Abstract
Insulin resistance causes diminished glucose uptake in similar regions of the brain in Alzheimer’s disease (AD) and type 2 diabetes mellitus (DM2). Brain tissue studies suggested that insulin resistance is caused by low insulin receptor signaling attributable to its abnormal association with more phospho (P)-serine-type 1 insulin receptor substrate (IRS-1) and less P-tyrosine-IRS-1. Plasma exosomes enriched for neural sources by immunoabsorption were obtained once from 26 patients with AD, 20 patients with DM2, 16 patients with frontotemporal dementia (FTD), and matched case control subjects. At 2 time points, they were obtained from 22 others when cognitively normal and 1 to 10 yr later when diagnosed with AD. Mean exosomal levels of extracted P-serine 312-IRS-1 and P-pan-tyrosine-IRS-1 by ELISA and the ratio of P-serine 312-IRS-1 to P-pan-tyrosine-IRS-1 (insulin resistance factor, R) for AD and DM2 and P-serine 312-IRS-1 and R for FTD were significantly different from those for case control subjects. The levels of R for AD were significantly higher than those for DM2 or FTD. Stepwise discriminant modeling showed correct classification of 100% of patients with AD, 97.5% of patients with DM2, and 84% of patients with FTD. In longitudinal studies of 22 patients with AD, exosomal levels of P-serine 312-IRS-1, P-pan-tyrosine-IRS-1, and R were significantly different 1 to 10 yr before and at the time of diagnosis compared with control subjects. Insulin resistance reflected in R values from this blood test is higher for patients with AD, DM2, and FTD than case control subjects; higher for patients with AD than patients with DM2 or FTD; and accurately predicts development of AD up to 10 yr prior to clinical onset.—Kapogiannis, D., Boxer, A., Schwartz, J. B., Abner, E. L., Biragyn, A., Masharani, U., Frassetto, L., Petersen, R. C., Miller, B. L., Goetzl, E. J. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease.
Keywords: insulin resistance, dementia, senescence
Normal tissue responses to insulin include enhanced glucose uptake, altered metabolism, and changes in cellular functions. A diverse range of reduced responses to insulin in brain and peripheral tissues is designated insulin resistance (1). Binding of insulin and type 1 insulin-like growth factor (IGF-1) to their receptors in brain and peripheral tissues evokes a proximal signaling cascade of receptor tyrosine phosphorylation, association of the adaptor insulin receptor substrate (IRS) with the ligand-occupied receptors through its SH2 domains, and phosphorylation of multiple IRS tyrosines (2–5). These events activate distal signaling pathways, including those recruited by MAPKs and PI3K. Patterns of phosphorylation of IRS-1/-2 regulate its stability, receptor association, and signaling capacity (6). Phosphorylation of several tyrosine residues of IRS-1/-2 promotes signaling functions, whereas dephosphorylation of tyrosine and/or phosphorylation of some serine and threonine residues are physiologic mechanisms for dissociation of IRS-1/-2 from the insulin receptor and reduced insulin signaling (7–10). Thus, dysregulated phosphorylation of tyrosine and serine/threonine in IRS-1/-2 may lead to the pathologic state of insulin resistance (11–14).
Patients with AD and normal peripheral tissue insulin responsiveness may show regional decreases in cerebral glucose metabolism similar to those detected in brains of cognitively normal patients with early DM2 and cognitively normal healthy elderly subjects (15). Brain tissues from patients with AD have abnormal expression of receptors for insulin and IGF-1, as well as altered phosphorylation of IRS-1/-2 in a pattern characteristic of insulin resistance. The predominant abnormalities of brain IRS-1/-2 detected in AD were decreased total levels and increased extent of phosphorylation of IRS-1 serine 312 and serine 616, which would result in diminished signaling by insulin receptors (16). The signaling of PI3K by insulin receptors also was reduced in association with increased levels of P-serine-IRS-1/-2 (17). The extent of IRS-1/-2 abnormalities appeared to increase with the progression of mild cognitive impairment (MCI) to probable AD and with further worsening of AD (17).
To assess brain insulin resistance in AD by studies of blood constituents alone, the present study focuses on the level of IRS-1 and its state of phosphorylation in neural-derived plasma exosomes. These exosomes are absorptively isolated by binding to anti-neural cell adhesion molecule-1 (NCAM-1) or anti-neural cell adhesion molecule L1 (L1CAM) antibodies and have served as a source of quantifiable AD-pathogenic proteins (18). Levels of total IRS-1 and representative phosphorylated forms of IRS-1 in such neural-derived exosomes from patients in the preclinical stage of AD or with manifest AD were compared with those of matched cognitively normal healthy control subjects and patients with FTD or DM2.
MATERIALS AND METHODS
Experimental design and patient evaluation
For cross-sectional studies, we retrospectively identified 26 patients with amnestic mild cognitive impairment (aMCI, n = 16) or mild to moderate dementia (n = 10) attributable to AD, who had donated blood at 1 time point in the Clinical Research Unit of the National Institute on Aging (CRU-NIA) of Harbor Hospital (Baltimore, MD) or at the Jewish Home of San Francisco (JHSF) (San Francisco, CA) (Table 1). Each CRU-NIA patient had mental status and neurophysiologic testing, a lumbar puncture for determination of cerebrospinal fluid (CSF) Aβ1-42, total tau and P-T181-tau, and a 2-h oral glucose tolerance test after a 12 h fast. For longitudinal studies, 22 additional patients with AD were identified who had provided blood at 2 time points in studies at the Mayo Clinic or the University of Kentucky, first when cognitively intact and later when diagnosed with AD. There were 12 men and 10 women with a mean age (±sd) of 78.0 ± 1.69 yr at the time of the second blood sample. The diagnosis at the onset of cognitive loss was aMCI for 11 patients and dementia for 11. Intervals between the 2 blood samples (number of patients) were as follows: 1 yr (n = 1), 2 yr (n = 1), 3 yr (n = 5), 4 yr (n = 1), 5 yr (n = 2), 6 yr (n = 1), 7 yr (n = 3), 8 yr (n = 3), 9 yr (n = 2), and 10 yr (n = 3). Longitudinal studies of plasma exosome proteins were completed without knowledge of patient characteristics.
