Abstract
Background: Air pollution is linked to low lung function and to respiratory events, yet little is known of associations with lung structure.
Objectives: We examined associations of particulate matter (PM2.5, PM10) and nitrogen oxides (NOx) with percent emphysema-like lung on computed tomography (CT).
Methods: The Multi-Ethnic Study of Atherosclerosis (MESA) recruited participants (45–84 years of age) in six U.S. states. Percent emphysema was defined as lung regions < –910 Hounsfield Units on cardiac CT scans acquired following a highly standardized protocol. Spirometry was also conducted on a subset. Individual-level 1- and 20-year average air pollution exposures were estimated using spatiotemporal models that included cohort-specific measurements. Multivariable regression was conducted to adjust for traditional risk factors and study location.
Results: Among 6,515 participants, we found evidence of an association between percent emphysema and long-term pollution concentrations in an analysis leveraging between-city exposure contrasts. Higher concentrations of PM2.5 (5 μg/m3) and NOx (25 ppb) over the previous year were associated with 0.6 (95% CI: 0.1, 1.2%) and 0.5 (95% CI: 0.1, 0.9%) higher average percent emphysema, respectively. However, after adjustment for study site the associations were –0.6% (95% CI: –1.5, 0.3%) for PM2.5 and –0.5% (95% CI: –1.1, 0.02%) for NOx. Lower lung function measures (FEV1 and FVC) were associated with higher PM2.5 and NOx levels in 3,791 participants before and after adjustment for study site, though most associations were not statistically significant.
Conclusions: Associations between ambient air pollution and percentage of emphysema-like lung were inconclusive in this cross-sectional study, thus longitudinal analyses may better clarify these associations with percent emphysema.
Citation: Adar SD, Kaufman JD, Diez-Roux AV, Hoffman EA, D’Souza J, Stukovsky KH, Rich SS, Rotter JI, Guo X, Raffel LJ, Sampson PD, Oron AP, Raghunathan T, Barr RG. 2015. Air pollution and percent emphysema identified by computed tomography in the Multi-Ethnic Study of Atherosclerosis. Environ Health Perspect 123:144–151; http://dx.doi.org/10.1289/ehp.1307951
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the 10 most debilitating illnesses worldwide (Vos et al. 2012). In 2010, 329 million people were estimated to have COPD, with nearly 29,000 productive person-years lost each year. Recent estimates suggest that COPD is currently the world’s third leading cause of death and the fifth leading cause of years lived with disability (Lozano et al. 2013; Vos et al. 2012).
COPD is defined physiologically by airflow limitation that is not fully reversible (Celli et al. 2004; Vestbo et al. 2013). Pulmonary emphysema is defined anatomically by destruction of interalveolar septae and loss of lung tissue and overlaps only partially with COPD. Although smoking is a leading cause of emphysema (Hogg 2004), only weak associations have been documented between emphysema severity and pack-years of cigarette smoking in the general population and in COPD patients (Hogg et al. 1994; Powell et al. 2013). In addition, emphysema has been shown to also develop in never-smokers (Auerbach et al. 1972). Thus, questions remain as to risk factors for the etiology of emphysema.
Exposures to airborne particulate matter (PM) in outdoor, indoor, and workplace air may contribute to the development of emphysema. Epidemiological studies have consistently linked short-term peaks of PM with respiratory outcomes including morbidity and mortality of individuals with COPD (Kelly and Fussell 2011). Greater long-term exposures to air pollution have also been associated with slowed lung growth in children (Avol et al. 2001; Gauderman et al. 2004; Rojas-Martinez et al. 2007) and more rapid decline in lung function in adults (Detels et al. 1991; Downs et al. 2007; Tashkin et al. 1994). Studies have similarly shown that greater long-term levels of PM and traffic-related air pollution are associated with higher incident and prevalent COPD (Andersen et al. 2011; Chen et al. 2005; Karakatsani et al. 2003; Lindgren et al. 2009; Schikowski et al. 2005; Sunyer 2001). To our knowledge, however, there has been no direct assessment of the relationship of ambient air pollution to pulmonary emphysema in an epidemiologic study.
Computed tomography (CT) provides an opportunity to assess pulmonary emphysema and changes in lung structure in vivo even among those with normal lung function.(Sanders et al. 1988). Here we examine the associations between long-term exposure to airborne PM ≤ 2.5 and ≤ 10 μm in aerodynamic diameter (PM2.5, PM10) and oxides of nitrogen (NOx; an indicator of traffic pollution) with emphysema-like lung on CT in a large, multi-ethnic cohort of adults. In secondary analyses, we also assessed associations with lung function.
Methods
Study sample. The Multi-Ethnic Study of Atherosclerosis (MESA) recruited 6,814 white, black, Hispanic, and Chinese men and women in Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan, New York; and St. Paul, Minnesota, between 2000 and 2002 (Bild et al. 2002). Participants, 45–84 years of age, were free of clinical cardiovascular disease at baseline. The MESA Air ancillary study recruited 257 additional participants from Rockland County, New York, and Los Angeles and Riverside Counties, California, in 2006–2007 using the same inclusion criteria (Kaufman et al. 2012). The MESA Family ancillary study recruited 1,542 additional black and Hispanic participants at all MESA centers in 2004–2007. Institutional review board approval and informed participant consent were obtained. Participants without consent for address geocoding and those without complete outcome, exposure, and key covariate data were excluded from statistical analysis.
Emphysema-like lung (percent emphysema). Two sequential axial scans were collected during each participant’s baseline visit using a highly standardized protocol following breath-holds at full inspiration (Carr et al. 2005). Cardiac scans were collected using a multidetector or electron-beam CT, depending on the technology available at each study site, and included approximately 70% of the lung volume from the carina to the lung bases. As described previously (Guo et al. 2002), percent emphysema was quantified by one of several blinded image analysts at a central reading center using the Pulmonary Analysis Software Suite (Guo et al. 2008), which was modified to read the lung fields of a cardiac CT. This measure of emphysema relies on image brightness, which can be used to differentiate tissue from air. Given past pathology research and the mild degree of emphysema in this population, we a priori defined percent emphysema as the number of voxels less than –910 Hounsfield Units (HU) divided by the total number of voxels in the lung field (Coxson et al. 1995; Gevenois et al. 1995). Sensitivity analyses explored a –950 HU threshold, which reflects more severe emphysema-like lung regions.
All measures were calibrated using the observed attenuation of air surrounding the body versus a theoretical attenuation of –1,000 HU. Scans with the largest air volume were selected unless there were image quality issues, in which case the higher-quality scan was selected (Hoffman et al. 2009). In a study of 119 participants, excellent agreement for percent emphysema was documented on replicate scans [intraclass correlation coefficient (ICC): 0.89–0.93 at follow-up exams and baseline exams, respectively]. Paired measurements from 10 individuals who were sequentially scanned using both multi-detector and electron beam CTs also demonstrated high correlation (r = 0.94) and very small mean differences (< 1%). Finally, validation of 24 individuals with cardiac CT and full lung scans using multi-detector scanners also demonstrated excellent agreement for percent emphysema (ρ = 0.93) (Hoffman et al. 2009).
