Abstract
The limited regenerative capacity of articular cartilage and deficiencies of current treatments have motivated the investigation of new repair technologies. In vitro cartilage generation using primary cell sources is limited by cell availability and expansion potential. Pluripotent stem cells possess the capacity for chondrocytic differentiation and extended expansion, providing a potential future solution to cell-based cartilage regeneration. However, despite successes in producing cartilage using adult and embryonic stem cells, the translation of these technologies to the clinic has been severely limited. This review discusses recent advances in stem cell-based cartilage tissue engineering and the major current limitations to clinical translation of these products. Concerns regarding appropriate animal models and studies, stem cell manufacturing, and relevant regulatory processes and guidelines will be addressed. Understanding the significant hurdles limiting the clinical use of stem cell-based cartilage may guide future developments in the fields of tissue engineering and regenerative medicine.
Keywords: regenerative medicine, osteoarthritis, cartilage tissue engineering, clinical trials, stem cell treatments
Introduction
Articular cartilage has a unique extracellular matrix (ECM) composition and structure enabling it to withstand the high loads of joints like the knee. Trauma or degenerative conditions, such as osteoarthritis, can permanently damage cartilage, which has an intrinsically limited healing capacity, and subsequently reduce joint mobility. The tremendous burden resulting from arthritis, projected to affect 67 million individuals by 2030 (Hootman and Helmick, 2006), has spurred the investigation of new treatment strategies. Disadvantages of current surgical treatments for cartilage healing—including donor site morbidity and biomechanically inferior fibrocartilage formation (Horas et al., 2003)—have prompted the investigation of tissue engineering, which aims to produce neocartilage that can function in the native environment and thus restore joint function. The resulting new treatments should avoid requiring the patient’s own cells or tissue, discourage multiple surgeries, and emphasize functionality upon implantation by recapitulating articular cartilage properties.
Cartilage regenerative medicine encompasses approaches to repair, regenerate, or treat defects or pathologies via stem cell use or induction. Examples include MSC injections or chemo-attraction of neighboring stem cells. The classical tissue engineering approach involves scaffolds, cells, and signals. Tissue engineering and regenerative medicine overlap where tissue implants are engineered using stem cells. Cell sourcing issues, including limited expansion potential (Darling and Athanasiou, 2005) and scarcity of primary chondrocytes, hinder clinical translation of cartilage tissue engineering technologies, which often require large cell numbers. This limitation spurs the investigation of stem cell-based cartilage tissue engineering. Chemical (e.g. TGF-β superfamily growth factors) and mechanical (e.g. compressive or tensile loading) signals are typically used to differentiate stem cells down a chondrocytic lineage, before and/or after scaffold placement.
Human embryonic stem cells (hESCs) constitute a promising cell source that can provide large cell numbers and circumvent primary cell sourcing issues. hESCs and induced pluripotent stem cells (iPSCs) are immortal and pluripotent, but require extensive manipulation prior to obtaining chondrogenic cells. Multipotent adult mesenchymal stem cells (MSCs) are also investigated for cartilage regeneration. MSCs with the capacity to differentiate into cartilage have been isolated from bone marrow, adipose tissue (ASCs), umbilical cord matrix, skin, and synovial tissue. In an allogeneic clinical treatment, employing MSCs or hESCs would eliminate the need for harvesting patient cells and reduce the treatment turnaround time. In addition, production of allogeneic stem cell banks increases the product’s commercial potential.
To determine the success of stem cell-based neocartilage engineering, native tissue must provide benchmarks. Hyaline articular cartilage consists of a solid phase, interstitial fluid, and mobile ions. The solid phase mainly consists of ECM, including collagen (50–75%/dw) and proteoglycans (15–30%/dw); chondrocytes occupy 1–5% of the tissue by volume (Hu and Athanasiou, 2003; Little, Bawolin, and Chen, 2011). The rest is primarily water and dissolved solutes, comprising 60–85% of cartilage by wet weight (Mow, Ratcliffe, and Poole, 1992). Glycosaminoglycans (GAGs) associate with aggrecan molecules and confer a large negative charge to cartilage, sequestering water and creating osmotic pressure that resists compressive loads, giving cartilage a compressive modulus up to 2·10MPa (Schinagl et al., 1997). Because the fluid phase bears the initial load, hydrostatic pressure is generated during joint loading. As water leaves the joint, the compressive load is transferred to the solid phase. Cartilage exhibits a strong structure-function relationship: the mechanical properties are intimately linked to ECM composition and organization. Therefore, reproducing the matrix is crucial for attaining native cartilage properties.
Although there have been successes in stem cell-based cartilage tissue engineering, clinical translation of these technologies for cartilage repair and regeneration remains severely limited. According to ClinicalTrials.gov, a global registry of public and private clinical trials, 21 clinical trials intended for stem cell-based cartilage repair are currently registered as of 6/10/2013 (search terms: “stem cells” and “cartilage”). All of these trials are MSC-based, with 24% of these trials using allogeneic MSCs. Aside from ethical and political concerns, there are major hurdles to translating stem cell products: sufficient pre-clinical data availability; production and facility expenses; and government product regulations. This review will discuss the successes and shortcomings of the current field of stem cell-based neocartilage engineering, clinical translation requirements for these technologies, and how these requirements can inform the field’s future directions. It will also address acceptable cellular processing methods, product implantation, and manufacturing obstacles of stem cell-based cartilage products.
Stem cell-based cartilage regeneration
Tissue engineering and regenerative medicine
Various stem cell-based tissue engineering methods have independently achieved promising biochemical and mechanical properties toward those of native cartilage (Huang et al., 2010; Meyer et al., 2011; Li et al., 2010); however, the lack of an optimal stimulation regimen limits the progression of neotissues to the clinic. Tables 1 and 2 provide an overview of the most commonly used biochemical and mechanical stimulation methods in tissue engineering of neocartilage from stem cell sources (see “Search strategy and selection criteria”). Cartilage tissue engineers must reach a consensus regarding the best magnitude, application time, duration, and frequency of these signals. Rather than investigating singular chemical or mechanical conditions, a systematic evaluation of these factors would define a successful platform upon which to build, allowing the field to ultimately achieve neotissues matching native articular cartilage. Researchers investigating multiple stimuli must consider that increasing the number of manipulations needed to differentiate stem cells before implantation increases translational barriers (e.g. scale-up).
Table 1. Mesenchymal Stem Cell (MSC) Chondrodifferentiation Techniques.
