopic miR-200 expression prevented the invasion and metastasis of cells from these mice71 by repressing the mesenchymal markers zinc finger E-box-binding homeobox 1 (ZEB1) and ZEB2, thus restoring E-cadherin expression69,70,74 (FIG. 4). These studies support the use of miR-200 restoration therapy for aggressive cancers.
LIN28 and let-7
LIN28, which is involved in stem cell maintenance75–77 and is also a marker of cancer stem cells, is often activated in advanced stage and high-grade tumours78. In concordance with this, let-7 and LIN28 have recently been placed in a metastasis-signalling cascade involving the RAF kinase inhibitory protein (RKIP)78. RKIP negatively regulates metastasis by inhibiting RAF kinase signalling and downstream MYC activation. Because MYC transcriptionally induces LIN28, RKIP indirectly suppresses LIN28 leading to enhanced let-7 function (including the suppression of oncogenic HMGA2 and RAS family members) (FIG. 4). Indeed, overexpressing LIN28 in vivo restores metastasis in RKIP-overexpressing tumours.
DICER
In vivo evidence supports the regulation of DICER expression by the miR-103/107 family, including a role for these miRNAs in metastasis. Piccolo and colleagues66 screened DICER1 for potential miRNA binding sites owing to its unusually long 3′ untranslated region (UTR), and confirmed direct negative regulation by miR-103 and miR-107. miR-103 and miR-107 levels are inversely correlated with DICER levels in human breast cancer samples, and miR-103 and miR-107 upregulation is associated with metastasis66,79. In vivo silencing of miR-103 and miR-107 inhibited metastasis, whereas overexpression induced metastasis of otherwise non-metastatic cells66. Interestingly, upregulation of miR-103 or miR-107 in human samples occurs more often (37%) than copy number variations of DICER1 (18%), suggesting that reducing DICER levels by miRNAs is preferred to allelic loss66.
The regulation of DICER by the miR-103/107 family was genetically linked to reduced miR-200 levels during EMT66 (FIG. 4). Because miR-103 and miR-107 reduce DICER expression, most miRNAs, including miR-200, are downregulated when miR-103 and miR-107 are overexpressed. (This is also the case when let-7 is overexpressed because it also targets DICER expression80.) Bridging these pathways together, silencing of miR-103 and miR-107 restored miR-200 expression and prevented EMT, whereas miR-200 overexpression reverted EMT that was induced by miR-103 and miR-107.
miR-31 and miR-10b
Only a few miRNAs have been shown to have a clear role in metastasis without affecting the other steps of carcinogenesis; these include miR-31 and miR-10b. miR-31 expression is decreased in metastatic human breast cancer samples and has been confirmed to have an important role in metastasis81–83. miR-31 overexpression causes the regression of metastases with no effect on the primary tumour82. Inducing miR-31 expression at various times following the transplantation of metastatic MDA-MB-231 human breast cancer cells did not alter primary tumour growth. However, miR-31 expression reverted the primary tumour from an invasive to a non-invasive phenotype through targeting prometastatic genes81,82 (FIG. 4). Similarly to treatment with traditional chemotherapies, miR-31 overexpression at later timepoints had no effect on the metastatic lesions. However, following primary tumour resection, miR-31 maintained its antimetastatic effects, thus pointing to the adjuvant use of miR-31. A similar scenario was observed for miR-10b, which is upregulated in — and seems to be restricted to — metastatic lesions (as discussed below in the ‘miRNA-based therapeutics’ section)84.
Other miRNAs
Additional miRNAs that are involved in metastasis (in particular in breast cancer) include miR-373, miR-520c, miR-126 and miR-335 (REFS 85,86). In a genetic screen using a non-metastatic human breast tumour cell line that was transduced with a miRNA expression library, miR-373 and miR520c emerged as positive metastatic regulators85. Overexpression of miR-373 and miR520c stimulated migration and invasion in vitro and in vivo, presumably through suppressing CD44, which is a cell surface marker of breast and prostate cancer stem cells. Conversely, loss of tumour-suppressive miRNAs miR-126, miR-335 and miR-206 was identified in tumours from patients with metastatic relapsing breast cancer86, and forced overexpression of miR-126 and miR-335 in malignant breast cancer cells suppressed metastasis to the lung and bone in mice. Although miR-126 reduced overall tumour growth and proliferation, miR-335 specifically inhibited metastatic invasion by targeting the expression of proteins that are involved in cell migration, such as the cell fate determinant SOX4 and the extracellular matrix component tenascin C (TNC) (FIG. 4).
miRNA-based therapeutics
It is well accepted that aberrant miRNA expression is linked to cancer, and the emerging mouse models described above provide evidence that miRNAs have a causal role in cancer. This implies that these molecules (in the case of tumour suppressors) or their antagomirs (in the case of oncomirs) might serve as effective therapeutics. Below we review some of the most promising individual miRNA-based therapeutic studies performed in vivo, which are summarized in TABLE 2.
Table 2.
Therapeutic use of miRNAs and antagomirs in vivo
| miRNA‡ | Delivery method | Model used | Phenotypes | Refs |
|---|---|---|---|---|
| let-7 | Intranasal delivery of viral particles | KrasG12D/+ autochthonous NSCLC mouse | Suppression of lung tumour initiation when delivered at the same time as transgene activation | 145, 146 |
| Intratumoral injection of lipid-based mimetic | Subcutaneous H460 NSCLC xenografts | Interfered with further tumour growth | 62 | |
| Intranasal delivery of viral particles | KrasG12D/+ autochthonous NSCLC mouse | Reduced burden of tumours that were allowed to preform 10 weeks before let-7 therapy | 62 | |
| Systemic delivery of lipid-based mimetic | KrasG12D/+ autochthonous NSCLC mouse | Reduced burden of tumours that were allowed to preform 10 weeks before let-7 therapy | 90 | |
| Transfected into cells before transplantation | Subcutaneous human U251 or U87 glioblastoma cells | Reduced tumour formation | 91 | |
| Transduced into cells before transplantation | Chemotherapy-resistant breast tumour initiating cells | Reduced tumour formation and metastasis | 92 | |
| miR-143 and miR-145 | Transduced into cells before transplantation | Subcutaneous MiaPaCa2 and Panc1 PDAC xenografts | Unable to form tumours | 94 |
| Systemic delivery of lipid-based expression vectors | Subcutaneous MiaPaCa2 PDAC xenografts | Inhibited growth | 96 | |
| Systemic delivery of lipid-based expression vectors | Orthotopic MiaPaCa2 PDAC xenografts | Inhibited growth | 96 | |
| miR-143 | Systemic delivery of anti-miR-143 | p21-HBx HCC mouse | Inhibited primary tumour and local and distant metastatic growth | 100 |
| miR-34 | Intratumoral delivery of lipid-based mimetic | Subcutaneous H460 NSCLC xenografts | Inhibited growth | 106 |
| Systemic delivery of lipid-based mimetic | Subcutaneous H460 and A549 NSCLC xenografts | Inhibited growth | 106 | |
| Systemic delivery of lipid-based mimetic | KrasG12D/+ autochthonous NSCLC mouse | Reduced burden of tumours that were allowed to preform 10 weeks before miR-34 therapy | 90 | |
| Transfected oligonucleotides into cells before transplantation | Subcutaneous prostate cancer xenografts (multiple cell lines) | Reduced tumour incidence | 107 | |
| Transduced cells with virus encoding precursor (pre)-miR-34 before transplantation | Subcutaneous prostate cancer xenografts (multiple cell lines) | Reduced tumour incidence | 107 | |
| Intratumoral injection of lipid-based mimetic | Subcutaneous PPC1 prostate cancer xenografts | Inhibited growth | 107 | |
| Systemic delivery of lipid-based mimetic | Orthotopic PC3 prostate cancer xenografts | Reduced tumour burden | 107 | |
| Systemic delivery of lipid-based mimetic | Orthotopic LAPC9 prostate cancer xenografts | Reduced lung metastasis without affecting primary tumour growth; extended survival | 107 | |
| Systemic delivery of lipid-based expression vectors | Subcutaneous and orthotopic MiaPaCa2 PDAC xenografts | Inhibited growth | 96 | |
| miR-122 | Transduced into cells before transplantation | Orthotopic SKHEP1 HCC xenografts | Reduced tumorigenesis, angiogenesis and intrahepatic metastasis | 111 |
| Systemic delivery of nucleic acid antagomir-miR-122 | HCV-infected non-human primates | Suppression of HCV viraemia and improved liver pathology | 112 | |
| Systemic delivery of lysine–lipid nanoparticle antagomir-miR-122 | C57BL/6J mice | Decreased plasma cholesterol levels | 113 | |
| Systemic delivery of antagomir-miR-122 | C57BL/6J mice | Decreased plasma cholesterol levels | 118 | |
| miR-26a | Systemic delivery of adeno-associated virus | HCC liver cancer model: MYC driven by the liver activator promoter | Inhibition of proliferation; induction of apoptosis; disease protection | 114 |
| miR-10b | Systemic delivery of antagomir-miR-10b | Implantation of 4T1 breast cancer cells into the mammary fat pad | Prevented metastasis with no effect on the primary tumour | 84 |
miRNAs are presented in the order in which they are discussed in the main text. miRNAs for which there is therapeutic evidence in endogenously occurring tumours or orthotopic implants are included. In some instances xenograft models are included but only as supporting evidence.