TABLE 1.
Characteristics of cross-sectional patients and control subjects
| Diagnosis | Total group |
MCI |
Dementia |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Male/female | Ages (yr), mean ± sd (range) | n | MMSE (mean ± sem) | ADAS-cog (mean ± sem) | n | MMSE (mean ± sem) | ADAS-cog (mean ± sem) | |
| AD | 26 | 13/13 | 74.3 ± 7.48 (62–90) | 16 | 28.3 ± 0.48 | 8.81 ± 1.00 | 10 | 20.5 ± 2.17* | 22.9 ± 2.92‡ |
| AC | 26 | 13/13 | 74.3 ± 7.48 (62–90) | 0 | 0 | ||||
| Mild Dementia | Moderate Dementia | ||||||||
| FTD | 16 | 12/4 | 63.1 ± 8.79 (48–79) | 9 | 26.7 ± 0.73 | 7 | 15.0 ± 3.65† | ||
| FTC | 16 | 12/4 | 63.7 ± 7.43 (48–79) | 0 | 0 | ||||
| Dementia | |||||||||
| DM2 | 20 | 9/11 | 73.0 ± 9.27 (58–91) | 0 | |||||
| DC | 20 | 9/11 | 73.0 ± 9.19 (58–91) | 0 | |||||
The significance of differences in values between the MCI/mild dementia and dementia/moderate dementia groups were calculated by an unpaired t test.
P < 0.001.
P < 0.01.
P < 0.0001.
Patients were classified as having aMCI according to the Petersen criteria and had a Clinical Dementia Rating (CDR) global score of 0.5 (19, 20). Those with probable AD and mild to moderate dementia were diagnosed by the Dubois criteria and had a CDR global score of 1.0 (21). A CSF level of Aβ1-42 < 192 pg/ml supported a diagnosis of AD (22). Standard mental status testing with the mini-mental state examination (MMSE) and the cognitive subscale of the AD assessment scale (ADAS-cog) was conducted as described (23, 24). Twenty-four of the 26 single-time sample patients with AD were taking an acetylcholinesterase inhibitor and/or memantine, and 5 were on antidepressant medications.
Sixteen patients with behavioral variant FTD had been evaluated and selected for study at the Memory and Aging Center of the Department of Neurology of the University of California–San Francisco (Table 1). Their diagnosis and assignment to mild dementia or moderate dementia groups (Table 1) were based on standard clinical, mental status, and psychiatric criteria, including discriminant analyses of neuropsychiatric elements, phonological performance, and object understanding that distinguish FTD from AD (25, 26). Seven of the patients with FTD were taking an antidepressant, 2 were taking an acetylcholinesterase inhibitor, and 1 was on memantine. Twenty elderly patients with DM2 and normal cognition had been diagnosed and evaluated at the UCSF Diabetes Center or the JHSF prior to giving a single sample of venous blood (Table 1). Eighty-one healthy subjects were recruited at the JHSF or CRU-NIA to be age- and gender-matched case control subjects for each individual in the AD, FTD, and DM2 groups of patients (3 served as control subjects for 2 clinical groups). Plasma samples had been collected 1 to 4 yr prior to the study from control subjects who had normal cognition at the times of collection and of the study.
Each individual studied and some patient-designates signed a consent form approved with the study protocol at each institution. Plasma was stored in 0.5 ml aliquots at −80°C. CSF levels of total tau, P-T181-tau and Aβ1-42 were quantified by Luminex xMAP technology using Innogenetics INNO-BIA Alz Bio3 kits (Fujirebio Corporation, Seguin, TX, USA).
Isolation of exosomes from plasma for extraction and ELISA quantification of exosome proteins
Aliquots (0.5 ml) of plasma were processed as described (18) with inclusion of suggested final concentrations of protease inhibitor cocktail (Roche Applied Sciences, Incorporated, Indianapolis, IN, USA) and phosphatase inhibitor cocktail (Pierce Halt, Thermo Fisher Scientific, Incorporated, Rockford, IL, USA) at each step. ExoQuick exosome solution (System Biosciences, Incorporated, Mountain View, CA, USA) was used to precipitate total exosomes as described (18). Each exosome pellet was resuspended in 150 μl distilled water with inhibitor cocktails before immunochemical enrichment of exosomes from neural sources. Each exosome suspension was incubated for 1 h at 4°C with 1 µg mouse anti-human CD171 (L1CAM) biotinylated antibody (clone 5G3; eBioscience, San Diego, CA, USA) in 50 µl 3% bovine serum albumin [BSA; 1:3.33 dilution of blocker BSA 10% solution in DBS−2 (Thermo Fisher Scientific, Incorporated)], followed by addition of 25 μl streptavidin-agarose resin (Thermo Fisher Scientific, Incorporated) with 50 µl 3% BSA and incubation for 30 min at 4°C. In some studies of cross-specificity, portions of exosome suspensions were incubated with mouse anti-human NCAM antibody (ERIC 1, sc-106; Santa Cruz Biotechnology, Santa Cruz, CA, USA) that had been biotinylated with the EZ-Link sulfo-NHS-biotin system (Thermo Fisher Scientific, Incorporated) in place of anti-CD171 antibody as described (18). Anti-human NCAM antibody bound over 95% of exosomes that had been enriched with anti-human CD171, as contrasted with a range of only 11 to 15% of total exosomes in plasmas from patients with AD (n = 6). After centrifugation at 200 × g for 10 min at 4°C and removal of the supernate, each pellet was suspended in 50 µl 0.05 M glycine-HCl (pH 3.0) by vortexing for 10 s. Each suspension then received 0.5 ml M-PER mammalian protein extraction reagent (Thermo Fisher Scientific, Incorporated) that had been adjusted to pH 8.0 with 1 M Tris-HCl (pH 8.6) and contained the cocktails of protease and phosphatase inhibitors. These suspensions were incubated at 37°C for 10 min and vortex-mixed for 15 s before storage at −80°C until use in ELISAs.