Commonly used growth factors and mechanical stimuli for inducing MSC chondrodifferentiation in cartilage tissue engineering. Most growth factors are applied continuously throughout culture. References selected based on PubMed search, as described in “Search strategy and selection criteria.”
| GROWTH FACTOR STIMULATION | ||||
|---|---|---|---|---|
| Source | Stimulus | Level | Culture method | Reference |
| BM | TGF-β3 | 20ng/mL | Collagen-GAG | (Matsiko et al., 2012) |
| TGF-β3 | 10ng/mL | PCL | (Abrahamsson et al., 2010) | |
| TGF-β3 | 10ng/mL | Hyaluronic acid | (Erickson et al., 2009) | |
| TGF-β3; BMP-2 | 10ng/mL; 50ng/mL | Pellet | (Perrier et al., 2011) | |
| TGF-β3; TGF-β1 | 10ng/mL; 20ng/mL | Gelatin/albumin | (Mohan, Nair, and Tabata, 2009) | |
| TGF-β1 | 10ng/mL | Agarose, alginate, gelatin | (Awad et al., 2004) | |
| TGF-β1 | 10ng/mL | PEG | (Nguyen et al., 2011) | |
| TGF-β1 | 10ng/mL | Gelatin | (Solorio et al., 2012) | |
| TGF-β1 | 10ng/mL | Collagen-GAG | (Liang et al., 2010) | |
| TGF-β1 | 10ng/mL | Hyaluronic acid | (Toh et al., 2012) | |
| TGF-β1; IGF-1 | 10ng/mL; 100ng/mL | PLLA | (Janjanin et al., 2008) | |
| Syn | TGF-β3; BMP-2 | 10ng/mL; 500ng/mL | Chitosan/alginate | (Qi et al., 2011) |
| TGF-β1; TGF-β3; BMP-2 | 10ng/mL; 10ng/mL; 10ng/mL | Gellan gum | (Fan et al., 2010) | |
| BMP-2 | 100ng/mL | Pellet | (Ando et al., 2007) | |
| AD | TGF-β1; IGF-1 | 10ng/mL; 100ng/mL | PLGA/chitosan | (Zhang et al., 2013) |
| TGF-β1; BMP-6 | 10ng/mL; 100ng/mL | Monolayer, alginate | (Lee et al., 2013) | |
| TGF-β1; BMP-6 | 10ng/mL; 10ng/mL | Pellet | (He and Pei, 2011) | |
| TGF-β1; BMP-2 | 10ng/mL; 50ng/mL | Pellet, hyaluronic acid | (Yoon et al., 2011) | |
| TGF-β3; BMP-6 | 10ng/mL; 10ng/mL | Pellet | (Hildner et al., 2010) | |
| AD, BM | TGF-β2; BMP-7 | 5ng/mL; 100ng/mL | Pellet | (Kim and Im, 2009) |
| MECHANICAL STIMULATION | ||||
| Source | Stimulus | Level | Culture method | Reference |
| BM | Compression | 4hrs/dy, 3dys @ 10% strain, 0.1, 0.5, or 1Hz | Fibrin | (Pelaez, Huang, and Cheung, 2009) |
| Compression; shear | 1hr/dy, 5dys/wk, 3wks @ 10–20%, 1Hz; ±25° | Fibrin-polyurethane | (Schatti et al., 2011) | |
| Ultrasound | 10m/12hrs, 1 or 4wks @ 200 mW/cm2, 1MHz | Fibrin-hyaluronic acid | (Choi et al., 2013) | |
| BM, AD | HP | 4hrs/dy, 21dys @ 7.5MPa, 1Hz | Agarose | (Puetzer et al., 2012) |
| AD | Compression | 4hrs/dy, 7dys @ 5%, 1Hz | Chitosan/gelatin | (Li et al., 2012) |
| Syn | HP | 1hr @ 1 or 5MPa, 0.5Hz | Alginate | (Sakao et al., 2008) |
| COMBINED GROWTH FACTOR AND MECHANICAL STIMULATION | ||||
| Source | Stimulus | Level | Culture method | References |
| BM | TGF-β1 Compression | 0, 1, 10ng/mL 1hr/dy, 7dys @ 10–20%, 1Hz | Fibrin-polyurethane | (Li et al., 2010) |
| TGF-β1 Compression | 10ng/mL 4hrs/dy, 7dys @ 8MPa, 0.33Hz | Hyaluronan/gelatin | (Angele et al., 2004) | |
| TGF-β1 Compression | 10ng/mL 4hrs/dy, 3, 4, or 7dys @ 10%, 1Hz | Pellet, agarose | (Huang et al., 2004) | |
| TGF-β3 Compression | 10ng/mL 1hr/d, 3 or 6 wks @ 10%, 1Hz | Agarose | (Thorpe et al., 2010) | |
| TGF-β3 Compression | 10ng/mL 1 or 4hrs/dy, 5dys/wk, 3wks @ 10%, 1Hz | Agarose | (Huang et al., 2010) | |
| TGF-β3 Compression | 10ng/mL 180m/dy, 5dys @ 10%, 1Hz | Agarose | (Mauck et al., 2007) | |
| TGF-β3 Compression | 10ng/mL 1.5hrs/6hrs, 24hrs/dy, 8dys @ 15%, 1Hz | Alginate | (Campbell, Lee, and Bader, 2006) | |
| TGF-β1 Tension | 10ng/mL 7dys @ 10%, 1Hz | Collagen-GAG | (McMahon et al., 2008) | |
| TGF-β3 HP | 10ng/mL 4hrs/dy, 5dy/wk, 3wks @ 10MPa, 1Hz | Agarose | (Steward, Wagner, and Kelly, 2013) | |
| TGF-β3 HP | 10ng/mL 4hrs/dy, 3, 7, or 14dys @ 0.1, 1, 10MPa, 1Hz | Pellet | (Miyanishi et al., 2006) | |
| TGF-β3 Fluid shear | 1ng/mL 4wks @ 100μL/m/fibre mesh | Chitosan/PBTA | (Alves da Silva et al., 2011) | |
| TGF-β1 Ultrasound | 10ng/mL 20 or 40m/dy, 7dys @ 30mW/cm2, 1.5MHz | Pellet or Hyaluronan/Gelatin | (Schumann et al., 2006) | |
| TGF-β1 Ultrasound | 10ng/mL 20m/dy, 7, 14, 21, 28dys @ 200mW/cm2, 1MHz | Monolayer | (Lai et al., 2010) | |
| TGF-β1 Ultrasound | 10ng/mL 20m/dy, 1 or 2wks @ 200mW/cm2, 1MHz | Alginate | (Lee et al., 2007) | |
| AD | TGF-β1 Compression | 5ng/mL 30m/2hrs, 16hrs/dy, 14dys @ 15%, 0.3Hz | PEGDA | (Steinmetz and Bryant, 2011) |
| TGF-β1 HP | 10ng/mL 1wk @ 0.5MPa, 0.5Hz | Collagen | (Ogawa, 2009) | |
| TGF-β1 HP | 10ng/mL 4hrs/dy, 7dys @ 5MPa, 0.5Hz | Pellet | (Safshekan et al., 2012) | |
| TGF-β3 HP | 10ng/mL 4hrs/dy, 5dy/wk, 3wks @ 0.4, 5MPa, 0.5Hz | Gellan gum | (Correia et al., 2012) | |
| AD, Syn | TGF-β3 HP | 1,10ng/mL 4hrs/dy, 14dys @ 10MPa, 1Hz | Pellet | (Vinardell et al., 2012) |
BM: bone marrow; Syn: synovium-derived; TGF: transforming growth factor; BMP: bone morphogenetic protein; IGF: insulin-like growth factor; HP: hydrostatic pressure; GAG: glycosaminoglycan; PCL: poly-ε-caprolactone; PEG: poly(ethylene glycol); PLLA: poly(L-lactic acid); PLGA: poly(l-glutamic acid); PBTA: poly(butylene terephthalate adipate); PEGDA: poly(ethylene glycol) diacrylate.