HCC, hepatocellular carcinoma; HCV, hepatitis C virus; miRNA, microRNA; NSCLC, non-small-cell lung cancer; PDAC, pancreatic ductal adenocarcinoma.
As the therapeutic potential of individual miRNAs is explored, we note that combinatorial miRNA therapeutics will probably follow. Targeting an individual gene or a subset of genes with multiple tumour-suppressive miRNAs should enhance the therapeutic effect by reducing resistance. For example, a single mutation in the 3′ UTR of an oncogene could disrupt the binding of a particular miRNA; however, combining two or three miRNAs that target the same gene would decrease the likelihood of mutation-induced resistance (FIG. 3). In addition, simultaneous targeting of upregulated miRNAs with antagomir technology and replacement of lost tumour suppressor miRNAs may prove beneficial.
Replacing tumour suppressor miRNAs
let-7
Re-expressing tumour-suppressive miRNAs has great promise for cancer therapy. A prime example is the miRNA let-7, which is downregulated in multiple cancers (reviewed in REF. 87). Genomic loss of let-7 and impaired let-7 processing are two factors that reduce mature let-7 levels7,75,88,89. Therefore, restoring let-7 may be beneficial in cancers that have genomic instability, abundant inhibitors of let-7 processing or aberrantly expressed let-7 targets (for example, KRAS, HMGA2, MYC, LIN28, cyclin-dependent kinase 6 (CDK6), CDC25A and cyclin D2 (CCND2)). Indeed, in the inducible KrasLSL-G12D/+ autochthonous model of non-small-cell lung cancer (NSCLC), delivering let-7a (REF. 88) or let-7g (REF. 89) to the lungs at the same time as KrasG12D expression reduced tumour burden by as much as 66%. The burden of preformed xenografts was reduced by intratumoral delivery of let-7b (REF. 62), as was the burden of KrasG12D/+ lung tumours by the intranasal delivery of let-7a by lentiviral particles62 or by the systemic delivery of let-7b using a neutral lipid-based delivery system62,90. Other xenograft studies further support the therapeutic potential of let-7 replacement91,92.
miR-143 and miR-145
Additional miRNAs also target oncogenic KRAS93–95. Constitutively active KRAS activates RAS-responsive element binding protein 1 (RREB1), which directly inhibits the transcription of the miRNA cluster encoding miR-143 and miR-145. In a feed-forward mechanism, miR-143 and miR-145 repress the expression of RREB1 and KRAS, respectively94; forced expression of miR-143 and miR-145 through cellular transduction94 or lipid-based systemic delivery96 in subcutaneous and orthotopic xenografts downregulated both KRAS and RREB1. A fine-tuned balance between the expression levels of the miR-143~145 cluster and KRAS is needed in quiescent cells. Loss of this cluster, as occurs in pancreatic, bladder, lung and colorectal carcioinomas,94,96–99 or elevations in KRAS expression can perturb this balance, thus leading to carcinogenesis.
As with some other miRNAs, the role of miR-143 and miR-145 depends on cellular context. In hepatocellular carcinoma (HCC), the expression of miR-143 is elevated and its suppression through systemic delivery of antagomirs prevented local and distant metastasis100. Similarly, the expression of miR-143 was significantly elevated in breast cancer tissue from individuals who relapsed101. However, a detailed analysis of 744 cancer samples identified miR-143~145 as a commonly deleted cluster, suggesting that the oncogenic roles of this cluster may be the exception rather than the norm102. Clearly, individualized profiles need to be performed before using many of these contradictory miRNAs as therapeutics.
miR-34
miRNAs of the p53 pathway have also been evaluated for therapeutic intervention. Transcriptionally induced by p53, miR-34 stimulates apoptosis or cellular senescence, induces G1 arrest and prevents migration. These effects are achieved through inhibiting the expression of silent information regulator 1 (SIRT1), BCL-2, CD44, various cyclins and CDKs and the proto-oncoproteins MYC and MYCN (reviewed in REFS 103,104). Similarly to let-7, miR-34 is repressed by multiple mechanisms, including epigenetic silencing owing to promoter methylation, allelic loss caused by genomic instability, and mutation of p53 (reviewed in REFS 103,105). Because miR-34 levels are frequently decreased in cancers, various laboratories have examined the therapeutic benefit of miR-34 replacement therapy. A miR-34 mimetic, delivered either intratumorally or systemically through tail vein injection, impaired tumorigenesis of NSCLC xenografts90,106, and systemic delivery reduced the burden of preformed KrasLSL-G12D/+ lung tumours90 by reducing proliferation and inducing apoptosis.
