Abstract
Rod tetrameric arrestin 1 (tet-ARR1), stored in the outer nuclear layer/inner segments in the dark, modulates photoreceptor synaptic activity; light exposure stimulates a reduction via translocation to the outer segments for terminating G-protein coupled phototransduction signaling. Here, we test the hypothesis that intraretinal spin-lattice relaxation rate in the rotating frame (1/T1ρ), an endogenous MRI contrast mechanism, has high potential for evaluating rod tet-ARR1 and its reduction via translocation. Dark- and light-exposed mice (null for the ARR1 gene, overexpressing ARR1, diabetic, or wild type with or without treatment with Mn2+, a calcium channel probe) were studied using 1/T1ρ MRI. Immunohistochemistry and single-cell recordings of the retinas were also performed. In wild-type mice with or without treatment with Mn2+, 1/T1ρ of avascular outer retina (64% to 72% depth) was significantly (P < 0.05) greater in the dark than in the light; a significant (P < 0.05) but opposite pattern was noted in the inner retina (<50% depth). Light-evoked outer retina Δ1/T1ρ was absent in ARR1-null mice and supernormal in overexpressing mice. In diabetic mice, the outer retinal Δ1/T1ρ pattern suggested normal dark-to-light tet-ARR1 translocation and chromophore content, conclusions confirmed ex vivo. Light-stimulated Δ1/T1ρ in inner retina was linked to changes in blood volume. Our data support 1/T1ρ MRI for noninvasively assessing rod tet-ARR1 and its reduction via protein translocation, which can be combined with other metrics of retinal function in vivo.—Berkowitz, B. A., Gorgis, J., Patel, A., Baameur, F., Gurevich, V. V., Craft, C. M., Kefalov, V. J., Roberts, R. Development of an MRI biomarker sensitive to tetrameric visual arrestin 1 and its reduction via light-evoked translocation in vivo.
Keywords: diabetes, magnetic resonance imaging, retina, rod photoreceptors
Healthy vision relies on light-induced activation and subsequent rapid inactivation of the phototransduction signaling cascade. Key for the timely shutoff of these light-driven responses is the inactivation of visual pigment. This is achieved in a 2-step process that involves partial inactivation via phosphorylation by G-protein receptor kinase 1 (GRK1 in mouse) (1) and subsequent complete inactivation by monomers of visual rod arrestin-1 (ARR1) and cone arrestin-4 (ARR4). The dominant form of arrestin in the mouse photoreceptors is ARR1, expressed in rods (the vast majority of their photoreceptor complement) and coexpressed with ARR4 in cones (2, 3). In darkness, most of ARR1 is localized in the outer nuclear layer and inner segments of photoreceptors, where it self-assembles into large tetramers (i.e., a storage form). Exposure to bright light induces a dramatic reduction in this tetrameric ARR1 (tet-ARR1) concentration in these compartments due to translocation of its monomers to the outer segments to bind to rhodopsin (4, 5). Importantly, reduction of tet-ARR1 via translocation to the outer segments is tightly integrated into photoreceptor function. For example, translocation only occurs if visual cycle regeneration of rhodopsin is normal (6). Another important function of tet-ARR1 is its modulation in the dark of exocytotic activity at the first retinal synapse activity via N-ethylmaleimide sensitive factor (7, 8). Defects in ARR1 are implicated in the sight-threatening Oguchi disease, a nonprogressive form of congenital stationary night blindness (9). Current evaluation of tet-ARR1 level and its reduction via translocation in mammals is primarily performed in excised retinal tissue. The inability to noninvasively monitor this tet-ARR1 physiology in vivo has greatly limited translational studies in normal, mutant, and disease mouse models. The role of tet-ARR1 in photoreceptor physiology in humans and its regulation in diseased retinas also remains enigmatic because of this technical limitation. Thus, it is highly desirable to develop a noninvasive method for evaluating tet-ARR1 level and its reduction via translocation in vivo.
1/T1ρ, the spin-lattice relaxation rate in the rotating frame, governs the decay of the transverse magnetization out of a spin-lock radiofrequency field and is directly modulated first by the macromolecular content in avascular tissues [a property exploited in, e.g., clinical evaluation of cartilage biochemical composition (10, 11)] and second by vascular volume in well-perfused tissue (10, 12–16). These sensitivities have not yet been tested in the retina. We hypothesized that the difference between avascular outer nuclear layer/inner segment tet-ARR1 in the dark plus the reduction of this storage form upon light exposure via translocation would be detectable as a change in 1/T1ρ (i.e., Δ1/T1ρ) in the outer retina in vivo. In addition, we also examined the known sensitivity of 1/T1ρ to functional blood volume change, a property that had not been investigated in the vascular inner retina and may complement the tet-ARR1 evaluation (13–16).
The results of this study are divided into 3 sections. In section A, we describe the general features of the light-evoked changes in intraretinal 1/T1ρ in untreated or manganese-treated mice; manganese imparts functional information about calcium channels as measured by manganese-enhanced MRI (MEMRI), the imaging modality of choice when performing studies of retinal ion activity (17, 18). The MRI axial resolution (in the present mouse study, 21.9 µm) used in all sections herein is sufficient to generate very high spatial resolution functional maps of central retina, as demonstrated in many publications from our group and others (19–25). In section B, we examine the sensitivity of 1/T1ρ MRI to purified tet-ARR1 in solution and in transgenic mice in vivo (11, 26). To begin to study 1/T1ρ MRI applicability to disease models, we also examined diabetic mice, which have been suggested to have an impairment in visual cycle activity and thus would be expected to have reduced ARR1 translocation. In section C, we investigate the sensitivity of 1/T1ρ MRI for detecting light-evoked vascular volume changes in the inner retinal circulation similar to those established in functional brain studies. Diabetic mice, which have impaired autoregulation of inner retinal blood volume between dark and light conditions (27, 28), were also examined.
MATERIALS AND METHODS
All animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and Institutional Animal and Care Use Committee authorization. Animals were housed and maintained in 12 h:12 h light–dark cycle laboratory lighting, unless otherwise noted.
Groups, treatment, and timing
The groups studied are summarized in Table 1. These groups contained a mix of male and female mice: normoglycemic and diabetic C57Bl/6J mice (wild-type [WT]; Jackson Laboratories, Bar Harbor, ME, USA), ARR1−/−, ARR1+/+-P48 mice expressing twice as much ARR1 as WT; it is presumed that the tet-ARR1 levels in the overexpressing mice is twice that of WT (29). Because ARR1−/− mice have retinal degeneration with daily light exposure, they were reared in the dark and then shipped to Wayne State University, where they were maintained in the dark; unavoidably, these mice were exposed to a variable but low light through the shipping container during the transport (30). ARR1+/+-P48 mice were also reared in the dark, but after shipping to Wayne State University, they were maintained in normal 12 h:12 h light–dark cycle laboratory lighting.
TABLE 1.
Group Summary (mean ± sem)
| Group, method of study | Wt (g) | A1c (%) | n | With Mn2+? |
|---|---|---|---|---|
| WT, MRI | 30.5 ± 0.5 | 6.99 ± 0.19 | 6 | No |
| WT, MRI | 30.4 ± 0.4 | 7.0 ± 0.16 | 7 | Yes |
| STZ, MRI | 22.8 ± 0.6* | 13.2 ± 0.75* | 5 | Yes |
| WT, dark, IHC | 28 ± 1 | 6.03 ± 0.08 | 3 | No |
| WT, light, IHC | 27 ± 0.6 | 5.65 ± 0.23 | 3 | No |
| STZ, dark, IHC | 21 ± 1* | 12.92 ± 0.24* | 3 | No |
| STZ, light, IHC | 22 ± 0.3* | 13.33 ± 0.34* | 3 | No |
| WT, electrophysiology | 6.58 ± 0.41 | 3 | No | |
| STZ, electrophysiology | 14.11 ± 0.43* | 6 | No | |
| Arr1−/−, MRI | 17.3 ± 1.69 | 7 | Yes | |
| P48, MRI | 20.6 ± 0.75 | 5 | Yes |
A1c (%), % of hemoglobin that is glycated; WT, wild-type C57Bl/6J mouse; STZ, diabetic C57Bl/6J mouse; Arr1−/−, arrestin-1 null mouse; P48, Arr1+/+-P48 mouse.