Table 2. Pluripotent Stem Cell Chondrodifferentiation Techniques.
Commonly used stimuli for inducing pluripotent stem cell chondrodifferentiation in cartilage tissue engineering. Most growth factors are applied continuously throughout culture. References selected based on PubMed search, as described in “Search strategy and selection criteria.”
| GROWTH FACTOR STIMULATION | ||||
|---|---|---|---|---|
| Source | Stimulus | Level | Culture method | Reference |
| ESCs | TGF-β1 | 10ng/mL | Monolayer | (Hwang, Polak, and Mantalaris, 2008) |
| TGF-β1 | 10ng/mL | Micromass | (Toh et al., 2010) | |
| TGF-β1; BMP-2 | 10ng/mL; 10ng/mL | Gelatin | (Alfred et al., 2011) | |
| TGF-β1; BMP-2 | 10ng/mL; 25ng/mL | Pellet | (Hwang et al., 2006) | |
| TGF-β1; TGF-β3; IGF-1; BMP-2 | 10ng/mL; 10ng/mL; 100ng/mL; 10ng/mL | EB | (Koay, Hoben, and Athanasiou, 2007) | |
| TGF-β1; BMP-2 | 2ng/mL; 2, 10ng/mL | EB | (zur Nieden et al., 2005) | |
| TGF-β1; BMP-7 | 10ng/mL; 300ng/mL | Pellet | (Nakagawa, Lee, and Reddi, 2009) | |
| TGF-β3 | 10ng/ml | EB | (Jukes et al., 2009) | |
| TGF-β3; BMP-2 | 10ng/mL; 10ng/mL | EB | (Bai et al., 2010) | |
| BMP-4 | 0.5ng/mL; 30–100ng/mL | EB; monolayer | (Craft et al., 2013) | |
| ESCs, iPSCs | TGF-β1; BMP-2 | 10ng/mL; 10ng/mL | Micromass | (Yamashita et al., 2013) |
| iPSCs | BMP-4; TGF-β3 | 50ng/mL; 10ng/mL | Micromass, pellet | (Diekman et al., 2012) |
| BMP-2 | 100ng/mL | Micromass | (Guzzo et al., 2012) | |
| BMP-2 | 100ng/mL | EB, agarose, PCL | (Kim et al., 2011) | |
| GROWTH FACTOR AND MECHANICAL STIMULATION | ||||
| Source | Stimulus | Level | Culture method | References |
| MSCs, ESCs | TGF-β1 Compression | 10ng/mL 1, 2, 2.5, 4hrs/d, 7, 14, 21dys @ 10%, 1Hz | Pellet, PEGDA | (Terraciano et al., 2007) |
ESCs: embryonic stem cells; iPSCs: induced pluripotent stem cells; TGF: transforming growth factor; BMP; bone morphogenetic protein; IGF: insulin-like growth factor; EB: embryoid body; PCL: polycaprolactone; PEGDA: poly(ethylene glycol)-diacrylate.
Scaffold and scaffold-free approaches
A wide variety of scaffolds have been employed to assist chondrogenic differentiation of stem cells, including agarose, collagen, and hyaluronic acid (Chung and Burdick, 2008; Leddy, Awad, and Guilak, 2004) (Tables 1 and 2). MSCs seeded in agarose produce neocartilages with compressive properties nearing 45% of native tissue (Huang et al., 2009; Athanasiou et al., 1991). Native cartilage ECM scaffolds (i.e. collagen type II) can produce better cartilage than synthetic scaffolds (Bosnakovski et al., 2006). These results illustrate the importance of recapitulating an appropriate stem cell niche that promotes and maintains a chondrocytic phenotype. Certain scaffolds that promote cell attachment and spreading may result in altered cellular phenotype (Li et al., 2003). Cartilage’s avascularity may also prevent removal of toxic scaffold by-products, ultimately hampering clinical translation.
Circumventing scaffold-associated issues, scaffold-free approaches may recapitulate cartilage morphogenesis (Ofek et al., 2008), generating neotissues with compressive properties nearly 65% of native tissue values (Ando et al., 2007). Scaffoldless technologies avoid scaffold-associated stress-shielding and reduced cell-cell communication. Scaffoldless methods help maintain a chondrocytic phenotype, which is particularly important concerning stem cell plasticity. Without scaffolds that hinder cell proliferation or matrix deposition, scaffoldless neocartilage may integrate better post-implantation. However, scaffoldless approaches require a comparatively high number of cells, which can be addressed by refining chondrodifferentiation strategies to produce more viable cells.
Stem cell sourcing for tissue engineering
Endogenous therapies are based on the recruitment of the body’s own stem cells to the cartilage defect, whereas exogenous approaches use stem cells that are first prepared in vitro and then delivered in situ. Endogenous stem cells have been shown to migrate to defect sites (or home) and are efficacious in initiating cartilage healing in vivo (Erggelet et al., 2007; Lee et al., 2010). Exogenous stem cell injection can similarly initiate repair; however, it is unclear whether injected or recruited cells are the major contributors to tissue repair. More information regarding the role of endogenous stem cells can be found elsewhere (Gerter, Kruegel, and Miosge, 2012).
MSC chondrodifferentiation can be achieved using scaffolds and growth factors to up-regulate aggrecan and collagen II gene expression, indicating their potential for neocartilage formation toward cartilage tissue engineering (Diekman et al., 2009) (Table 1). Due to their immunoprivilege, MSC use in cartilage repair may alleviate concerns of a host immune response (Beyth et al., 2005). On the other hand, autologous MSC-based cartilage therapies must take into account that MSCs exhibit age-dependent limitations, with MSC numbers declining with age (Caplan, 2007). Applying chondrodifferentiation protocols to autologous MSCs that decrease in availability has broad implications for an aging population prone to cartilage afflictions; thus, allogeneic sources may be best in these cases.
Compared to MSC chondrodifferentiation work, there is a dearth of studies regarding hESC differentiation to chondroprogenitors (Table 2). No direct, systematic comparison between the growth factor-induced chondrocytic potential of these sources has been performed, and a study that determines the differentiation efficiency of MSCs versus hESCs given similar stimuli would greatly direct the field. A single study illustrates hESC-derived MSCs as more sensitive to mechanical loading than MSCs (Terraciano et al., 2007). Knowledge obtained from hESC work could be applied toward the use of iPSCs for cartilage tissue engineering, shifting the entire field into the realm of personalized medicine (Diekman et al., 2012).