Although these pioneering studies examined the use of miR-34 in lung cancer, miR-34 replacement therapy should also be considered for other cancers that have p53 mutations or attenuated miR-34 expression, including prostate and pancreatic cancers96,107. miR-34 levels were decreased in a subset of prostate cancer stem cells that were purified from xenografts and primary tumours107. Enforced expression of miR-34 in the cancer stem cell population repressed clonogenic expansion, tumour regeneration and metastasis. Most notably, prostate cancer metastasis was impaired and mouse survival was extended through systemic delivery of lipid-based miR-34 mimetics. In another model, systemic delivery of a lipid-based nanovector expressing miR-34 inhibited the growth of subcutaneous and orthotopic MiaPaCa2 pancreatic tumour xenografts by suppressing proliferation and inducing apoptosis and tissue necrosis96. Reduced expression of target genes SIRT1 and CD44 confirmed that sufficient miR-34 had reached the tumour tissue following systemic delivery; because suboptimal blood flow is a characteristic of pancreatic lesions, the delivery of therapeutics to these tumours is a frequent concern108.
miR-122
Replacement of miR-122, a miRNA that is often correlated with high plasma cholesterol levels, is postulated to reduce HCC metastasis109–111. Reduced miR-122 levels are correlated with intrahepatic metastasis and the loss of crucial liver functions in HCC, supporting the therapeutic potential of re-introducing miR-122. Although re-expressing miR-122 in cells reduced tumorigenesis, angiogenesis and metastatic potential in an orthotopic liver cancer model, systemic delivery of miR-122 has not yet been reported in cancer111. However, antagomirs of miR-122 have been delivered systemically in hepatitis C virus (HCV)-infected non-human primates to suppress HCV viraemia112, and in mice to decrease plasma cholesterol levels113. The pleiotropic role of miR-122 in multiple pathophysiologies suggests that caution should be exercised before using miR-122 therapeutically.
miR-26a
Levels of miR-26a are also decreased in HCC114,115. Systemic administration of miR-26a in a HCC model using an adeno-associated virus inhibited cancer cell proliferation, induced apoptosis and protected animals from disease progression114. Likewise, levels of miR-26a are also reduced in nasopharyngeal carcinoma and lymphoma116,117. In both tumour types, miR-26 restoration reduced proliferation and colony formation through G1 arrest and through direct repression of the histone-lysine N-methyltransferase, EZH2, a global regulator of gene expression. Contrary to these findings, miR-26a was one of five miRNAs that independently promoted T-ALL through the inhibition of PTEN34. In the background of activating mutations in Notch1, miR-26a overexpression decreased the latency of T-ALL more than the other miRNAs in the signature, including the bona fide oncomirs miR-20, miR-19 and miR-92. Indeed, the contextual nature of miR-26 function needs to be considered when advancing miR-26 therapies in vivo.
Targeting oncogenic miRNAs
miR-10b
Although miRNAs are frequently downregulated in cancer, some upregulated oncomirs have been therapeutically targeted in vivo with antagomirs. Proof-of-concept was established in 2005 when intravenous administration of antagomirs in mice impaired the functions of several miRNAs in multiple organs118. Also, miRNA silencing in HCV-infected non-human primates was successful with no toxicity112. In vivo evidence supporting the use of antagomirs in cancer has also recently been established. Systemic intravenous delivery of antagomir-miR-10b — the effects of which are constrained to metastases — to tumour-bearing mice reduced miR-10b levels and suppressed the metastasis of 4T1 breast cancer cells to the lung84. Antagomir-miR-10b treatment had no effect on the primary tumour but reduced pulmonary metastases by >80%. The crucial stages of metastasis targeted by antagomir-miR-10b were proposed to be the steps before extravasation (FIG. 4) because systemic antagomir-miR-10b did not prevent metastasis following the tail vein injection of 4T1 cells. Therefore, antagomir-miR-10b may prevent metastasis of highly invasive cancers containing elevated miR-10b levels, but may not be beneficial against already established metastatic lesions.
Sensitizing tumours to chemotherapy
Owing to the ability of miRNAs to target signalling pathways that are often perturbed in cancer, the potential of miRNAs or antagomirs to sensitize resistant cells to commonly used cancer therapies is being evaluated. Because this is an emerging area, limited in vivo analysis is available; however, based on promising in vitro experiments, more miRNA-based sensitization studies in preclinical animal models will probably be reported soon.
Multi-drug resistance
miRNAs that target genes that encode proteins involved in multi-drug resistance in tumours could sensitize otherwise impervious tumour cells. Multi-drug resistance usually involves the increased excretion of a drug through ATP-binding cassette (ABC) transporters. Two of these, ABCC3 and ABCC6, are induced directly by SOX2 (REF. 119). As such, when SOX2 expression is elevated, cells indirectly dispense drugs back into the extracellular environment. Kim and colleagues119 recently identified miR-9 as a negative regulator of SOX2. Forced expression of miR-9⋆ (miR-9 star strand) in a chemotherapy-resistant glioma stem cell line suppressed SOX2 expression, which led to reduced ABC transporter expression and hence drug retention. SOX2 is also one of a few transcription factors that is capable of inducing pluripotent stem cells. Therefore, miR-9⋆ replacement therapy has the potential to decrease stemness as well as to reduce drug efflux.
Tamoxifen
miRNAs have also been shown to sensitize cancer cells to tamoxifen, the most widely used selective oestrogen receptor modulator (SERM). Tamoxifen treatment, although effective for many individuals, can result in recurrent breast cancer. Truncations of ERα or loss of its expression contribute to resistance. This is especially problematic in individuals whose tumours overexpress the receptor tyrosine kinase ERBB2, which promotes growth and differentiation. One mechanism of ERBB2-induced survival involves the direct repression of the tumour-suppressive miRNAs miR-15 and miR-16 to restore anti-apoptotic BCL-2 expression120. Accordingly, reintroduction of miR-15 and miR-16 decreases BCL-2 levels, thus sensitizing cells to tamoxifen. ERBB2 also induces the oncomir miR-21, suggesting that antagonizing miR-21 may further sensitize cells to tamoxifen. Similar findings were identified for miR-342, which was downregulated in tamoxifen-resistant cells; overexpression sensitized cells to tamoxifen-induced apoptosis121,122. Lastly, overexpression of the miR-221~222 cluster was associated with tamoxifen resistance through downregulation of the cell cycle inhibitor, p27KIP1; ectopic p27KIP1 expression restored tamoxifen-induced cell death122. If these data are confirmed in vivo, miR-15, miR-16, miR-342 or anti-miR-221~222 could be used to enhance tamoxifen sensitivity.
Gefitinib
Levels of miRNAs that target cell survival signalling pathways are often decreased in chemotherapy-resistant cells; their re-expression affords the chemotherapy a better chance at treating the tumour. For example, forced overexpression of miR-126 sensitized cells to the small molecule epidermal growth factor receptor (EGFR) inhibitor gefitinib123. Gefitinib is often a first-line treatment for EGFR-positive cancers, especially those of the lung and breast, to suppress downstream signalling by RAS and PI3K and thus to prevent uncontrollable growth and proliferation. However, point mutations within EGFR, or changes that activate the RAS and PI3K signalling network, can reduce gefitinib efficacy (reviewed in REF. 124). miRNAs targeting these pathways are often altered in cancers, especially in those that are resistant to therapeutics such as gefitinib. For example, levels of miR-126, which targets the PI3K pathway, are decreased in cancer; however, its overexpression was recently reported to resensitize two gefitinib-resistant NSCLC cell lines123. Curiously, levels of miR-126 were also decreased in docetaxel-resistant breast cancer cells125, so miR-126 replacement could also sensitize cells to conventional chemotherapeutics. There is also a rationale for using let-7 to sensitize tumours to gefitinib, especially those with constitutively active KRAS alleles, as let-7 can repress this pathway126 and is known to radiosensitize lung cancer cells in vitro127.
