Abstract
Nutritional imbalance is emerging as a causative factor of hearing loss. Epidemiologic studies have linked hearing loss to elevated plasma total homocysteine (tHcy) and folate deficiency, and have shown that folate supplementation lowers tHcy levels potentially ameliorating age-related hearing loss. The purpose of this study was to address the impact of folate deficiency on hearing loss and to examine the underlying mechanisms. For this purpose, 2-mo-old C57BL/6J mice (Animalia Chordata Mus musculus) were randomly divided into 2 groups (n = 65 each) that were fed folate-deficient (FD) or standard diets for 8 wk. HPLC analysis demonstrated a 7-fold decline in serum folate and a 3-fold increase in tHcy levels. FD mice exhibited severe hearing loss measured by auditory brainstem recordings and TUNEL-positive-apoptotic cochlear cells. RT-quantitative PCR and Western blotting showed reduced levels of enzymes catalyzing homocysteine (Hcy) production and recycling, together with a 30% increase in protein homocysteinylation. Redox stress was demonstrated by decreased expression of catalase, glutathione peroxidase 4, and glutathione synthetase genes, increased levels of manganese superoxide dismutase, and NADPH oxidase-complex adaptor cytochrome b-245, α-polypeptide (p22phox) proteins, and elevated concentrations of glutathione species. Altogether, our findings demonstrate, for the first time, that the relationship between hyperhomocysteinemia induced by folate deficiency and premature hearing loss involves impairment of cochlear Hcy metabolism and associated oxidative stress.—Martínez-Vega, R., Garrido, F., Partearroyo, T., Cediel, R., Zeisel, S. H., Martínez-Álvarez, C., Varela-Moreiras, G., Varela-Nieto, I., and Pajares, M. A. Folic acid deficiency induces premature hearing loss through mechanisms involving cochlear oxidative stress and impairment of homocysteine metabolism.
Keywords: apoptosis, dietary restriction, hair cell loss, hyperhomocysteinemia, methionine cycle
According to recent World Health Organization data, moderate-to-profound hearing loss affects over 360 million people worldwide. Its incidence varies in each population segment, affecting approximately 10% of children, rising to 30% of adults over 65 (age-related hearing loss; ARHL) and increasing further with age (1, 2). It is caused by a combination of genetic and environmental factors and has a tremendous impact on quality of life of the elderly (2, 3). In contrast with congenital deafness, little is known about the genetic factors that contribute to ARHL; most of the available information is derived from the study of mouse models (4, 5). Environmental factors, such as noise and ototoxic drugs, are among the well-known stressors that induce early hearing loss (6, 7), but a few reports have recently suggested a role for nutritional status in premature hearing impairment. Indeed, decreased levels of essential nutrients, such as several vitamins, have been shown to correlate with hearing loss (8–14). Among micronutrients, reduced folic acid concentrations have been found in ARHL and sudden sensorineural hearing loss. This decrease correlates with either reduced vitamin B12 status (11, 12) or increased homocysteine (Hcy) levels (13). Accumulating evidence from epidemiologic research has shown the association between atherosclerosis in the inner ear and poor hearing, as well as the relationship between risk factors of vascular disease and ARHL (15–17). The intimate relationship between Hcy, an independent risk factor for cardiovascular disease, and folic acid metabolism provides the basis by which supplementation with the vitamin lowers plasma Hcy levels (18), an intervention that has been reported to ameliorate ARHL in a randomized trial (19).
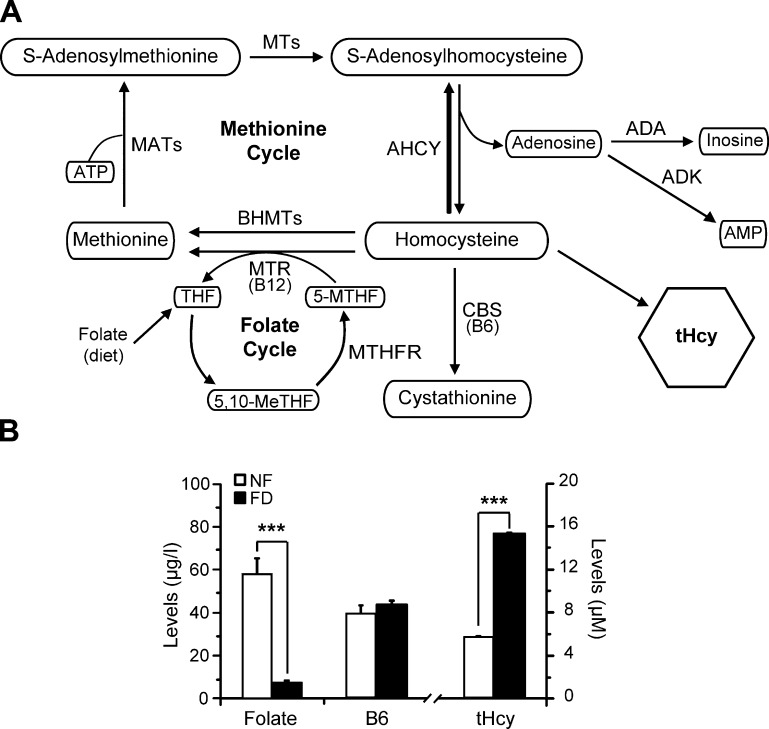
Hcy constitutes a metabolic branch point linking the methionine and folate cycles and the transsulfuration pathway (Fig. 1A). Hcy remethylation is catalyzed by either cobalamin-dependent methionine synthase (MTR) or betaine homocysteine methyltransferase (BHMT), enzymes that use 5′-methyltetrahydrofolate and betaine as methyl donors, respectively (20). Both reactions generate methionine that is, in turn, used to synthesize S-adenosylmethionine, the main methyl donor for cellular transmethylations. Donation of the methyl group renders S-adenosylhomocysteine, which is hydrolyzed by S-adenosylhomocysteine hydrolase (AHCY) to Hcy and adenosine in a reversible reaction. Elimination of Hcy also takes place by conjugation with serine in a B6-dependent reaction catalyzed by cystathionine β-synthase (CBS) or by export into the plasma. The correct function of the pathway depends on a continuous supply of 5′-methyltetrahydrofolate that is recycled in the folate cycle, where methylenetetrahydrofolate reductase (MTHFR) exerts a key role. Epidemiologic studies have analyzed the alleged relationship of the MTHFR C677T mutation with hearing loss, yielding contradictory results (21–23). Animal models have generated data that, collectively taken, support a central role for Hcy metabolism in hearing pathophysiology. For example, Cbs+/− mutant mice have increased hearing thresholds, high concentrations of Hcy in the stria vascularis and the spiral ligament, oxidative stress, and reduction of vessel density, effects that were prevented by the administration of folic acid in the drinking water (24). Hearing impairment has been also described in Connexin 30 (Cx30−/−) null mice, where the stria vascularis exhibited down- and up-regulation of Bhmt and Ahcy expression, respectively, and elevated levels of Hcy immunostaining (25).
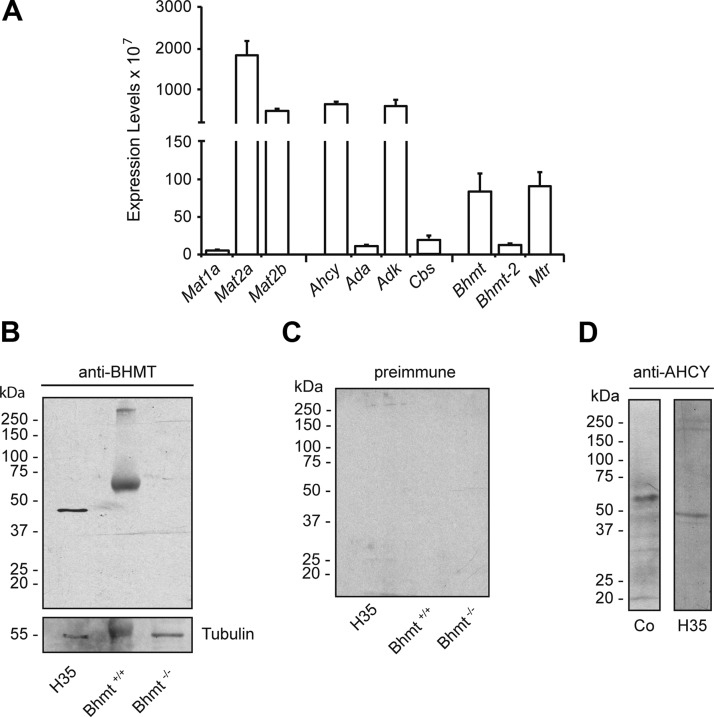
Figure 1.
Pathways involved in Hcy metabolism and systemic metabolite levels. A) Scheme of Hcy metabolism and related pathways. Metabolites appear in white boxes, and the enzymes listed include the following: ADA, ADK, AdoHcy hydrolase (AHCY), BHMTs, AdoMet-dependent methyltransferases (MTs), MATs, vitamin B6-dependent CBS, MTHFR, and vitamin B12-dependent MTR. B) Serum and plasma metabolite levels from all animals in both normal (NF) and FD groups are shown. Serum folic acid [50.85 ± 22.03 (n = 10) vs. 7.46 ± 3.88 μg/L (n = 11)], tHcy [5.05 ± 2.45 (n = 33) vs. 14.70 ± 3.19 μM (n = 69)], and plasma vitamin B6 levels [40.00 ± 15.71 (n = 11) vs. 43.71 ± 8.74 μg/L (n = 18)] were measured as specified under Materials and Methods. The figure shows the mean ± SEM for each group, and differences were considered significant when P ≤ 0.05; ***P < 0.0001.
Altogether, human epidemiology and mouse genetic data reinforce the hypothesis that folic acid deficiency and Hcy metabolism play an important role in hearing disorders, although the mechanisms by which cochlear function is affected remain poorly understood. In this study, we used a rodent model for folic acid deficiency (26, 27) to show that reduced intake of this essential vitamin causes cochlear impairment of Hcy metabolism, oxidative stress, severe cellular damage, and apoptotic cell death, leading to accelerated hearing loss.