Chemical and mechanical stimulation
TGF-β, BMP-6, and dexamethasone, among other soluble factors, have been widely used to chondrodifferentiate MSCs and hESCs (Estes, Wu, and Guilak, 2006; Mehlhorn et al., 2007; Hwang et al., 2008b; Koay, Hoben, and Athanasiou, 2007) (Tables 1 and 2). While these potent stimuli enhance neocartilage properties, their dosing and temporal use requires optimization. Applying an abundance of chemical stimuli should be avoided, as excess use can result in unwanted differentiation, overgrowth of tissue, or undesirable hypertrophy of cells. Furthermore, in implanted constructs, residual growth factors may adversely impact the native joint environment. Alternatively, the stem cells within the implant may not survive in the joint without in vitro growth factor levels.
Mechanical stimuli—such as dynamic compression, hydrostatic pressure, and tension—have been applied as effective chondrodifferentiation agents (Baker et al., 2011; Kisiday et al., 2009) (Tables 1 and 2). Applied at physiologic levels, these stimuli mimic natural joint biomechanics. For example, dynamic compression mimics the cyclic loading of the joint and elicits cellular biosynthesis. As with chemical stimulation, mechanics-based protocols differ in loading magnitude, duration, time of application, duty cycle, and frequency. Variations in loading protocols and equipment prevent the direct comparison of successful studies, thus limiting optimization and ultimately hampering the progression of the field toward clinical applications. As with chemical stimuli, commercialization of neotissues generated using mechanical loading are susceptible to scale-up considerations, requiring large bioreactor development.
Despite successes in using chemical and mechanical stimuli independently, the interactive effects and overall benefit of combined treatments are difficult to decipher. The importance of mechanical stimuli in chondrodifferentiation is itself a contentious topic. It is postulated that mechanical loads are transduced through mechanotransductive elements (e.g. ion channels, integrin receptors) to affect chondrogenesis; an alternative hypothesis is that loading may simply allow for improved fluid-borne transport of chondro-inductive chemicals. Thus, mechanical and chemical stimulation regimens are difficult to decouple. However, understanding the difference has broader implications for translation, as the use of soluble factors is more amenable to scale-up considerations than mechanical regimens. The discovery and use of chemical equivalents to mechanical stimuli may alleviate these concerns and facilitate translation of stem cell-based neocartilage.
Evaluation of stem cell-based cartilage therapies in animal models and veterinary medicine
Existing animal models
The USA Food and Drug Administration (FDA) guidance document for products intended to repair or replace knee cartilage acknowledges that “no perfect animal model of articular cartilage injury exists” (FDA, 2011a). Both small and large animal models should be used to show safety, efficacy, and durability of response. Small animal models are less expensive and can provide an initial indication of safety (e.g. biocompatibility) and efficacy of stem cell-based treatments; the rabbit is the most popular model for cartilage defects. However, it is generally accepted that spontaneous healing of cartilage defects occurs in rabbits, potentially confounding the results of such studies, but not in large animals or humans. Therefore, a large animal model (e.g. sheep, horse) is necessary to further evaluate efficacy, especially if the product is intended for load-bearing joints such as the knee (Zscharnack et al., 2010; Guo et al., 2004; McIlwraith et al., 2011).
Lasting repair is shown by durability studies, best conducted in large animals that better approximate the biomechanics and scale of human diarthrodial joints. Large animals also offer the potential for long-term follow-up. Furthermore, efficacy in small animals does not necessarily translate to large animals. For example, in 12/12 rabbits with full-thickness cartilage defects, an ASC/fibrin scaffold treatment resulted in hyaline-like repair (Dragoo et al., 2007). In horses, a similar defect model and treatment yielded positive results initially, but durability was not seen 8 months after implantation (Wilke, Nydam, and Nixon, 2007). These discrepancies demonstrate that preclinical animal studies may require multiple animal models to evaluate durable cartilage repair.
Results from animal studies are most meaningful when a model is chosen to reflect human pathology or injury and when a standard set of data are reported. While many defect and pathology models have been generated in large animals, their fidelity to human conditions varies and few, if any, are widely adopted. This is especially true for diseases like osteoarthritis where complex tissue interactions exist. To facilitate comparison of such models, a minimum, standardized set of data should be reported; while the FDA’s guidance document suggests a list of parameters, not all published studies report them. For example, the FDA guidance document suggests reporting the animal model (i.e. joint size and load, age, skeletal maturity) and articular cartilage defect type (i.e. location, size, depth), as well as a description of methods regarding defect preparation, gross and histological assessments, and mechanical evaluations. Better characterization of appropriate animal models is needed, and publications reporting a standard set of parameters are expected to improve model development.
Stem cells, too, present challenges for choosing an animal model. For a proposed therapy containing human cells, animal studies should either 1) use the intended human cells in combination with immunosuppressives or 2) use animal-derived cells that are analogous to “the ultimate clinical product in phenotype and biologic activity” (FDA, 2011a). Immunosuppressives are costly, may increase morbidity, and potentially influence treatment efficacy. The potency of analogous, animal-derived cells may also differ from the final human product. When an intended human product contains autologous cells, should autologous cells also be necessary in an animal study, given that there is no evidence that autologous cells perform differently than allogeneic cells in animal models? Considering that allogeneic cells would reduce cost and number of procedures, are allogeneic cells sufficient to prove efficacy, even if the intended product is autologous? Ultimately, some proposed treatments may require evaluation via multiple large animal models prior to first-in-human studies.
Clinical use of stem cells to treat cartilage disease in veterinary medicine
The promise of stem cell therapy has driven an industry in veterinary use. Pre-clinical studies in dogs and horses have facilitated rapid translation of stem cell therapies into veterinary medicine, creating a gap between human and veterinary markets. ASCs have been commercially available for use in veterinary clinics since 2003; commercial and academic institutions offer fat and bone marrow processing for autologous re-implantation to treat osteoarthritis, bone fractures, and tendon or ligament injuries. Stem cell therapies have also been developed for other diseases, including renal failure in cats. While one company reports more than 8,000 animals treated with their stem cell product, there is notably little published information documenting efficacy in clinical veterinary patients.
Blinded, controlled trials of a commercially available product administered by intra-articular injection have demonstrated improvement in clinical signs (e.g. lameness, pain, and range of motion) associated with elbow and coxofemoral osteoarthritis in dogs (Black et al., 2008; Black et al., 2007). There is no evidence that the treatment repaired or regenerated cartilage. Intra-articular injection of the same product did not result in significant improvement in clinical, histological, or biochemical parameters associated with cartilage repair in an induced model of osteoarthritis in horses (Frisbie et al., 2009). Such discrepancies illustrate the need for more rigorous examination of stem cell therapies in veterinary medicine to yield greater insight on the role of stem cells in modulating idiopathic osteoarthritis.
For translation to humans, companies commercializing veterinary products must be incentivized to publish methods and results. Such transparency may minimize the gap between human and veterinary markets, ultimately accelerating the establishment of stem cell-based cartilage repair for human afflictions. The existing use of stem cells in veterinary medicine creates an exciting opportunity for collaboration between veterinarians and physicians to advance the treatment of injury and disease in both human and animal patients alike.