Taxanes
Taxanes are used to treat multiple cancers, and although individuals initially respond favourably, resistance can develop. Treatment with taxanes alters the expression of various genes that confer drug resistance. Many of these genes have since been found to be regulated by miRNAs, such as the oncogenic targets, mitogen- and stress-activated protein kinase 1 (MSK1; which is regulated by miR-148a), CASP8 and FADD-like apoptosis regulator (CFLAR, also known as c-FLIP; which is inhibited by miR-512-3p), BCL-2 antagonist killer 1 (BAK1; which is repressed by miR-125b), SIRT1 and BCL-2 (which are inhibited by miR-34) and inositol monophosphatase 1 (IMPA1; which is inhibited by let-7 family members)128–132. In all of these cases, forced expression of the individual tumour-suppressive miRNAs sensitized cells to taxanes. Conversely, levels of the oncomir miR-135a were significantly elevated in ovarian, NSCLC and uterine cells that were resistant to the taxane paclitaxel. Anti-miR-135a treatment in paclitaxel-resistant NSCLC xenografts restored sensitivity to paclitaxel, in part through the direct inhibition of adenomatous polyposis coli (APC) expression133.
5-Fluorouracil
Based on studies showing that 5-fluorouracil (5-FU) can alter protein-coding gene abundance, a comprehensive study was carried out to determine whether miRNA expression was altered in colorectal cancer cells that were treated with 5-FU. Among others, miR-21 levels were predictive of treatment response; high miR-21 levels correlated with poor therapeutic outcome134. Some of the therapeutic resistance was attributed to miR-21-directed downregulation of core mismatch repair mutator genes135. Similar findings were reported in HCC136 and pancreatic cancer137. In concordance, antagomirs directed against miR-21 were able to sensitize cultured cells to 5-FU treatment137, suggesting that antagomir-miR-21 may sensitize tumours that are otherwise refractory to 5-FU.
Other chemotherapies
Many other studies have reported the ability of miRNAs and antagomirs to sensitize cells to cancer chemotherapeutics. These include antagonizing miR-21 and miR-221, or overexpressing miR-200 and let-7, in cells that are resistant to gemcitabine138,139. In the case of cisplatin, overexpressing miR-34, miR-200c, miR-181 or miR-630 sensitized cells, as did antagonizing PTEN translation using miR-214 (REFS 140–142). The advancement of this knowledge in in vivo systems will help to guide the clinical development of miRNA-based therapies for sensitization to chemotherapy.
The forefront of miRNA-based therapeutics
It is likely, in light of recent advances in the field, that we will see the transition of therapeutics that are based on miRNAs into the clinic in the not-so-distant future. These master gene regulators are no longer the novel molecules once described as ‘oddities’143. They are similar to protein-coding genes in that they regulate many survival-signalling pathways, are themselves subject to mutagenesis and often have conflicting roles in various disease states. They differ however in their therapeutic potential. In essence, miRNA replacement therapies have the capacity to do what protein-coding gene replacement therapies have tried to but with fewer obstacles to overcome. miRNAs are much smaller and less antigenic than their protein-coding counterparts and, as such, cellular delivery is possible without the use of potentially harmful viral-based delivery mechanisms that are needed for the cellular uptake of larger protein-coding genes.
Likewise, effective tools for systemically silencing miRNAs have been developed that specifically and safely target miRNAs. These antagomirs act as small sponges that soak-up miRNAs, resulting in subsequent miRNA degradation and thus the upregulation of predicted targets with an in vivo effect that can be sustained for over 3 weeks118. This is in contrast to standard antisense miRNA targeting, which has a limited ability to suppress miRNA function and often leads to toxicity. Thus far, antagomirs have proven to efficiently and selectively silence miRNA function with limited side effects. Prime examples include systemically delivered antagomir-miR-122 in both primate and mouse models112,113,118 and antagomir-miR-10b against metastatic cancer in mice84.
Indeed, multiple groups have explored effective mechanisms to deliver miRNAs in both a targeted and ubiquitous fashion with a good deal of success. Because many of these delivery strategies have recently been reviewed for small interfering RNAs (siRNAs)144, we cover them minimally. Although the initial proof-of-principle studies using miRNAs as therapeutics took advantage of adenoviral-based145 and lentiviral-based62,146 delivery methods, translation into clinical practice requires the development of safer delivery vehicles. These include packaging mature miRNAs into lipid-based nanoparticles (neutral or charged) that can be delivered locally to the tumour tissue or systemically, in which case they have been found to accumulate and to therapeutically regulate their targets in the lung90,106, pancreas96 and prostate107. Expanding on this, physical and chemical moieties of the particles that facilitate the targeted distribution and the controlled and sustained release of miRNAs — including liposomes, polymers and dendrimer conjugates — are under clinical investigation147. Furthermore, external moieties, such as aptamers and ligands that enhance cellular uptake by cancer cells, are being developed to direct the particles to a particular tissue148,149. The challenge is to further refine the chemistry to allow the crossing of tissue barriers, especially in the case of treating glioblastoma with miRNAs, which has shown promise in vitro and in xenograft studies but is hindered owing to the inability of miRNAs to cross the blood–brain barrier91.
Certainly we must be cautious of the possible side effects of these molecules in human trials, independently of the side effects that could be associated with the delivery agents. Even in situations in which the miRNA is delivered directly to the tumour, miRNAs can escape from the tumour cells and become systemic, either through microvesicle exocytosis or secretion of miRNA–Argonaute 2 complexes that can be delivered to (and induce silencing of mRNA in) other cells150–153. Furthermore, although there is value in overexpressing tumour-suppressive miRNAs, it is also important that this is monitored. It is plausible that miRNA overexpression could lead to the saturation of the miRNA machinery and non-specific effects. Previous reports suggest that the functions of two key miRNA silencing components, exportin 5 and Argonaute 2, are diminished when miRNA or siRNA levels are elevated154,155. Furthermore, because DICER functions as a haploinsufficient tumour suppressor64, overexpressing miRNAs may dampen DICER function and induce a tumour-prone environment. However, decreased DICER function due to the overexpression of a single miRNA has yet to be reported. Furthermore, increasing the cellular concentration of miRNAs may suppress lower-affinity targets that might not be targeted at endogenous miRNA levels. Similarly to current chemotherapies, concentration and dosing schedules need to be evaluated for efficacy and toxicity, and the long-term effects following treatment must be assessed. The literature reviewed here suggests limited toxicity of some of these miRNAs in various model systems, whereas other miRNAs and antagomirs still need to be evaluated. Finally, clinical trials will have to evaluate whether this preclinical promise will be recapitulated in human patients.
At a glance.
Recently generated germline transgenic and knockout mice have provided a detailed view of the implication of the gain or loss of individual microRNAs (miRNAs), miRNA clusters and the miRNA processing machinery in cancer. These have been classified as oncogenic (such as miR-155, miR-21 and miR-17~92), tumour-suppressive (such as miR-15~16, LIN28, DICER) or context-dependent (such as miR-146 and miR-29).
miRNAs and the miRNA processing machinery are involved in all stages of metastatic disease. Some — such as miR-200, the LIN28–let-7 interaction and DICER — contribute to both metastasis and primary tumour development, whereas others, such as miR-31 and miR-10b, are unique to metastasis.
Studies uncovering miRNA function have led to their therapeutic application. Delivering tumour-suppressive miRNAs and silencing oncogenic miRNAs with antagomirs has been successful in various mouse models. Many of these studies began with overexpression and knockdown strategies and have since progressed to delivering miRNA-based molecules intranasally, intratumorally or systemically.
Expanding on their therapeutic application, miRNAs are also being evaluated for their ability to sensitize cancers to chemotherapeutics. Much of this work is being accomplished in cell culture, with the hope that it will soon transition into preclinical model systems.
Acknowledgements
A.L.K. was supported by a US National Institutes of Health (NIH) grant (1F32CA153885-01) and an American Cancer Society Postdoctoral Fellowship (120,766-PF-11-244-01-TBG). F.J.S. was supported by a grant from the US National Cancer Institute (NCI) (1R01CA131301).