Exosome proteins were quantified by ELISA kits for human P-serine 312-IRS-1 (Life Technologies Corporation, Carlsbad, CA, USA), human P-pan-tyrosine-IRS-1 (Cell Signaling Technology, Danvers, MA, USA), human total IRS-1 (AMSBIO, Incorporated, Cambridge, MA, USA), and tetraspanning exosome marker human CD81 (Hölzel Diagnostika-Cusabio, Cologne, Germany) with verification of the CD81 antigen standard curve using human purified recombinant CD81 antigen (Origene Technologies, Incorporated, Rockville, MD, USA), according to suppliers’ directions. The mean value for all determinations of CD81 in each assay group was set at 1.00 and the relative values for individual samples used to normalize their recovery.
Statistical analyses
The statistical significance of differences between group means for patients with AD or FTD or DM2 and between patient groups and their respective matched control groups was determined with an unpaired t test, including a Bonferroni correction in the interpretation (Prism 6; GraphPad Software, Incorporated, La Jolla, CA, USA). Separate discriminant classifier analyses were conducted to define the best simple linear models for comparing group AD with its control group AC, DM2 with its control group DC, and FTD with its control group FTC. The discriminant analyses were performed stepwise with the Wilks’ lambda method. In each step, only variables with a minimum partial F of 3.84 to enter and 2.71 to remove were retained. Prior probabilities were considered equal for all groups. Fisher function coefficients and within-group covariances also were computed. Receiver operating characteristics (ROC) analyses were conducted under the nonparametric distribution assumption for standard error of area to determine the performance of the models for discriminating AD from AC, FTD from FTC, DM2 from DC, and AD from DM2 and FTD. Discriminant and ROC analyses were conducted with SPSS v21.0 (IBM, Armonk, NY, USA). To assess significance of associations between CSF biomarkers in patients with AD and IRS-1 proteins in neurally enriched exosomes, we computed zero-order Pearson correlations and partial correlations (controlling for age and gender). For longitudinal analyses, the significance of differences between serial values for patients with AD taken before and after onset of aMCI or dementia was calculated with a paired t test (GraphPad Software, Incorporated).
RESULTS
Patient characteristics
Characteristics of control subjects in the AC, FTC, and DC groups were the same as those of patients with AD, FTD, and DM2 with whom they were matched in cross-sectional studies (Table 1). Patients and control subjects in the AD/AC and DM2/DC clusters had similar descriptors, whereas those in the FTD/FTC cluster showed younger age and greater male predominance. Results of MMSE and ADAS-cog testing confirmed significantly more profound cognitive losses for the dementia than the aMCI patients with AD, and results of the MMSE confirmed greater cognitive losses for the moderately than mildly demented patients with FTD.
Neural-derived plasma exosomal IRS-1 and P-IRS-1 levels
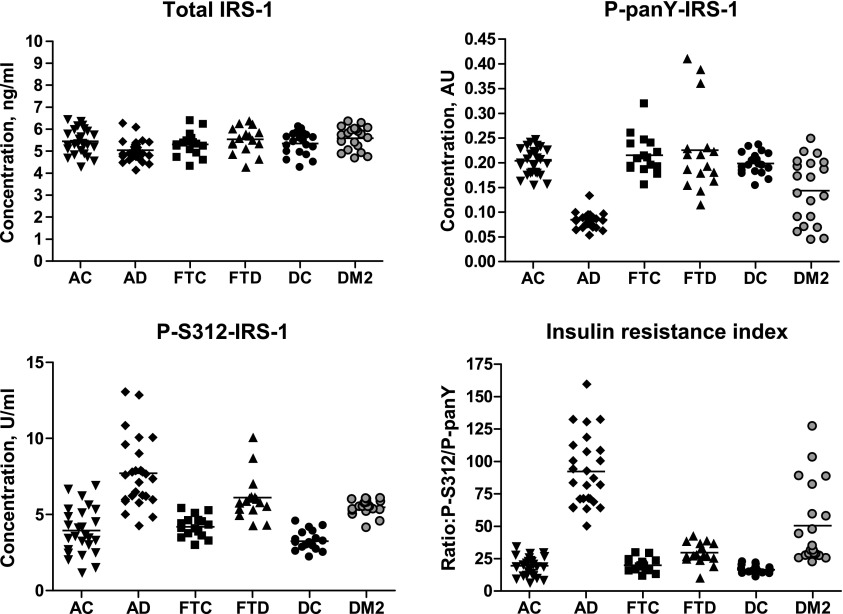
Mean ± sem total concentrations of IRS-1 in exosomal extracts of patients with AD, FTD, and DM2 were 5.04 ± 0.10, 5.54 ± 0.15, and 5.59 ± 0.12 ng/ml, respectively, compared with 5.45 ± 0.11, 5.30 ± 0.14, and 5.34 ± 0.12 ng/ml for their corresponding AC, FTC, and DC case control subjects (Fig. 1). Of these clusters, only the difference between the values for AD and AC was significant after Bonferroni correction (P = 0.0081). In contrast, the differences between AD and AC and between DM2 and DC in exosomal extract concentrations of P-serine 312-IRS-1 and P-pan-tyrosine-IRS-1, as well as in the ratio of P-serine 312-IRS-1 to P-pan-tyrosine-IRS-1 (R), termed the “insulin resistance index,” were highly significant (Fig. 1). Mean ± sem levels of P-serine 312-IRS-1 and P-pan-tyrosine-IRS-1 were 7.72 ± 0.45 U/ml (P < 0.0001) and 0.085 ± 0.003 AU (absorbance units at 450 nm; P < 0.0001) for AD and 3.94 ± 0.30 U/ml and 0.204 ± 0.005 AU for AC. Mean ± sem P-serine 312/P-pan-tyrosine ratio (R) values were 92.2 ± 5.34 for AD and 19.4 ± 1.44 for AC (P < 0.0001). Furthermore, the immunoabsorbed neural-derived level of P-serine 312-IRS-1 was a mean of 68% of the total in nonenriched AD plasma exosome extracts (n = 5), suggesting that dysfunctional phosphorylation of IRS-1 was predominantly in exosomes from the nervous system. Mean ± sem levels of P-serine 312-IRS-1 and P-pan-tyrosine-IRS-1 