MATERIALS AND METHODS
Mouse handling and experimental design
Two-month-old C57BL/6J female mice were purchased from Harlan Interfauna Ibérica S.A. (Barcelona, Spain) and housed under standard conditions. The C57BL/6J mouse strain is a well-characterized model of progressive hearing loss due to the Ahl alleles present in its genome. These mice demonstrate ARHL from the age of 6 mo onward (28, 29). Mice were randomly divided into 2 experimental groups (n = 65 each) that were fed different types of diets ad libitum for 8 wk. The normal folate (NF) group received a maintenance diet containing standard folate levels (A04/A04C/R04; Scientific Animal Food & Engineering, Panlab, Cornellá de Llobregat, Spain), whereas the folate-deficient (FD) group received a folate-depleted diet (folic acid ≤0.1–0.2 mg/kg) as previously described (26, 27). At least 6 different mouse cohorts including both experimental groups were studied independently. All experiments were approved by the CSIC Bioethics Committee and carried out in full accordance with European Community guidelines (2010/63/EU) and Spanish regulations (RD 53/2013) for the use of laboratory animals. Additionally, certain experiments required the use of Bhmt−/− null mice previously described (30), as specified below. Bhmt−/− mice were continuously backcrossed to C57BL/6J mice and fed the NF diet.
Hearing assessment
Hearing was evaluated by auditory brainstem response (ABR) analysis to broadband click and 8, 16, 20, 28, and 40 kHz pure tone frequencies recorded at an intensity range from 90 to 20 dB sound pressure level (SPL) in 5–10 dB steps, as previously reported (31–33). Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg). The electrical responses were amplified and averaged to determine hearing thresholds for each stimulus. Peak and interpeak latencies were analyzed at 15–20 dB SPL above the hearing threshold after click stimulation. The FD group showed either moderate or profound hearing loss in 2 well-defined populations. Therefore, further determinations in the FD group were restricted to those animals exhibiting profound hearing loss.
Blood and tissue extraction
Mice were sacrificed by CO2 asphyxiation for fresh tissue removal, and the isolated tissues were immediately frozen in liquid nitrogen for protein or RNA extraction. Blood samples were collected by cardiac puncture and placed directly in either regular or heparin (Laboratorios Farmacéuticos Rovi, Madrid, Spain)-coated tubes, centrifuged at 2500 × g for 10 min, and the respective serum and plasma fractions were isolated and stored at −80°C until use.
For histologic analysis, mice were injected with a pentobarbital overdose and perfused with cold PBS and 4% (v/v) paraformaldehyde (PFA; Merck, Darmstadt, Germany). Cochleae were then dissected out from the temporal bone as described previously (34, 35). Isolated tissues were immediately frozen in liquid nitrogen or fixed for immunohistochemistry studies. Fixation was carried out overnight in 4% (v/v) PFA at 4°C followed by decalcification with EDTA (Sigma-Aldrich, Tres Cantos, Madrid, Spain) for 10 d (31, 32). Decalcified samples were dehydrated and embedded in paraffin wax (Panreac Química, Castellar del Vallés, Barcelona, Spain) or cryoprotected in sucrose, embedded in a sucrose/gelatin solution, and frozen in isopentane at −80°C as previously described (36).
Cochlear morphology and immunohistochemistry
Cochlear cytoarchitecture was evaluated using representative paraffin sections (7 μm thick) stained with hematoxylin and eosin (H&E; Sigma-Aldrich) and Masson’s Trichrome (Sigma-Aldrich). Phalloidin histochemistry was performed on frozen sections using Alexa 488–conjugated phalloidin (1:100 v/v; Molecular Probes, Eugene, OR, USA). For paraffin immunohistochemistry, slides were dewaxed, rehydrated, and the endogenous peroxidase was inactivated for 20 min with 3% (v/v) hydrogen peroxide (Merck). For immunohistofluorescence, cryostat frozen sections (15 µm) were prepared using Cryocut 1900 (Leica Microsystems, Deerfield, IL, USA). All slides were permeabilized in 0.05% (v/v) Triton X-100 (Calbiochem, La Jolla, CA, USA) in PBS (T-PBS), and nonspecific tissue-binding sites were blocked for 40 min using 5% (v/v) normal rabbit or donkey serum (Sigma-Aldrich) in PBS. Samples were incubated with the primary antibodies overnight at 4°C, washed with T-PBS, and incubated with the corresponding secondary antibody for 2 h at room temperature (Table 1). Slides were then washed with T-PBS and covered with a tertiary ExtrAvidin peroxidase-conjugated antibody for 90 min (1:200 v/v; Sigma-Aldrich). Immunodetection was carried out with 3,3′-diaminobenzidine (DAB; Sigma-Aldrich). When indicated, sections were dehydrated and mounted with Entellan (Merck) or Prolong Gold with DAPI (Invitrogen, Carlsbad, CA, USA). All bright-field images were obtained using an Olympus DP70 digital camera (Melville, NY, USA) mounted on a Zeiss microscope (Jena, Germany). Immunofluorescence images were produced with a confocal microscope (Leica TCS SP2; Leica, Wetzlar, Germany).
TABLE 1.
Antibodies used in this study
| Primary antibody | Source | Dilution (v/v) | Secondary antibody | Source | Dilution (v/v) | Technique |
|---|---|---|---|---|---|---|
| Chicken anti-myelin P0 | Novus Biologicals (NB100-1607) | 1:100 | IgG Rabbit anti-chicken biotin conjugated | Acris Antibodies (R1300B) | 1:300 | IC-Pa |
| Rabbit anti–3-NT | Millipore (AB5411) | 1:200 | IgG goat anti-rabbit biotin conjugated | Chemicon-Millipore (AQ132B) | 1:300 | IC-Pa |
| Rabbit anti-Kir4.1 | Chemicon (AB5818) | 1:200 | IgG donkey anti-rabbit Alexa Fluor 488 | Molecular Probes (A21206) | 1:400 | IHF |
| Mouse anti–β-actin | Sigma-Aldrich (A5441) | 1:5000 | IgG sheep anti-mouse HRP conjugated | GE Healthcare (NA931) | 1:5000 | WB |
| Mouse anti–α-tubulin | Sigma-Aldrich (T9026) | 1:2500 | IgG sheep anti-mouse HRP conjugated | GE Healthcare (NA931) | 1:20,000 | WB |
| Mouse anti-CBS | Abnova (H00000875-A01) | 1:2500 | IgG sheep anti-mouse HRP conjugated | GE Healthcare (NA931) | 1:20,000 | WB |
| Rabbit anti-BHMT | González et al. [37] | 1:5000 | IgG goat anti-rabbit HRP conjugated | Bio-Rad Laboratories (170-6515) | 1:5000 | WB |
| Rabbit anti-ADK | Abcam (ab88903) | 1:500 | IgG goat anti-rabbit HRP conjugated | Bio-Rad Laboratories (170-6515) | 1:5000 | WB |
| Rabbit anti-Hcy | Chemicon-Millipore (AB5512) | 1:1000 | IgG goat anti-rabbit HRP conjugated | Bio-Rad Laboratories (170-6515) | 1:3000 | WB |
| Rabbit anti-p22phox | Santa Cruz Biotechnology (sc-20781) | 1:500 | IgG goat anti-rabbit HRP conjugated | Bio-Rad Laboratories (170-6515) | 1:3000 | WB |
| Rabbit anti-MnSOD | Upstate Millipore (06-984) | 1:2500 | IgG goat anti-rabbit HRP conjugated | Bio-Rad Laboratories (170-6515) | 1:3000 | WB |
| Rabbit anti-NOX3 | Abcam (ab82708) | 1:1000 | IgG goat anti-rabbit HRP conjugated | Bio-Rad Laboratories (170-6515) | 1:5000 | WB |
| Goat anti-AHCY | Santa Cruz Biotechnology (sc-66759) | 1:1000 | IgG donkey anti-goat HRP conjugated | Santa Cruz Biotechnology (sc-2020) | 1:10,000 | WB |
| Goat anti-MTR | Abnova (PAB6022) | 1:250 | IgG donkey anti-goat HRP conjugated | Santa Cruz Biotechnology (sc-2020) | 1:10,000 | WB |
| Goat anti-ADA | Santa Cruz Biotechnology (sc-7451) | 1:2500 | IgG donkey anti-goat HRP conjugated | Santa Cruz Biotechnology (sc-2020) | 1:10,000 | WB |
| Goat anti-NOX4 | Santa Cruz Biotechnology (sc-21860) | 1:500 | IgG donkey anti-goat HRP conjugated | Santa Cruz Biotechnology (sc-2020) | 1:3000 | WB |
The table lists the antibodies used in this work indicating the dilutions used for the different techniques. IC-P, immunohistochemistry-paraffin; IHF, immunohistofluorescence; WB, Western blotting. aTertiary extrAvidin peroxidase-conjugated antibody (1:300 v/v; Sigma-Aldrich) was needed in this case.
Photomicrographs and densitometry for 3-nitrotyrosine (3-NT) quantification were performed with a Zeiss Axiophot microscope (Carl Zeiss, Jena, Germany) as reported, using a minimum of 4 animals per group (32). Freehand-delimited areas for the stria vascularis and the cochlear ganglion were used for optical density estimation using ImageJ software v1.43m (NIH, Bethesda, MD, USA). Three consecutive serial slides were studied, and for each section, at least 2 pictures per structure were taken. The same procedure was carried out for myelin P0 and Kir4.1 immunohistochemistry quantification. The relative intensity:area ratio of the cochlear ganglion was performed on paraffin sections stained with H&E using ImageJ software. Every fifth slide was analyzed so that at least 4 representative sections for each animal and cochlear region were included.
Metabolite determination
Total Hcy was determined after derivatization using the Reagent kit for HPLC analysis of Hcy in plasma/serum (Chromsystems Instruments & Chemicals Gesellschaft mit beschränkter Haftung, Munich, Germany). Derivatized samples (50 µl) were injected into the HPLC column, and fluorescence was measured at 515 nm after excitation at 385 nm.
Plasma vitamin B6 levels were measured using a commercial kit (Chromsystems Instruments & Chemicals Gesellschaft mit beschränkter Haftung), and HPLC analysis coupled to fluorescence detection was performed following the manufacturer’s instructions. Total serum folate was determined using the microbiological method described by Horne and Patterson (38) with the modifications introduced by Tamura (39).
Reduced (GSH) and oxidized glutathione (GSSG) levels were measured by the Tietze method as modified by Rahman et al. (40). For this purpose, 2 cochleae were homogenized in 0.1 M potassium phosphate buffer (pH 7.5), containing 5 mM EDTA and 0.6% (w/v) sulfosalicylic acid (250 μl), centrifuged at 8000 × g for 10 min at 4°C, and the corresponding extract was used for GSH (1:10 v/v) and GSSG (1:2 v/v) determinations.