P < 0.05 compared to WT data.
Diabetes was induced in WT mice with starting weights of 18 to 22 g by streptozotocin (60 mg/kg; 10 mM citrate buffer, pH 4.5) intraperitoneal injection once a day for 5 consecutive days. Body weight and blood glucose levels were monitored twice weekly. Insulin (neutral protamine Hagedorn), administered to mice as needed on the basis of body weight and blood glucose levels but not more than twice weekly, allowed slow weight gain while maintaining hyperglycemia (blood glucose levels higher than 400 mg/dl). Mice that lost weight and/or had blood glucose levels greater than 600 mg/dl were given 0.2 units of Lilly Humulin N insulin. Normal rodent chow (TestDiet 5001; Purina, Richmond, IN, USA; contains 11.2% fat, 26% protein, and 62.7% carbohydrate) and water were provided ad libitum. In 2 mo old diabetic mice, the percentage of hemoglobin that was glycated [A1c (%)] was measured from blood collected after their MEMRI experiment (Glyco-Tek affinity columns, kit 5351; Helena Laboratories, Beaumont, TX, USA). Blood was drawn from the left ventricle, after puncture, into a capillary tube and stored in an Eppendorf tube with a small amount of heparin to prevent coagulation. The blood was kept in the refrigerator until analysis within 1 week after the MRI experiment.
MRI procedures
Our general animal preparation for high-resolution MRI of the retina in vivo is well established (22). All animals were maintained in darkness for at least 16 h before and during the first part of the MRI examination (i.e., before turning on the light). In some mouse groups, MnCl2 was administered, under dim red light or in darkness, as an intraperitoneal injection (66 mg MnCl2·4H2O/kg) on the right side of awake mice. After this injection, mice were maintained in the dark for another 3.5 to 4 h. MRI data were acquired on a 7 T MRI system (Clinscan; Bruker, Billerica, MA, USA) using a receive-only surface coil (1.0 cm diameter) centered on the left eye. The end of a fiber optic bundle was attached to a light source (Mark II Light Source; Prescott’s Inc., Monument, CO, USA) placed caudal to the eye, projecting at a white paper placed ∼1 cm from eye, as previously described (23). Depending on knob position, we could expose the eye to either 0 or ∼500 lux [with these values confirmed outside the magnet using a Traceable Dual-Range Light Meter (Control Company, Friendswood, TX, USA) placed against a 1 cm diameter aperture; measured this way, lighting is ∼300 lx]. Aside from the fiber optic light source, all lights in the MRI room were turned off. In all groups, immediately before the MRI experiment, mice were anesthetized with urethane (36% solution intraperitoneally; 0.083 ml/20 g animal weight, prepared fresh daily; Aldrich, Milwaukee, WI, USA) and treated topically with atropine to ensure maximal dilation of the iris during light exposure.
1/T1ρ
Two 1/T1ρ data sets were collected, first in the dark and then 20 min after turning on the light to allow for ARR1 translocation (4). 1/T1ρ data were collected using a modified spin-echo sequence and a compensated spin-lock pulse (31) with spin-lock (500 Hz) durations of 1, 10, 20, 30, 40, 70, and 100 ms; TR 2000 ms; TE 13 ms; slice thickness 600 µm; final matrix size 320 × 320; FOV 7 × 7 mm2; and axial resolution 21.875 µm. In some mouse groups, after removal from the MRI, a final blood sample was obtained for glycated hemoglobin analysis. In all cases, animals were humanely euthanatized as detailed in our Division of Laboratory Animal Resources-approved protocol.
Monocrystalline iron oxide nanocolloids
While under dim red light, mice were anesthetized with urethane [to maintain systemic physiology (32, 33)] and a tail vein catheter inserted for monocrystalline iron oxide nanocolloid (MION) (20 mg/kg) injection; similar images were collected from uninjected control mice. Mice were maintained in the dim red light, and after careful positioning in the magnet, a conventional 2D gradient-echo image was collected through the midline of the eye (center of lens, center of optic nerve head) with the following parameters: flip angle 25, number of repetitions 16, spectral width = 28 kHz, TR = 150 ms, TE = 5 ms, FOV = 6 × 6 mm, slice thickness = 600 mm, final matrix = 320 × 320; and axial resolution 18.75 µm. The 16 images were then registered and averaged for later analysis.
Isolating and purifying ARR1 monomers and tetramers
Mouse ARR1, both WT and self-association-deficient mutant ARR1-(Phe86Ala, Phe198Ala), were expressed in Escherichia coli and purified as previously described (34).
Arrestin 1 immunohistochemistry
ARR1 was visualized in fixed 30 μm sections, prepared as previously described (35). Sections were blocked in PBS/0.3% Triton X-100/5% BSA for 1 h at room temperature with mild agitation. The sections were incubated with affinity-purified rabbit polyclonal peptide antibody raised against C10C10 bovine S-antigen/ARR1 epitope (36) overnight at 4°C in PBS/0.03% Triton X-100/1% BSA (incubation buffer), washed with PBS for 15-20 min, and then stained for 1 h at room temperature in incubation buffer with biotinylated anti-rabbit antibodies (Jackson Labs), followed by washing with PBS for 15 to 20 min, and incubated with green fluorescent Streptavidin-Alexa488 (Invitrogen), washed with PBS as above, and mounted on Vectabond-coated slides with Vectashield. Mounted sections were analyzed by confocal microscopy (×40 oil objective) (LSM510; Zeiss, Oberkochen, Germany) (36, 37). Although immunohistochemistry is not a quantitative approach, this assay is useful for visualizing the translocation of ARR1.
Single-cell recordings
After overnight dark adaptation, mice were euthanized in dim red illumination by CO2 asphyxiation followed by cervical dislocation. The eye was removed, and the retina was isolated and finely sliced under infrared illumination. Small pieces of the retina were placed in the recording chamber on the stage of an inverted microscope and perfused with bicarbonate-buffered solution [112.5 mM NaCl, 3.6 mM KCl, 2.4 mM MgCl2, 1.2 mM CaCl2, 10 mM HEPES (pH 7.4), 20 mM NaHCO3, 3 mM sodium succinate, 0.5 mM sodium glutamate, 0.02 mM EDTA, and 10 mM glucose] bubbled with 95% O2/5% CO2 and warmed to 36–38°C. Membrane current was recorded with a suction electrode from a single rod outer segment protruding from a piece of retina. The recording electrode was filled with 140 mM NaCl, 3.6 mM KCl, 2.4 mM MgCl2, 1.2 mM CaCl2, 3 mM HEPES (pH 7.4), 0.02 mM EDTA, and 10 mM glucose. Flashes (20 ms) were delivered from a calibrated light source via computer-controlled shutters. Light intensity and wavelength were changed by using calibrated neutral-density and interference filters. The current was amplified, low-pass filtered at 30 Hz, digitized at 1 kHz, and stored on a computer for subsequent analysis.