Glossary
- miR-17~92
This is a cluster of miRNAs containing miR-17, miR-20a, miR-18a, miR-19a, miR-19b and miR-92a. Clusters are expressed as a polycistronic message from a single promoter; this is indicated in the text by a tilde (~).
- Paralogues
Genes that are derived from the same ancestral gene but that reside in different locations in the same genome.
- DICER
An endoribonuclease that cleaves precursor microRNAs (pre-miRNAs) into 20–25 nucleotide double-stranded RNAs.
- Adaptive immune response
The adaptive immune response — also known as specific or acquired immunity — is mediated by antigen-specific lymphocytes and antibodies.
- Innate immune response
The innate immune system provides an immediate, non-specific defence against pathogens until an adaptive, pathogen-specific immune response is able to develop.
- Nestin promoter
This promoter drives the expression of genes in the central and peripheral nervous system and in myogenic and other tissues.
- DMBA–TPA model
A two-stage chemical skin carcinogenesis model using a single dose of the genotoxic carcinogen, 7,12-dimethylbenz(a) anthracene (DMBA), followed by multiple doses of a non-genotoxic tumour-promoter, 12-O-tetradecanoylphorbol-13-acetate (TPA).
- Papillomas
Benign tumours of epithelial origin that grow outwards.
- Polycistronic
A single transcript that carries the information of several genes.
- Fragile site
A site in a chromosome that is susceptible to chromosome breakage and fusion with other chromosomes.
- Host gene
A gene containing another gene (such as a microRNA within an intron of a protein-coding gene).
- Seed region
Nucleotides 2–7 of the microRNA, typically having 100% complementarity with the target gene.
- Xenografts
Grafts of cells or tissues that are transplanted from one species to another.
- Haploinsufficient
A phenotype that arises in diploid organisms owing to the loss of only one allele.
- Hemizygous
A state of having a single copy of a gene (in a diploid organism).
- Epithelial to mesenchymal transition
(EMT). The conversion of polarized, immotile epithelial cells to motile mesenchymal cells. It is characterized by the loss of adhesion, the repression of E-cadherin, the expression of mesenchymal markers and increased cell motility.
- Adjuvant
Treatment given in addition to the primary therapy. For cancer treatment, this typically refers to the therapy given after the surgical resection of a tumour.
- Antagomirs
Chemically engineered, cholesterol-conjugated single-stranded RNA oligonucleotides that are complementary to microRNAs.
- Autochthonous
Formed from endogenous tissue in the correct anatomical location.
- Orthotopic
Transplanted into the correct anatomical location.
- Silent information regulator 1
(SIRT1). A class-III histone deacetylase that regulates apoptosis in response to genotoxic and oxidative stress.
- star strand
(miRNA*). The passenger strand of the mature microRNA (miRNA), originally thought not to be involved in miRNA-induced silencing; however, deep sequencing followed by functional studies have determined that the some of the miRNA* products are abundant and functional.
- Selective oestrogen receptor modulator
(SERM). An agent that targets the oestrogen receptors, ERα and ERβ, which are often upregulated in breast cancer.
- Docetaxel
A cytotoxic antimicrotubule agent that is used to treat breast, ovarian, prostate and non-small-cell lung cancer.
- Gemcitabine
A nucleoside analogue that is used to treat multiple forms of cancer.
- Cisplatin
A platinum-containing cytotoxic cancer drug.
Footnotes
Competing interests statement
F.J.S. declares competing financial interests. See Web version for details.
DATABASES
National Cancer Institute Drug Dictionary:
http://www.cancer.gov/drugdictionary cisplatin | docetaxel | 5-fluorouracil | gefitinib | gemcitabine | paclitaxel | tamoxifen
FURTHER INFORMATION
Frank J. Slack’s homepage: http://www.yale.edu/slack
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Chalfie M, Horvitz HR, Sulston JE. Mutations that lead to reiterations in the cell lineages of C. elegans. Cell. 1981;24:59–69. doi: 10.1016/0092-8674(81)90501-8. [DOI] [PubMed] [Google Scholar]
- 2.Hodgkin J, Horvitz HR, Brenner S. Nondisjunction mutants of the nematode Caenorhabditis elegans. Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 4.Reinhart BJ, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 5.Slack FJ, et al. The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the LIN-29 transcription factor. Mol. Cell. 2000;5:659–669. doi: 10.1016/s1097-2765(00)80245-2. [DOI] [PubMed] [Google Scholar]
- 6.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–D157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calin GA, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl Acad. Sci. USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: the implications for cancer research. Nature Rev. Cancer. 2010;10:389–402. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nature Rev. Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 10.Esquela-Kerscher A, Slack FJ. Oncomirs — microRNAs with a role in cancer. Nature Rev. Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 11.Costinean S, et al. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in Eμ-miR155 transgenic mice. Proc. Natl Acad. Sci. USA. 2006;103:7024–7029. doi: 10.1073/pnas.0602266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connell RM, et al. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33:607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez A, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thai TH, et al. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 15.Tili E, et al. Mutator activity induced by microRNA-155 (miR-155) links inflammation and cancer. Proc. Natl Acad. Sci. USA. 2011;108:4908–4913. doi: 10.1073/pnas.1101795108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 17.Landgraf P, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volinia S, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl Acad. Sci. USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatley ME, et al. Modulation of K-Ras-dependent lung tumorigenesis by MicroRNA-21. Cancer Cell. 2010;18:282–293. doi: 10.1016/j.ccr.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma X, et al. Loss of the miR-21 allele elevates the expression of its target genes and reduces tumorigenesis. Proc. Natl Acad. Sci. USA. 2011;108:10144–10149. doi: 10.1073/pnas.1103735108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86–90. doi: 10.1038/nature09284. In addition to references 19 and 20, this study generated miR-21 transgenic mice. All three studies report miR-21 as an oncogene in different contexts.
- 22.Wang J, et al. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev. Res. (Phila) 2009;2:807–813. doi: 10.1158/1940-6207.CAPR-09-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ventura A, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao C, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17–92 expression in lymphocytes. Nature Immunol. 2008;9:405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castellano L, et al. The estrogen receptor-alpha-induced microRNA signature regulates itself and its transcriptional response. Proc. Natl Acad. Sci. USA. 2009;106:15732–15737. doi: 10.1073/pnas.0906947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashita Y, et al. A polycistronic microRNA cluster, miR-17–92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 27.He L, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanza G, et al. mRNA/microRNA gene expression profile in microsatellite unstable colorectal cancer. Mol. Cancer. 2007;6:54. doi: 10.1186/1476-4598-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mestdagh P, et al. MYCN/c-MYC-induced microRNAs repress coding gene networks associated with poor outcome in MYCN/c-MYC-activated tumors. Oncogene. 2010;29:1394–1404. doi: 10.1038/onc.2009.429. [DOI] [PubMed] [Google Scholar]
- 30.O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 31.Poliseno L, et al. Identification of the miR-106b ~ 25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci. Signal. 2010;3:ra29. doi: 10.1126/scisignal.2000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mu P, et al. Genetic dissection of the miR-17 ~ 92 cluster of microRNAs in Myc-induced B-cell lymphomas. Genes Dev. 2009;23:2806–2811. doi: 10.1101/gad.1872909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olive V, et al. miR-19 is a key oncogenic component of mir-17–92. Genes Dev. 2009;23:2839–2849. doi: 10.1101/gad.1861409. References 32 and 33 isolated the oncogenic role of miR-19 from the miR-17~92 cluster. Both studies confirmed that miR-19 was sufficient to promote MYC-induced lymphomagenesis.