were 5.48 ± 0.11 U/ml (P < 0.0001) and 0.143 ± 0.015 AU (P < 0.001) for DM2 and 3.24 ± 0.14 U/ml and 0.199 ± 0.005 AU for DC. Mean ± sem R levels were 50.4 ± 6.93 for DM2 and 16.4 ± 0.67 for DC (P < 0.0001). The immunoabsorbed neural-derived level of P-serine 312-IRS-1 was a mean of 27% of the total in nonenriched DM2 plasma exosome extracts (n = 5), suggesting that dysfunctional phosphorylation of exosomal IRS-1 had broader systemic distribution than in AD. A significant difference between values for FTD and FTC was observed for P-serine 312-IRS-1 and R but not for P-pan-tyrosine-IRS-1 (Fig. 1). Mean ± sem levels of P-serine 312-IRS-1 and P-pan-tyrosine-IRS-1 were 6.12 ± 0.37 U/ml (P < 0.0001) and 0.226 ± 0.087 AU (P = 0.666) for FTD and 4.19 ± 0.18 U/ml and 0.215 ± 0.010 AU for FTC. Mean ± sem R levels were 29.5 ± 2.08 for FTD and 19.9 ± 1.30 for FTC (P = 0.0005).
Figure 1.
Cross-sectional analysis of altered levels of phosphorylated IRS-1 in AD, DM2, and FTD. AU, absorbance units at 450 nm; insulin resistance index, ratio of P-serine 312-IRS-1 (P-S312-IRS-1)/P-pan-tyrosine-IRS-1 (P-panY-IRS-1) (R); horizontal line in each cluster of individual values is the mean level for that group.
An increase in R, which reflects the level of altered regulation of proximal signaling by the insulin receptor, was the most sensitive of the variables determined for distinguishing between disease and control groups. Each disease group was significantly distinguished from its corresponding control by this index (Fig.1, bottom right frame), there was no overlap of values for AC vs. AD and an overlap of only 1 value for DC vs. DM2. The mean level of R for AD was significantly higher than those for DM2 and FTD, and there was no overlap of values for AD vs. FTD.
Stepwise discriminant analysis of the data that distinguished AD from AC resulted in a model that incorporated first P-pan-tyrosine-IRS-1 and second P-serine 312-IRS-1 but not total IRS-1. The final model achieved a Wilks’ lambda of 0.105 and an exact F of 207.9 (P < 0.001) and correctly classified 100% of patients with aMCI/AD and AC subjects. In the ROC analysis of classification of the AD and AC groups, individual subject scores from the final model achieved a perfect area under the curve (AUC) of 1 (asymptotic significance < 0.001; Supplemental Fig. 1). The AUC values were 0.703, 0.932, and 1.000, respectively, for total IRS-1, P-serine 312-IRS-1, and P-pan-tyrosine-IRS-1. Stepwise discriminant analysis of data that distinguished DM2 from DC resulted in a model that incorporated first P-pan-tyrosine-IRS-1 and second P-serine 312-IRS-1 but not total IRS-1. The final model achieved a Wilks’ lambda of 0.168 and an exact F of 91.8 (P < 0.001) and correctly classified 97.5% of patients with DM2 and DC subjects. In ROC analysis of classification of the DM2 and DC groups, individual subject scores from the final model achieved an AUC of 1. The AUC values were 0.741 (asymptotic significance = 0.009) and 0.999 (asymptotic significance < 0.001), respectively, for P-pan-tyrosine-IRS-1 and P-serine 312-IRS-1. Stepwise discriminant analysis of data that distinguished FTD from FTC resulted in a model that incorporated only P-serine 312-IRS-1 but not P-pan-tyrosine-IRS-1 or total IRS-1. The final model achieved a Wilks’ lambda of 0.576 and an exact F of 22.1 (P < 0.001) and correctly classified 84% of patients with FTD and FTC subjects. In the ROC analysis of classification of the FTD and FTC groups, P-serine 312-IRS-1 achieved an AUC of 0.928 (asymptotic significance < 0.001).
Lack of relationship of levels of IRS-1 proteins in neural-derived plasma exosomes to severity and stage of AD
A comparison of the results for 16 patients with AD with aMCI with the 10 patients with AD with dementia at presentation showed no differences in the exosomal levels of P-serine 312-IRS-1, P-pan-tyrosine-IRS-1, total IRS-1, or R (Table 2). There also were no significant correlations between levels of any of the IRS-1 proteins in neural-enriched exosomes and cognitive function determined by MMSE or ADAS-cog (Table 1). This lack of correlation with clinical severity at presentation suggested that increased exosomal levels of 1 or more of the forms of IRS-1 might be detectable early in the preclinical course. Therefore, blood exosomal IRS-1 proteins were measured for an additional group of 22 patients with AD at 2 time points, the first was preclinical at 1 to 10 yr before their diagnosis (PC-AD), and the second at the time of initial diagnosis of AD (AD).
TABLE 2.