Limitations of bench-to-bedside translation of cartilage therapies
Culture and processing methods
Commercialization of current chondrodifferentiation techniques may be subject to scale-up limitations, excessive costs, and regulatory hurdles (as discussed in the “Regulation of Stem Cell-Based Cartilage Products” section). For instance, hydrostatic pressure bioreactors are easily scaled-up, unlike direct compression bioreactors which are more challenging. Commercialization of stem cell therapies is subject to the design of novel bioreactors that can sustain large-scale production of stem cells while using minimal space and maintaining low costs (Marolt et al., 2006; Alfred et al., 2011; Tao et al., 2011). The challenge is that development of culturing techniques potentially distinct from those used in basic research might be required to maintain the desired cellular phenotype to keep the product efficacious on an industrial scale; for example, clinical-grade flow sorting may be used to ensure homogeneity of the product when used in a Good Manufacturing Practices (GMP)-rated facility that follows appropriate manufacturing guidelines (Hare et al., 2009; Jung, Bauer, and Nolta, 2012; Koç et al., 2000). Large-scale use of exogenous growth factors may prove prohibitively expensive. In addition to cost and production logistics, regulations also delay translation. Examples of regulatory issues include techniques that employ bacterial plasmids or viral vector-mediated genetic engineering. While chondrogenic genes may be up-regulated effectively using such methods, extensive regulatory oversight due to biosafety concerns exist (e.g. a 15-year follow-up of all treated patients by the FDA and recombinant advisory committee, RAC). Thus, due to unique circumstances related to stem cells, feasibility at the industrial level should be considered even during the research phase for tissue-engineered cartilage products.
Product implantation
According to the FDA, implantation in humans must be preceded by sufficient evidence for differentiation efficiency, integration, safety, and long-term viability and functionality. Appropriate differentiation and efficacy must be demonstrated in vitro and in vivo as the initial “proof-of-concept” stage (Figure 1). Then, strong data supporting biosafety must be demonstrated with appropriate record-keeping. The biosafety of hESC chondrodifferentiated constructs should be evaluated exhaustively in immune-deficient mice for at least six months to “rule-out teratomas,” (Gruenloh et al., 2011) since teratomas are tell-tale signs of incomplete chondrodifferentiation processes that had left potentially dangerous pluripotent stem cells in the implant. In contrast to ESCs, adult stem cells such as MSCs have a strong safety profile in many clinical trials to-date (Hare et al., 2009; Newman et al., 2009; Griffin, Ritter, and Mahon, 2010). This demonstrated biosafety makes the regulatory path for MSCs shorter, requiring instead a thorough examination of all tissues in recipient animals since MSCs often migrate to multiple tissues post-implantation. Long-term proof-of-efficacy in animal models with absence of teratomas would move hESC-based products past the proof-of-concept stage.
Fig. 1.
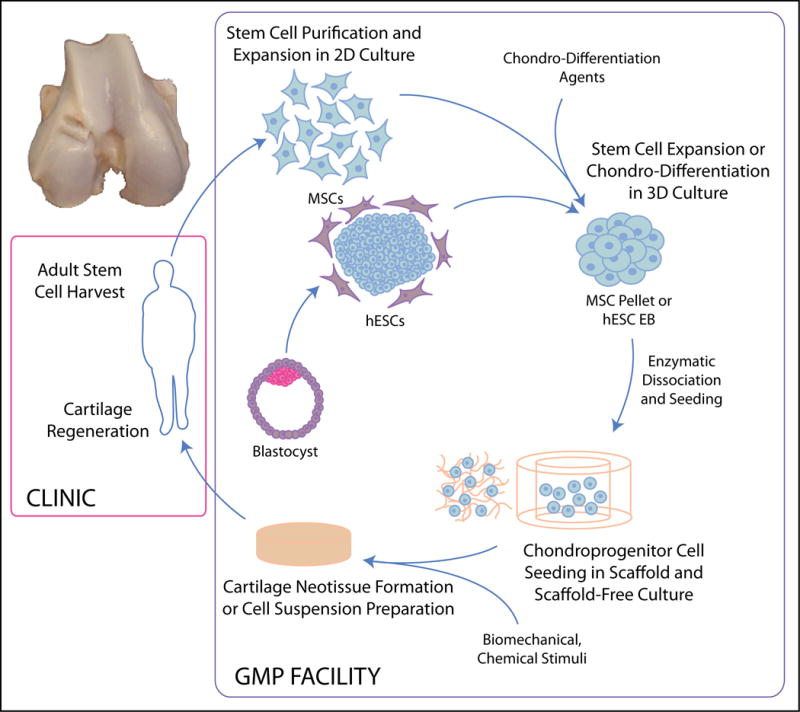
Overview of stem cell-based neocartilage formation. Patient-derived adult stem cells or patient-matched ESC-derived cells are sorted and expanded in 2D culture before 3D aggregate differentiation. Cells are typically dissociated and seeded into scaffolds or used in a scaffoldless approach to generate mechanically and biochemically robust neocartilage for in vivo implantation. For clinical translation, cellular manipulations must be performed in facilities compliant with Good Manufacturing Practices (GMP).
Fixation and integration of stem cell-based neocartilage may rely on suturing, tissue glues, and/or cell infiltration. Loose implants are rapidly destroyed with loading, and effective integration methods must be identified during early product development. Implants must also provide mechanical function. Tissue-engineered cartilage can be immediately functional if neotissue and native tissue properties match. Temporary immobilization may be necessary for a joint receiving an implant that is expected to mature to functionality in vivo. Establishing a robust mode of neocartilage implantation at early stages of product development accelerates clinical translation and commercialization.
Manufacturing
The lack of sufficient FDA-compliant manufacturing sites impedes clinical translation of stem cell-based cartilage therapies. Such facilities need to operate under FDA guidance documents concerning product specifications, manufacture, and regulation (FDA, 2011b). Additionally, companies should consult the FDA on a case-by-case basis as different products’ regulations and manufacturing requirements may vary. Continual communication with the FDA is an absolute necessity for establishing manufacturing sites and passing facilities inspections.
Good Tissue Practice (GTP) and other manufacturing requirements, described in Code of Federal Regulations Title 21, Sections 1270 and 1271 (21 CFR 1270, 1271), must be followed by any facility producing human cells, tissues, and cellular- and tissue-based products (HCT/Ps). GTP compliance falls within GMP guidelines and ensures the manufacture of a sterile, efficacious, and uncontaminated HCT/P. To generate products used in clinical trials or for commercialization purposes, the manufacturing facility must operate and be maintained in compliance with GTP standards. Although designing a GTP-compliant enterprise to manufacture stem cell-based cartilage is expensive and represents a major hurdle in clinical translation, more of these establishments are needed.