Lung function. Between 2004 and 2007 spirometry was performed on a subset of MESA (n = 3,835) and MESA Family (n = 92) participants, and on all MESA Air participants (n = 257). Participants were randomly selected for spirometry in MESA if they had consented to genetic analysis and had baseline measures of endothelial function; Chinese Americans were also oversampled to ensure adequate sample size for stratified and adjusted analyses (Rodriguez et al. 2010). Spirometry was conducted in accordance with the American Thoracic Society/European Respiratory Society guidelines (Miller et al. 2005) using a dry-rolling seal spirometer (Occupational Marketing, Inc., Houston, TX), and all tests were read by one investigator (Hankinson et al. 2010). Replicate testing of 10% of study participants within 2 weeks of the same examination yielded an average inter- and intratechnician ICC for forced expiratory volume in 1 sec (FEV1) and forced vital capacity (FVC) of 0.99. Airflow limitation was defined as having an FEV1/FVC and FEV1 less than the lower limit of normal (LLN) with a sensitivity analysis definition of only the FEV1/FVC ratio less than the LLN (Gläser et al. 2010). LLN were defined using reference equations from the National Health and Nutrition Examination Survey III (Hankinson et al. 1999; Miller et al. 2005) with a 0.88 correction for Asians (Hankinson et al. 2010).
Participant characteristics. Participant health data were collected during each examination, including anthropometry measures such as height and weight as well as self-reported information on demographics, medical history, medication use, and smoking exposures (Bild et al. 2002). Urinary cotinine levels were also measured on participants with spirometry. Residential addresses were assigned geographic coordinates using ArcGIS v9.1 (ESRI, Redlands, CA) and the Dynamap 2000 street network (TeleAtlas, Boston, MA).
Exposure assignment. Long-term ambient air pollution concentrations were estimated for all participant addresses using residential history data and area-specific prediction models that incorporated time-varying trends and spatial effects using a large suite of spatial covariates detailed elsewhere (Cohen et al. 2009; Raghunathan et al. 2006; Sampson et al. 2011; Szpiro et al. 2010). Our main analyses used modeled-based estimates of average PM2.5 and NOx concentrations at participants’ residences during the year before the baseline exam, which were estimated using intensive MESA-specific measurements as well as more spatially limited data from the U.S. Environmental Protection Agency’s Air Quality System (AQS; http://www.epa.gov/ttn/airs/airsaqs/). Because these estimates were not available before 1999, we used these 1-year average exposure estimates as proxies of long-term exposures. We also estimated associations between outcomes and average PM2.5 and PM10 concentrations between 1980 and 2000 (referred to as 20-year average exposures) that were estimated in a prior MESA ancillary study using models constructed on AQS data for PM10 and a PM2.5/PM10 ratio (Raghunathan et al. 2006). These estimates had more temporal but less spatial information, so they were explored in secondary analyses. For sensitivity analyses we also obtained PM2.5 concentrations at AQS monitoring stations and meteorological data from the National Oceanic and Atmospheric Administration (http://www.ncdc.noaa.gov) on the day before each clinical exam.
Data analysis. Multivariable regression modeling was performed with SAS v9.2 (SAS Institute Inc., Cary, NC) to examine cross-sectional associations between percent emphysema and long-term exposures to air pollutants. Percent emphysema had a strongly skewed distribution, but because alternate distributions (e.g., the gamma distribution) generated results with similar directionality and significance to our main findings (data not shown), we modeled the outcome as an untransformed variable. Linear regression was used for FEV1, FVC, and the ratio of FEV1/FVC, and logistic regression was used for airflow limitation (present versus absent).
Modeling was performed with increasing levels of control for potential confounders defined at the time of the examination. All models were adjusted for continuous age and height (with a linear term for percent emphysema models and square terms for pulmonary function models), body mass index (with squared and cubic terms for percent emphysema models and a linear term for pulmonary function models), and pollution as a linear term. Categorical variables in all models included sex, race/ethnicity (white, black, Chinese American, Hispanic), education (< high school, high school degree, some college without a degree, technical or associates degree, bachelors degree, advanced degree), birth location (United States, Puerto Rico, other country), smoking status (never, former, current), pack-years (0, > 0 to 10, > 10 to 20, > 20), cigarettes per day (0 to < 5, 5 to < 10, 10 to < 20, > 20), and exposure to active or secondhand smoke (yes or no). Models for percent emphysema also included a categorical term for CT scanner (electron-beam, non-Siemens multidetector, Siemens multidetector) and an interaction between body weight (≤ 220 lb or > 220 lb) and CT scanner since the radiation was increased 25% for individuals > 220 lb. For our lung function and airflow limitation models, we also controlled for household size and MESA examination (2000–2002, 2004–2005, 2005–2007) and binary variables for hay fever, secondhand smoke exposures (ever or never) in childhood, the workplace, and at home as well as workplace exposures to dust, fumes, or vapors (ever or never). These data (i.e., hay fever, childhood and workplace exposures) were incomplete in the larger cohort, but sensitivity analyses indicated that adjustment did not influence associations between air pollution and percent emphysema. Associations between air pollutants and all outcomes were also robust to adjustment for 1-day average PM2.5 concentrations, temperature, and relatively humidity, personal wealth, neighborhood socioeconomic status, asthma before 45 years of age, family history of emphysema, cotinine, cigar and pipe smoking, medication use (i.e., anticholinergics, beta2-agonists, and inhaled steroids), so these covariates were not included in our models in the interest of parsimony. All analyses were controlled for metropolitan area as a fixed effect in the final model to explore potential confounding by study location, though this was expected to reduce power because between-center differences in pollutant levels were known to be large. Mixed models with random effects for site and generalized estimating equations with robust standard errors were also tested in sensitivity analysis but were not presented because they had similar conclusions with respect to direction, magnitude, and significance of the associations and are less able to reliably estimate between-site variability with only six study sites.
Modification of the associations by age (categorized by decade of age), race/ethnicity, sex, education, smoking status, and metropolitan area was also explored using interaction terms and global F-tests. Statistical significance was defined based on a p-value < 0.05. We furthermore tested the sensitivity of our results to restriction to nonmovers (> 10 years of residential stability).
Results
Of the 7,014 participants with percent emphysema assessments who consented to geocoding, 6,515 had complete 1-year average exposure and covariate information. Because 20-year estimates of PM10 and PM2.5 were available in the main MESA cohort only, we investigated these exposures among 4,813 participants. For lung function, we included 3,791 of the 4,182 participants who consented to geocoding based on complete 1-year average exposure and covariate information. Of those, 2,811 had 20-year exposure estimates. For detailed counts of individuals for each analysis, see Supplemental Material, Figure S1.
As shown in Table 1, there were roughly equal numbers of male and female participants with a mean age of 62 years at the time of CT scanning. Approximately 50% were former or current smokers, and 30% had smoked > 10 pack-years. The mean percent emphysema (–910 HU) was 20%. Average percent predicted was approximately 94% for FEV1 and 95% for FVC. Approximately 6% of the cohort had airflow limitation by either definition considered. Those included in the secondary analyses of the 20-year exposures were generally similar to those in the primary cohort (Table 1).
Table 1.