Lack of relationship between plasma neurally derived exosome forms of IRS-1 and severity of dementia or presence of impaired glucose tolerance in AD
| Patient group | n | P-S312-IRS-1 (U/ml) | P-panY-IRS-1 (AU) | Total IRS-1 (ng/ml) | P-S312/P-panY |
|---|---|---|---|---|---|
| AD, MCI | 16 | 8.03 ± 0.59 | 0.084 ± 0.006 | 5.03 ± 0.12 | 91.7 ± 7.01 |
| AD, dementia | 10 | 7.12 ± 0.66 | 0.078 ± 0.005 | 5.06 ± 0.18 | 93.2 ± 8.44 |
| AD, normal glucose tolerance | 13 | 7.25 ± 0.61 | 0.078 ± 0.009 | 5.15 ± 0.12 | 87.1 ± 6.52 |
| AD, impaired glucose tolerance | 13 | 8.17 ± 0.66 | 0.085 ± 0.002 | 4.93 ± 0.15 | 97.4 ± 8.50 |
All values are mean ± sem. Impaired glucose tolerance is defined as fasting glucose level >100 mg/dl and/or glucose level at 2 hours after oral load >140 mg/dl. None of the differences between MCI and dementia groups or between normal and impaired glucose tolerance groups is significant. AU, absorbance units at 450 nm; P-S312, P-serine 312-IRS-1; P-panY, P-pan-tyrosine-IRS-1.
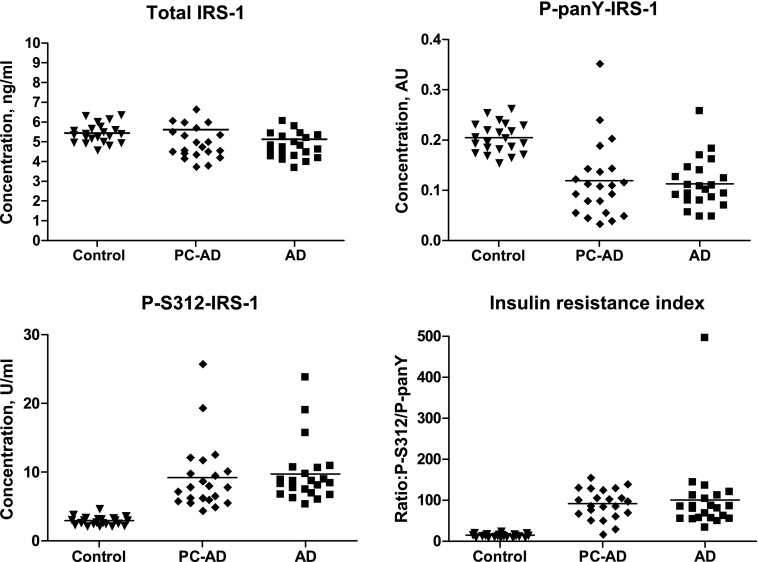
As for the single time-point values (Fig. 1), longitudinal studies showed mean levels (±sem) of P-pan-tyrosine-IRS-1 (0.113 ± 0.011 AU), P-serine 312-IRS-1 (9.70 ± 0.95 U/ml), and R (101 ± 19.8) at the time of diagnosis of AD (AD) that were all significantly different (P < 0.0001) from those of their case control subjects (0.205 ± 0.006 AU, 2.93 ± 0.13 U/ml, and 14.7 ± 0.89, respectively; Fig. 2). It was expected that the mean level of total IRS-1 for the patients with AD at the time of diagnosis (5.13 ± 0.37 ng/ml) was no different from that of their control group (5.49 ± 0.10 ng/ml). Furthermore, the mean PC-AD levels of total IRS-1 (5.62 ± 0.65 ng/ml), P-pan-tyrosine-IRS-1 (0.119 ± 0.016 AU), P-serine 312-IRS-1 (9.22 ± 1.07 U/ml), and R (92.0 ± 7.72) were no different from those of the manifest AD group. Thus, distinctly different exosomal levels of all forms of IRS-1 were clearly detectable in a preclinical cognitively normal PC-AD group and had attained a plateau as early as 10 yr before clinical diagnosis of manifest AD. A comparison of all PC-AD and AD exosomal IRS-1 and P-IRS-1 levels of patients converting to aMCI with those converting to AD did not show any significant differences. Furthermore, a comparison of all PC-AD and AD exosomal protein levels of patients converting to aMCI or AD after 1 to 5 yr with those converting after 6 to 10 yr also did not show any significant differences.
Figure 2.
Longitudinal analysis of the development of altered levels of phosphorylated IRS-1 in AD. PC-AD, preclinical values 1 to 10 yr before diagnosis for patients with AD; control, values for cognitively normal healthy subjects matched by age and gender with each patient with AD at the time of diagnosis. P-S312-IRS-1, P-serine 312-IRS-1; P-panY-IRS-1, P-pan-tyrosine-IRS-1.
Lack of relationship of levels of IRS-1 proteins in neural-derived plasma exosomes to CSF biomarkers or glucose tolerance
There were no significant correlations for patients with AD in the cross-sectional study between any of the forms of IRS-1 or R in neural-enriched blood exosomes, and either CSF total-tau or CSF P-T181-tau. CSF levels of Aβ1-42 showed a weak negative correlation with the P-serine 312/P-pan-tyrosine R ratio both before (R = −0.370; P = 0.038, not significant after Bonferroni correction) and after controlling for age and gender (R = −0.36; P = 0.047, not significant after Bonferroni correction).
Patients with AD in the cross-sectional study with normal glucose tolerance in oral challenge testing had levels of total IRS-1, P-serine 312-IRS-1, P-pan-tyrosine-IRS-1, and R indistinguishable from those with impaired glucose tolerance, reflecting insulin resistance (Table 2).