Data analysis
Open eyelid and iris positions on MRI were confirmed to ensure an unimpeded light pathway in each animal. MRI data of central retina (±0.04 to 1 mm from the center of the optic nerve) were analyzed as follows: all 7 images for each mouse per lighting condition were first registered (rigid body) across spin-lock durations. As summarized in Supplemental Fig. 1, high-order polynomials spanning from ora serrata to ora serrata were fit to the provisional retina/vitreous border through a semiautomated process. Then retinal signal intensities were sampled regularly along lines perpendicular to those polynomials and organized as a linearized image (Supplemental Fig. 1B). After linearization, a moving-window approach (1.5 mm width) was used to ensure the retina–vitreous border, determined using the previously published half-height method (38), was well-aligned across TRs. In all cases, the same central retinal region of interest (±0.4 to 1 mm from the center of the optic nerve head) was analyzed by calculating 1/T1ρ maps by first fitting to a 3-parameter decaying exponential equation {y = a + b × [exp(−c) × (spin-lock time)]}, where a, b, and c are fitted parameters, on a pixel-by-pixel basis using R software, v.2.9.0 (R Foundation for Statistical Computing, Vienna, Austria), scripts developed in house, and the minpack.lm package (v.1.1.1, Timur V. Elzhov and Katharine M. Mullen minpack.lm: R interface to the Levenberg-Marquardt nonlinear least-squares algorithm found in Minpack, R package version 1.1-1.). Central retinal 1/T1ρ profiles (and retinal thickness) were determined for each mouse, similar to that detailed elsewhere (19, 20). Linearized data were binned by percentage of retinal extent into 10 equal segments per hemiretina. For each bin, the average signal intensity as a function of retinal depth was then found (Supplemental Fig. 1C).
Within each data bin, the vitreous–retina and retina–choroid borders were found with the half-height method used elsewhere (19, 38, 39) and subtracted to calculate retinal thickness. In this approach, the border between adjacent structures (e.g., vitreous and retina) is found at the signal intensity midway between the local minimum (vitreous) and maximum (retina) (Supplemental Fig. 1D). Linearized data were organized by distance from the vitreous–retina border according to the percentage of the retinal thickness within each bin (Supplemental Fig. 1E). In this configuration, all retinal data are spatially normalized in both dimensions (percentage retinal extent by percentage retinal thickness). In rodents, each retinal layer occupies a similar percentage of the whole-retinal thickness throughout development and across retinal eccentricities (40), making subsequent functional comparisons straightforward. Next, high-order polynomials were fit to the vitreoretinal border of the superior, then to the inferior, hemiretina. Retinal thickness data were then remapped on to the in situ image of the eye (Supplemental Fig. 1G) and used to calculate polynomial best-fit lines for the retina/choroid border of each hemiretina. The above polynomial functions could be integrated about the central axis of the eye to estimate retinal volume and surface area, but these measurements were not part of this study.
For functional comparisons, only data from the central retina—from 10% to 30% of the total retinal extent (Supplemental Fig. 1)—were analyzed. The lower limit (10% extent) was set to avoid the optic nerve head. The upper limit (30% extent) was set to limit the influence of surface coil signal intensity gradients on functional measurements. These artifacts are caused by relative tissue distance from the surface coil, and although they are largely removed through calculation of tissue T1ρ, the upper limit (30% extent) is retained. At stark structural borders, however, signal and calculated T1ρ depends heavily on each tissue's exact contribution to a partial-volume average. The average noise standard deviation was also determined from a background region of interest in each of the 7 images and used to calculate the Gaussian white noise standard deviation used in making the SQEXP modification to minimize noise contribution and optimize the accuracy of the fit to the T1ρ data (41). Finally, Δ1/T1ρ profiles were calculated from the difference of the paired light and dark 1/T1ρ data at each retinal location for each mouse.
For the MION data, after coregistering the average uninjected and injected mouse eyes, a retinal blood volume index map was calculated (ImageJ; http://rsbweb.nih.gov/ij/) pixel by pixel as ΔR2* = −ln(S/So)/TE, where S/So is the signal from MION-treated images relative to the value in controls and TE is the echo time (42). An adaptive threshold method was used to segment the retinal circulation (https://sites.google.com/site/qingzongtseng/adaptivethreshold). These segmented data were then analyzed for either pixel area (retinal blood volume index area) or relative signal intensity (relative to dark values) (42).
For suction-electrode recordings, dark current in individual rods was measured as the amplitude of a saturating light response. Sensitivity was estimated from the intensity–response curve for each cell as the light intensity required to produce a half-saturating response. Time to peak was measured as the time between the midpoint of the flash and the peak of the response. Integration time was calculated as the time integral of the dim-flash response divided by its peak amplitude.
Statistical analysis
The body weights, glycated hemoglobin, and suction-electrode recording data were consistent with a normal distribution and were compared, when possible, using a 1-way ANOVA analysis with post hoc 2-tailed t test analyses. Comparison of Δ1/T1ρ MRI data between groups was performed using individual t tests at different locations of the intraretinal profiles. A generalized estimating equation approach was also used to compare selected location ranges identified from the t tests as significant (43, 44). The generalized estimating equation performs a general linear regression analysis using contiguous locations in each subject and accounts for the within-subject correlation between contiguous locations. In all cases, P ≤ 0.05 was considered statistically significant. Data are presented as mean ± standard error of the mean (sem) unless otherwise noted.
RESULTS
Section A
Δ1/T1ρ MRI measures light- and retinal layer–dependent functional contrast
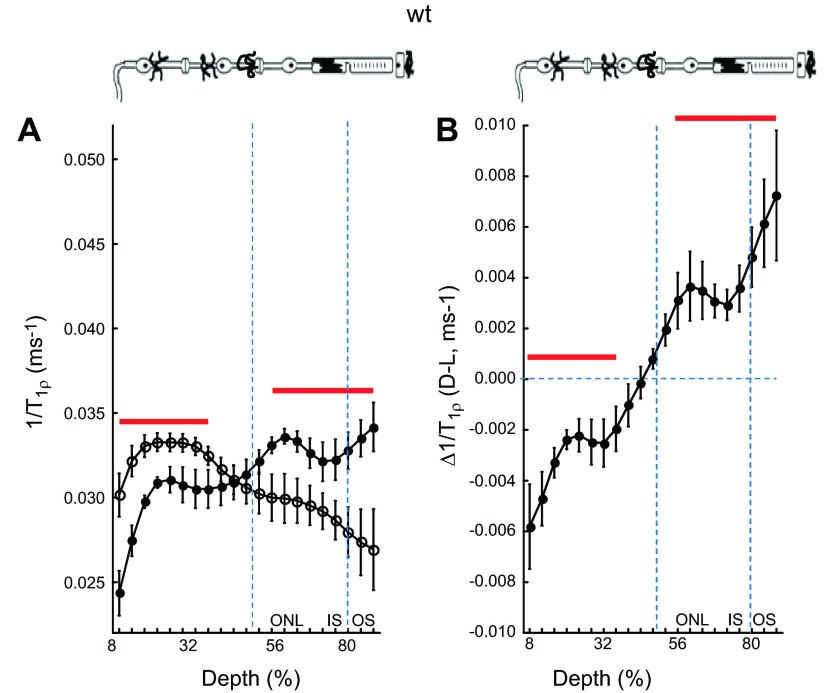
Initially, we characterized the light sensitivity of high-resolution 1/T1ρ retinal maps in untreated WT mice. As shown in Fig. 1A, in this group average presentation, the avascular outer retina (i.e., > 50% depth) 1/T1ρ was significantly (P < 0.05) greater in the dark than in the light. The opposite pattern (P < 0.05) was noted in the inner retina (i.e., < 50% depth into the retina). A dark–light paired analysis of these data demonstrates significant (P < 0.05) differences from zero in similar retinal regions (Fig. 1B).
Figure 1.