Regulation of stem cell-based cartilage products
Product classification
HCT/Ps are classified under section 361 or 351 of the Public Health Service Act (PHS). Section 361 focuses on preventing the introduction, transmission, and spread of communicable diseases, while section 351 regulates drugs, devices, and/or biological products. HCT/Ps are further regulated under section 21 CFR 1271. A HCT/P regulated under PHS 361 must be minimal manipulated, intended for homologous use, and uncombined with another article. Homologous use products perform the same basic function in the recipient as in the donor; e.g. hematopoietic stem cells that reconstitute the blood. Stem cell-based cartilage products will likely exceed minimal manipulation to result in mature, implantable cartilage and will most likely be regulated as a drug, device, and/or biological product, falling under PHS 351.
To determine HCT/P classification as a biologic, device, or combination product, the FDA provides several guidance documents (FDA, 2011d) offering definitions and examples of each type. Exact definitions for biologic, device, and combination products are set forth in 21 CFR 1271 Part 3. Stem cell-loaded scaffolds, drug-eluting meshes, and chemical-secreting cells all fall within the combination product category. Cartilage tissue engineering approaches using stem cells generally produce combination products. If classification of a cartilage repair or replacement HCT/P is still ambiguous given the definition, questions can be directed to the Office of Combination Products (OCP).
Regulation as a biologic, device, or combination product
Stem cell-based cartilage products will likely require three tiers of testing: initial development and “proof-of-concept” studies, preclinical studies done under Good Laboratory Practices (GLP), and clinical studies. Preclinical work in animal models should demonstrate safety and the biological response, durability, toxicology, and dose response of the technology. The product used in animals should be nearly identical to the human product. Analogous alternatives exist, e.g. if the product uses autologous human MSCs, the animal study may employ autologous animal MSCs. Despite the large number of veterinary patients treated with stem cell-based products, their results are not necessarily usable in lieu of preclinical animal work.
Several FDA centers evaluate the safety and efficacy of new technologies. Potential new products, considered biologics, devices, or both, are regulated by different centers that guide and oversee the approval process (Figure 2). The Center for Devices and Radiological Health (CDRH) and the Center for Biologics Evaluation and Research (CBER) are responsible for devices and biologics, respectively. Technologies with both device and biologic components (e.g. scaffold-seeded stem cells) are regulated as combination products. Per the guidance documents, these products are assigned to the most relevant FDA center and must conform to the regulations of both centers.
Fig. 2.
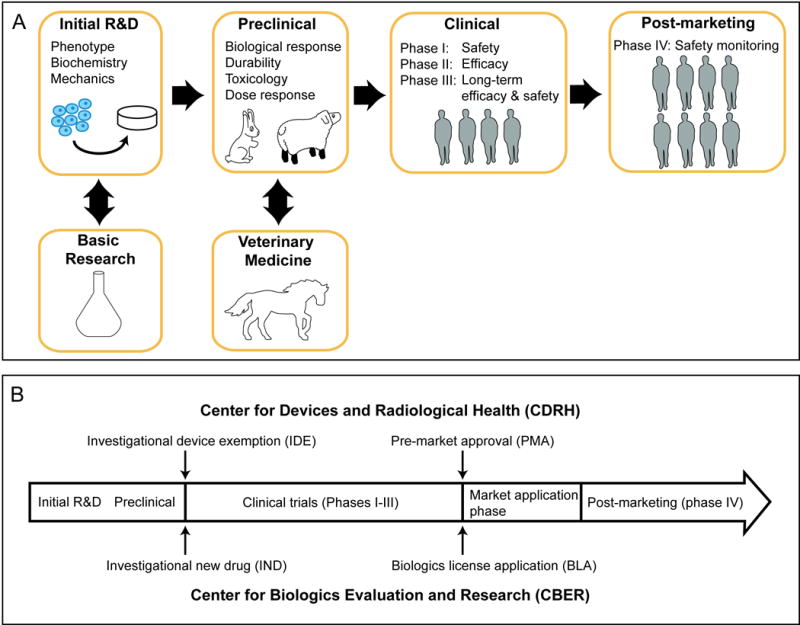
Translation of stem cell products for cartilage regeneration. (A) FDA regulation of biologics requires stem cell products to exhibit purity, reproducibility, and stability. Neocartilage is evaluated in preclinical trials for biological response, durability, toxicology, and dose response. Multi-phase clinical studies are used to evaluate dosage, efficacy, and safety. (B) Two major regulatory centers exist: CDRH for devices and CBER for drugs and biologics. Prior to initiating clinical trials, a product must receive an IDE or IND. After clinical trials, market approval enables clinical application of the product.
Prior to using a product for clinical trials, there must be an approved investigational device exemption (IDE) or investigational new drug (IND) application, in addition to institutional review board (IRB) approval. In general, market approval for biologics require INDs while devices require IDEs, with both pathways ultimately aiming to ensure product safety and effectiveness. Deciphering product classification and the relevant pathways may increase interaction with the FDA, which in turn, may facilitate the process of obtaining approval.
Several laws and published FDA documents govern the regulatory approval of stem cell products for cartilage repair. A technology using human stem cells, even if the product is de-cellularized, is a HCT/P that needs to be evaluated for purity, reproducibility, and stability. The FDA considers products intended to repair knee cartilage, whether cellular or not, as significant risk devices requiring IDE and/or IND submission. Though the FDA provides a template to follow to register a product, it is still not clear whether one needs to regenerate articular cartilage in order to achieve improved patient outcome. It may be that simply repairing articular cartilage may provide a benefit tantamount to regenerating articular cartilage. The expectation at this point, however, is that by following the FDA guidance documents, not only will functional repair or regenerative neocartilage be produced, but that the tissue will also provide improved patient outcomes in terms of pain relief and restoration of joint function. Although there are FDA guidance documents and laws relevant to cartilage regeneration technologies, the approval process is product-specific and requires continual communication with the FDA.
Clinical studies are carried out in several phases to demonstrate product safety and efficacy (Figure 2). After clinical trials conclude, the product’s lead center can approve the product for marketing by providing a biologics license application (BLA) or a pre-market approval (PMA) via CBER or CDRH, respectively. Post-marketing meetings with the FDA to review clinical data ensure that the product is safe and effective.
Case studies: Chondrogen, RepliCart, and Cartistem
US-based Osiris’s Chondrogen illustrates the commercial development process of stem cell-based cartilage regeneration. Preclinical studies demonstrated that intra-articular MSC injection promotes regeneration following meniscectomy, prompting a Phase I/II clinical trial testing two dosages (50 or 150 million human MSCs) for safety and efficacy. Chondrogen was found to significantly improve patient pain in a dose-dependent manner, improving pain scores from 6 months to 1 year (Osiris Therapeutics, 2012).
Similarly, Australian company Mesoblast demonstrated in preclinical sheep studies that osteoarthritic joints receiving RepliCart, an allogeneic MSC product, experienced significantly greater tissue thickness and “biomechanical strength” over control joints (Ghosh et al., 2009). RepliCart is now in clinical trials to evaluate safety at 12 months, and prevention of osteoarthritis and cartilage loss at a second time point. Chondrogen and RepliCart both mirror veterinary successes in illustrating the potential of cell suspension-based products.