- 34.Mavrakis KJ, et al. A cooperative microRNA-tumor suppressor gene network in acute T-cell lymphoblastic leukemia (T-ALL) Nature Genet. 2011;43:673–678. doi: 10.1038/ng.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calin GA, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl Acad. Sci. USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raveche ES, et al. Abnormal microRNA-16 locus with synteny to human 13q14 linked to CLL in NZB mice. Blood. 2007;109:5079–5086. doi: 10.1182/blood-2007-02-071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salerno E, et al. Correcting miR-15a/16 genetic defect in New Zealand Black mouse model of CLL enhances drug sensitivity. Mol. Cancer Ther. 2009;8:2684–2692. doi: 10.1158/1535-7163.MCT-09-0127. [DOI] [PubMed] [Google Scholar]
- 38. Klein U, et al. The DLEU2/miR-15a/16-11 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 2010;17:28–40. doi: 10.1016/j.ccr.2009.11.019. In this study, the tumour-suppressive contribution of miR-15a~16 was teased apart from its host gene Dleu2. Overexpression of miR15a~16 could reduce cellular proliferation in Dleu2;miR-15a~16 double knockout mice.
- 39.Musumeci M, et al. Control of tumor and microenvironment cross-talk by miR-15a and miR-16 in prostate cancer. Oncogene. 2011;30:4231–4242. doi: 10.1038/onc.2011.140. [DOI] [PubMed] [Google Scholar]
- 40.Roccaro AM, et al. MicroRNAs 15a and 16 regulate tumor proliferation in multiple myeloma. Blood. 2009;113:6669–6680. doi: 10.1182/blood-2009-01-198408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acevedo VD, Ittmann M, Spencer DM. Paths of FGFR-driven tumorigenesis. Cell Cycle. 2009;8:580–588. doi: 10.4161/cc.8.4.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dahiya N, et al. MicroRNA expression and identification of putative miRNA targets in ovarian cancer. PLoS ONE. 2008;3:e2436. doi: 10.1371/journal.pone.0002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He H, et al. The role of microRNA genes in papillary thyroid carcinoma. Proc. Natl Acad. Sci. USA. 2005;102:19075–19080. doi: 10.1073/pnas.0509603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, et al. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS ONE. 2008;3:e2557. doi: 10.1371/journal.pone.0002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boldin MP, et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J. Exp. Med. 2011;208:1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Starczynowski DT, et al. MicroRNA-146a disrupts hematopoietic differentiation and survival. Exp. Hematol. 2011;39:167–178. doi: 10.1016/j.exphem.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 47. Zhao JL, et al. NF- κB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc. Natl Acad. Sci. USA. 2011;108:9184–9189. doi: 10.1073/pnas.1105398108. A careful analysis of miR-146a-knockout mice. Mice developed myeloid sarcomas and lymphomas. A link between miR-146a and NF-κB was also described.
- 48.Garcia AI, et al. Down-regulation of BRCA1 expression by miR-146a and miR-146b-5p in triple negative sporadic breast cancers. EMBO Mol. Med. 2011;3:279–290. doi: 10.1002/emmm.201100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl Acad. Sci. USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dickson KM, Bhakar AL, Barker PA. TRAF6-dependent NF-kB transcriptional activity during mouse development. Dev. Dyn. 2004;231:122–127. doi: 10.1002/dvdy.20110. [DOI] [PubMed] [Google Scholar]
- 51.Thomas JA, et al. Impaired cytokine signaling in mice lacking the IL-1 receptor-associated kinase. J. Immunol. 1999;163:978–984. [PubMed] [Google Scholar]
- 52.Fabbri M, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl Acad. Sci. USA. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mott JL, Kobayashi S, Bronk SF, Gores GJ. miR-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pekarsky Y, et al. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res. 2006;66:11590–11593. doi: 10.1158/0008-5472.CAN-06-3613. [DOI] [PubMed] [Google Scholar]
- 55.Zhao JJ, et al. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood. 2010;115:2630–2639. doi: 10.1182/blood-2009-09-243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garzon R, et al. MicroRNA 29b functions in acute myeloid leukemia. Blood. 2009;114:5331–5341. doi: 10.1182/blood-2009-03-211938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pekarsky Y, Croce CM. Is miR-29 an oncogene or tumor suppressor in CLL? Oncotarget. 2010;1:224–227. doi: 10.18632/oncotarget.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han YC, et al. microRNA-29a induces aberrant self-renewal capacity in hematopoietic progenitors, biased myeloid development, and acute myeloid leukemia. J. Exp. Med. 2010;207:475–489. doi: 10.1084/jem.20090831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santanam U, et al. Chronic lymphocytic leukemia modeled in mouse by targeted miR-29 expression. Proc. Natl Acad. Sci. USA. 2010;107:12210–12215. doi: 10.1073/pnas.1007186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu H, et al. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nature Genet. 2010;42:626–630. doi: 10.1038/ng.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu H, et al. The Lin28/let-7 Axis Regulates Glucose Metabolism. Cell. 2011;147:81–94. doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Trang P, et al. Regression of murine lung tumors by the let-7 microRNA. Oncogene. 2009;29:1580–1587. doi: 10.1038/onc.2009.445. This was the first indication that let-7 could be used to reduce preformed lung tumours in mice, in support of its clinical application.
- 63.Lu J, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 64.Kumar MS, et al. Dicer1 functions as a haploinsufficient tumor suppressor. Genes Dev. 2009;23:2700–2704. doi: 10.1101/gad.1848209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lambertz I, et al. Monoallelic but not biallelic loss of Dicer1 promotes tumorigenesis in vivo. Cell Death Differ. 2010;17:633–641. doi: 10.1038/cdd.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Martello G, et al. A MicroRNA targeting dicer for metastasis control. Cell. 2010;141:1195–1207. doi: 10.1016/j.cell.2010.05.017. This paper describes a direct link between high miR-103 and miR-107 expression and low DICER expression in cancer. miR-103 and miR-107 abrogate DICER expression, leading to reduced miRNA biogenesis and accelerated metastasis.
- 67. Su X, et al. TAp63 suppresses metastasis through coordinate regulation of Dicer and miRNAs. Nature. 2010;467:986–990. doi: 10.1038/nature09459. This paper reports that TAp63 suppresses tumorigenesis and metastasis by transactivating DICER expression.