Protein extraction and Western blotting
Whole cochlear protein was prepared as reported previously (34, 41). Protein concentrations were measured using the Pierce BCA Protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA) and bovine serum albumin as standard. Samples (200 μg) were loaded onto 10% SDS-PAGE gels and electrotransferred onto a membrane for incubation with the corresponding dilutions of primary and secondary antibodies for 1 h as specified in Table 1. Signals were developed using Western Lightning ECL (PerkinElmer Life and Analytical Sciences, Waltham, MA, USA). Blots underwent densitometric scanning using ImageJ, and the values were normalized against α-tubulin or β-actin signals. Mean values for the NF group were considered to be 100%.
Real-time RT-PCR
Total cochlear RNA was isolated from NF and FD mice using an RNeasy kit (QIAGEN, Hilden, Germany), and the quality and quantity were determined spectrophotometrically with a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA). RT-PCR was performed as previously described using 1.25 μg total RNA as template and the High Capacity Archive kit (Applied Biosystems, Foster City, CA, USA) (42). cDNAs (10 ng) were amplified in triplicate using gene-specific primers (Table 2) and Power SYBR Green PCR Master Mix (Applied Biosystems) for the evaluation of Hcy metabolism. In addition, analysis of Cat (catalase), Foxm1 (Forkhead box M1), Foxp3 (Forkhead box P3), Gap43 (growth-associated protein 43), Gclc (glutamate-cysteine ligase catalytic subunit), Gpx1 (glutathione peroxidase 1), Gpx4 (glutathione peroxidase 4), Gsr (glutathione reductase), Gss (glutathione synthetase), NOX3 (NADPH oxidase), NOX4, p22phox (cytochrome b-245, α-polypeptide), Mef2a (myocyte enhancer factor 2A), and Mef2d (myocyte enhancer factor 2D) genes was carried out with commercial TaqMan probes (Life Technologies S.A., Alcobendas, Madrid, Spain). Assays were performed on the ABI 7900HT Real-Time PCR System (Applied Biosystems). Primers for Cbs and adenosine kinase (Adk) amplification were designed to allow amplification of the 2 splicing forms of each gene reported to date. Fluorescent signals were collected after each extension step, and the curves were analyzed with SDS 2.2.2 software (Applied Biosystems). Relative expression ratios were normalized to the geometric mean of Rn18s or Rplp0 (ribosomal protein large P0) genes. Experimental efficiencies were calculated for each transcript and used to obtain fold changes according to Pfaffl (43).
TABLE 2.
Primers designed for real-time RT-PCR experiments with SYBR Green
| Gene | Bases | Forward primer (5′–3′) | Bases | Reverse primer (5′–3′) | nM |
|---|---|---|---|---|---|
| Ada | 641–663 | GGGATGAGACCATTGAAGGAAGT | 709–689 | TCTTTACTGCGCCCTCATAGG | 300 |
| Adk | 883–903 | AGAGGCAGAGGACCGTGATCT | 946–925 | TCATTCTCTGCAGCCACTATGG | 300 |
| Bhmt | 672–692 | CAGAATTCCCCTTTGGATTGG | 742–721 | GGCCTCTCTGGCATATTTTTGA | 300 |
| Bhmt2 | 143–166 | CTCCAGAAGCAGTGGTAGAACATC | 217–198 | CATCAGCTCCCGCTCTCAAG | 300 |
| Cbs | 1274–1292 | GCAGCGCTGTGTGGTCATC | 1337–1312 | GTCACTCAGGAACTTGGACATGTAGT | 300 |
| Ahcy | 1531–1554 | TCGAAGTGTCCAATGTTACAGACA | 1592–1575 | CTTGGCCGGCACTTTGAG | 300 |
| Mtr | 2006–2026 | CGCGATCAAGTTTGGTATGGA | 2080–2057 | TCCTTGTGGATAGCATCATACACA | 300 |
| Mat1a | 1285–1307 | GGTGTTATTGTCAGGGACTTGGA | 1347–1329 | GCCGTAGCACGCAGTCTTC | 300 |
| Mat2a | 1057–1079 | GGAGGGTTCTTGTTCAGGTCTCT | 1123–1102 | TGGAAAATGGAGATCGACAATG | 300 |
| Mat2b | 134–153 | GCTGGTGGAGGAGGAAGTGA | 192–173 | CAGTGGCACCGGTAATGAGA | 300 |
| Rn18s | 1645–1666 | CCAGTAAGTGCGGGTCATAAGC | 1737–1716 | CCTCACTAAACCATCCAATCGG | 100 |
Appropriate primers for genes in the methionine cycle and related pathways were designed using the Program Primer Express 3.0 and the mouse gene sequences available in the data bank with references NC_000068.6 (Ada), NC_000080.5 (Adk), NW_000085.1 (Bhmt), NC_000079.5 (Bhmt2), NC_000083 (Cbs), NT_039207 (Ahcy), NC_000079 (Mtr), NC_000080.5 (Mat1a), NC_000072.5 (Mat2a), NC_000077.5 (Mat2b), and NC_000072.6 (Rn18s). Base numbers indicate the location of the primer sequences in the corresponding mRNA; primers for Cbs and Adk were designed in regions common to the 2 splicing forms reported. The concentrations of the primers used for SYBR Green detection are shown in the right column.
TUNEL assay
The presence of apoptotic cells was evaluated in cochleae using the ApopTag Plus Peroxidase In Situ Apoptosis Detection kit (S7100; Millipore Iberica S.A.U., Madrid, Spain) for TUNEL assay, according to manufacturer’s instructions. In brief, samples were fixed in 1% PFA (v/v) for 10 min at room temperature, washed twice in PBS, and postfixed at −20°C in ethanol:acetic acid (2:1, v/v) for 5 min. Slides were then washed, endogenous peroxidase was blocked with hydrogen peroxide (5%, v/v) for 20 min, and washed again, and the equilibration buffer was added for 10 min. Next, samples were incubated at 37°C for 1 h with terminal deoxynucleotidyl transferase (TdT), and the reaction was stopped and further incubated using the digoxigenin/antidigoxigenin antibody system. The final reaction was monitored with a microscope using DAB as the chromogen. Afterwards, samples were dehydrated and mounted with Entellan (Merck). DNase I–treated normal cochleae and rat postweaning mammary gland tissue (S7115; Millipore) were used as positive controls; negative controls were obtained by omitting the TdT enzyme. The number of TUNEL-positive cells was evaluated as described above in 4 sections per animal (n = 6 mice per group), and the resulting counts were averaged for comparison.
Statistical analysis
SPSS version 19.0 software package (SPSS, Chicago, IL, USA) and Prism v 5.0 (GraphPad Software Incorporated, San Diego, CA, USA) were used for statistical analysis. Statistical significance was determined by Student’s t test for unpaired samples between NF and FD groups. All results were expressed as the mean ± SEM, and differences were considered significant when P ≤ 0.05.
RESULTS
Plasma concentrations of selected metabolites were measured in mice receiving normal (NF) or FD diets in order to assess the effect of the treatment (Fig. 1B). As expected, a severe reduction in serum folate levels (∼7-fold) was observed in the FD group compared with the NF group. Additionally, plasma total homocysteine (tHcy) concentrations were elevated (∼3-fold) in the FD group, whereas plasma vitamin B6 levels were similar in both dietary groups. Hence, the diet was effective at causing systemic folate deficiency and hyperhomocysteinemia.
FD mice show early signs of severe sensorineural hearing loss
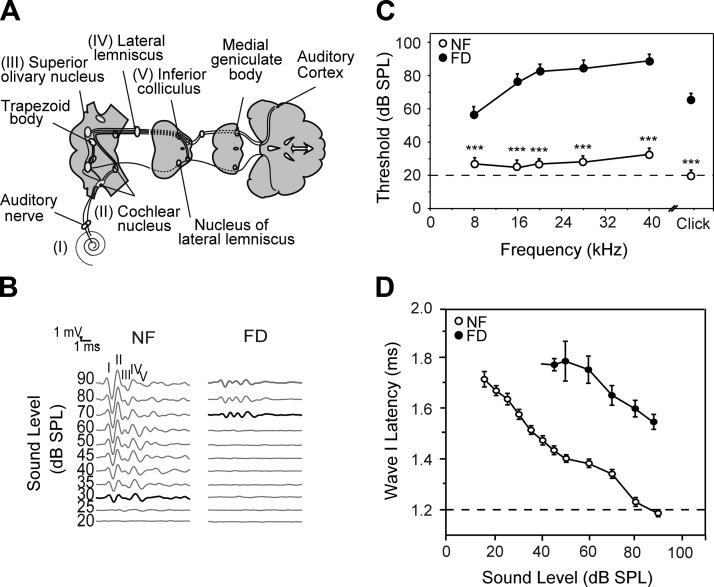
On average, mice in the NF group had normal hearing, whereas animals in the FD group had moderate (34%) or profound (66%) hearing loss; only the latter group was further studied and referred to as FD (Fig. 2A–C). An average increase of 45 dB SPL in ABR thresholds was found in FD mice when compared to the NF group in response to click stimuli. Accordingly, there was a significant increase in hearing thresholds at all studied frequencies, although the effect was more acute at high (50 dB SPL threshold shift) than at low frequencies (20 dB SPL threshold shift) (Fig. 2C). Click ABR wave analysis revealed a significant delay in the latency of wave I in FD mice when compared to the NF group (Fig. 2D), as well as a dramatic reduction of the amplitude of wave I (Supplemental Fig. 1A). No further differences were observed in ABR interpeak latencies (I–II, II–IV, and I–IV) (Supplemental Fig. 1B), and the wave IV latency-intensity function when these parameters were measured at intensities of 20 dB SPL above threshold. However, a progressive decrease in the wave IV amplitude:intensity ratio was observed in the FD mice group (Supplemental Fig. 1C, D).
Figure 2.
FD mice show early signs of hearing loss. The auditory response was analyzed in mice of normal (NF) and FD groups. A) Scheme of the auditory pathway adapted from Murillo-Cuesta et al. (32). B) Representative ABR recordings obtained in response to click stimuli of NF (showing normal hearing) and FD mice (showing profound hearing loss). C) ABR thresholds in response to click and tone burst stimuli in NF (○) and FD (•) mice, after 8 wk of diet (n = 21 for each group). D) Latency-intensity functions for wave I of NF and FD mice showing a delay in the appearance of the wave. Data are shown as the mean ± SEM. ***P < 0.001.