A third example involves a tissue-engineered product which, unlike cell suspensions, employs a biomaterial in conjunction with stem cells. The Korean company Medipost actively markets and sells Cartistem, an umbilical cord blood-derived MSC and semi-solid polymer-based treatment, to treat arthritis and to heal cartilage injury. This allogeneic product received approval for sale and clinical use in Korea in early 2012; the FDA recently approved Phase I/IIa USA clinical trials for Cartistem. USA approval of Cartistem will pave the way for future stem cell-based, tissue-engineered products for cartilage repair and regeneration.
Future directions and conclusions
Various strategies can promote chondrogenesis of both hESCs and MSCs for regenerating cartilage. Using the expansion capabilities and flexible lineage potential of MSCs and hESCs, researchers employ 3D culture techniques, tissue engineering scaffolds, and scaffold-less differentiation methods to promote chondrogenesis. The ultimate goal of chondrodifferentiating stem cells is to provide a cell source that can be used to engineer neocartilage capable of functioning in strenuous joint environments. Success criteria based on native cartilage physiology, in vivo cartilage development, and the associated regulatory pathways will inform future development of stem cell-based tissue engineering technologies.
Many stimuli have been used to chondrodifferentiate stem cells for tissue engineering. However, the efficiencies among studies in recapitulating native values are often not discussed. Selecting an optimal regimen for engineering neocartilage is therefore difficult as published data are often not normalized to native tissue values. Furthermore, for statistical optimization of differentiation or tissue engineering protocols, the existence of diverse success criteria necessitates a variable that can evaluate them simultaneously. Establishment of a quantitative parameter, such as a “functionality index” that equally weighs biomechanical and biochemical properties normalized to native tissue (Elder and Athanasiou, 2009), will enable optimization and key comparisons of various protocols.
To create clinically applicable neocartilage, larger constructs must be formed by improving stem cell expansion and efficiency of differentiation techniques to obtain larger cell numbers. A modular approach of stem cell differentiation followed by tissue engineering of lineage-committed cells may alleviate these size considerations. In the first module, any stem cell can be chondrodifferentiated. In the second module, chondrodifferentiated cells can be used in tissue engineering to obtain robust neocartilage with clinically significant dimensions. Enhanced chondrodifferentiation and subsequent protein synthesis may result from applying optimized stimuli during each phase. By investigating the phases independently, differential effects of each regimen can be identified.
Appreciation of FDA guidelines for products, facilities, manufacturing, and regulatory processes, as outlined in guidance documents, will expedite the development of stem cell-based treatments for cartilage diseases. By enhancing dialogue with the FDA, necessary design characteristics can be integrated into early iterations of a product, speeding the time to clinical trials, with the goal of market approval and product commercialization. Integrating an understanding of the major hurdles impeding clinical translation of stem-cell based cartilage products with basic or translational research could ultimately lead to the first U.S. licensed, stem cell-based cartilage repair product.
Search strategy and selection criteria
Review content was identified via searches of PubMed, FDA.gov, and relevant articles using the search terms: “clinical stem cell cartilage,” “cartilage tissue engineering,” and “stem cell animal model cartilage.” Only articles published from 1992 to 2013 in English were considered. Tables 1 and 2 were populated based on a PubMed search of “stem cells” AND cartilage AND X, where X is a growth factor (e.g. TGF-β1, BMP-2) or mechanical stimulus (e.g. compression, tension). With a balance between space constraints and scholarship, up to 5 unique laboratories per stem cell type per growth factor or mechanical stimulus were reported from the past 10 years. By consulting the works cited in these tables, the reader can readily gather additional factors that are used by multiple groups, such as dexamethasone. To avoid redundancy, these commonly used factors have not been included in this table.
Acknowledgments
The authors make no acknowledgements.
The authors gratefully acknowledge funding support from NIH R01 AR053286, R01 AR047839, R01 DE019666, R01 DE015038, R01 GM099688, T32 GM008799, and CIRM TR3-05709. KAA is on the Board of Histogenics, but does not hold any shares or receive compensation from this company.
Footnotes
Declaration of interest
In the preparation of this Review, the authors have no other declarations of interest to declare.
References
- Abrahamsson CK, Yang F, Park H, Brunger JM, Valonen PK, Langer R, Welter JF, Caplan AI, Guilak F, Freed LE. Chondrogenesis and Mineralization During In Vitro Culture of Human Mesenchymal Stem Cells on Three-Dimensional Woven Scaffolds. Tissue Engineering Part A. 2010;16:3709–18. doi: 10.1089/ten.tea.2010.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfred R, Taiani JT, Krawetz RJ, Yamashita A, Rancourt DE, Kallos MS. Large-scale production of murine embryonic stem cell-derived osteoblasts and chondrocytes on microcarriers in serum-free media. Biomaterials. 2011;32:6006–16. doi: 10.1016/j.biomaterials.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Alves da Silva ML, Martins A, Costa-Pinto AR, Correlo VM, Sol P, Bhattacharya M, Faria S, Reis RL, Neves NM. Chondrogenic differentiation of human bone marrow mesenchymal stem cells in chitosan-based scaffolds using a flow-perfusion bioreactor. J Tissue Eng Regen Med. 2011;5:722–32. doi: 10.1002/term.372. [DOI] [PubMed] [Google Scholar]
- Ando W, Tateishi K, Hart DA, Katakai D, Tanaka Y, Nakata K, Hashimoto J, Fujie H, Shino K, Yoshikawa H, Nakamura N. Cartilage repair using an in vitro generated scaffold-free tissue-engineered construct derived from porcine synovial mesenchymal stem cells. Biomaterials. 2007;28:5462–70. doi: 10.1016/j.biomaterials.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Angele P, Schumann D, Angele M, Kinner B, Englert C, Hente R, Fuchtmeier B, Nerlich M, Neumann C, Kujat R. Cyclic, mechanical compression enhances chondrogenesis of mesenchymal progenitor cells in tissue engineering scaffolds. Biorheology. 2004;41:335–46. [PubMed] [Google Scholar]
- Athanasiou KA, Rosenwasser MP, Buckwalter JA, Malinin TI, Mow VC. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J Orthop Res. 1991;9:330–40. doi: 10.1002/jor.1100090304. [DOI] [PubMed] [Google Scholar]
- Awad HA, Quinn Wickham M, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–22. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- Bai HY, Chen GA, Mao GH, Song TR, Wang YX. Three step derivation of cartilage like tissue from human embryonic stem cells by 2D–3D sequential culture in vitro and further implantation in vivo on alginate/PLGA scaffolds. J Biomed Mater Res A. 2010;94:539–46. doi: 10.1002/jbm.a.32732. [DOI] [PubMed] [Google Scholar]