1/T1ρ MRI measures light- and retinal layer-dependent functional contrast. A) Layer-specific analysis of mean group central retinal 1/T1ρ profiles during darkness (filled circles, D) and light (open circles, L) in untreated WT mice. Profiles are spatially normalized to retinal thickness (0% = vitreous/retina border, 100% = vitreous /choroid border). Regions less than 8% and greater than 88% are not included as a result of partial volume averaging with signal from outside of the retina. Approximate retinal layers (ONL, outer nuclear layer; IS, inner segments; OS, outer segments) are indicated (vertical dotted blue lines) and are assigned based on the fact that in rodents, each retinal layer occupies a similar percentage of the whole-retinal thickness throughout development (40); for example, 80% depth is considered a reasonable estimate of where the IS/OS border is located in the mouse. Red lines = regions with significant differences (P < 0.05) between profiles. The top graph provides simplified schematic of retina and support circulations (58–60). B) Paired-difference profile between dark and light 1/T1ρ (i.e., Δ1/T1ρ) profiles in WT mice using the data in (A). Red lines indicate regions with significant differences (P < 0.05) from zero (horizontal dotted blue line).
A powerful high-resolution functional imaging method, manganese-enhanced MRI (MEMRI), measures the functional topography of retinal calcium channel activity from awake and freely moving mice but does not monitor tet-ARR1 level and its reduction via translocation (18). To determine the feasibility of combining MEMRI and 1/T1ρ MRI, we examined mice treated with manganese in light and dark conditions and observed that the 1/T1ρ profiles shifted upward (cf. Fig. 1A and Fig. 2A; Supplemental Fig. 2). This shift likely reflects the fact that 1/T1ρ is sensitive to paramagnetic manganese-induced reductions in T1 (14, 16). Figure 3A illustrates a dark–light paired difference plot from manganese-treated mice illustrating significant (P < 0.05) differences from zero in inner and outer retinal regions; these regions were similar to, but not exactly the same as, those regions in Fig. 1 (no manganese) and Fig. 2 (mean group analysis) that are different from zero.
Figure 2.
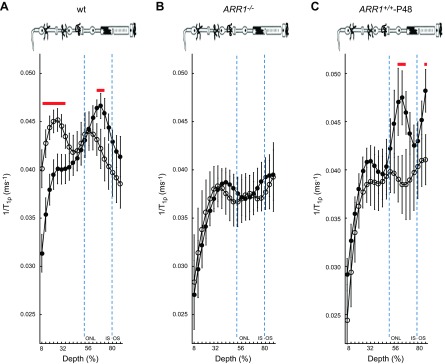
Outer retina 1/T1ρ MRI is sensitive to ARR1 level and its reduction via translocation. Layer-specific analysis of mean group central retinal 1/T1ρ profiles during darkness (filled circles, D) and light (open circles, L) in manganese-treated A) WT mice, B) ARR1-null mice, and C) ARR1-overexpressing mice. For 1/T1ρ at 64% to 72% depth, null mice, but not overexpressing mice, were functionally unresponsive, consistent with our hypothesis that this region reflects tet-ARR1. Data are presented using the conventions in Fig. 1.
Figure 3.
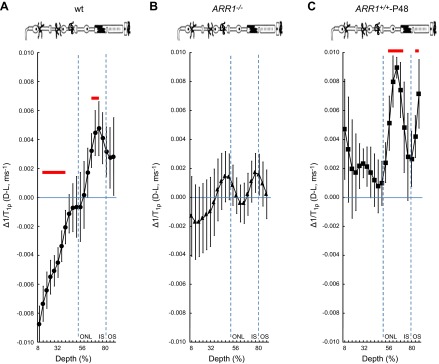
Paired-difference analysis of outer retina Δ1/T1ρ MRI. Layer-specific analysis of paired-difference profiles of central retinal 1/T1ρ in manganese-treated A) WT mice, B) ARR1-null mice, and C) ARR1-overexpressing mice. For Δ1/T1ρ at 64% to 72% depth, null mice but not overexpressing mice were functionally unresponsive, consistent with our hypothesis that this region reflects tet-ARR1. Data are presented using the conventions in Fig. 1.
To address why the overlap in retinal regions between the untreated and treated groups was not more extensive, we note that manganese seemed to increase variability in the 1/T1ρ plots (cf. Figs. 1, 2, and 3). In fact, a quantitative analysis supported the observation that variability increased with manganese treatment [e.g., standard deviation in the untreated data from 64% to 72% depth ranged from 0.0021 (dark) to 0.0028 (light)] relative to that in the treated groups [0.0031 (dark), 0.0052 (light)]. The light-dependent change in 1/T1ρ (Δ1/T1ρ) at 64% to 72% depth was not different (P < 0.05) between untreated (0.0032 ± 0.00065 ms−1, mean ± sem) and manganese-treated mice (0.0041 ± 0.0014 ms−1). The manganese-induced increased variability in the 1/T1ρ plots likely limited detection sensitivity to the following retinal regions with the largest light-evoked differences: 8% to 36% in the inner retina and 64% to 72% in the outer retina. Nonetheless, these data suggest that 1/T1ρ and MEMRI studies can be performed in the same animal (18).
Section B
1/T1ρ MRI in the outer retina (64% to 72% depth into the retina) is sensitive to tet-ARR1 and its reduction via translocation
Because 1/T1ρ is sensitive to the presence of large cross-linked molecules, the fact that the dark-to-light transition decreased 1/T1ρ in the avascular outer nuclear/inner segment region (>50% depth) suggested a reduction in such a macromolecule, such as that known to occur for tet-ARR1. To begin to test this hypothesis, in purified protein solutions, we found that 1/T1ρ of ARR1 monomer (0.0013 ms−1) was smaller than that of tet-ARR1 (0.0023 ms−1); both of these were larger than that in distilled water (0.0007 ms−1). These data support the current hypothesis, but limited sample availability precluded statistical analysis. We next examined the following genetically modified mice treated with manganese: ARR1−/− (the kind gift of Dr. Jeannie Chen, University of Southern California) and ARR1+/+-P48, where ARR1 is expressed at twice the WT level (29) (kind gift of Dr. Jeannie Chen). As summarized in Figs. 2 and 3, ARR1−/− mice had an Δ1/T1ρ at 64% to 72% depth that did not respond to light (P < 0.05), whereas in ARR1+/+-P48 mice, Δ1/T1ρ was significantly (P < 0.05) different from zero at 64% to 72% depth (i.e., within outer nuclear layer and inner segments where the ARR1 tetramers are located). We also noted a 1.8-fold difference in Δ1/T1ρ value at this depth between WT (0.0041 ± 0.0014 ms−1) and ARR1+/+-P48 (0.0075 ± 0.0017 ms−1). Together, these data strongly suggest that light-sensitive Δ1/T1ρ at 64% to 72% depth is sensitive to tet-ARR1 levels and reflects its light-evoked movement.