DISCUSSION
Many associations between the insulin resistance of DM2 and increased incidence of AD have been documented in epidemiologic studies (27–29). Direct neuropathological consequences of insulin resistance include increased Aβ1-42 oligomerization and accumulation, greater tau phosphorylation, and the capacity of compensatory higher insulin levels in the brain to competitively inhibit protease degradation of Aβ1-42 (30–32). Many patients with AD express brain metabolic abnormalities similar to those of patients with DM2, of which insulin resistance appears most likely to contribute to the pathophysiology of AD (16, 17). Although numerous defects in insulin production, transport into the central nervous system, and biodegradation have been detected in AD, the major basis for insulin resistance appears to be deficient signaling by the insulin receptor largely due to altered phosphorylation of IRS-1/-2 (33–36). It has not been possible previously to determine the prevalence of brain insulin resistance in living patients with AD or to establish a temporal relationship between the onset of insulin resistance and clinically evident signs of AD.
The capacity to quantify IRS-1 and its phosphorylated forms in neurally derived plasma exosomes of living patients with AD now has permitted direct verification of the abnormalities initially detected in brain tissues at autopsy. The exosomal levels of P-serine 312-IRS-1 and P-pan-tyrosine-IRS-1 as well as the ratio of P-serine 312-IRS-1 to P-pan-tyrosine-IRS-1 (R or insulin resistance index) all were significantly different in patients with AD than in age- and gender-matched control subjects (Fig. 1). These exosomal IRS-1 levels also were significantly different in patients with AD than in age- and gender-matched patients with FTD and DM2. Furthermore, these differences were identified in preclinical AD up to 10 yr before the onset of clinically recognizable AD, and the protein levels were similar in the preclinical and manifest stages of AD irrespective of whether the presentation was MCI or dementia (Fig. 2).
The extent of enrichment of neurally derived exosomes from total plasma exosomes has not been fully quantified, but it is substantial based on several observations. Anti-human NCAM antibodies and anti-CD171 (L1CAM) antibodies separately bind fewer than 15% of total plasma exosomes. After immunoabsorptive enrichment by either anti-human neural adhesive protein antibody, over 95% of the exosomes recovered bind to the other antibody, suggesting successful enrichment. Furthermore, ELISA quantification of the amount of human neuron-specific enolase-2 (γ-enolase; R&D Systems, Minneapolis, MN, USA) extracted from anti-CD171antibody-enriched exosomes was a mean (n = 6) 26-fold higher than the level extracted from the same number of total plasma exosomes. At this point, however, it is not possible to resolve exosomes of the CNS from those of other neural sources in plasma.
Several biochemical links between insulin resistance and the neurodegeneration of AD have been investigated at the molecular level. One example is the negative regulation of activity of glycogen synthase kinase 3 (GSK3) by insulin through activation of protein kinase B or Akt. Insulin resistance in the central nervous system results in greater GSK3 activity and consequent hyperphosphorylation of tau. This hyperphosphorylation of tau by GSK has been demonstrated in vitro and in transgenic mice conditionally overexpressing GSK (37). In mice with neuron-selective disruption of the insulin receptor, activation of Akt is strikingly reduced with parallel increases in GSK activity and tau hyperphosphorylation (38). However, the higher level of insulin resistance (R) in the patients with DM2 compared with the DC control subjects (Fig.1) was not here associated with elevated exosomal levels of AD-related pathogenic proteins, suggesting a multifactorial etiology. Mean ± sem neural-derived plasma exosome levels of Aβ1-42 and P-T181-tau in the 20 patients with DM2 were 5.67 ± 0.40 and 47.7 ± 8.29 pg/ml, respectively, which were no different than the corresponding levels of 5.18 ± 0.47 and 34.9 ± 5.80 pg/ml for matched control subjects and very significantly lower than parallel levels of 19.7 ± 1.39 and 193 ± 15.8 pg/ml for patients with AD. These complex interactions between multiple pathogenic factors and cellular susceptibility in situ will need to be examined further in mouse models of AD and studies of human exosomal biomarkers.
It will be important now to investigate in detail the insulin receptor signaling pathways distal to IRS-1/-2 in plasma exosomal extracts. These studies will encompass the PI3K pathway to Akt and mammalian target of rapamycin and the Ras-MAPK pathway to Erk1/2 to evaluate differences in insulin effects on protein synthesis (39). Further clinical studies will include selection of subjects with signs of significant insulin resistance in the preclinical phase from results of analyses of exosomal P-IRS-1 for longitudinal determination of their metabolic, endocrine, and neurologic courses relative to subjects with little or no evidence of insulin resistance. The availability of plasma exosome-based assays of forms of neural P-IRS-1 will permit such clinical studies as well as objective documentation of effects of therapeutic agents.
Supplementary Material
Acknowledgments
The authors are grateful to Lynn Kane (JHSF), Anna Karydas (UCSF MAC), Dana Swenson-Dravis and Matthew Miller (Mayo Clinic), Sonya Anderson (University of Kentucky), and Olga Carlson and Melissa Swaby Intramural Research Program of the National Institute on Aging (NIA) for organizing and distributing clinical materials and data and for providing access to analytical systems. They also thank Judith H. Goetzl for expert preparation of graphic illustrations. This study was funded by the U.S. National Institutes of Health (NIH) NIA (to A.Bi., D.K.), United Kingdom Alzheimer’s Disease Center grant P30-AG028383 (to E.L.A.), and an unrestricted supply grant for methodologic development from Nanosomix, Incorporated (to E.J.G.). D.K., J.B.S., E.L.A., A.Bi., U.M., L.F., R.C.P., and B.L.M. declare no potential conflicts of interest. E.J.G. has filed a provisional application with the U.S. Patent Office for the methodology described in this report; Nanosomix, Incorporated, supports the research but provides no salary, expense reimbursement, or fees and does not influence the processes of research or publication. A.B. reports grants from the NIH/NIA; grants from Tau Research Consortium; grants from Corticobasal Degeneration Solutions; grants, personal fees, and nonfinancial support from Archer Biosciences; grants from Allon Therapeutics; personal fees from Acetylon; personal fees from Ipierian; grants from Genentech; grants from Bristol-Myers Squibb; grants from TauRx; grants from Alzheimer's Association; grants from Bluefield Project to Cure FTD; grants from Association for Frontotemporal Degeneration; grants from Alzheimer's Drug Discovery Foundation; grants from EnVivo; grants from C2N Diagnostics; grants from Pfizer; grants from Eli Lilly for support outside the submitted work.