Descriptive characteristics (mean ± SD or %) of study participants.
| Characteristic | Emphysema cohort | Lung function cohort | ||
|---|---|---|---|---|
| 1-year estimate(n= 6,515) | 20-year estimate(n = 4,813) | 1-year estimate(n = 3,791) | 20-year estimate(n = 2,811) | |
| Percent emphysema (%), –910 HU | 19.9 ± 13.4 | 20.5 ± 13.6 | 20.2 ± 13.3 | 20.7 ± 13.5 |
| Airflow limitation (%)a | 5.7 | 5.8 | 5.9 | 5.9 |
| Percent predicted FEV1 | 93.9 ± 17.8 | 93.5 ± 18.1 | 93.8 ± 17.9 | 93.4 ± 18.2 |
| Percent predicted FVC | 95.5 ± 16.2 | 95.2 ± 16.3 | 95.4 ± 16.2 | 95.2 ± 16.3 |
| Percent predicted FEV1/FVC | 98.5 ± 10.7 | 98.4 ± 10.9 | 98.4 ± 10.7 | 98.3 ± 10.9 |
| FEV1 (L) | 2.4 ± 0.7 | 2.4 ± 0.7 | 2.4 ± 0.7 | 2.4 ± 0.7 |
| FVC (L) | 3.2 ± 1.0 | 3.2 ± 1.0 | 3.2 ± 1.0 | 3.2 ± 1.0 |
| FEV1/FVC (%) | 75.1 ± 8.5 | 75.0 ± 8.7 | 75.0 ± 8.5 | 74.9 ± 8.6 |
| Age (years) | 62 ± 10 | 62 ± 10 | 61 ± 10 | 62 ± 10 |
| Female (%) | 54 | 53 | 51 | 50 |
| Race/ethnicity (%) | ||||
| White | 37 | 43 | 36 | 39 |
| Black | 28 | 30 | 24 | 28 |
| Chinese | 11 | 7 | 15 | 10 |
| Hispanic | 24 | 21 | 25 | 22 |
| Education (%) | ||||
| Less than high school | 17 | 15 | 18 | 15 |
| High school | 18 | 19 | 17 | 19 |
| Higher education | 47 | 47 | 46 | 46 |
| Advanced degree | 18 | 19 | 19 | 20 |
| Any smoke exposure (%) | 48 | 50 | 46 | 49 |
| Smoking status (%) | ||||
| Never | 51 | 49 | 48 | 46 |
| Former | 36 | 38 | 42 | 44 |
| Current | 13 | 13 | 10 | 10 |
| Pack-years of smoking (%) | ||||
| 0 | 52 | 50 | 54 | 52 |
| ≤ 10 | 19 | 19 | 16 | 15 |
| > 10 and ≤ 20 | 10 | 10 | 9 | 9 |
| > 20 | 20 | 21 | 21 | 23 |
| Residential stability (years) | ||||
| ≥ 10 | 69 | 75 | 68 | 75 |
| ≥ 20 | 45 | 52 | 44 | 51 |
| Study site (%) | ||||
| Winston-Salem, NC | 15 | 17 | 13 | 15 |
| New York, NY | 18 | 16 | 23 | 19 |
| Baltimore, MD | 14 | 16 | 11 | 14 |
| St. Paul, MN | 15 | 17 | 13 | 15 |
| Chicago, IL | 18 | 18 | 18 | 19 |
| Los Angeles, CA | 20 | 16 | 23 | 17 |
| Air pollution | ||||
| PM2.5 (μg/m3) | 16.3 ± 3.7 | 22.0 ± 5.0 | 14.2 ± 2.4 | 22.2 ± 5.0 |
| PM10 (μg/m3) | NA | 34.3 ± 7.7 | NA | 34.7 ± 7.7 |
| NOx (ppb) | 48.3 ± 25.2 | NA | 41.1 ± 21.1 | NA |
| NA, not applicable. aAir flow restriction defined as an FEV1/FVC and FEV1 less than the lower limit of normal (LLN). | ||||
Long-term estimates of each air pollutant are presented in Table 1. Concentrations declined over time, such that the 20-year averages of PM2.5 were consistently higher than the more recent 1-year average levels. Spatial contrasts in PM2.5 were consistent over time, however, with the highest concentrations in Los Angeles and the lowest concentrations in St. Paul (Figure 1). PM10 followed similar spatial patterns and was highly correlated with PM2.5 in the overall data (ρ: 0.7–0.9) but weakly correlated after stratification by metropolitan area (average ρ: 0.1–0.3). NOx had lower correlations with PM10 and PM2.5 (overall ρ: 0.5–0.6; area-specific ρ: 0.1–0.3). Similar concentrations of PM2.5 and NOx were found between the 1-year and 20-year cohorts except for New York and Los Angeles, where additional study subjects reduced the mean concentrations slightly and increased the overall variability (results not shown).
Figure 1.
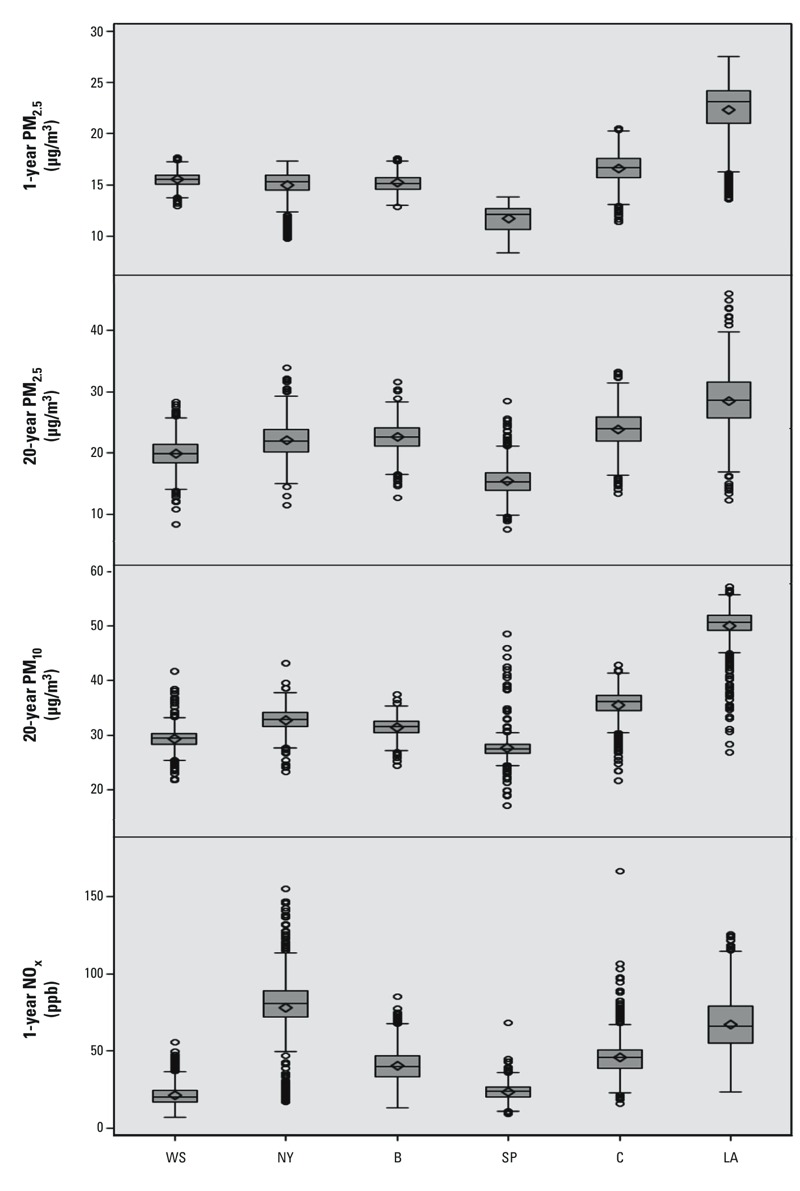
Distribution of individual-level estimates of long-term PM2.5, PM10, and NOx concentrations at participant residences by city and averaging period. Abbreviations: B, Baltimore; C, Chicago; LA, Los Angeles; NY, New York; SP, St. Paul; WS, Winston-Salem. Scales vary by plot. Boxes extend from the 25th to the 75th percentile, horizontal bars represent the median, diamonds represent the means, whiskers extend 1.5 times the length of the interquartile range above and below the 75th and 25th percentiles, respectively, and outliers are represented as points.