Δ1/T1ρ MRI measurements predicts intact dark-to-light ARR1 translocation in a model of diabetic retinopathy
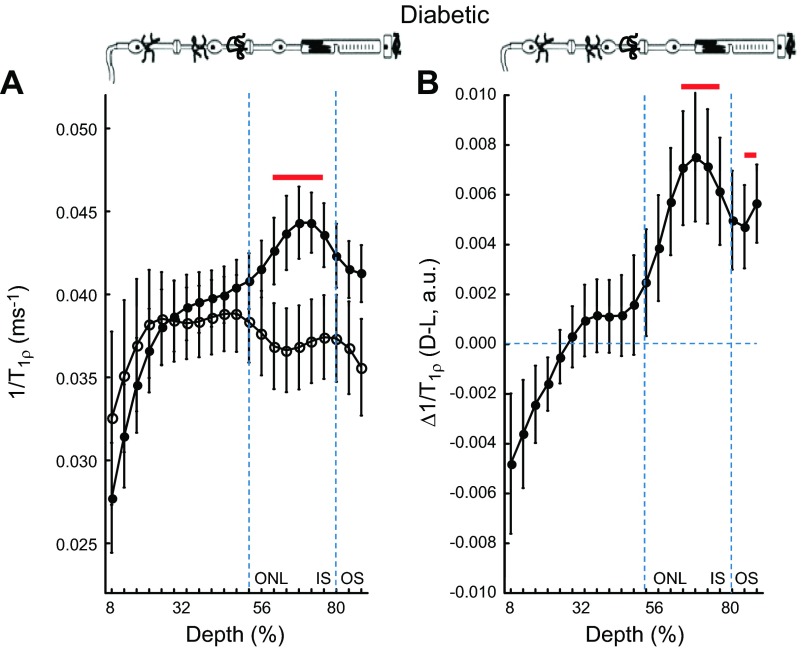
Several previous studies have reported indirect evidence suggesting that the diabetic mouse model suffers from an impaired rod visual cycle (6, 22, 27, 45–48). If true, little to no reduction in tet-ARR1 with light would be expected (6). To test this idea, and to begin to evaluate the potential value of 1/T1ρ measurements in disease, we compared 1/T1ρ MRI of diabetic mice to standard ex vivo assays of ARR1 immunohistochemistry and single-cell recordings. Given the literature evidence suggesting impaired visual cycle, our prediction of impaired ARR1 translocation was, surprisingly, not supported. At the 64% to 72% depth in diabetic mice, we observed significant light-dependent group 1/T1ρ differences and a Δ1/T1ρ different from zero, suggesting normal tet-ARR1 levels with reduction via translocation similar to that of nondiabetic mice (cf. Fig. 2, Fig. 3, and Fig. 4); no correlation (r2 ∼ 0) between A1c (%) and dark or light 1/T1ρ values was noted (data not shown). To verify that the dark-to-light translocated ARR1 is intact in 2 mo old diabetic mice, immunohistochemical analysis was performed (Fig. 5A). The results of this gold standard immunologic assay support the Δ1/T1ρ findings. To directly evaluate the chromophore level in the diabetic mice, we performed single-cell recordings from isolated rods and found normal light sensitivity (Fig. 5B, Table 2). Collectively, these results strongly argue against impairment of the chromophore content in the diabetic mice (6, 22, 27). Furthermore, these results suggest that 1/T1ρ MRI will be useful in mouse models of disease to assay tet-ARR1 level and its reduction via translocation.
Figure 4.
1/T1ρ MRI measurements predict normal light-evoked tet-ARR1 translocation and visual cycle activity in a model of diabetic retinopathy. A) Layer-specific analysis of mean group central retinal 1/T1ρ profiles during darkness (solid circle, D) and light (open circle, L) in manganese-treated diabetic mice. B) Paired-difference profile between dark and light 1/T1ρ (i.e., Δ1/T1ρ) profiles in diabetic mice using the data in (A). Data are presented using the conventions in Fig. 1. In the outer retina, 1/T1ρ data indicates normal responses, suggesting that diabetes has little effect on light-dependent ARR1 translocation—and thus visual cycle activity—at this duration of disease; this prediction was confirmed by the data in Fig. 5. We also note that the Δ1/T1ρ profile in the inner retina of the diabetic mice was unresponsive to light. Our data suggest (section C) that 1/T1ρ profile in the inner retina is an independent measure of functional change in retinal blood volume (i.e., autoregulation); this complements, and is not necessarily related to, ARR1 translocation evaluation. We previously established impaired autoregulation in diabetic mice (27), and the present data provide additional support for this phenotype.
Figure 5.
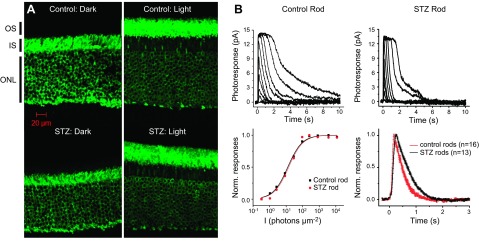
Confirming predications ex vivo made by 1/T1ρ MRI functional topography about tet-ARR1 and its reduction translocation in the diabetic mouse. A) Immunohistochemical analysis performed using anti-S-antigen/ARR1-peptide-specific rabbit polyclonal antibodies of ARR1 translocation in control (top row) and diabetic (bottom row) mouse retinas collected either in the dark (left column) or 20 min after light exposure (right column). These data support the results shown in Fig. 4B. We also investigated visual cycle activity in the diabetic murine retina, which has been suggested to have an impaired visual cycle that may in turn affect ARR1 translocation (6, 22). Representative families of flash responses from control (top left) and STZ diabetic (top right) mouse rods. Flash intensities for both families of responses increased from 0.34 photons µm−2 for the dimmest flash in increments of 0.5 log units. The corresponding intensity-response data (bottom left) were fit by the Naka-Rushton function, R/Rmax = I/(I + I1/2), where R is the response amplitude, Rmax is the saturated rod response amplitude, I is the test flash intensity flash intensity, and I1/2 is the flash intensity required to produce a half-maximal response. Bottom right panel shows the population-averaged normalized dim flash responses, with their rising phases shown in detail in the inset. Error bars are ±sem. These data further support the results in Fig. 4.
TABLE 2.
Rod response parameters from single-cell recordings
| Idark (pA) | I1/2 (phot μm−2) | Tpeak (ms) | Tintegr (ms) | τrec (ms) | |
|---|---|---|---|---|---|
| Control rods (n = 16) | 14.1 ± 0.9 | 18.1 ± 1.6 | 255 ± 12 | 554 ± 20 | 381 ± 16 |
| STZ rods (n = 16) | 13.9 ± 1.0 | 20.8 ± 3.8 | 189 ± 11 | 405 ± 37 | 284 ± 24 |
| t test | NS | NS | P < 0.0005 | P < 0.001 | P < 0.005 |
Experimental parameters: Idark, dark current measured from saturated responses; I1/2, half-saturating light intensity; time to peak (Tpeak) and integration time (Tintegr) refer to responses whose amplitudes were <0.2 Idark and fell within the linear range; τrec, time constant of single-exponential decay of dim flash response recovery phase. STZ, diabetic C57Bl/6J mouse. Values are means ± sem. NS indicates P > 0.05 between control and STZ diabetic rod values.
Section C
Δ1/T1ρ MRI is sensitive to light-evoked changes in inner retinal blood volume
In addition to the changes in the outer retina, 1/T1ρ of the inner retina was also observed to be regulated by light. It has been well established in brain studies that 1/T1ρ in blood is much shorter than in tissue, and this forms the basis of its use in functional brain studies (13–16). Furthermore, it is known that outer retinal oxygen consumption is significantly larger in dark than in light and ∼10% of the oxygen needs of the outer retina are supplied from the inner retinal circulation (42, 49–51). Thus, we speculated that the decrease in net 1/T1ρ in the well-vascularized inner retina that we measure in the dark (vs. light) reflected an increase in inner retinal blood volume (13–16).
To test if the established sensitivity of 1/T1ρ to vascular volume, as documented in brain studies, translates into the retina, an initial study was performed using MRI and the blood-pool contrast agent MION, a method previously used to study the rat retinal circulation (42). As shown in Fig. 6, we observed the predicted light-evoked and time-dependent decrease in inner retinal blood volume. We found significant differences throughout the inner retina [8% to 36% depth (untreated mice) and 8% to 32% depth (manganese-treated mice, which have relatively noisier data than untreated mice)]. These data do not support a differential regulation between the deep plexus and the superficial plexi to our simple light provocation. More work in future studies is needed to examine their responses to different types of challenges.
Figure 6.