Glossary
- AC
Alzheimer’s disease case control subjects
- AD
Alzheimer’s disease
- ADAS-cog
cognitive subscale of the Alzheimer’s disease assessment scale
- aMCI
amnestic MCI
- AUC
area under the curve
- CDR
clinical dementia rating
- DC
DM2 case controls
- DM2
type 2 diabetes mellitus
- FTC
frontotemporal dementia case controls
- FTD
frontotemporal dementia
- GSK3
glycogen synthase kinase 3
- IGF-1
type 1 insulin-like growth factor
- IRS
insulin receptor substrate
- MMSE
mini-mental state examination
- PC-AD
cases of preclinical AD
- R
insulin resistance factor
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Cersosimo E., DeFronzo R. A. (2006) Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab. Res. Rev. 22, 423–436 [DOI] [PubMed] [Google Scholar]
- 2.Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. (1991) Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature 352, 73–77 [DOI] [PubMed] [Google Scholar]
- 3.Yamada M., Ohnishi H., Sano Si., Nakatani A., Ikeuchi T., Hatanaka H. (1997) Insulin receptor substrate (IRS)-1 and IRS-2 are tyrosine-phosphorylated and associated with phosphatidylinositol 3-kinase in response to brain-derived neurotrophic factor in cultured cerebral cortical neurons. J. Biol. Chem. 272, 30334–30339 [DOI] [PubMed] [Google Scholar]
- 4.Dhe-Paganon S., Ottinger E. A., Nolte R. T., Eck M. J., Shoelson S. E. (1999) Crystal structure of the pleckstrin homology-phosphotyrosine binding (PH-PTB) targeting region of insulin receptor substrate 1. Proc. Natl. Acad. Sci. USA 96, 8378–8383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amoui M., Craddock B. P., Miller W. T. (2001) Differential phosphorylation of IRS-1 by insulin and insulin-like growth factor I receptors in Chinese hamster ovary cells. J. Endocrinol. 171, 153–162 [DOI] [PubMed] [Google Scholar]
- 6.Gual P., Le Marchand-Brustel Y., Tanti J. F. (2005) Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 87, 99–109 [DOI] [PubMed] [Google Scholar]
- 7.Goldstein B. J., Bittner-Kowalczyk A., White M. F., Harbeck M. (2000) Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. J. Biol. Chem. 275, 4283–4289 [DOI] [PubMed] [Google Scholar]
- 8.Aguirre V., Werner E. D., Giraud J., Lee Y. H., Shoelson S. E., White M. F. (2002) Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 277, 1531–1537 [DOI] [PubMed] [Google Scholar]
- 9.Carlson C. J., White M. F., Rondinone C. M. (2004) Mammalian target of rapamycin regulates IRS-1 serine 307 phosphorylation. Biochem. Biophys. Res. Commun. 316, 533–539 [DOI] [PubMed] [Google Scholar]
- 10.Yi Z., Langlais P., De Filippis E. A., Luo M., Flynn C. R., Schroeder S., Weintraub S. T., Mapes R., Mandarino L. J. (2007) Global assessment of regulation of phosphorylation of insulin receptor substrate-1 by insulin in vivo in human muscle. Diabetes 56, 1508–1516 [DOI] [PubMed] [Google Scholar]
- 11.Herschkovitz A., Liu Y. F., Ilan E., Ronen D., Boura-Halfon S., Zick Y. (2007) Common inhibitory serine sites phosphorylated by IRS-1 kinases, triggered by insulin and inducers of insulin resistance. J. Biol. Chem. 282, 18018–18027 [DOI] [PubMed] [Google Scholar]
- 12.Copps K. D., White M. F. (2012) Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55, 2565–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuel V. T., Shulman G. I. (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148, 852–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hançer N. J., Qiu W., Cherella C., Li Y., Copps K. D., White M. F. (2014) Insulin and metabolic stress stimulate multisite serine/threonine phosphorylation of insulin receptor substrate 1 and inhibit tyrosine phosphorylation. J. Biol. Chem. 289, 12467–12484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker L. D., Cross D. J., Minoshima S., Belongia D., Watson G. S., Craft S. (2011) Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch. Neurol. 68, 51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moloney A. M., Griffin R. J., Timmons S., O’Connor R., Ravid R., O’Neill C. (2010) Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol. Aging 31, 224–243 [DOI] [PubMed] [Google Scholar]
- 17.Talbot K., Wang H. Y., Kazi H., Han L. Y., Bakshi K. P., Stucky A., Fuino R. L., Kawaguchi K. R., Samoyedny A. J., Wilson R. S., Arvanitakis Z., Schneider J. A., Wolf B. A., Bennett D. A., Trojanowski J. Q., Arnold S. E. (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Invest. 122, 1316–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiandaca M. S., Kapogiannis D., Mapstone M., Boxer A., Eitan E., Schwartz J. B., Abner E. L., Petersen R. C., Federoff H. J., Miller B. L., Goetzl E. J.. Identification of pre-clinical Alzheimer’s disease by a profile of pathogenic proteins in neurally-derived blood exosomes: a case-control study. Alzheimers Dement. DOI:10.1016/j.jalz.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen R. C. (2004) Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256, 183–194 [DOI] [PubMed] [Google Scholar]
- 20.Sperling R. A., Aisen P. S., Beckett L. A., Bennett D. A., Craft S., Fagan A. M., Iwatsubo T., Jack C. R. Jr., Kaye J., Montine T. J., Park D. C., Reiman E. M., Rowe C. C., Siemers E., Stern Y., Yaffe K., Carrillo M. C., Thies B., Morrison-Bogorad M., Wagster M. V., Phelps C. H. (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubois B., Feldman H. H., Jacova C., Dekosky S. T., Barberger-Gateau P., Cummings J., Delacourte A., Galasko D., Gauthier S., Jicha G., Meguro K., O’brien J., Pasquier F., Robert P., Rossor M., Salloway S., Stern Y., Visser P. J., Scheltens P. (2007) Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746 [DOI] [PubMed] [Google Scholar]