Table 2 presents relationships between percent emphysema with the different air pollutants and averaging times examined. Without adjustment for study site, higher levels of all pollutants were associated with greater percent emphysema. For example, 5 μg/m3 greater PM2.5 and 25 ppb higher NOx concentrations over the year preceding the clinical visit were associated with 0.6 [95% confidence interval (CI): 0.1, 1.2%] and 0.5 (95% CI: 0.1, 0.9%) higher average percent emphysema. However, after adjustment for study site the associations were –0.6% (95% CI: –1.5, 0.3%) for PM2.5 and –0.5% (95% CI: –1.1, 0.02%) for NOx.
Table 2.
Associations (95% CIs, p-values) between long-term concentrations of pollutants and percent emphysema on CT.
| Model | 1-year average | 20-year average | ||
|---|---|---|---|---|
| PM2.5 (n = 6,515) | NOx (n = 6,515) | PM2.5 (n = 4,813) | PM10 (n = 4,813) | |
| Minimal control (demographics) | 0.4 (–0.1, 0.8) | 0.3 (0.0, 0.6) | 1.0 (0.7, 1.4) | 0.4 (0.1, 0.6) |
| Moderate control (risk factors) | 0.6 (0.1, 1.2) | 0.5 (0.1, 0.9) | 1.0 (0.6, 1.4) | 0.4 (0.1, 0.7) |
| Full control (site adjusted) | –0.6 (–1.5, 0.3) | –0.5 (–1.1, 0.0) | 0.2 (–0.3, 0.7) | –0.5 (–1.2, 0.2) |
| Associations were scaled to 5 μg/m3 for PM and 25 ppb for NOx. Minimal control models were adjusted for age, race/ethnicity, and sex. Moderate control models added height, body mass index, education, household size, birth location, smoking, examination, scanner, and scanner by body size. Full control models incorporated site adjustment using a fixed effect. | ||||
Closer inspection of the data suggested that associations observed before adjustment for study site were strongly influenced by statistically significantly lower mean percent emphysema in St. Paul (see Supplemental Material, Table S1), where air pollution levels were also lowest. The importance of between-city contrasts can be visualized in Figure 2, where the average percent emphysema for each city after controlling for other risk factors is plotted against the city-average 1-year PM2.5 concentrations. In fact, positive associations between percent emphysema and pollution levels were not observed in models excluding St. Paul (results not shown) or for within-city contrasts in any of the study sites (Figure 3).
Figure 2.
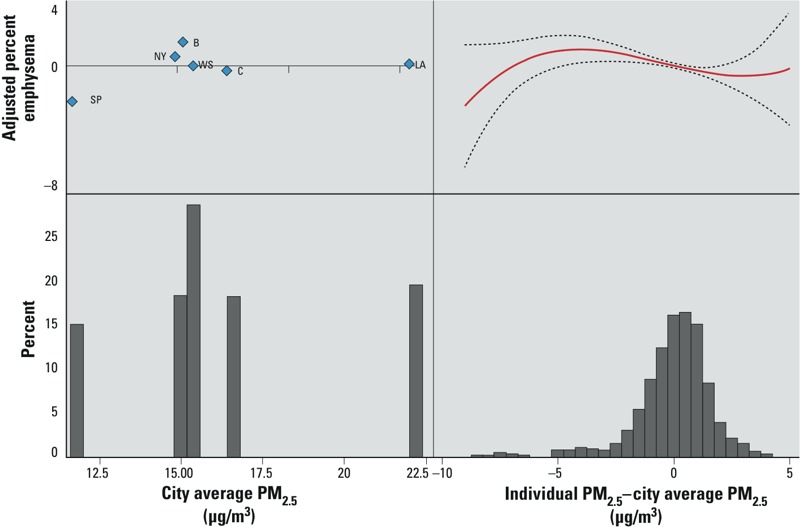
Adjusted relationships between percent emphysema and 1-year PM2.5 concentrations expressed as between-site (city average) and within-site (individual concentration–city average) gradients. The left panel illustrates adjusted city mean emphysema vs. city average PM2.5 concentrations. This reflects the information provided by between-city contrasts. The right panel illustrates the continuous dose–response relationship (in red; 95% CI in dashed lines) between adjusted percent emphysema vs. within-city contrasts in exposures. In both panels, the bottom of the figure represents a frequency distribution of exposures. Abbreviations: B, Baltimore; C, Chicago; LA, Los Angeles; NY, New York; SP, St. Paul; WS, Winston-Salem. All models were adjusted for age, race/ethnicity, sex, height, body mass index, education, household size, birth location, smoking, examination, scanner, and multiple detector computed tomography scanner by body size.
Figure 3.
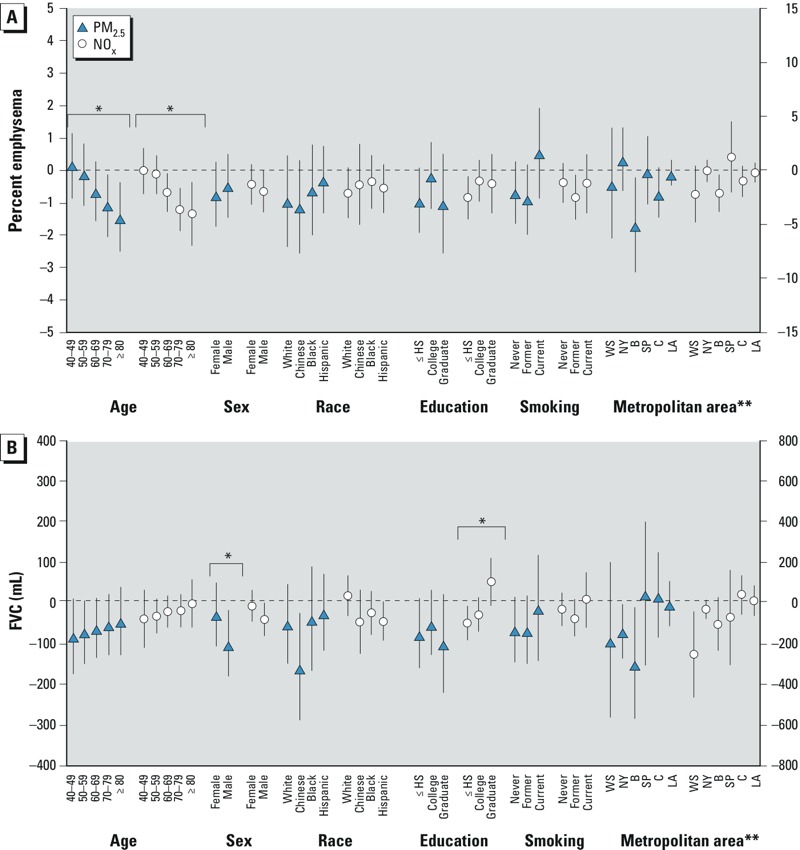
Associations (95% CIs) between 1-year average PM2.5 and NOx concentrations with percent emphysema and FVC by selected personal factors. Models were adjusted for age, race/ethnicity, sex, height, body mass index, education, household size, birth location, smoking, examination, and site. Percent emphysema was further adjusted for scanner and MDCT scanner by body size. Lung function was further adjusted for detailed smoke exposures, workplace exposures, and hay fever. Abbreviations: B, Baltimore; C, Chicago; HS, high school; LA, Los Angeles; NY, New York; SP, St. Paul; WS, Winston-Salem. *Significant effect modification (F-test p-value < 0.05). **Metropolitan area results are presented on secondary (right-hand) axis.