- 68.Nicoloso MS, Spizzo R, Shimizu M, Rossi S, Calin GA. MicroRNAs — the micro steering wheel of tumour metastases. Nature Rev. Cancer. 2009;9:293–302. doi: 10.1038/nrc2619. [DOI] [PubMed] [Google Scholar]
- 69.Gregory PA, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 70.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gibbons DL, et al. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes Dev. 2009;23:2140–2151. doi: 10.1101/gad.1820209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kong D, et al. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS ONE. 2010;5:e12445. doi: 10.1371/journal.pone.0012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wellner U, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 74.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J. Biol. Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Viswanathan SR, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nature Genet. 2009;41:843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 78.Dangi-Garimella S, et al. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J. 2009;28:347–358. doi: 10.1038/emboj.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li X, et al. MicroRNA-107, an oncogene microRNA that regulates tumor invasion and metastasis by targeting DICER1 in gastric cancer: miR-107 promotes gastric cancer invasion and metastasis. J. Cell. Mol. Med. 2011;15:1887–1895. doi: 10.1111/j.1582-4934.2010.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tokumaru S, Suzuki M, Yamada H, Nagino M, Takahashi T. let-7 regulates Dicer expression and constitutes a negative feedback loop. Carcinogenesis. 2008;29:2073–2077. doi: 10.1093/carcin/bgn187. [DOI] [PubMed] [Google Scholar]
- 81.Valastyan S, Chang A, Benaich N, Reinhardt F, Weinberg RA. Concurrent suppression of integrin α5, radixin, and RhoA phenocopies the effects of miR-31 on metastasis. Cancer Res. 2010;70:5147–5154. doi: 10.1158/0008-5472.CAN-10-0410. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82. Valastyan S, Chang A, Benaich N, Reinhardt F, Weinberg RA. Activation of miR-31 function in already-established metastases elicits metastatic regression. Genes Dev. 2011;25:646–659. doi: 10.1101/gad.2004211. A comprehensive study evaluating the antimetastatic role of miR-31 and suggesting the potential use of miR-31 for the treatment of metastatic disease.
- 83.Valastyan S, et al. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137:1032–1046. doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84. Ma L, et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nature Biotech. 2010;28:341–347. doi: 10.1038/nbt.1618. The only report of systemically delivering antagomirs for cancer therapy. In mice, anti-miR-10b treatment suppressed metastasis without affecting primary tumour development.
- 85.Huang Q, et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat. Cell Biol. 2008;10:202–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 86.Tavazoie SF, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008;18:505–516. doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 88.Dangi-Garimella S, Strouch MJ, Grippo PJ, Bentrem DJ, Munshi HG. Collagen regulation of let-7 in pancreatic cancer involves TGF-β1-mediated membrane type 1-matrix metalloproteinase expression. Oncogene. 2011;30:1002–1008. doi: 10.1038/onc.2010.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang M, Hu Y, Amatangelo MD, Stearns ME. Role of ribosomal protein RPS2 in controlling let-7a expression in human prostate cancer. Mol. Cancer Res. 2011;9:36–50. doi: 10.1158/1541-7786.MCR-10-0158. [DOI] [PubMed] [Google Scholar]
- 90. Trang P, et al. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol. Ther. 2011;19:1116–1122. doi: 10.1038/mt.2011.48. This paper reports the use of systemically delivered miRNAs (let-7 and miR-34) to treat preformed lung tumours. Systemically delivered miRNAs were well tolerated and effective.
- 91.Lee ST, et al. Let-7 microRNA inhibits the proliferation of human glioblastoma cells. J. Neurooncol. 2010;102:19–24. doi: 10.1007/s11060-010-0286-6. [DOI] [PubMed] [Google Scholar]
- 92.Yu F, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 93.Hirai H, et al. Activation of the c-K-ras oncogene in a human pancreas carcinoma. Biochem. Biophys. Res. Commun. 1985;127:168–174. doi: 10.1016/s0006-291x(85)80140-6. [DOI] [PubMed] [Google Scholar]
- 94.Kent OA, et al. Repression of the miR-143/145 cluster by oncogenic Ras initiates a tumor-promoting feed-forward pathway. Genes Dev. 2010;24:2754–2759. doi: 10.1101/gad.1950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nakano H, et al. Isolation of transforming sequences of two human lung carcinomas: structural and functional analysis of the activated c-K-ras oncogenes. Proc. Natl Acad. Sci. USA. 1984;81:71–75. doi: 10.1073/pnas.81.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pramanik D, et al. Restitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol. Cancer Ther. 2011;10:1470–1480. doi: 10.1158/1535-7163.MCT-11-0152. This study reports the systemic delivery of miR-34, miR-143 and miR-145 to orthotopic pancreatic tumours in vivo. Tumour growth was inhibited with no signs of toxicity. This is especially interesting because blood flow to the pancreas is thought to be low.
- 97.Earle JS, et al. Association of microRNA expression with microsatellite instability status in colorectal adenocarcinoma. J. Mol. Diagn. 2010;12:433–440. doi: 10.2353/jmoldx.2010.090154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao W, et al. Deregulated expression of miR-21, miR-143 and miR-181a in non small cell lung cancer is related to clinicopathologic characteristics or patient prognosis. Biomed. Pharmacother. 2010;64:399–408. doi: 10.1016/j.biopha.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 99.Han Y, et al. MicroRNA expression signatures of bladder cancer revealed by deep sequencing. PLoS ONE. 2011;6:e18286. doi: 10.1371/journal.pone.0018286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang X, Liu S, Hu T, He Y, Sun S. Up-regulated microRNA-143 transcribed by nuclear factor kappa B enhances hepatocarcinoma metastasis by repressing fibronectin expression. Hepatology. 2009;50:490–499. doi: 10.1002/hep.23008. [DOI] [PubMed] [Google Scholar]
- 101.Marchini S, et al. Association between miR-200c and the survival of patients with stage I epithelial ovarian cancer: a retrospective study of two independent tumour tissue collections. Lancet Oncol. 2011;12:273–285. doi: 10.1016/S1470-2045(11)70012-2. [DOI] [PubMed] [Google Scholar]
- 102.Volinia S, et al. Reprogramming of miRNA networks in cancer and leukemia. Genome Res. 2010;20:589–599. doi: 10.1101/gr.098046.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2009;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 104.Kasinski AL, Slack FJ. Potential microRNA therapies targeting Ras, NFκB and p53 signaling. Curr. Opin. Mol. Ther. 2010;12:147–157. [PubMed] [Google Scholar]
- 105.Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nature Rev. Cancer. 2009;9:701–713. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- 106.Wiggins JF, et al. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010;70:5923–5930. doi: 10.1158/0008-5472.CAN-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Liu C, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nature Med. 2011;17:211–215. doi: 10.1038/nm.2284. This study showed that systemic miR-34 delivery inhibited prostate cancer metastasis and increased survival. CD44 was validated as a causal target of the miR-34 therapeutic effect.