- 22.Shaw L. M., Vanderstichele H., Knapik-Czajka M., Clark C. M., Aisen P. S., Petersen R. C., Blennow K., Soares H., Simon A., Lewczuk P., Dean R., Siemers E., Potter W., Lee V. M., Trojanowski J. Q.; Alzheimer’s Disease Neuroimaging Initiative (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 65, 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irizarry M. C., Webb D. J., Bains C., Barrett S. J., Lai R. Y., Laroche J. P., Hosford D., Maher-Edwards G., Weil J. G. (2008) Predictors of placebo group decline in the Alzheimer’s disease Assessment Scale-cognitive subscale (ADAS-Cog) in 24 week clinical trials of Alzheimer’s disease. J. Alzheimers Dis. 14, 301–311 [DOI] [PubMed] [Google Scholar]
- 24.Cano S. J., Posner H. B., Moline M. L., Hurt S. W., Swartz J., Hsu T., Hobart J. C. (2010) The ADAS-cog in Alzheimer’s disease clinical trials: psychometric evaluation of the sum and its parts. J. Neurol. Neurosurg. Psychiatry 81, 1363–1368 [DOI] [PubMed] [Google Scholar]
- 25.Rascovsky K., Hodges J. R., Knopman D., Mendez M. F., Kramer J. H., Neuhaus J., van Swieten J. C., Seelaar H., Dopper E. G., Onyike C. U., Hillis A. E., Josephs K. A., Boeve B. F., Kertesz A., Seeley W. W., Rankin K. P., Johnson J. K., Gorno-Tempini M. L., Rosen H., Prioleau-Latham C. E., Lee A., Kipps C. M., Lillo P., Piguet O., Rohrer J. D., Rossor M. N., Warren J. D., Fox N. C., Galasko D., Salmon D. P., Black S. E., Mesulam M., Weintraub S., Dickerson B. C., Diehl-Schmid J., Pasquier F., Deramecourt V., Lebert F., Pijnenburg Y., Chow T. W., Manes F., Grafman J., Cappa S. F., Freedman M., Grossman M., Miller B.L. (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorno-Tempini M. L., Hillis A. E., Weintraub S., Kertesz A., Mendez M., Cappa S. F., Ogar J. M., Rohrer J. D., Black S., Boeve B. F., Manes F., Dronkers N. F., Vandenberghe R., Rascovsky K., Patterson K., Miller B. L., Knopman D. S., Hodges J. R., Mesulam M. M., Grossman M. (2011) Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrijvers E. M., Witteman J. C., Sijbrands E. J., Hofman A., Koudstaal P. J., Breteler M. M. (2010) Insulin metabolism and the risk of Alzheimer disease: the Rotterdam Study. Neurology 75, 1982–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crane P. K., Walker R., Hubbard R. A., Li G., Nathan D. M., Zheng H., Haneuse S., Craft S., Montine T. J., Kahn S. E., McCormick W., McCurry S. M., Bowen J. D., Larson E. B. (2013) Glucose levels and risk of dementia. N. Engl. J. Med. 369, 540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu C., Sigurdsson S., Zhang Q., Jonsdottir M. K., Kjartansson O., Eiriksdottir G., Garcia M. E., Harris T. B., van Buchem M. A., Gudnason V., Launer L. J. (2014) Diabetes, markers of brain pathology and cognitive function: the Age, Gene/Environment Susceptibility-Reykjavik Study. Ann. Neurol. 75, 138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craft S., Watson G. S. (2004) Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 3, 169–178 [DOI] [PubMed] [Google Scholar]
- 31.Planel E., Tatebayashi Y., Miyasaka T., Liu L., Wang L., Herman M., Yu W. H., Luchsinger J. A., Wadzinski B., Duff K. E., Takashima A. (2007) Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J. Neurosci. 27, 13635–13648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao W. Q., Lacor P. N., Chen H., Lambert M. P., Quon M. J., Krafft G. A., Klein W. L. (2009) Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric abeta. J. Biol. Chem. 284, 18742–18753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duarte A. I., Moreira P. I., Oliveira C. R. (2012) Insulin in central nervous system: more than just a peripheral hormone. J. Aging Res. 2012, 384017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neumann K. F., Rojo L., Navarrete L. P., Farías G., Reyes P., Maccioni R. B. (2008) Insulin resistance and Alzheimer’s disease: molecular links & clinical implications. Curr. Alzheimer Res. 5, 438–447 [DOI] [PubMed] [Google Scholar]
- 35.Schiöth H. B., Craft S., Brooks S. J., Frey W. H. II, Benedict C. (2012) Brain insulin signaling and Alzheimer’s disease: current evidence and future directions. Mol. Neurobiol. 46, 4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bosco D., Fava A., Plastino M., Montalcini T., Pujia A. (2011) Possible implications of insulin resistance and glucose metabolism in Alzheimer’s disease pathogenesis. J. Cell. Mol. Med. 15, 1807–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucas J. J., Hernández F., Gómez-Ramos P., Morán M. A., Hen R., Avila J. (2001) Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 20, 27–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schubert M., Gautam D., Surjo D., Ueki K., Baudler S., Schubert D., Kondo T., Alber J., Galldiks N., Küstermann E., Arndt S., Jacobs A. H., Krone W., Kahn C. R., Brüning J. C. (2004) Role for neuronal insulin resistance in neurodegenerative diseases. Proc. Natl. Acad. Sci. USA 101, 3100–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taniguchi C. M., Emanuelli B., Kahn C. R. (2006) Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7, 85–96 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.