Decreased lung function was consistently observed with higher concentrations of PM2.5 and NOx with and without adjustment for site, although many of the associations did not meet statistical significance (Table 3; see also Supplemental Material, Figure S2). The relationships of the greatest magnitude were between the 1-year average PM2.5 concentrations and FVC with –54 mL (95% CI: –91, –18 mL) and –59 mL (95% CI: –132, 13 mL) lower FVC per 5 μg/m3 before and after control for site, respectively. The 1-year PM2.5 concentration was also more strongly associated with FEV1 than 20-year PM2.5 concentrations, with –24 mL (95% CI: –54, 6 mL) and –20 mL (95% CI: –80, 41 mL) lower FEV1 per 5 μg/m3 before and after control for site, respectively. Higher PM2.5 concentrations (5 μg/m3) over the previous day were associated with lower FEV1 (–5 mL; 95% CI: –13, 4 mL) and FVC (–3 mL; 95% CI: –13, 7 mL) though these could not be distinguished from no association. Associations between all lung function metrics and PM10 were positive but with wide confidence intervals. No consistent associations were observed with the ratio of FEV1/FVC or airflow limitation.
Table 3.
Associations (95% CIs) between pollutants and lung function.
| Model | 1-year average | 20-year average | ||
|---|---|---|---|---|
| PM2.5 (n = 3,791) | NOx (n = 3,791) | PM2.5 (n = 2,811) | PM10 (n = 2,811) | |
| Difference in mean FEV1 (mL) | ||||
| Minimal control (demographics) | –27 (–58, 4) | –22 (–40, –4) | –4 (–21, 13) | 13 (1, 24) |
| Moderate control (risk factors) | –24 (–54, 6) | –12 (–30, 7) | –15 (–31, 2) | 6 (–5, 18) |
| Full control (site adjusted) | –20 (–80, 41) | –4 (–33, 25) | –13 (–37, 11) | 1 (–30, 32) |
| Difference in mean FVC (mL) | ||||
| Minimal control (demographics) | –64 (–101, –26) | –20 (–42, 2) | –9 (–29, 12) | 12 (–2, 26) |
| Moderate control (risk factors) | –54 (–91, –18) | –9 (–31, 14) | –19 (–39, 0) | 6 (–8, 20) |
| Full control (site adjusted) | –59 (–132, 13) | –21 (–55, 14) | –6 (–35, 22) | 19 (–29, 45) |
| Difference in mean FEV1/FVC (%) | ||||
| Minimal control (demographics) | 0.6 (0.0, 1.1) | –0.3 (–0.8, 0.0) | 0.1 (–0.2, 0.4) | 0.1 (–0.1, 0.3) |
| Moderate control (risk factors) | 0.4 (–0.2, 1.0) | –0.3 (–0.5, 0.0) | 0.0 (–0.3, 0.3) | 0.1 (–0.2, 0.3) |
| Full control (site adjusted) | 0.2 (–0.9, 1.3) | 0.3 (–0.3, 0.8) | –0.3 (–0.7, 0.2) | 0.3 (–0.8, 0.4) |
| Odds of airflow limitation | ||||
| Minimal control (demographics) | 1.2 (0.9, 1.6) | 1.3 (1.1, 1.5) | 1.1 (0.9, 1.3) | 1.0 (0.9, 1.1) |
| Moderate control (risk factors) | 1.2 (0.9, 1.6) | 1.3 (1.0, 1.5) | 1.2 (1.0, 1.4) | 1.1 (0.9, 1.2) |
| Full control (site adjusted) | 0.9 (0.5, 1.7) | 1.1 (0.8, 1.4) | 1.1 (0.8, 1.5) | 1.1 (0.8, 1.6) |
| Associations were scaled to 5 μg/m3 for PM and 25 ppb for NOx. Minimal control models included age, race/ethnicity, and sex. Moderate control models added height, body mass index, education, household size, birth location, smoking, examination, detailed smoke exposures, workplace exposures, and hay fever. Full control included site adjustment using a fixed effect. | ||||
In secondary analyses, we found limited evidence of effect modification of associations by personal characteristics (Figure 3). The most consistent findings across pollutants and outcomes were increasingly negative associations between air pollution and percent emphysema and increasingly positive associations with lung function measures among persons of greater age in models adjusting for study site. There was also some evidence of significant effect modification of the relationship between NOx and FVC as well as FEV1 (results not shown) by sex and education, but the same was not true for PM2.5. Other sensitivity analyses indicated that all results were qualitatively robust (similar magnitude, direction, and significance) to using an alternate definition of airflow limitation and restricting to individuals who had not moved in the previous 10 years (results not shown). Significant positive associations were also demonstrated between percent emphysema defined using a –950 HU threshold with the 1-year average of NOx and 20-year average of PM2.5 before adjustment for study site, though less consistent findings were found with the other pollutants. All associations with percent emphysema defined by –950 HU had similar directionality and significance after controlling for study site (results not shown).
Discussion
In this large, multi-center study, we found weak evidence of an association between long-term exposures to air pollution and emphysema. Higher long-term PM2.5, PM10, and NOx concentrations between study sites were associated with greater percent emphysema, though these findings were driven by differences between study sites and were not replicated for within-site exposure contrasts. Suggestive but imprecise associations were also identified between air pollution and lung function, with lower FEV1 and FVC observed among persons with higher long-term levels of PM2.5 and NOx.
This research is unique in its use of percent emphysema on CT scan to study associations between air pollution exposures and respiratory health in a large cohort. CT scans may be a valuable tool for air pollution epidemiology studies because they allow for quantification of early changes in lung structure, as opposed to lung function, which is assessed by traditional spirometry testing. This may lead to important contributions because a recent review of the associations between air pollution and COPD (Schikowski et al. 2014) discussed the limitations of existing studies in their ability to characterize subclinical phenotypes and progression of COPD. Although careful consideration must be made given the additional cost and radiation exposure to participants, albeit small, percent emphysema may also have clinical importance because it has been linked with increased risks of mortality in several, though not all, studies (Dawkins et al. 2003; Haruna et al. 2010; Johannessen et al. 2013; Martinez et al. 2006; Sverzellati et al. 2012).