FD mice show aberrant cochlear cytoarchitecture and altered expression of cell-type markers
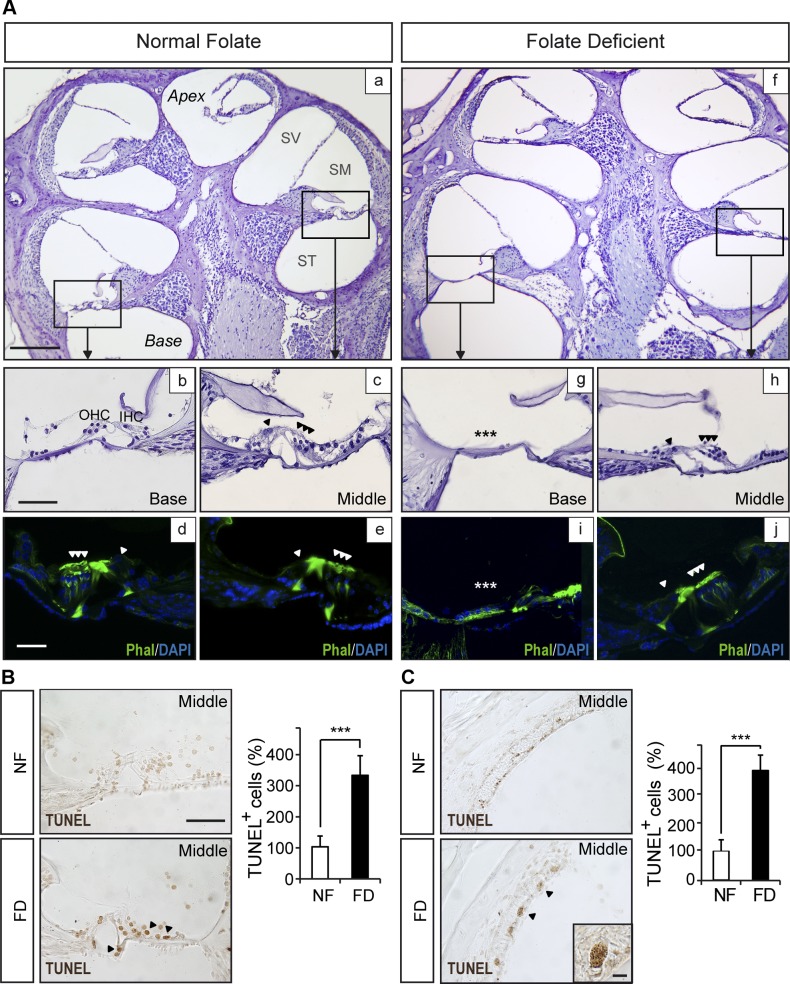
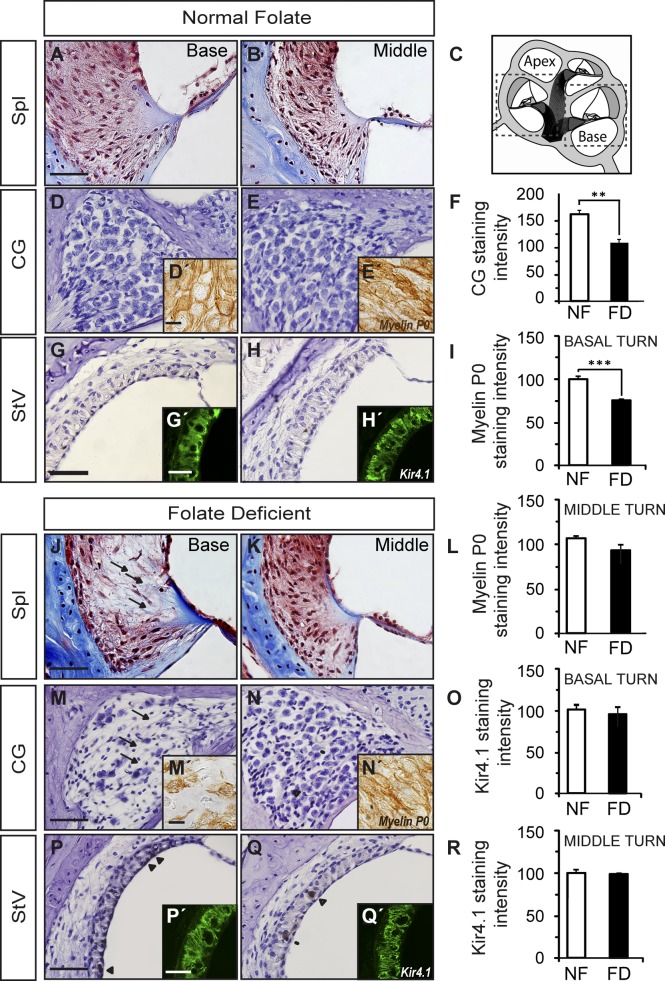
Cochlear architecture differed between groups, with cellular loss in the basal turn seen in FD mice compared with the NF group (Figs. 3 and 4). The organ of Corti structure in the NF mice remained normal along all cochlear turns (Fig. 3A, a–e). In contrast, the cochlea of FD mice showed a flat cochlear epithelium in the basal turn (Fig. 3A, f–j). TUNEL-positive cells were evident in the organ of Corti and stria vascularis in the middle turn of the FD cochlea, but not in NF (Fig. 3B, C). At the time of the study, apoptotic cells were not observed in the flat basal turn or in the normal apex (data not shown). Additionally, other cellular populations in the basal and middle turns of the cochlea of FD mice were affected when compared to NF (Fig. 4). Cellular aberrations included loss of type IV fibrocytes (compare Fig. 4A, B with J, K) and a 33% reduction of ganglionar density and myelin P0 levels (P < 0.001; compare Fig. 4D, E with M, N and quantification in F, I, L). Myelin P0 levels from the middle turn toward the apex remained unchanged for both dietary groups (Fig. 4L). Disorganization of the striatal capillaries and accumulation of melanin granules were also observed in 5 of 9 mice from the FD group. In contrast, none of the NF group mice showed this phenotype (compare Fig. 4G, H with P, Q; quantification in Fig. 4O, R). Kir4.1 levels did not show evident differences between the dietary groups (P > 0.05; compare Fig. 4G′, H′ with P′, Q′). Furthermore, gene expression levels in the organ of Corti were analyzed (Supplemental Fig. 1E). Mef2a and Mef2d showed a small but statistically significant decrease in the FD group compared with the NF group; Foxp3 levels were significantly increased (P < 0.05), and there were no differences in Gap43 and Foxm1 levels.
Figure 3.
FD mice showed altered cochlear morphology and apoptotic cells. Cochlear morphology from normal (NF) and FD mice was studied by histochemistry and immunohistofluorescence. A) Representative micrographs show basal and middle turn details of the organ of Corti in sections of NF (a–e; n = 6) and FD mice (f–j; n = 9). Phalloidin (Phal) staining of the organ of Corti appears in panels d, e, i, and j. Asterisks denote the flat epithelium of the organ of Corti in panel g. Asterisks indicate the absence of hair cells in panel i. B) Representative images and quantification of cell death by TUNEL assay of the organ of Corti per region of interest (ROI) from the middle turn of both groups. C) Percentage of TUNEL-positive cells/ROI in the stria vascularis from FD mice compared to NF (n = 6 mice studied per experimental group). IHC, inner hair cell; OHC, outer hair cell; SV, scala vestibulae; ST, scala tympani; SM, scala media. Scale bars, 500 µm (a and f), 50 µm (d, e, i, and j), and 25 µm (b, c, g, and h).
Figure 4.
FD mice showed altered cochlear cytoarchitecture. Sections of cochleae from mice on normal (A–H; n = 6) or FD (J–Q; n = 9) diets were stained with Masson’s Trichrome, H&E, or used for immunofluorescence. Basal and middle turn details of the ligament (Spl), cochlear ganglion (CG), and stria vascularis (StV) are shown. Immunohistochemistry results of the cochlear ganglion neurons labeled with myelin protein zero are shown at higher magnification in the insets to panels (D), (E), (M), and (N). Details of the stria vascularis immunolabeled with Kir4.1 are included as insets to panels (G), (H), (P), and (Q). Arrows indicate the absence of cells (J and M). Panel (C) depicts a scheme of the cochlea where basal and middle turns are indicated by squares. The intensity of the signals (0–256 gray scale) was quantified for normal (NF; n = 4) and FD n = 6) samples, and the results are shown as the mean ± SEM in (F), (I), (L), (O), and (R) histograms. **P < 0.01; ***P < 0.001. Scale bars, 25 µm (A–H, J–Q, and insets to G, H, P, and Q) and 10 µm (insets to D, E, M, and N).
Cochlear Hcy metabolism showed specific expression features
Systemic changes in folate and Hcy levels may reflect alterations in cochlear metabolism, which has not been studied previously. Therefore, real-time RT-quantitative PCR (RT-qPCR) assays were carried out using cochleae of mice in the NF group to define the control expression pattern (Fig. 5A). Normalization of the results using the Rn18s housekeeping gene showed different levels of expression among genes in this pathway. Mat2a expression was the highest, followed by that of Mat2b, Ahcy, and Adk. Intermediate expression levels were detected for Bhmt and Mtr, whereas low levels were found for Mat1a, Ada, Cbs, and Bhmt2. These results confirmed the expected extrahepatic expression pattern, except in Bhmt and Mtr genes.
Figure 5.
Cochlear Hcy metabolism in control mice. Cochleae from mice receiving an NF diet were used to characterize Hcy metabolism and related reactions. A) Expression levels of the genes of interest were determined by RT-qPCR using total cochlear RNA; the data were normalized using the Rn18s gene as reference (n = 6). B–D) Total cochlear proteins from wild-type and Bhmt−/− null mice (200 µg), were used to verify the specificity of bands detected on Western blots. Membranes were incubated with (B) rabbit anti-BHMT (37) and mouse anti-tubulin, (C) preimmune serum, and (D) goat anti-AHCY. Cytosols from hepatoma H35 cells (3 μg) were used as reference for the expected mobility. Size of the prestained markers is indicated on the left side of the blots.