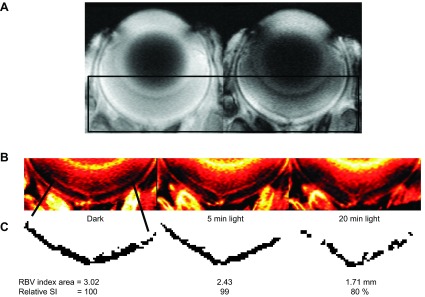
Supporting a prediction that inner retinal 1/T1ρ reflects light-evoked changes in retinal vascular volume. A) Gradient-echo images in a dark adapted uninjected mouse (left) and MION-injected (20 mg Fe/kg) mouse (right). Black box indicates region of interest highlighted in (B). B) ΔR2* maps calculated from the images in (A) representing retinal blood volume index maps with dark adaptation, and at 5 and 20 min after light exposure. C) Segmentation of inner retina circulation and analysis results.
Interestingly, neither the ARR1−/− nor the ARR1+/+-P48 mice demonstrated significant changes in inner retinal 1/T1ρ suggesting a lack of a light-stimulated change in blood volume. Previous studies have shown that sub- and supraphysiologic ARR1 level negatively impacts photoreceptor synaptic function and photoreceptor health, respectively (8, 29). We speculate that these problems may be reflected in photoregulation of the inner retinal circulation, although more work is needed to explore this issue. On the other hand, 2 mo old diabetic mice have impaired autoregulation of inner retinal blood flow (27). Thus, we predicted, and in fact observed, light-insensitive 1/T1ρ data in the inner retina (Fig. 4) (27).
Collectively, these results suggest that inner retinal 1/T1ρ provides a noninvasive measure of light-evoked blood volume change that complements the measure of tet-ARR1 and its reduction via translocation in the outer retina.
DISCUSSION
In this study, we present evidence suggesting that high-resolution functional 1/T1ρ topography of mouse retina is a useful new approach for monitoring retinal tet-ARR1 level and its reduction via translocation in vivo. We suggest that an additional feature of the 1/T1ρ profile is that it contains information about functional changes in inner retinal blood volume (section C). Furthermore, our data imply that 1/T1ρ MRI can be performed in concert with MEMRI studies and in retinal disease models. The present results suggest that tet-ARR1- and blood volume-sensitive 1/T1ρ account for much of the light-evoked changes of 1/T1ρ intraretinal profile; additional work is needed to explore the contribution of other factors. Despite much progress, the mechanism or mechanisms and biologic role of ARR1 translocation are still hotly debated (7). Although ARR1 translocation has been studied in detail in several model organisms using mostly sectioned eyes, immunohistochemistry and immunoblot analysis, both its extent and kinetics in humans remains unexplored as a result of a lack of suitable noninvasive methods. We expect that the new technology described in this study will motivate future studies that can address this major gap in our knowledge.
Brain studies have suggested that increased neuronal activity lowers tissue pH and that this alone is sufficient to lower 1/T1ρ; however, most investigators have concluded that blood volume dominates the activation-induced changes in 1/T1ρ (13–16). Our results shed some light on this issue. In the cat retina, light/dark state changes can produce fluctuations in pH of up to 0.2 pH units in the outer nuclear layer (52). If applied to the mouse retina, such pH change would correspond to a Δ1/T1ρ of ∼0.0004 ms−1, based on the calibration curve of previously published data (16), which is an order of magnitude smaller than the changes measured in the outer nuclear layer (Fig. 1) (52). Given the order-of-magnitude discrepancy between our data and that prediction, it is unlikely that changes in pH could explain the present data. Moreover, the avascular outer retinal change in 1/T1ρ cannot be due to blood flow.
We also considered if other prominent molecules in rod photoreceptors, such as α-transducin-1 (Tα), might contribute to the light dependence of the 1/T1ρ profile. Tα, an essential retinal G-protein subunit involved in activation of rhodopsin in rod phototransduction, also translocates with light exposure but in the opposite direction to, and independently of, ARR1 (4, 6, 35). Another difference from ARR1 is that to our knowledge, Tα does not self-assemble into a larger macromolecule, and thus, on first principles, would not be expected to substantially contribute to the 1/T1ρ profile. In an initial study of Tα knockout mice (GNAT1−/−) (n = 4, kind gift of Dr. J. Lem, Tufts University, Medford, MA, USA) (30, 53, 54), we observed significant (P < 0.05) changes from 0 at 64% to 72% of Δ1/T1ρ (0.0048 ± 0.0029 ms−1), consistent with the expected light-evoked reduction in tet-ARR1 level; this Δ1/T1ρ was similar to that in controls (0.0041 ± 0.0014 ms−1). Δ1/T1ρ in the inner retina was different from zero consistent with functional changes in blood volume in these mice (data not shown). Thus, we did not find evidence that Δ1/T1ρ profiles were sensitive to Tα, although more work is needed to fully evaluate its contribution.
In general, there are few methods for noninvasively evaluating murine retinal function in a colocalized manner in vivo. This has greatly limited physiologic and drug-discovery efforts that could take advantage of the vast number of transgenic mouse models available for hypothesis testing. One established technique involves retinal electrophysiology, which measures functional changes in photoreceptor cells but not morphology. Retinal electrophysiology represents an integrated response from whole retina and thus is not sensitive to focal lesions. In principle, multifocal electroretinographic methods address this latter concern; however, the small size of the rodent retina produces stray light artifacts (55, 56). This confounds interpretation because localization of the functional signal is not unambiguous as a result of inappropriate stimulation of areas outside those covered by the hexagonal regions of interest (55, 56). Another method is optical coherence tomography, which provides exquisite high-resolution imaging of retinal anatomy over a limited field of view. However, optical coherence tomography information was found to be hard to interpret in the retina of the patient with Oguchi disease with genetic defects in ARR1 (57). Here, we present data supporting a novel high-resolution imaging approach for evaluating a critically important aspect of central rod function in vivo: tet-ARR1 level and its reduction via translocation that can be measured simultaneously with other aspects of retinal function (i.e., Δ1/T1ρ MRI blood volume and MEMRI-based assessment of calcium channels). A measure of retinal thickness is also provided as an inherent outcome metric of high-spatial-resolution MRI (18). In conclusion, retinal Δ1/T1ρ is an innovative functional imaging technique with exciting potential uses in a diverse range of studies in normal and retinal disease states and with possible diagnostic application in future clinical studies.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the contributions of Y. Ye for writing the T1ρ acquisition code, S.A. Vishnivetskiy for his careful preparation of purified arrestin monomer and tetramer solutions, K.V. for expert tail vein catheterization under dim red light conditions, and B.M. Brown for his technical expertise. This work was supported by the U.S. National Institutes of Health (NIH) National Eye Institute (Grants EY015851 to C.M.C., EY011500 to V.V.G., EY021126 and EY019312 to V.J.K. and EY002687 to the Department of Ophthalmology and Visual Sciences at Washington University), NIH Animal Models of Diabetic Complications Consortium and Mouse Metabolic Phenotyping Centers Pilot and Feasibility Programs (B.A.B.), an unrestricted grant from Research to Prevent Blindness (Kresge Eye Institute), the Mary D. Allen Foundation (C.M.C.), and Research to Prevent Blindness (University of Southern Californiaand Washington University). C.M.C. is the Mary D. Allen Chair in Vision Research, Doheny Eye Institute.