Although little is known of air pollution’s impacts on emphysema, past research generally supports a link between the inhalation of ambient pollutants and adverse impacts on the pulmonary system (Kelly and Fussell 2011). Biologically, this is hypothesized to occur via several interconnected mechanisms including pulmonary oxidative stress and inflammation (Adar et al. 2007; Budinger et al. 2011; Happo et al. 2010; Stringer and Kobzik 1998), alterations in airway ciliary activity (Calderón-Garcidueñas et al. 2001), as well as enhanced susceptibility to respiratory infections (Stern et al. 2013), which can ultimately lead to long-term damage to the lungs including loss of alveolar tissue (i.e., emphysema). Although the larger inhaled particles of tobacco smoke or ambient PM are deposited higher in the airways and likely result in a more classically bronchitic phenotype, PM2.5 deposits more heavily in the alveoli, likely resulting in more parenchymal rather than airway damage (U.S. Environmental Protection Agency 2009).
Consistent with the toxicological literature, epidemiology studies similarly show evidence of increased respiratory symptoms and hospitalizations with air pollution exposure (Bayer-Oglesby et al. 2006; Brauer et al. 2007; Dominici et al. 2006; Martins et al. 2002) as well as evidence of slowed lung growth among cohorts of children followed over time in several different countries (Gauderman et al. 2004; Horak et al. 2002; Mölter et al. 2013). The SAPALDIA study (Swiss Study on Air Pollution and Lung Diseases in Adults) similarly demonstrated slower age-related declines in FEV1 with larger reductions in pollution over time in approximately 10,000 Swiss adults (Downs et al. 2007), though no association was reported between NO2 and FEV1 decline among 2,644 British adults (Pujades-Rodríguez et al. 2009). Higher long-term concentrations of air pollutants, including particles and traffic-related pollutants, have also been associated with increased odds of COPD in Germany (Schikowski et al. 2005) and risk of incident COPD hospitalizations in Denmark and Canada (Andersen et al. 2011; Gan et al. 2013). A smaller study of approximately 400 German women further reported lower prevalent COPD with larger reductions in PM10 over time (Schikowski et al. 2010). Occupational settings have shown linkages between particulate exposures, emphysema, and COPD even after control for cigarette smoking (Coggon and Newman Taylor 1998; Diaz-Guzman et al. 2012; Green et al. 1998). Although one analysis of long-term exposure to PM2.5 linked higher concentrations with lower risk of COPD death in the United States, this work relied on death certificates for outcome ascertainment, and it was hypothesized that this unexpected apparent protective relationship may have been an artifact of competing risks, because pneumonia and cardiovascular events were positively associated with air pollution (Pope et al. 2004).
In this study, we also found consistent evidence of inverse associations between air pollution and emphysema among the oldest participants (70–79 and ≥ 80 years) for both PM2.5 and NOx as well as weaker associations between pollution and lung function among the oldest participants. These unexpected findings can likely be explained by the unique population of MESA, which recruited older adults without clinical cardiovascular disease at baseline. Given that air pollution has also been linked to cardiovascular disease (Brook et al. 2010), our findings of increasingly negative associations with greater age may simply reflect the selection of older individuals in the study who are healthier and less susceptible to air pollution than the general population.
Within MESA, exposure and outcomes varied substantially between study sites, and these differences were especially influential in models for emphysema. As a result, our results for percent emphysema but not lung function were sensitive to adjustment for study site. Importantly, our results remained largely insensitive to control for personal-level socioeconomic status including education, household size, and a wealth index. Nevertheless, there remains the possibility for residual confounding by unmeasured factors. Regional differences may have played an important role: A detailed investigation of our findings suggests that our overall results for percent emphysema were strongly influenced by data from St. Paul, which had low levels of COPD and low levels of pollution. Interestingly, scanner technology cannot explain these differences because the same scanner used in St. Paul was also used at another study site, and the differences in mean percent emphysema were found even after control for scanner. Although control for study site is likely warranted, even if only to properly estimate our standard errors, including such control reduced the exposure variability given the large contrasts in exposure between locations. Thus, there may be power issues in detecting differences within-city.
An additional possible weakness of this work is that percent emphysema was measured using cardiac scans, which do not include the lung apices and hence may have underestimated the degree of emphysema compared with a full-lung scan. However percent emphysema measurements on MESA cardiac scans have been previously validated against full-lung scans (Hoffman et al. 2009) and health outcomes (Barr et al. 2010, 2012).
A major strength of this study was that we used a well-defined cohort with rich estimates of PM and traffic-related pollutants in outdoor air that capture both spatial and temporal trends. Individual-level 1-year average concentrations were derived using data from intensive monitoring campaigns in participants’ comunities and homes. These estimates were complemented by 20-year estimates, which inform us of long-term exposures over a participant’s long-term residential history, although they have substantially less precision for fine-scale spatial variability. Generally consistent findings were observed for the 1-year and 20-year estimates. In addition, our results were robust among persons with long-term (> 10 years) residential stability.
In summary, this cross-sectional analysis of a large, multi-center, population-based cohort found some suggestive evidence to support the hypothesis that higher long-term air pollution exposures are associated with emphysema. Because results were dominated by contrasts between study sites, however, future work is required to confirm our findings.
Supplemental Material
Acknowledgments
We gratefully acknowledge the other Multi-Ethnic Study of Atherosclerosis (MESA) investigators, institutions, staff, and participants (http://www.mesa-nhlbi.org).
Footnotes
This work was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute, National Institutes of Health (NHLBI/NIH). MESA and Air Pollution (MESA Air) is supported by a U.S. Environmental Protection Agency (EPA) STAR research assistance agreement, RD831697. MESA Family is supported by NHLBI grants and contracts R01HL071051, R01HL071205, R01HL071250-R01HL071252, R01HL071258, and R01HL071259. The MESA Lung studies are funded by NHLBI grants R01HL077612, RC1HL100543, and HL093081. Additional support was provided by U.S. EPA grants RD833741010 and K24 ES013195.
Although supported in part by the U.S. EPA, this work has not been formally reviewed by the U.S. EPA and the views in this document are solely those of the authors. The U.S. EPA does not endorse any products or commercial services mentioned.
R.G.B. receives funding from the Alpha-1 Foundation and royalties from UpToDate, and has received an unrestricted gift of a nutritional supplement for an NIH-funded clinical trial from Cenestra Health. The funding sources did not influence the design, collection, analysis, interpretation of the data, or decision to publish this work. The other authors declare they have no actual or potential competing financial interests.