Proteins involved in cochlear Hcy metabolism were detected by Western blotting, and most of them showed the expected mobility. In contrast, cochlear anti-BHMT and anti-AHCY signals were detected as proteins with calculated molecular masses of 68 and 58 kDa, respectively (Fig. 5B, D). This altered mobility represents an increase of 10–15 kDa in the size of either protein as compared to the single band (∼45 kDa) detected in H35 cytosols (Fig. 5B, D). No such new bands could be detected in liver samples even when using larger amounts of protein or upon film overexposure. The specificity of the BHMT band was demonstrated by the lack of signal observed with either preimmune rabbit serum or cochlear extracts obtained from Bhmt−/− null mice (Fig. 5B, C). Again, the data demonstrate specific features of Hcy metabolism in the cochlea.
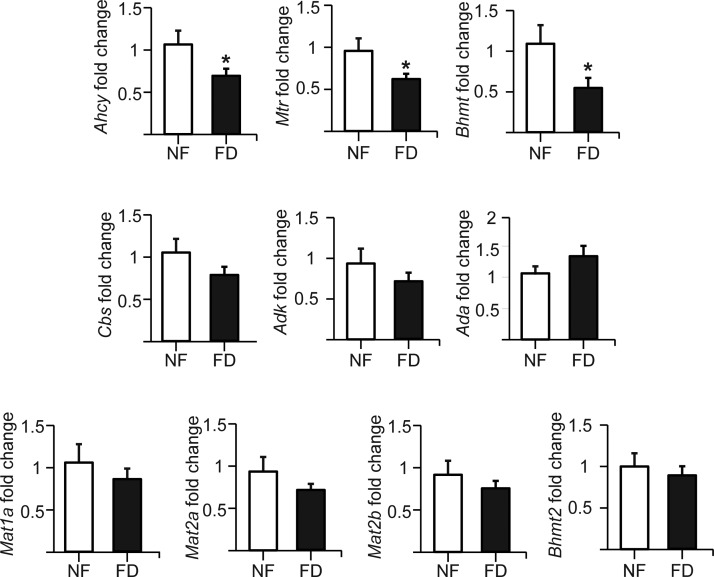
Folate deficiency induced impairment of cochlear Hcy metabolism
Systemic changes in folate and Hcy levels may reflect alterations in this part of cochlear metabolism. Therefore, RT-qPCR assays were carried out to analyze the putative changes induced by folate deficiency (Fig. 6). mRNA levels of enzymes involved in Hcy production (Ahcy) and remethylation (Bhmt and Mtr) reduced their expression in FD mice (30–50%) compared with NF animals. In contrast, mRNA levels of enzymes involved in elimination of S-adenosylhomocysteine products showed no change (Cbs and Adk), or a tendency to increase (Ada; P = 0.08). In addition, no significant alterations in mRNA levels for Mat subunits were detected, nor were alterations in Bhmt2 detected. Therefore, expression changes induced by folate deficiency were directed toward reduction of Hcy production and toward favoring its elimination in the plasma or through the transsulfuration pathway.
Figure 6.
Folate deficiency alters expression of genes involved in Hcy metabolism. Total cochlear RNA of mice on normal (NF, n = 11) or FD (n = 12) diets was used to analyze expression levels of the genes of interest by real-time RT-PCR using the Rn18s gene as reference. The results are shown as the mean ± SEM of determinations made in triplicate for each animal sample. Statistical analysis by Student’s t test was carried out using GraphPad Prism, and data are considered significant when *P ≤ 0.05.
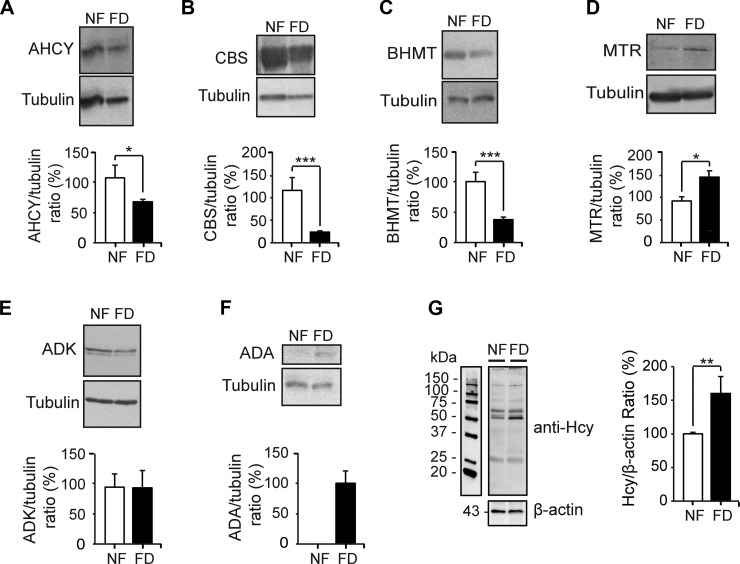
However, cochlear expression levels may not reflect the actual protein levels that were analyzed by Western blotting. Compared to NF samples, decreased protein levels were found in the FD group for the AHCY 58 kDa band (∼40%), the double bands ∼60–65 kDa corresponding to CBS isoenzymes (∼70%), and the BHMT 68 kDa band (∼60%) (Fig. 7A–C). In contrast, ADK (41 kDa) showed no change, whereas increased signals were found for the 138 kDa MTR band (∼40%) and the 41 kDa adenosine deaminase (ADA) protein in FD cochleae (Fig. 7D–F). This last protein gave no signal in NF samples. Again, the results suggest an effort to reduce Hcy production and toward its elimination in the plasma. However, the combined effects on expression and protein levels in FD mice may not be enough to avoid the increase in intracellular Hcy levels. A consequence of such an intracellular enhancement of Hcy could be protein homocysteinylation, a protein modification that can be followed by Western blotting using total cochlear extracts and anti-Hcy (Fig. 7G). The results showed a 50% increase in immunostaining of FD cochlear proteins, compared with NF extracts, indicative of cochlear hyperhomocysteinemia.
Figure 7.
Effects of folate deficiency on cochlear protein levels of enzymes involved in Hcy metabolism. Total cochlear protein (200 μg/lane) of animals on normal (NF; n = 11) and FD (n = 20) diets was analyzed by Western blot using the following antibodies: A) anti-AHCY, B) anti-CBS, C) anti-BHMT, D) anti-MTR, E) anti-ADK, and F) anti-ADA. Representative immunoblots for each antibody are shown, together with quantifications (mean ± SEM) carried out with ImageJ software, normalized using tubulin as the loading control. For graphic purposes, the mean of the NF group ratio was considered 100% in each case. G) Representative image of anti- Hcy Western blot of NF and FD mice and its densitometric analysis. Mean data of the NF group are presented as 100% for graphic purposes; ratios are 3.92 ± 0.80 (NF; n = 4) and 6.28 ± 1.06 (FD; n = 5). Statistical analysis was carried out using Student’s t test and differences were considered significant when P ≤ 0.05. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
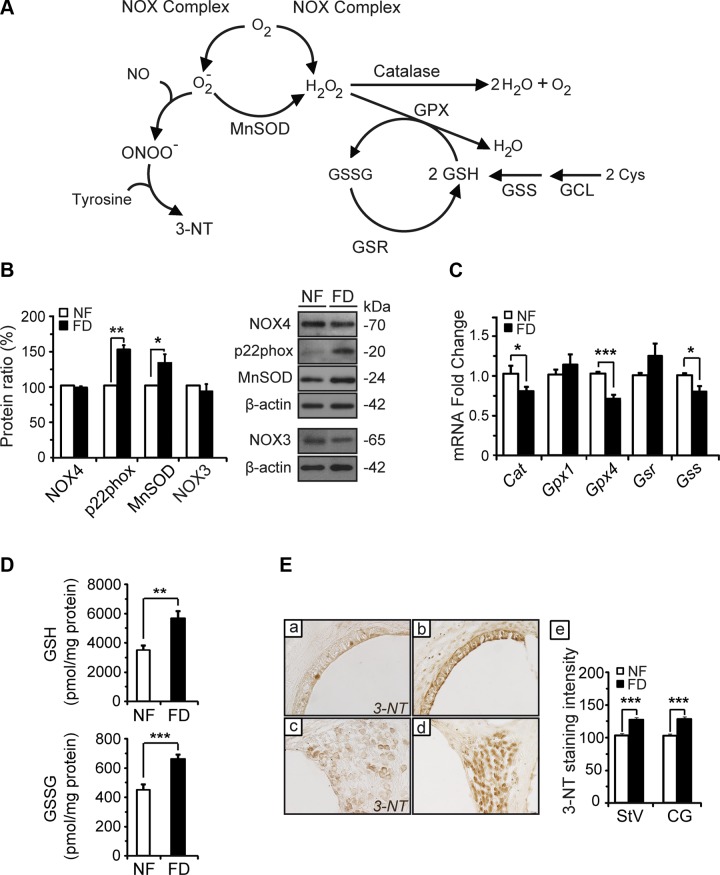
FD mice showed oxidative imbalance in the cochlea
Hearing loss was previously associated with redox imbalance; hence, we next studied the putative effect of the FD diet on parameters of the cellular oxidative response in the cochlea (Fig. 8A). Protein levels of the NOX complex (NOX4, NOX3, and p22phox) and manganese superoxide dismutase (MnSOD) were analyzed by Western blotting (Fig. 8B). Similar NOX4 and NOX3 protein levels were found in both mouse groups, whereas a significant increase in p22phox (∼1.5-fold) and MnSOD (∼1.7-fold) levels was detected in FD cochleae as compared to NF samples. Expression levels of genes involved in redox control were also analyzed by RT-qPCR in both dietary groups (Fig. 8C). Reduced transcript levels were detected for Cat (20%), Gpx4 (30%), and Gss (15%) in the FD group, whereas no change was observed for Gsr, Gpx1, and Gclc between dietary groups. No changes between groups were found in either p22phox, Nox3 or Nox4 expression levels (data not shown). GPX proteins require GSH and generate GSSG; hence, cochlear glutathione levels were also determined. Increased GSH (60%) and GSSG levels (50%) were measured in FD as compared to NF samples (Fig. 8D), but these changes did not significantly alter the GSH:GSSG ratio (7.76 ± 0.36 vs. 8.49 ± 0.56; P = 0.29). A consequence of superoxide metabolism is 3-NT synthesis, a metabolite that can be immunostained in cochlear sections (Fig. 8E). FD mice showed strong immunoreactivity for 3-NT in the stria vascularis and the cochlear ganglion, whereas mice on the NF diet exhibited low basal signals as described previously (44).