- 108.Komar G, et al. Decreased blood flow with increased metabolic activity: a novel sign of pancreatic tumor aggressiveness. Clin. Cancer Res. 2009;15:5511–5517. doi: 10.1158/1078-0432.CCR-09-0414. [DOI] [PubMed] [Google Scholar]
- 109.Bai S, et al. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J. Biol. Chem. 2009;284:32015–32027. doi: 10.1074/jbc.M109.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Burchard J, et al. microRNA-122 as a regulator of mitochondrial metabolic gene network in hepatocellular carcinoma. Mol. Syst. Biol. 2010;6:402. doi: 10.1038/msb.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsai WC, et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2009;49:1571–1582. doi: 10.1002/hep.22806. [DOI] [PubMed] [Google Scholar]
- 112.Lanford RE, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Su J, Baigude H, McCarroll J, Rana TM. Silencing microRNA by interfering nanoparticles in mice. Nucleic Acids Res. 2011;39:e38. doi: 10.1093/nar/gkq1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kota J, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ji J, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N. Engl. J. Med. 2009;361:1437–1447. doi: 10.1056/NEJMoa0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu J, et al. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res. 2011;71:225–233. doi: 10.1158/0008-5472.CAN-10-1850. [DOI] [PubMed] [Google Scholar]
- 117.Sander S, Bullinger L, Wirth T. Repressing the repressor: a new mode of MYC action in lymphomagenesis. Cell Cycle. 2009;8:556–559. doi: 10.4161/cc.8.4.7599. [DOI] [PubMed] [Google Scholar]
- 118.Krutzfeldt J, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 119.Jeon HM, et al. ID4 imparts chemoresistance and cancer stemness to glioma cells by derepressing miR-9*-mediated suppression of SOX2. Cancer Res. 2011;71:3410–3421. doi: 10.1158/0008-5472.CAN-10-3340. [DOI] [PubMed] [Google Scholar]
- 120.Cittelly DM, et al. Oncogenic HER2Δ16 suppresses miR-15a/16 and deregulates BCL-2 to promote endocrine resistance of breast tumors. Carcinogenesis. 2010;31:2049–2057. doi: 10.1093/carcin/bgq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cittelly DM, et al. Downregulation of miR-342 is associated with tamoxifen resistant breast tumors. Mol. Cancer. 2010;9:317. doi: 10.1186/1476-4598-9-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miller TE, et al. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J. Biol. Chem. 2008;283:29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhong M, Ma X, Sun C, Chen L. MicroRNAs reduce tumor growth and contribute to enhance cytotoxicity induced by gefitinib in non-small cell lung cancer. Chem. Biol. Interact. 2010;184:431–438. doi: 10.1016/j.cbi.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 124.Harris TJ, McCormick F. The molecular pathology of cancer. Nature Rev. Clin. Oncol. 2010;7:251–265. doi: 10.1038/nrclinonc.2010.41. [DOI] [PubMed] [Google Scholar]
- 125.Kastl L, Brown I, Schofield AC. miRNA-34a is associated with docetaxel resistance in human breast cancer cells. Breast Cancer Res. Treat. 2011 Mar 12; doi: 10.1007/s10549-011-1424-3. [DOI] [PubMed] [Google Scholar]
- 126.Johnson SM, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 127.Weidhaas JB, et al. MicroRNAs as potential agents to alter resistance to cytotoxic anticancer therapy. Cancer Res. 2007;67:11111–11116. doi: 10.1158/0008-5472.CAN-07-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Boyerinas B, et al. Let-7 modulates acquired resistance of ovarian cancer to Taxanes via IMP-1-mediated stabilization of MDR1. Int. J. Cancer. 2011 May 26; doi: 10.1002/ijc.26190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen F, et al. Inhibition of c-FLIP expression by miR-512-3p contributes to taxol-induced apoptosis in hepatocellular carcinoma cells. Oncol. Rep. 2010;23:1457–1462. doi: 10.3892/or_00000784. [DOI] [PubMed] [Google Scholar]
- 130.Fujita Y, et al. MiR-148a attenuates paclitaxel resistance of hormone-refractory, drug-resistant prostate cancer PC3 cells by regulating MSK1 expression. J. Biol. Chem. 2010;285:19076–19084. doi: 10.1074/jbc.M109.079525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kojima K, Fujita Y, Nozawa Y, Deguchi T, Ito M. MiR-34a attenuates paclitaxel-resistance of hormone-refractory prostate cancer PC3 cells through direct and indirect mechanisms. Prostate. 2010;70:1501–1512. doi: 10.1002/pros.21185. [DOI] [PubMed] [Google Scholar]
- 132.Zhou M, et al. MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J. Biol. Chem. 2010;285:21496–21507. doi: 10.1074/jbc.M109.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Holleman A, et al. miR-135a contributes to paclitaxel resistance in tumor cells both in vitro and in vivo. Oncogene. 2011;30:4386–4398. doi: 10.1038/onc.2011.148. This study identified upregulation of miR-135a in paclitaxel-resistant tumours. Antagonizing miR-135a in vivo resensitized paclitaxel resistant tumours to this therapy.
- 134.Schetter AJ, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Valeri N, et al. MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA MutS homolog 2 (hMSH2) Proc. Natl Acad. Sci. USA. 2010;107:21098–21103. doi: 10.1073/pnas.1015541107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tomimaru Y, et al. MicroRNA-21 induces resistance to the anti-tumour effect of interferon-alpha/5-fluorouracil in hepatocellular carcinoma cells. Br. J. Cancer. 2010;103:1617–1626. doi: 10.1038/sj.bjc.6605958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hwang JH, et al. Identification of microRNA-2 1 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS ONE. 2010;5:e10630. doi: 10.1371/journal.pone.0010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li Y, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Park JK, Lee EJ, Esau C, Schmittgen TD. Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas. 2009;38:e190–e199. doi: 10.1097/MPA.0b013e3181ba82e1. [DOI] [PubMed] [Google Scholar]
- 140.Ceppi P, et al. Loss of miR-200c expression induces an aggressive, invasive, and chemoresistant phenotype in non-small cell lung cancer. Mol. Cancer Res. 2010;8:1207–1216. doi: 10.1158/1541-7786.MCR-10-0052. [DOI] [PubMed] [Google Scholar]
- 141.Galluzzi L, et al. miR-181a and miR-630 regulate cisplatin-induced cancer cell death. Cancer Res. 2010;70:1793–1803. doi: 10.1158/0008-5472.CAN-09-3112. [DOI] [PubMed] [Google Scholar]
- 142.Weeraratne SD, et al. miR-34a confers chemosensitivity through modulation of MAGE-A and p53 in medulloblastoma. Neuro Oncol. 2011;13:165–175. doi: 10.1093/neuonc/noq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 144.Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nature Rev. Cancer. 2011;11:59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Esquela-Kerscher A, et al. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle. 2008;7:759–764. doi: 10.4161/cc.7.6.5834. [DOI] [PubMed] [Google Scholar]
- 146.Kumar MS, et al. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc. Natl Acad. Sci. USA. 2008;105:3903–3908. doi: 10.1073/pnas.0712321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Duncan R. The dawning era of polymer therapeutics. Nature Rev. Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 148.Estevez MC, et al. Nanoparticle-aptamer conjugates for cancer cell targeting and detection. Methods Mol. Biol. 2010;624:235–248. doi: 10.1007/978-1-60761-609-2_16. [DOI] [PubMed] [Google Scholar]
- 149.Chen Y, Zhu X, Zhang X, Liu B, Huang L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol. Ther. 2010;18:1650–1656. doi: 10.1038/mt.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Arroyo JD, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl Acad. Sci. USA. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hunter MP, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS ONE. 2008;3:e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 154.Grimm D, et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- 155.Grimm D, et al. Argonaute proteins are key determinants of RNAi efficacy, toxicity, and persistence in the adult mouse liver. J. Clin. Invest. 2010;120:3106–3119. doi: 10.1172/JCI43565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen K, Rajewsky N. Natural selection on human microRNA binding sites inferred from SNP data. Nature Genet. 2006;38:1452–1456. doi: 10.1038/ng1910. [DOI] [PubMed] [Google Scholar]
- 157.Chin LJ, et al. A SNP in a let-7 microRNA complementary site in the KRAS 3′ untranslated region increases non-small cell lung cancer risk. Cancer Res. 2008;68:8535–8540. doi: 10.1158/0008-5472.CAN-08-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jazdzewski K, et al. Common SNP in pre-miR-146a decreases mature miR expression and predisposes to papillary thyroid carcinoma. Proc. Natl Acad. Sci. USA. 2008;105:7269–7274. doi: 10.1073/pnas.0802682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hoffman AE, et al. microRNA miR-196a-2 and breast cancer: a genetic and epigenetic association study and functional analysis. Cancer Res. 2009;69:5970–5977. doi: 10.1158/0008-5472.CAN-09-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nature Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]