References
- Adar SD, Adamkiewicz G, Gold DR, Schwartz J, Coull BA, Suh H.2007Ambient and microenvironmental particles and exhaled nitric oxide before and after a group bus trip. Environ Health Perspect 115507–512.; 10.1289/ehp.9386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ZJ, Hvidberg M, Jensen SS, Ketzel M, Loft S, Sørensen M, et al. Chronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution a cohort study. Am J Respir Crit Care Med. 2011;183:455–461. doi: 10.1164/rccm.201006-0937OC. [DOI] [PubMed] [Google Scholar]
- Auerbach O, Hammond EC, Garfinkel L, Benante C. Relation of smoking and age to emphysema—whole-lung section study. New Engl J Med. 1972;286:853–857. doi: 10.1056/NEJM197204202861601. [DOI] [PubMed] [Google Scholar]
- Avol EL, Gauderman WJ, Tan SM, London SJ, Peters JM. Respiratory effects of relocating to areas of differing air pollution levels. Am J Respir Crit Care Med. 2001;164:2067–2072. doi: 10.1164/ajrccm.164.11.2102005. [DOI] [PubMed] [Google Scholar]
- Barr RG, Ahmed FS, Carr JJ, Hoffman EA, Jiang R, Kawut SM, et al. Subclinical atherosclerosis, airflow obstruction and emphysema: the MESA Lung Study. Eur Respir J. 2012;39:846–854. doi: 10.1183/09031936.00165410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. New Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer-Oglesby L, Schindler C, Hazenkamp-von Arx ME, Braun-Fahrländer C, Keidel D, Rapp R, et al. Living near main streets and respiratory symptoms in adults: the Swiss Cohort Study on Air Pollution and Lung Diseases in Adults. Am J Epidemiol. 2006;164:1190–1198. doi: 10.1093/aje/kwj338. [DOI] [PubMed] [Google Scholar]
- Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- Brauer M, Hoek G, Smit HA, de Jongste JC, Gerritsen J, Postma DS, et al. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J. 2007;29:879–888. doi: 10.1183/09031936.00083406. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Budinger GRS, McKell JL, Urich D, Foiles N, Weiss I, Chiarella SE, et al. 2011Particulate matter-induced lung inflammation increases systemic levels of PAI-1 and activates coagulation through distinct mechanisms. PloS One 6e18525; 10.1371/journal.pone.0018525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Valencia-Salazar G, Rodríguez-Alcaraz A, Gambling TM, Garcia R, Osnaya N, et al. Ultrastructural nasal pathology in children chronically and sequentially exposed to air pollutants. Am J Respir Cell Mol Biol. 2001;24:132–138. doi: 10.1165/ajrcmb.24.2.4157. [DOI] [PubMed] [Google Scholar]
- Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- Celli BR, MacNee W, Agusti A, Anzueto A, Berg B, Buist AS, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang QY, Krewski D, Burnett RT, Shi YL, McGrail KM. The effect of coarse ambient particulate matter on first, second, and overall hospital admissions for respiratory disease among the elderly. Inhal Toxicol. 2005;17:649–655. doi: 10.1080/08958370500189420. [DOI] [PubMed] [Google Scholar]
- Coggon D, Newman Taylor A. Coal mining and chronic obstructive pulmonary disease: a review of the evidence. Thorax. 1998;53:398–407. doi: 10.1136/thx.53.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MA, Adar SD, Allen RW, Avol E, Curl CL, Gould T, et al. Approach to estimating participant pollutant exposures in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Environ Sci Technol. 2009;43:4687–4693. doi: 10.1021/es8030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxson HO, Mayo JR, Behzad H, Moore BJ, Verburgt LM, Staples CA, et al. Measurement of lung expansion with computed-tomography and comparison with quantiative histology. J Appl Physiol (1985) 1995;79:1525–1530. doi: 10.1152/jappl.1995.79.5.1525. [DOI] [PubMed] [Google Scholar]
- Dawkins PA, Dowson LJ, Guest PJ, Stockley RA. Predictors of mortality in α1-antitrypsin deficiency. Thorax. 2003;58:1020–1026. doi: 10.1136/thorax.58.12.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detels R, Tashkin DP, Sayre JW, Rokaw SN, Massey FJ, Jr, Coulson AH, et al. The UCLA population studies of CORD: X. A cohort study of changes in respiratory function associated with chronic exposure to SOx, NOx, and hydrocarbons. Am J Public Health. 1991;81:350–359. doi: 10.2105/ajph.81.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Guzman E, Aryal S, Mannino DM. Occupational chronic obstructive pulmonary disease: an update. Clin Chest Med. 2012;33:625–636. doi: 10.1016/j.ccm.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SH, Schindler C, Liu LJ, Keidel D, Bayer-Oglesby L, Brutsche MH, et al. Reduced exposure to PM10 and attenuated age-related decline in lung function. New Engl J Med. 2007;357:2338–2347. doi: 10.1056/NEJMoa073625. [DOI] [PubMed] [Google Scholar]
- Gan WQ, FitzGerald JM, Carlsten C, Sadatsafavi M, Brauer M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am J Respir Crit Care Med. 2013;187:721–727. doi: 10.1164/rccm.201211-2004OC. [DOI] [PubMed] [Google Scholar]
- Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. The effect of air pollution on lung development from 10 to 18 years of age. New Engl J Med. 2004;351:1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152(2):653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- Gläser S, Schäper C, Obst A, Ittermann T, Völzke H, Felix SB, et al. Impact of different definitions of airflow limitation on the prevalence of chronic obstructive pulmonary disease in the general population. Respiration. 2010;80:292–300. doi: 10.1159/000282171. [DOI] [PubMed] [Google Scholar]
- Green FHY, Brower PL, Vallyathan V, Attfield M. In: Advances in the Prevention of Occupational Respiratory Diseases, Vol. 1153 (Chiyotani K, Hosoda Y, Aizawa Y, eds). Amsterdam:Elsevier Science BV, 948–953; 1998. Coal mine dust exposure and type of pulmonary emphysema in coal workers. [Google Scholar]
- Guo J, Fuld MK, Alford SK, Reinhardt JM, Hoffman EA. Pulmonary Analysis Software Suite 9.0: integrating quantitative measures of function with structural analyses. 2008. Available: http://www.lungworkshop.org/2009/proc2008/29-guo.pdf [accessed 12 January 2015]
- Guo J, Reinhardt JM, Kitaoka H, Zhang L, Sonka M, McLennan G, et al. In: 2002 International Symposium on Biomedical Imaging, 871–874; 2002. Integrated system for CT-based assessment of parenchymal lung disease. [Google Scholar]
- Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Chest. 2010;137:138–145. doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Happo MS, Salonen RO, Hälinen AI, Jalava PI, Pennanen AS, Dormans JA, et al. Inflammation and tissue damage in mouse lung by single and repeated dosing of urban air coarse and fine particles collected from six European cities. Inhal Toxicol. 2010;22:402–416. doi: 10.3109/08958370903527908. [DOI] [PubMed] [Google Scholar]