Figure 8.
FD mice showed oxidative imbalance in the cochlea. Cochleae from mice on normal (NF) and FD diets were used to evaluate several oxidative stress markers. A) Schematic representation of the role of the oxidative stress markers analyzed in this work. B) Representative immunoblots for NOX4 (n = 3 NF and n = 4 FD), MnSOD (n = 6 NF and n = 9 FD), and p22phox (n = 5 NF and n = 9 FD). The histograms show the mean ± SEM of densitometric scanning results after normalization using β-actin levels. C) Expression levels of Cat, Gpx1, GPx4, Gsr, Gclc, and Gss evaluated by real-time RT-PCR using Rplp0 as reference. D) Evaluation of cochlear GSSG and GSH levels (n = 6 mice per group). E) Representative images of 3-NT levels detected by immunohistochemistry in the stria vascularis (StV; a and b) and cochlear ganglion (SG; c and d) of NF (a and c; n = 3) and FD (b and d; n = 6) mice. e) Quantification of the 3-NT signal is shown in the histograms as the mean ± SEM. Statistical evaluation of the data was performed by Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
Hearing loss is one of numerous health problems associated with micronutrient deficiencies worldwide (8–13). Its origin lies not only in malnutrition but also in an inadequate nutrient density in the so-called western societies. Low serum and red blood cell folate levels have been detected in several types of hearing impairment, and its association with elevated tHcy concentrations has been also reported in some cases (11–13). However, the underlying mechanisms linking folate deficiency and hearing loss remained unidentified. For this reason, we used a nutritional mouse model that presented both low serum folate levels and hyperhomocysteinemia, thus making it a suitable model for analysis of the association of these alterations and hearing loss.
Evaluation of the auditory response shows higher thresholds with more acute losses at high frequencies in FD mice, hence demonstrating a direct association between folate deficiency, hyperhomocysteinemia and profound hearing loss. Wave I amplitude decrement confirms premature impairment at the hair cell auditory nerve synapse, similar to that described in hearing loss associated with oxidative stress (45). Histopathologic analyses of hearing loss in FD mice indicate that different cochlear populations are affected. Severe damage was evident at the organ of Corti, the spiral ganglion, the stria vascularis, and cells of the spiral ligament. Moreover, cochlear damage displays a gradient, with severe cellular loss in the basal turn, signs of damage in the middle turn (including the presence of apoptotic cells), and very light or no phenotypic differences in the apex. Cochlear cell populations exhibit differences in susceptibility against ototoxic drug treatments with salicylate (46) or cisplatin (47). This chemotherapy drug induces a loss of cochlear outer hair cells that is larger at the base than at the apex.
Folic acid is needed for Hcy remethylation, but knowledge of this portion of cochlear metabolism is very limited, therefore requiring characterization in control tissues. Most genes of Hcy metabolism show the expected expression profile for extrahepatic (high Mat2a expression) and nonlymphoid tissues (Adk vs. Ada expression) (48, 49). However, remethylation genes exhibit a specific pattern with similar expression levels for Mtr and Bhmt, and lower levels for Bhmt2, thus making the cochlea a different subtype among extrahepatic tissues. The corresponding proteins were also detected in immunoblots, commonly at very low levels. Anomalous mobilities for BHMT and AHCY bands were also observed, which were compatible with their posttranslational modification and suggest changes in their behavior and function. Large-scale mass spectrometry studies detected a number of such modifications in these proteins (50), although their impact on BHMT and AHCY function has been scarcely explored (20).
Folic acid deficiency alters the cochlear gene expression pattern, reducing the expression of Ahcy, Mtr, and Bhmt. Reduced Bhmt expression was previously described in the stria vascularis of Cx30−/− null mice; however, in that model of hearing loss, Ahcy showed a modest up-regulation (25). This transcriptomic study did not report additional changes in genes of these pathways, neither were Adk mRNA nor protein levels altered in rats exposed to broadband noise (25, 49). Expression results in FD cochleae correlate with those of immunoblots for AHCY, BHMT, and ADA, whereas levels of CBS splicing forms and MTR signals follow different patterns. Lack of correlation between mRNA and protein steady-state levels is not uncommon and may be due to changes in their half-lives. Consistent with this line of evidence, cobalamin binding to MTR protein has been reported to stabilize this enzyme (51), an effect that could be expected in FD mice in an effort to recycle the low amounts of folate available. Altogether, changes induced by folic acid deficiency on Hcy metabolism are directed toward decreasing Hcy synthesis (precluding the reversibility of the AHCY reaction through augmented adenosine utilization), reducing Hcy flux through remethylation and decreasing its use in transsulfuration. In this scenario, increased adenosine elimination may limit protection against cochlear oxidative stress through adenosine signaling (52), although sparing purine for nucleic acid and NADH/NADPH synthesis. An additional drawback for cochlear function may derive from the reduced flux through transsulfuration. CBS and cystathionase are able to synthesize H2S, whose protective role as cochlear vasodilator has been reported in noise-induced hearing loss (53). A reduced production of H2S may contribute to the changes detected in striatal capillaries in FD mice, in concordance with the decreased vessel density previously reported for Cbs+/− mice cochleae (24).
Taken together, the aforementioned alterations in Hcy metabolism suggest the presence of elevated Hcy levels in the cochleae of FD mice, an increase that was confirmed by detection of enhanced Hcy immunostaining in FD protein lanes. Increased Hcy immunostaining was previously reported in the stria vascularis of Cx30−/− null mice (25), also associated with reduced Bhmt expression, both data alluding to BHMT as the key target for the regulation of cochlear Hcy levels. Again, these results suggest several mechanisms that could explain cochlear dysfunction and that involve increased Hcy levels: 1) elevated protein homocysteinylation, a posttranslational modification leading to inactivation and aggregation of proteins with the consequent impairment of their function (54); 2) reduction of blood flow due to atherosclerosis in the inner ear, for which elevated Hcy levels are a determinant, as suggested by several epidemiologic reports (15–17); and 3) overexcitation of N-methyl-d-aspartate receptors by the Hcy agonist, a process associated with hearing impairment (55, 56).
Oxidative stress is a condition associated with rapid aging and that correlates with hearing loss caused by noise or other noxious stimuli (45). Hearing loss caused by folate deficiency also concurs with cochlear oxidative stress and accumulation of reactive oxygen and nitrogen species, as shown by several indicators, namely the following: 1) increased p22phox and MnSOD protein levels; 2) decreased expression of Cat, Gpx4, and Gss; 3) increased 3-NT levels in the stria vascularis and the spiral ganglion; and 4) increased GSH and GSSG levels. Accumulation of reactive species and metabolites of cellular redox buffers (i.e., NADP+, GSSG) may contribute to impair cochlear Hcy metabolism. In fact, oxidative stress is known to alter the expression and function of several enzymes of this pathway, as has been demonstrated mainly in liver disease (20, 42, 57, 58). Maintenance of cell function requires elimination of damaging reactive species (59–61), a process involving signaling pathways. Results obtained from FD cochleae suggest increased reactive oxygen species signaling involving the NOX complex, which in turn may cause the high level of apoptotic cells detected.
NOX is expressed in the rat cochlea and regulated by noxious, stimuli, such as noise (62). During oxidative stress, NOX4 and NOX3 protein levels remain unchanged. NOX activator subunits, such as p22phox, are the main proteins involved in stress response (63, 64). Parallel to NOX activation, the increase in MnSOD suggests an effort to eliminate the highly reactive superoxide ion, producing peroxide with lower reactivity. MnSOD is a marker of neuroprotection that is triggered as a compensatory mechanism against stress (65). Interestingly, Sod1−/− null mice are deaf (66). However, the decrease in Cat expression in FD mice suggests a putative impairment in peroxide elimination through this reaction, an effect that could be larger if we considered Hcy inhibition of the enzyme previously reported (67). Compensatory peroxide catabolism can be obtained through GPX, mainly GPX1, leading to increased GSSG production (68). GPX1 is able to use either GSH or the γ-glutamylcysteine produced in the first step of GSH synthesis (68), a fact that can overcome the small reduction detected in Gss expression in FD mice. Additionally, expression and enzyme levels may not correlate, given the complex regulation of glutathione synthesis that involves antioxidant response elements in gene promoters, posttranslational modifications, and feedback inhibition by GSH, among others (69). Therefore, a combination of γ-glutamylcysteine accumulation, increased GSH synthesis by stabilization of GCLC protein, and/or augmented association to glutamate-cysteine ligase or γ-glutamylcysteine synthetase, modifier subunit (lowering its Michaelis constant for glutamate and raising the inhibition constant for GSH), together with elevated GSSG synthesis through GPX and imbalanced recycling through GSR, may explain conservation of a normal cochlear GSH:GSSG ratio during folate deficiency. The existence of a profound redox imbalance in the cochleae of FD mice was further confirmed by the detection of elevated 3-NT immunostaining. This metabolite is a product of peroxynitrite action, whose levels have been found augmented during oxidative stress in the aging ear (44).
Oxidative stress is also known to alter methionine and folate cycles at different levels. Several enzymes of these pathways are susceptible to this condition given their need for cofactors and metals (20, 41, 58). Therefore, a reduction in folate levels, together with the cochlear oxidative stress detected, not only remodels expression and protein levels, but is expected to modify enzyme activities, subcellular distribution, and oligomerization as occur in several experimental settings (57, 70–72). Along this line, the increased p22phox levels in FD cochleae are expected to produce NOX activation and, hence, lead to elevated NADP+ concentrations. This metabolite is known to favor trimerization of methionine adenosyltransferase (MAT) II, reducing its affinity for methionine (58), an effect that in turn would guarantee the S-adenosylmethionine synthesis needed for epigenetic remodeling during nutritional stress. This effect may not be essential for FD mice whose diets supply enough methionine and cysteine for synthesizing the methyl donor and glutathione. However, this effect may derive from an effort to correct the altered methylation index resulting from decreased cochlear AHCY levels that, in turn, would lead to increased S-adenosylhomocysteine concentrations and inhibition of transmethylations. An additional effect of impaired cochlear Hcy metabolism, precisely lower BHMT levels, is betaine accumulation. This increase in osmolyte concentrations might be beneficial in maintaining the function of thin cochlear basal epithelia in FD mice.