Glossary
- 1/T1ρ
spin-lattice relaxation rate in the rotating frame
- ARR1
arrestin-1
- ARR4
arrestin-4
- GRK1
G-protein receptor kinase 1
- MEMRI
manganese-enhanced MRI
- MION
monocrystalline iron oxide nanocolloid
- tet-ARR1
tetrameric arrestin-1
- Tα
α-transducin-1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Chen C. K., Burns M. E., Spencer M., Niemi G. A., Chen J., Hurley J. B., Baylor D. A., Simon M. I. (1999) Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc. Natl. Acad. Sci. USA 96, 3718–3722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu J., Dodd R. L., Makino C. L., Simon M. I., Baylor D. A., Chen J. (1997) Prolonged photoresponses in transgenic mouse rods lacking arrestin. Nature 389, 505–509 [DOI] [PubMed] [Google Scholar]
- 3.Nikonov S. S., Brown B. M., Davis J. A., Zuniga F. I., Bragin A., Pugh E. N. Jr., Craft C. M. (2008) Mouse cones require an arrestin for normal inactivation of phototransduction. Neuron 59, 462–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Philp NJ., Chang W., Long K. (1987) Light-stimulated protein movement in rod photoreceptor cells of the rat retina. FEBS Lett. 225:127–132. [DOI] [PubMed] [Google Scholar]
- 5.Hanson S. M., Vishnivetskiy S. A., Hubbell W. L., Gurevich V. V. (2008) Opposing effects of inositol hexakisphosphate on rod arrestin and arrestin2 self-association. Biochemistry 47, 1070–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendez A., Lem J., Simon M., Chen J. (2003) Light-dependent translocation of arrestin in the absence of rhodopsin phosphorylation and transducin signaling. J. Neurosci. 23, 3124–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurevich V. V., Hanson S. M., Song X., Vishnivetskiy S. A., Gurevich E. V. (2011) The functional cycle of visual arrestins in photoreceptor cells. Prog. Retin. Eye Res. 30, 405–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang S. P., Brown B. M., Craft C. M. (2010) Visual arrestin 1 acts as a modulator for N-ethylmaleimide-sensitive factor in the photoreceptor synapse. J. Neurosci. 30, 9381–9391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith W. C. (2013) The role of arrestins in visual and disease processes of the eye. In: Progress in Molecular Biology and Translational Science, Volume 118, The Molecular Biology of Arrestins (Louis, M. L., ed.), pp. 243–265, Academic Press, New York. [DOI] [PubMed] [Google Scholar]
- 10.Regatte R. R., Akella S. V. S., Borthakur A., Reddy R. (2003) Proton spin-lock ratio imaging for quantitation of glycosaminoglycans in articular cartilage. J. Magn. Reson. Imaging 17, 114–121 [DOI] [PubMed] [Google Scholar]
- 11.Akella S. V. S., Regatte R. R., Gougoutas A. J., Borthakur A., Shapiro E. M., Kneeland J. B., Leigh J. S., Reddy R. (2001) Proteoglycan-induced changes in T1ρ-relaxation of articular cartilage at 4T. Magn. Reson. Med. 46, 419–423 [DOI] [PubMed] [Google Scholar]
- 12.Li X., Benjamin Ma C., Link T. M., Castillo D. D., Blumenkrantz G., Lozano J., Carballido-Gamio J., Ries M., Majumdar S. (2007) In vivo T(1ρ) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage 15, 789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hulvershorn J., Borthakur A., Bloy L., Gualtieri E. E., Reddy R., Leigh J. S., Elliott M. A. (2005) T1ρ contrast in functional magnetic resonance imaging. Magn. Reson. Med. 54, 1155–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin T., Kim S. G. (2013) Characterization of non-hemodynamic functional signal measured by spin-lock fMRI. Neuroimage 78, 385–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kettunen M. I., Gröhn O. H. J., Silvennoinen M. J., Penttonen M., Kauppinen R. A. (2002) Effects of intracellular pH, blood, and tissue oxygen tension on T1ρ relaxation in rat brain. Magn. Reson. Med. 48, 470–477 [DOI] [PubMed] [Google Scholar]
- 16.Magnotta V. A., Heo H. Y., Dlouhy B. J., Dahdaleh N. S., Follmer R. L., Thedens D. R., Welsh M. J., Wemmie J. A. (2012) Detecting activity-evoked pH changes in human brain. Proc. Natl. Acad. Sci. USA 109, 8270–8273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos de Carvalho J. E., Verbraak F. D., Aalders M. C., van Noorden C. J., Schlingemann R. O. (2014) Recent advances in ophthalmic molecular imaging. Surv. Ophthalmol. 59, 393–413 [DOI] [PubMed] [Google Scholar]
- 18.Berkowitz B. A., Bissig D., Dutczak O., Corbett S., North R., Roberts R. (2013) MRI biomarkers for evaluation of treatment efficacy in preclinical diabetic retinopathy. Expert Opin. Med. Diagn. 7, 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkowitz B. A., Roberts R., Bissig D. (2010) Light-dependant intraretinal ion regulation by melanopsin in young awake and free moving mice evaluated with manganese-enhanced MRI. Mol. Vis. 16, 1776–1780 [PMC free article] [PubMed] [Google Scholar]
- 20.Bissig D., Berkowitz B. A. (2011) Same-session functional assessment of rat retina and brain with manganese-enhanced MRI. Neuroimage 58, 749–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkowitz B. A., Bissig D., Ye Y., Valsadia P., Kern T. S., Roberts R. (2012) Evidence for diffuse central retinal edema in vivo in diabetic male Sprague Dawley rats. PLoS ONE 7, e29619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berkowitz B. A., Bissig D., Patel P., Bhatia A., Roberts R. (2012) Acute systemic 11-cis-retinal intervention improves abnormal outer retinal ion channel closure in diabetic mice. Mol. Vis. 18, 372–376 [PMC free article] [PubMed] [Google Scholar]
- 23.Bissig D., Berkowitz B. A. (2012) Light-dependent changes in outer retinal water diffusion in rats in vivo. Mol. Vis. 18, 2561–xxx [PMC free article] [PubMed] [Google Scholar]
- 24.Bissig D., Goebel D., Berkowitz B. A. (2013) Diminished vision in healthy aging is associated with increased retinal L-type voltage gated calcium channel ion influx. PLoS ONE 8, e56340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duong T. Q. (2014) Magnetic resonance imaging of the retina: from mice to men. Magn. Reson. Med. 71, 1526–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kettunen M. I., Mäkelä H. I., Penttonen M., Pitkänen A., Lukkarinen J. A., Kauppinen R. A., Kauppinen R. A., Gröhn O. H. J. (2000) Early detection of irreversible cerebral ischemia in the rat using dispersion of the magnetic resonance imaging relaxation time, T1ρ. J. Cereb. Blood Flow Metab. 20, 1457–1466 [DOI] [PubMed] [Google Scholar]
- 27.Kern T. S., Tang J., Berkowitz B. A. (2010) Validation of structural and functional lesions of diabetic retinopathy in mice. Mol. Vis. 16, 2121–2131 [PMC free article] [PubMed] [Google Scholar]
- 28.Berkowitz B. A., Roberts R. (2008) Prognostic MRI biomarkers of treatment efficacy for retinopathy. NMR Biomed. 21, 957–967 [DOI] [PubMed] [Google Scholar]
- 29.Song X., Vishnivetskiy S. A., Seo J., Chen J., Gurevich E. V., Gurevich V. V. (2011) Arrestin-1 expression level in rods: balancing functional performance and photoreceptor health. Neuroscience 174, 37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J., Simon M. I., Matthes M. T., Yasumura D., LaVail M. M. (1999) Increased susceptibility to light damage in an arrestin knockout mouse model of Oguchi disease (stationary night blindness). Invest. Ophthalmol. Vis. Sci. 40, 2978–2982 [PubMed] [Google Scholar]