- Haruna A, Muro S, Nakano Y, Ohara T, Hoshino Y, Ogawa E, et al. CT scan findings of emphysema predict mortality in COPD. Chest. 2010;138:635–640. doi: 10.1378/chest.09-2836. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, et al. Reproducibility and validity of lung density measures from cardiac CT scans—the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16:689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- Hogg J, Wright J, Wiggs B, Coxson H, Opazo Saez A, Paré PD. Lung structure and function in cigarette smokers. Thorax. 1994;49:473–478. doi: 10.1136/thx.49.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak F, Jr, Studnicka M, Gartner C, Spengler JD, Tauber E, Urbanek R, et al. Particulate matter and lung function growth in children: a 3-yr follow-up study in Austrian schoolchildren. Eur Respir J. 2002;19:838–845. doi: 10.1183/09031936.02.00512001. [DOI] [PubMed] [Google Scholar]
- Johannessen A, Skorge TD, Bottai M, Grydeland TB, Nilsen RM, Coxson H, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187:602–608. doi: 10.1164/rccm.201209-1722OC. [DOI] [PubMed] [Google Scholar]
- Karakatsani A, Andreadaki S, Katsouyanni K, Dimitroulis I, Trichopoulos D, Benetou V, et al. Air pollution in relation to manifestations of chronic pulmonary disease: a nested case–control study in Athens, Greece. Eur J Epidemiol. 2003;18:45–53. doi: 10.1023/a:1022576028603. [DOI] [PubMed] [Google Scholar]
- Kaufman JD, Adar SD, Allen RW, Barr RG, Budoff MJ, Burke GL, et al. Prospective study of particulate air pollution exposures, subclinical atherosclerosis, and clinical cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Am J Epidemiol. 2012;176:825–837. doi: 10.1093/aje/kws169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly F, Fussell J. Air pollution and airway disease. Clin Exp Allergy. 2011;41:1059–1071. doi: 10.1111/j.1365-2222.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- Lindgren A, Stroh E, Montnémery P, Nihlén U, Jakobsson K, Axmon A.2009Traffic-related air pollution associated with prevalence of asthma and COPD/chronic bronchitis. A cross-sectional study in southern Sweden. Int J Health Geogr 82; 10.1186/1476-072X-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173:1326–1334. doi: 10.1164/rccm.200510-1677OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins LC, Latorre M, Saldiva PHD, Braga ALF. Air pollution and emergency room visits due to chronic lower respiratory diseases in the elderly: an ecological time-series study in São Paulo, Brazil. J Occup Environ Med. 2002;44:622–627. doi: 10.1097/00043764-200207000-00006. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Mölter A, Agius RM, de Vocht F, Lindley S, Gerrard W, Lowe L, et al. 2013Long term exposure to PM10 and NO2 in association with lung volume and airway resistance in the MAAS birth cohort. Environ Health Perspect 1211232–1238.; 10.1289/ehp.1205961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, III, Hansen ML, Long RW, Nielsen KR, Eatough NL, Wilson WE, et al. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004;112:339–345. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell R, Davidson D, Divers J, Manichaikul A, Carr JJ, Detrano R, et al. Genetic ancestry and the relationship of cigarette smoking to lung function and per cent emphysema in four race/ethnic groups: a cross-sectional study. Thorax. 2013;68:634–642. doi: 10.1136/thoraxjnl-2012-202116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujades-Rodríguez M, McKeever T, Lewis S, Whyatt D, Britton J, Venn A.2009Effect of traffic pollution on respiratory and allergic disease in adults: cross-sectional and longitudinal analyses. BMC Pulm Med 942; 10.1186/1471-2466-9-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan T, Diez-Roux AV, Chen W. Predicting cumulative particulate matter exposure using space-time models and historical monitor data [Abstract]. Epidemiology. 2006;17(6) suppl:S250. [Google Scholar]
- Rodriguez J, Jiang R, Johnson WC, MacKenzie BA, Smith LJ, Barr RG. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med. 2010;152:201–210. doi: 10.1059/0003-4819-152-4-201002160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Martinez R, Perez-Padilla R, Olaiz-Fernandez G, Mendoza-Alvarado L, Moreno-Macias H, Fortoul T, et al. Lung function growth in children with long-term exposure to air pollutants in Mexico City. Am J Respir Crit Care Med. 2007;176:377–384. doi: 10.1164/rccm.200510-1678OC. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Szpiro AA, Sheppard L, Lindström J, Kaufman JD. Pragmatic estimation of a spatio-temporal air quality model with irregular monitoring data. Atmos Environ. 2011;45(36):6593–6606. [Google Scholar]
- Sanders C, Nath PH, Bailey WC. Detection of emphysema with computed tomography. Correlation with pulmonary function tests and chest radiography. Invest Radiol. 1988;23:262–266. doi: 10.1097/00004424-198804000-00004. [DOI] [PubMed] [Google Scholar]
- Schikowski T, Mills IC, Anderson HR, Cohen A, Hansell A, Kauffmann F, et al. Ambient air pollution: a cause of COPD? Eur Respir J. 2014;43(1):250–263. doi: 10.1183/09031936.00100112. [DOI] [PubMed] [Google Scholar]
- Schikowski T, Ranft U, Sugiri D, Vierkötter A, Brüning T, Harth V, et al. 2010Decline in air pollution and change in prevalence in respiratory symptoms and chronic obstructive pulmonary disease in elderly women. Respir Res 11113; 10.1186/1465-9921-11-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikowski T, Sugiri D, Ranft U, Gehring U, Heinrich J, Wichmann HE, et al. 2005Long-term air pollution exposure and living close to busy roads are associated with COPD in women. Respir Res 61152; 10.1186/1465-9921-6-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern G, Latzin P, Röösli M, Fuchs O, Proietti E, Kuehni C, et al. A prospective study of the impact of air pollution on respiratory symptoms and infections in infants. Am J Respir Crit Care Med. 2013;187:1341–1348. doi: 10.1164/rccm.201211-2008OC. [DOI] [PubMed] [Google Scholar]
- Stringer B, Kobzik L. Environmental particulate-mediated cytokine production in lung epithelial cells (A549): role of pre-existing inflammation and oxidant stress. J Toxicol Environ Health A. 1998;55:31–44. doi: 10.1080/009841098158601. [DOI] [PubMed] [Google Scholar]
- Sunyer J. Urban air pollution and chronic obstructive pulmonary disease: a review. Eur Respir J. 2001;17:1024–1033. doi: 10.1183/09031936.01.17510240. [DOI] [PubMed] [Google Scholar]
- Sverzellati N, Cademartiri F, Bravi F, Martini C, Gira FA, Maffei E, et al. Relationship and prognostic value of modified coronary artery calcium score, FEV1, and emphysema in lung cancer screening population: the Mild trial. Radiology. 2012;262:460–467. doi: 10.1148/radiol.11110364. [DOI] [PubMed] [Google Scholar]
- Szpiro AA, Sampson PD, Sheppard L, Lumley T, Adar SD, Kaufman JD. Predicting intra-urban variation in air pollution concentrations with complex spatio-temporal dependencies. Environmetrics. 2010;21:606–631. doi: 10.1002/env.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashkin DP, Detels R, Simmons M, Liu H, Coulson AH, Sayre J, et al. The UCLA population studies of chronic obsutructive respiratory disease: XI. Impact of air pollution and smoking on annual change in forced expiratory volume in one second. Am J Respir Crit Care Med. 1994;149:1209–1217. doi: 10.1164/ajrccm.149.5.8173761. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. EPA/600/R-08/139F. Research Triangle Park, NC:U.S. EPA. 2009. Integrated Science Assessment for Particulate Matter (Final Report). [PubMed] [Google Scholar]
- Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