Altogether, our results confirm that insufficient folic acid intake induces severe impairment of cochlear Hcy metabolism, along with a profound oxidative imbalance, ultimately leading to hearing loss. The positive correlation between hyperhomocysteinemia and hearing loss in folate deficiency also suggests the potential of the former as a prognostic value. In addition, targeting Hcy metabolism by nutritional intervention could be a novel pathway to achieve therapeutic protection against hearing loss.
Supplementary Material
Acknowledgments
The authors thank Estela Maldonado from Complutense University of Madrid, Silvia Murillo-Cuesta from the Non-invasive Neurofunctional Evaluation facility, and the team from the Genomics facility (both from IIBM, CSIC-UAM) for the technical support provided. The authors also warmly thank their colleagues at the Neurobiology of Hearing group for sharing unpublished data and helpful discussions. R.M.-V. is supported by a fellowship from the JAE-CSIC predoctoral program. This work was supported by grants from the Ministerio de Ciencia e Innovación (BFU2009-08977 to M.A.P., SAF2011-24391 to I.V.-N., and PS09/01762 to C.M.-A.), the European Union (FP7-AFHELO and TARGEAR to I.V.-N.), U.S. National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (Grant DK056350 to S.H.Z.), and Puleva BioFoods (to I.V.-N., G.V.-M., and M.A.P.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no conflicts of interest.
Glossary
- 3-NT
3-nitrotyrosine
- ABR
auditory brainstem response
- ADA
adenosine deaminase
- ADK
adenosine kinase
- AHCY
adenosylhomocysteinase or S-adenosylhomocysteine hydrolase
- ARHL
age-related hearing loss
- BHMT
betaine homocysteine methyltransferase
- Cat
catalase
- CBS
cystathionine β-synthase
- Cx30
Connexin 30
- DAB
3,3′-diaminobenzidine
- FD
folate deficient
- Foxm1
Forkhead box M1
- Foxp3
Forkhead box P3
- Gap43
growth-associated protein 43
- Gclc
glutamate-cysteine ligase or γ-glutamylcysteine synthetase, catalytic subunit
- Gpx1
glutathione peroxidase 1
- Gpx4
glutathione peroxidase 4
- GSH
glutathione reduced form
- Gsr
glutathione reductase
- Gss
glutathione synthetase
- GSSG
glutathione oxidized form
- Hcy
homocysteine
- H&E
hematoxylin and eosin
- MAT
methionine adenosyltransferase
- Mef2a
myocyte enhancer factor 2A
- Mef2d
myocyte enhancer factor 2D
- MnSOD
manganese superoxide dismutase
- MTHFR
methylenetetrahydrofolate reductase
- MTR
methionine synthase
- NF
normal folate
- NOX
NADPH oxidase
- p22phox (Cyba)
cytochrome b-245, α-polypeptide
- PFA
paraformaldehyde
- Rplp0
ribosomal protein large P0
- RT-qPCR
RT-quantitative PCR
- SPL
sound pressure level
- TdT
terminal deoxynucleotidyl transferase
- tHcy
plasma total homocysteine
- T-PBS
Triton X-100 in PBS
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Roth T. N., Hanebuth D., Probst R. (2011) Prevalence of age-related hearing loss in Europe: a review. Eur. Arch. Otorhinolaryngol. 268, 1101–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li-Korotky H. S. (2012) Age-related hearing loss: quality of care for quality of life. Gerontologist 52, 265–271 [DOI] [PubMed] [Google Scholar]
- 3.Dror A. A., Avraham K. B. (2009) Hearing loss: mechanisms revealed by genetics and cell biology. Annu. Rev. Genet. 43, 411–437 [DOI] [PubMed] [Google Scholar]
- 4.Liu X. Z., Yan D. (2007) Ageing and hearing loss. J. Pathol. 211, 188–197 [DOI] [PubMed] [Google Scholar]
- 5.Ohlemiller K. K. (2006) Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 1091, 89–102 [DOI] [PubMed] [Google Scholar]
- 6.Tabuchi K., Nishimura B., Nakamagoe M., Hayashi K., Nakayama M., Hara A. (2011) Ototoxicity: mechanisms of cochlear impairment and its prevention. Curr. Med. Chem. 18, 4866–4871 [DOI] [PubMed] [Google Scholar]
- 7.Sliwinska-Kowalska M., Davis A. (2012) Noise-induced hearing loss. Noise Health 14, 274–280 [DOI] [PubMed] [Google Scholar]
- 8.Emmett S. D., West K. P. Jr (2014) Gestational vitamin A deficiency: a novel cause of sensorineural hearing loss in the developing world? Med. Hypotheses 82, 6–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attias J., Raveh E., Aizer-Dannon A., Bloch-Mimouni A., Fattal-Valevski A. (2012) Auditory system dysfunction due to infantile thiamine deficiency: long-term auditory sequelae. Audiol. Neurootol. 17, 309–320 [DOI] [PubMed] [Google Scholar]
- 10.Karli R., Gül A., Uğur B. (2013) Effect of vitamin B12 deficiency on otoacoustic emissions. Acta Otorhinolaryngol. Ital. 33, 243–247 [PMC free article] [PubMed] [Google Scholar]
- 11.Houston D. K., Johnson M. A., Nozza R. J., Gunter E. W., Shea K. J., Cutler G. M., Edmonds J. T. (1999) Age-related hearing loss, vitamin B-12, and folate in elderly women. Am. J. Clin. Nutr. 69, 564–571 [DOI] [PubMed] [Google Scholar]
- 12.Lasisi A. O., Fehintola F. A., Yusuf O. B. (2010) Age-related hearing loss, vitamin B12, and folate in the elderly. Otolaryngol. Head Neck Surg. 143, 826–830 [DOI] [PubMed] [Google Scholar]
- 13.Cadoni G., Agostino S., Scipione S., Galli J. (2004) Low serum folate levels: a risk factor for sudden sensorineural hearing loss? Acta Otolaryngol. 124, 608–611 [DOI] [PubMed] [Google Scholar]
- 14.Gok U., Halifeoglu I., Canatan H., Yildiz M., Gursu M. F., Gur B. (2004) Comparative analysis of serum homocysteine, folic acid and Vitamin B12 levels in patients with noise-induced hearing loss. Auris Nasus Larynx 31, 19–22 [DOI] [PubMed] [Google Scholar]
- 15.Johnsson L. G., Hawkins J. E. Jr (1972) Vascular changes in the human inner ear associated with aging. Ann. Otol. Rhinol. Laryngol. 81, 364–376 [DOI] [PubMed] [Google Scholar]
- 16.Makishima K. (1978) Arteriolar sclerosis as a cause of presbycusis. Otolaryngology 86, ORL322–ORL326 [DOI] [PubMed] [Google Scholar]
- 17.Rosen S., Olin P. (1965) Hearing loss and coronary heart disease. Bull. N. Y. Acad. Med. 41, 1052–1068 [PMC free article] [PubMed] [Google Scholar]
- 18.Jacques P. F., Selhub J., Bostom A. G., Wilson P. W., Rosenberg I. H. (1999) The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N. Engl. J. Med. 340, 1449–1454 [DOI] [PubMed] [Google Scholar]
- 19.Durga J., Verhoef P., Anteunis L. J., Schouten E., Kok F. J. (2007) Effects of folic acid supplementation on hearing in older adults: a randomized, controlled trial. Ann. Intern. Med. 146, 1–9 [DOI] [PubMed] [Google Scholar]
- 20.Pajares M. A., Pérez-Sala D. (2006) Betaine homocysteine S-methyltransferase: just a regulator of homocysteine metabolism? Cell. Mol. Life Sci. 63, 2792–2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fusconi M., Chistolini A., de Virgilio A., Greco A., Massaro F., Turchetta R., Benincasa A. T., Tombolini M., de Vincentiis M. (2012) Sudden sensorineural hearing loss: a vascular cause? Analysis of prothrombotic risk factors in head and neck. Int. J. Audiol. 51, 800–805 [DOI] [PubMed] [Google Scholar]
- 22.Uchida Y., Sugiura S., Ando F., Nakashima T., Shimokata H. (2011) Hearing impairment risk and interaction of folate metabolism related gene polymorphisms in an aging study. BMC Med. Genet. 12, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durga J., Anteunis L. J., Schouten E. G., Bots M. L., Kok F. J., Verhoef P. (2006) Association of folate with hearing is dependent on the 5,10-methylenetetrahdyrofolate reductase 677C—>T mutation. Neurobiol. Aging 27, 482–489 [DOI] [PubMed] [Google Scholar]
- 24.Kundu S., Munjal C., Tyagi N., Sen U., Tyagi A. C., Tyagi S. C. (2012) Folic acid improves inner ear vascularization in hyperhomocysteinemic mice. Hear. Res. 284, 42–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen-Salmon M., Regnault B., Cayet N., Caille D., Demuth K., Hardelin J. P., Janel N., Meda P., Petit C. (2007) Connexin30 deficiency causes instrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proc. Natl. Acad. Sci. USA 104, 6229–6234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maestro-de-las-Casas C., Pérez-Miguelsanz J., López-Gordillo Y., Maldonado E., Partearroyo T., Varela-Moreiras G., Martínez-Álvarez C. (2013) Maternal folic acid-deficient diet causes congenital malformations in the mouse eye. Birth Defects Res. A Clin. Mol. Teratol. 97, 587–596 [DOI] [PubMed] [Google Scholar]
- 27.Maldonado E., Murillo J., Barrio C., del Río A., Pérez-Miguelsanz J., López-Gordillo Y., Partearroyo T., Paradas I., Maestro C., Martínez-Sanz E., Varela-Moreiras G., Martinez-Alvarez C. (2011) Occurrence of cleft-palate and alteration of Tgf-β(3) expression and the mechanisms leading to palatal fusion in mice following dietary folic-acid deficiency. Cells Tissues Organs (Print) 194, 406–420 [DOI] [PubMed] [Google Scholar]
- 28.Ison J. R., Allen P. D., O’Neill W. E. (2007) Age-related hearing loss in C57BL/6J mice has both frequency-specific and non-frequency-specific components that produce a hyperacusis-like exaggeration of the acoustic startle reflex. J. Assoc. Res. Otolaryngol. 8, 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keithley E. M., Canto C., Zheng Q. Y., Fischel-Ghodsian N., Johnson K. R. (2004) Age-related hearing loss and the ahl locus in mice. Hear. Res. 188, 21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teng Y. W., Mehedint M. G., Garrow T. A., Zeisel S. H. (2011) Deletion of betaine-homocysteine S-methyltransferase in mice perturbs choline and 1-carbon metabolism, resulting in fatty liver and hepatocellular carcinomas. J. Biol. Chem. 286, 36258–36267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cediel R., Riquelme R., Contreras J., Díaz A., Varela-Nieto I. (2006) Sensorineural hearing loss in insulin-like growth factor I-null mice: a new model of human deafness. Eur. J. Neurosci. 23, 587–590 [DOI] [PubMed] [Google Scholar]