- 31.Charagundla S. R., Borthakur A., Leigh J. S., Reddy R. (2003) Artifacts in T(1ρ)-weighted imaging: correction with a self-compensating spin-locking pulse. J. Magn. Reson. 162, 113–121 [DOI] [PubMed] [Google Scholar]
- 32.Berkowitz B. A., Kowluru R. A., Frank R. N., Kern T. S., Hohman T. C., Prakash M. (1999) Subnormal retinal oxygenation response precedes diabetic-like retinopathy. Invest. Ophthalmol. Vis. Sci. 40, 2100–2105 [PubMed] [Google Scholar]
- 33.Berkowitz B. A., Penn J. S. (1998) Abnormal panretinal response pattern to carbogen inhalation in experimental retinopathy of prematurity. Invest. Ophthalmol. Vis. Sci. 39, 840–845 [PubMed] [Google Scholar]
- 34.Kim M., Hanson S. M., Vishnivetskiy S. A., Song X., Cleghorn W. M., Hubbell W. L., Gurevich V. V. (2011) Robust self-association is a common feature of mammalian visual arrestin-1. Biochemistry 50, 2235–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nair K. S., Hanson S. M., Mendez A., Gurevich E. V., Kennedy M. J., Shestopalov V. I., Vishnivetskiy S. A., Chen J., Hurley J. B., Gurevich V. V., Slepak V. Z. (2005) Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein–protein interactions. Neuron 46, 555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown B. M., Ramirez T., Rife L., Craft C. M. (2010) Visual arrestin 1 contributes to cone photoreceptor survival and light adaptation. Invest. Ophthalmol. Vis. Sci. 51, 2372–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knospe V., Donoso L. A., Banga J. P., Yue S., Kasp E., Gregerson D. S. (1988) Epitope mapping of bovine retinal S-antigen with monoclonal antibodies. Curr. Eye Res. 7, 1137–1147 [DOI] [PubMed] [Google Scholar]
- 38.Cheng H., Nair G., Walker T. A., Kim M. K., Pardue M. T., Thulé P. M., Olson D. E., Duong T. Q. (2006) Structural and functional MRI reveals multiple retinal layers. Proc. Natl. Acad. Sci. USA 103, 17525–17530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berkowitz B. A., Gradianu M., Bissig D., Kern T. S., Roberts R. (2009) Retinal ion regulation in a mouse model of diabetic retinopathy: natural history and the effect of Cu/Zn superoxide dismutase overexpression. Invest. Ophthalmol. Vis. Sci. 50, 2351–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braekevelt C. R., Hollenberg M. J. (1970) The development of the retina of the albino rat. Am. J. Anat. 127, 281–301 [DOI] [PubMed] [Google Scholar]
- 41.Raya J. G., Dietrich O., Horng A., Weber J., Reiser M. F., Glaser C. (2010) T2 measurement in articular cartilage: impact of the fitting method on accuracy and precision at low SNR. Magn. Reson. Med. 63, 181–193 [DOI] [PubMed] [Google Scholar]
- 42.Shih Y. Y., De la Garza B. H., Muir E. R., Rogers W. E., Harrison J. M., Kiel J. W., Duong T. Q. (2011) Lamina-specific functional MRI of retinal and choroidal responses to visual stimuli. Invest. Ophthalmol. Vis. Sci. 52, 5303–5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berkowitz B. A., Roberts R., Goebel D. J., Luan H. (2006) Noninvasive and simultaneous imaging of layer-specific retinal functional adaptation by manganese-enhanced MRI. Invest. Ophthalmol. Vis. Sci. 47, 2668–2674 [DOI] [PubMed] [Google Scholar]
- 44.Liang Z. (1986) Longitudinal data analysis using generalized linear models. Biometrika 73, 13–22 [Google Scholar]
- 45.Ostroy S. E. (1998) Altered rhodopsin regeneration in diabetic mice caused by acid conditions within the rod photoreceptors. Curr. Eye Res. 17, 979–985 [DOI] [PubMed] [Google Scholar]
- 46.Ostroy S. E., Frede S. M., Wagner E. F., Gaitatzes C. G., Janle E. M. (1994) Decreased rhodopsin regeneration in diabetic mouse eyes. Invest. Ophthalmol. Vis. Sci. 35, 3905–3909 [PubMed] [Google Scholar]
- 47.Garcia-Ramírez M., Hernández C., Villarroel M., Canals F., Alonso M. A., Fortuny R., Masmiquel L., Navarro A., García-Arumí J., Simó R. (2009) Interphotoreceptor retinoid-binding protein (IRBP) is downregulated at early stages of diabetic retinopathy. Diabetologia 52, 2633–2641 [DOI] [PubMed] [Google Scholar]
- 48.Kirwin S. J., Kanaly S. T., Hansen C. R., Cairns B. J., Ren M., Edelman J. L. (2011) Retinal gene expression and visually evoked behavior in diabetic long evans rats. Invest. Ophthalmol. Vis. Sci. 52, 7654–7663 [DOI] [PubMed] [Google Scholar]
- 49.Shih Y. Y., Wang L., De La Garza B. H., Li G., Cull G., Kiel J. W., Duong T. Q. (2013) Quantitative retinal and choroidal blood flow during light, dark adaptation and flicker light stimulation in rats using fluorescent microspheres. Curr. Eye Res. 38, 292–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ames A. III, Li Y. Y., Heher E. C., Kimble C. R. (1992) Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. J. Neurosci. 12, 840–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardarson S. H., Basit S., Jonsdottir T. E., Eysteinsson T., Halldorsson G. H., Karlsson R. A., Beach J. M., Benediktsson J. A., Stefansson E. (2009) Oxygen saturation in human retinal vessels is higher in dark than in light. Invest. Ophthalmol. Vis. Sci. 50, 2308–2311 [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto F., Borgula G. A., Steinberg R. H. (1992) Effects of light and darkness on pH outside rod photoreceptors in the cat retina. Exp. Eye Res. 54, 685–697 [DOI] [PubMed] [Google Scholar]
- 53.Burns M. E., Mendez A., Chen C. K., Almuete A., Quillinan N., Simon M. I., Baylor D. A., Chen J. (2006) Deactivation of phosphorylated and nonphosphorylated rhodopsin by arrestin splice variants. J. Neurosci. 26, 1036–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calvert P. D., Krasnoperova N. V., Lyubarsky A. L., Isayama T., Nicoló M., Kosaras B., Wong G., Gannon K. S., Margolskee R. F., Sidman R. L, Pugh E. N. Jr., Makino C. L., Lem J. (2000) Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha-subunit. Proc. Natl. Acad. Sci. USA 97, 13913–13918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ball S. L., Petry H. M. (2000) Noninvasive assessment of retinal function in rats using multifocal electroretinography. Invest. Ophthalmol. Vis. Sci. 41, 610–617 [PubMed] [Google Scholar]
- 56.Nusinowitz S., Ridder W. H. III, Heckenlively J. R. (1999) Rod multifocal electroretinograms in mice. Invest. Ophthalmol. Vis. Sci. 40, 2848–2858 [PubMed] [Google Scholar]
- 57.Godara P., Cooper R. F., Sergouniotis P. I., Diederichs M. A., Streb M. R., Genead M. A., McAnany J. J., Webster A. R., Moore A. T., Dubis A. M., Neitz M., Dubra A., Stone E. M., Fishman G. A., Han D. P., Michaelides M., Carroll J. (2012) Assessing retinal structure in complete congenital stationary night blindness and Oguchi disease. Am. J. Ophthalmol. 154, 987–1001, e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wangsa-Wirawan N. D., Linsenmeier R. A. (2003) Retinal oxygen: fundamental and clinical aspects. Arch. Ophthalmol. 121, 547–557 [DOI] [PubMed] [Google Scholar]
- 59.Zhi Z., Chao J. R., Wietecha T., Hudkins K. L., Alpers C. E., Wang R. K. (2014) Noninvasive imaging of retinal morphology and microvasculature in obese mice using optical coherence tomography and optical microangiography. Invest. Ophthalmol. Vis. Sci. 55, 1024–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paques M., Tadayoni R., Sercombe R., Laurent P., Genevois O., Gaudric A., Vicaut E. (2003) Structural and hemodynamic analysis of the mouse retinal microcirculation. Invest. Ophthalmol. Vis. Sci. 44, 4960–4967 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.