- 32.Murillo-Cuesta S., Camarero G., González-Rodríguez A., De La Rosa L. R., Burks D. J., Avendaño C., Valverde A. M., Varela-Nieto I. (2012) Insulin receptor substrate 2 (IRS2)-deficient mice show sensorineural hearing loss that is delayed by concomitant protein tyrosine phosphatase 1B (PTP1B) loss of function. Mol. Med. 18, 260–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodríguez-de la Rosa L., López-Herradón A., Portal-Núñez S., Murillo-Cuesta S., Lozano D., Cediel R., Varela-Nieto I., Esbrit P. (2014) Treatment with N- and C-terminal peptides of parathyroid hormone-related protein partly compensate the skeletal abnormalities in IGF-I deficient mice. PLoS One 9, e87536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez-Calderon H., Rodriguez-de la Rosa L., Milo M., Pichel J. G., Holley M., Varela-Nieto I. (2010) RNA microarray analysis in prenatal mouse cochlea reveals novel IGF-I target genes: implication of MEF2 and FOXM1 transcription factors. PLoS One 5, e8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camarero G., Avendano C., Fernandez-Moreno C., Villar A., Contreras J., de Pablo F., Pichel J. G., Varela-Nieto I. (2001) Delayed inner ear maturation and neuronal loss in postnatal Igf-1-deficient mice. J. Neurosci. 21, 7630–7641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aburto M. R., Magariños M., Leon Y., Varela-Nieto I., Sanchez-Calderon H. (2012) AKT signaling mediates IGF-I survival actions on otic neural progenitors. PLoS One 7, e30790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.González B., Campillo N., Garrido F., Gasset M., Sanz-Aparicio J., Pajares M. A. (2003) Active-site-mutagenesis study of rat liver betaine-homocysteine S-methyltransferase. Biochem. J. 370, 945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horne D. W., Patterson D. (1988) Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin. Chem. 34, 2357–2359 [PubMed] [Google Scholar]
- 39.Tamura T. (1990) Microbiological assays of folates. In Folic Acid Metabolism in Health and Disease (Piccairo M. F., Stokstad R., Gregory J. F., eds.), pp. 121–137, Wiley-Liss, Inc., New York [Google Scholar]
- 40.Rahman I., Kode A., Biswas S. K. (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 1, 3159–3165 [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-de la Rosa L., Fernandez-Sanchez L., Germain F., Murillo-Cuesta S., Varela-Nieto I., de la Villa P., Cuenca N. (2012) Age-related functional and structural retinal modifications in the Igf1-/- null mouse. Neurobiol. Dis. 46, 476–485 [DOI] [PubMed] [Google Scholar]
- 42.Delgado M., Pérez-Miguelsanz J., Garrido F., Rodríguez-Tarduchy G., Pérez-Sala D., Pajares M. A. (2008) Early effects of copper accumulation on methionine metabolism. Cell. Mol. Life Sci. 65, 2080–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang H., Talaska A. E., Schacht J., Sha S. H. (2007) Oxidative imbalance in the aging inner ear. Neurobiol. Aging 28, 1605–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kujawa S. G., Liberman M. C. (2009) Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. 29, 14077–14085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng H., Yin S. H., Tang A. Z. (2011) Blocking caspase-3-dependent pathway preserves hair cells from salicylate-induced apoptosis in the guinea pig cochlea. Mol. Cell. Biochem. 353, 291–303 [DOI] [PubMed] [Google Scholar]
- 47.Tropitzsch A., Arnold H., Bassiouni M., Müller A., Eckhard A., Müller M., Löwenheim H. (2014) Assessing cisplatin-induced ototoxicity and otoprotection in whole organ culture of the mouse inner ear in simulated microgravity. Toxicol. Lett. 227, 203–212 [DOI] [PubMed] [Google Scholar]
- 48.Pajares M. A., Markham G. D. (2011) Methionine adenosyltransferase (s-adenosylmethionine synthetase). Adv. Enzymol. Relat. Areas Mol. Biol. 78, 449–521 [DOI] [PubMed] [Google Scholar]
- 49.Vlajkovic S. M., Guo C. X., Dharmawardana N., Wong A. C., Boison D., Housley G. D., Thorne P. R. (2010) Role of adenosine kinase in cochlear development and response to noise. J. Neurosci. Res. 88, 2598–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hornbeck P. V., Kornhauser J. M., Tkachev S., Zhang B., Skrzypek E., Murray B., Latham V., Sullivan M. (2012) PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 40, D261–D270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamada K., Kawata T., Wada M., Isshiki T., Onoda J., Kawanishi T., Kunou A., Tadokoro T., Tobimatsu T., Maekawa A., Toraya T. (2000) Extremely low activity of methionine synthase in vitamin B-12-deficient rats may be related to effects on coenzyme stabilization rather than to changes in coenzyme induction. J. Nutr. 130, 1894–1900 [DOI] [PubMed] [Google Scholar]
- 52.Vlajkovic S. M., Guo C. X., Telang R., Wong A. C., Paramananthasivam V., Boison D., Housley G. D., Thorne P. R. (2011) Adenosine kinase inhibition in the cochlea delays the onset of age-related hearing loss. Exp. Gerontol. 46, 905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X., Mao X. B., Hei R. Y., Zhang Z. B., Wen L. T., Zhang P. Z., Qiu J. H., Qiao L. (2011) Protective role of hydrogen sulfide against noise-induced cochlear damage: a chronic intracochlear infusion model. PLoS One 6, e26728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jakubowski H., Głowacki R. (2011) Chemical biology of homocysteine thiolactone and related metabolites. Adv. Clin. Chem. 55, 81–103 [DOI] [PubMed] [Google Scholar]
- 55.Lipton S. A., Kim W. K., Choi Y. B., Kumar S., D’Emilia D. M., Rayudu P. V., Arnelle D. R., Stamler J. S. (1997) Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. USA 94, 5923–5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puel J. L., Ladrech S., Chabert R., Pujol R., Eybalin M. (1991) Electrophysiological evidence for the presence of NMDA receptors in the guinea pig cochlea. Hear. Res. 51, 255–264 [DOI] [PubMed] [Google Scholar]
- 57.Pajares M. A., Alvarez L., Pérez-Sala D. (2013) How are mammalian methionine adenosyltransferases regulated in the liver? A focus on redox stress. FEBS Lett. 587, 1711–1716 [DOI] [PubMed] [Google Scholar]
- 58.González B., Garrido F., Ortega R., Martínez-Júlvez M., Revilla-Guarinos A., Pérez-Pertejo Y., Velázquez-Campoy A., Sanz-Aparicio J., Pajares M. A. (2012) NADP+ binding to the regulatory subunit of methionine adenosyltransferase II increases intersubunit binding affinity in the hetero-trimer. PLoS One 7, e50329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radi R. (2013) Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 288, 26464–26472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banerjee R. (2012) Redox outside the box: linking extracellular redox remodeling with intracellular redox metabolism. J. Biol. Chem. 287, 4397–4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lambeth J. D., Neish A. S. (2014) Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu. Rev. Pathol. 9, 119–145 [DOI] [PubMed] [Google Scholar]
- 62.Vlajkovic S. M., Lin S. C., Wong A. C., Wackrow B., Thorne P. R. (2013) Noise-induced changes in expression levels of NADPH oxidases in the cochlea. Hear. Res. 304, 145–152 [DOI] [PubMed] [Google Scholar]
- 63.Kundu S., Tyagi N., Sen U., Tyagi S. C. (2009) Matrix imbalance by inducing expression of metalloproteinase and oxidative stress in cochlea of hyperhomocysteinemic mice. Mol. Cell. Biochem. 332, 215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bánfi B., Malgrange B., Knisz J., Steger K., Dubois-Dauphin M., Krause K. H. (2004) NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 279, 46065–46072 [DOI] [PubMed] [Google Scholar]
- 65.Sánchez-Alcázar J. A., Schneider E., Hernández-Muñoz I., Ruiz-Cabello J., Siles-Rivas E., de la Torre P., Bornstein B., Brea G., Arenas J., Garesse R., Solis-Herruzo J. A., Knox A. J., Navas P. (2003) Reactive oxygen species mediate the down-regulation of mitochondrial transcripts and proteins by tumour necrosis factor-alpha in L929 cells. Biochem. J. 370, 609–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keithley E. M., Canto C., Zheng Q. Y., Wang X., Fischel-Ghodsian N., Johnson K. R. (2005) Cu/Zn superoxide dismutase and age-related hearing loss. Hear. Res. 209, 76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Milton N. G. N. (2008) Homocysteine inhibits hydrogen peroxide breakdown by catalase. Open Enzym. Inhib. J. 1, 34–41 [Google Scholar]
- 68.Quintana-Cabrera R., Fernandez-Fernandez S., Bobo-Jimenez V., Escobar J., Sastre J., Almeida A., Bolaños J. P. (2012) γ-Glutamylcysteine detoxifies reactive oxygen species by acting as glutathione peroxidase-1 cofactor. Nat. Commun. 3, 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu S. C. (2013) Glutathione synthesis. Biochim. Biophys. Acta 1830, 3143–3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu S. C., Mato J. M. (2012) S-adenosylmethionine in liver health, injury, and cancer. Physiol. Rev. 92, 1515–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delgado M., Garrido F., Pérez-Miguelsanz J., Pacheco M., Partearroyo T., Pérez-Sala D., Pajares M. A. (2014) Acute liver injury induces nucleocytoplasmic redistribution of hepatic methionine metabolism enzymes. Antioxid. Redox Signal. 20, 2541–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mosharov E., Cranford M. R., Banerjee R. (2000) The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 39, 13005–13011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.