- Baker BM, Shah RP, Huang AH, Mauck RL. Dynamic Tensile Loading Improves the Functional Properties of Mesenchymal Stem Cell-Laden Nanofiber-Based Fibrocartilage. Tissue Engineering Part A. 2011;17:1445–55. doi: 10.1089/ten.tea.2010.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–19. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- Black LL, Gaynor J, Adams C, Dhupa S, Sams AE, Taylor R, Harman S, Gingerich DA, Harman R. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Veterinary therapeutics: research in applied veterinary medicine. 2008;9:192–200. [PubMed] [Google Scholar]
- Black LL, Gaynor J, Gahring D, Adams C, Aron D, Harman S, Gingerich DA, Harman R. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Veterinary therapeutics : research in applied veterinary medicine. 2007;8:272–84. [PubMed] [Google Scholar]
- Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: Influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnology and Bioengineering. 2006;93:1152–63. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Lee DA, Bader DL. Dynamic compressive strain influences chondrogenic gene expression in human mesenchymal stem cells. Biorheology. 2006;43:455–70. [PubMed] [Google Scholar]
- Caplan AI. Adult Mesenchymal Stem Cells for Tissue Engineering Versus Regenerative Medicine. Journal of Cellular Physiology. 2007;213:341–47. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- Choi JW, Choi BH, Park SH, Pai KS, Li TZ, Min BH, Park SR. Mechanical Stimulation by Ultrasound Enhances Chondrogenic Differentiation of Mesenchymal Stem Cells in a Fibrin-Hyaluronic Acid Hydrogel. Artif Organs. 2013;37:648–55. doi: 10.1111/aor.12041. [DOI] [PubMed] [Google Scholar]
- Chung C, Burdick JA. Influence of Three-Dimensional Hyaluronic Acid Microenvironments on Mesenchymal Stem Cell Chondrogenesis. Tissue Engineering Part A. 2008;15:243–54. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia C, Pereira AL, Duarte AR, Frias AM, Pedro AJ, Oliveira JT, Sousa RA, Reis RL. Dynamic culturing of cartilage tissue: the significance of hydrostatic pressure. Tissue Eng Part A. 2012;18:1979–91. doi: 10.1089/ten.tea.2012.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft AM, Ahmed N, Rockel JS, Baht GS, Alman BA, Kandel RA, Grigoriadis AE, Keller GM. Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development. 2013;140:2597–610. doi: 10.1242/dev.087890. [DOI] [PubMed] [Google Scholar]
- Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. Journal of Orthopaedic Research. 2005;23:425–32. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW, Guilak F. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109:19172–7. doi: 10.1073/pnas.1210422109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekman BO, Rowland CR, Lennon DP, Caplan AI, Guilak F. Chondrogenesis of Adult Stem Cells from Adipose Tissue and Bone Marrow: Induction by Growth Factors and Cartilage-Derived Matrix. Tissue Engineering Part A. 2009;16:523–33. doi: 10.1089/ten.tea.2009.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoo JL, Carlson G, McCormick F, Khan-Farooqi H, Zhu M, Zuk PA, Benhaim P. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng. 2007;13:1615–21. doi: 10.1089/ten.2006.0249. [DOI] [PubMed] [Google Scholar]
- Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2009;17:114–23. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erggelet C, Neumann K, Endres M, Haberstroh K, Sittinger M, Kaps C. Regeneration of ovine articular cartilage defects by cell-free polymer-based implants. Biomaterials. 2007;28:5570–80. doi: 10.1016/j.biomaterials.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Erickson IE, Huang AH, Sengupta S, Kestle S, Burdick JA, Mauck RL. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis Cartilage. 2009;17:1639–48. doi: 10.1016/j.joca.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes BT, Wu AW, Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis & Rheumatism. 2006;54:1222–32. doi: 10.1002/art.21779. [DOI] [PubMed] [Google Scholar]
- Fan J, Gong Y, Ren L, Varshney RR, Cai D, Wang DA. In vitro engineered cartilage using synovium-derived mesenchymal stem cells with injectable gellan hydrogels. Acta Biomater. 2010;6:1178–85. doi: 10.1016/j.actbio.2009.08.042. [DOI] [PubMed] [Google Scholar]
- FDA. Guidance for Industry: Preparation of IDEs and INDs for Products Intended to Repair or Replace Knee Cartilage. 2011a Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM288011.pdf.
- FDA. Inspection of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) 7341.002. 2011b Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/CompliancePrograms/UCM095216.pdf.
- FDA. Guidance for Industry: Current Good Tissue Practice (CGTP) and Additional Requirements for Manufacturers of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) 2011c Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM285223.pdf.
- FDA. Guidance for Industry and FDA Staff: Classification of Products as Drugs and Devices & Additional Product Classification Issues. 2011d Available at: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM258957.pdf.
- Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009;27:1675–80. doi: 10.1002/jor.20933. [DOI] [PubMed] [Google Scholar]
- Gerter R, Kruegel J, Miosge N. New insights into cartilage repair – The role of migratory progenitor cells in osteoarthritis. Matrix Biol. 2012;31:206–13. doi: 10.1016/j.matbio.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Itescu S, Read RA, Cake MA, Smith T, Little CB, Smith MM, Appleyard RC, Gronthos S, Zannettino A. Intra-articular Injection of Allogeneic Immunoselected STRO-3+ Mesenchymal Precursor Stem Cells into Ovine Joints with Pre-existing Osteoarthritis Improves Articular Cartilage Integrity 6 Months Post Administrationed. Orthopaedic Research Society; Las Vegas, Nevada: 2009. ˆeds. [Google Scholar]
- Griffin MD, Ritter T, Mahon BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Human gene therapy. 2010;21:1641–55. doi: 10.1089/hum.2010.156. [DOI] [PubMed] [Google Scholar]
- Gruenloh W, Kambal A, Sondergaard C, McGee J, Nacey C, Kalomoiris S, Pepper K, Olson S, Fierro F, Nolta JA. Characterization and in vivo testing of mesenchymal stem cells derived from human embryonic stem cells. Tissue Eng Part A. 2011;17:1517–25. doi: 10.1089/ten.tea.2010.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Wang C, Zhang Y, Xia R, Hu M, Duan C, Zhao Q, Dong L, Lu J, Qing Song Y. Repair of large articular cartilage defects with implants of autologous mesenchymal stem cells seeded into beta-tricalcium phosphate in a sheep model. Tissue Engineering. 2004;10:1818–29. doi: 10.1089/ten.2004.10.1818. [DOI] [PubMed] [Google Scholar]
- Guzzo RM, Gibson J, Xu RH, Lee FY, Drissi H. Efficient differentiation of human iPSC-derived mesenchymal stem cells to chondroprogenitor cells. J Cell Biochem. 2012;114:480–90. doi: 10.1002/jcb.24388. [DOI] [PubMed] [Google Scholar]
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Pei M. Extracellular matrix enhances differentiation of adipose stem cells from infrapatellar fat pad toward chondrogenesis. J Tissue Eng Regen Med. 2011;7:73–84. doi: 10.1002/term.505. [DOI] [PubMed] [Google Scholar]
- Hildner F, Peterbauer A, Wolbank S, Nurnberger S, Marlovits S, Redl H, van Griensven M, Gabriel C. FGF-2 abolishes the chondrogenic effect of combined BMP-6 and TGF-beta in human adipose derived stem cells. J Biomed Mater Res A. 2010;94:978–87. doi: 10.1002/jbm.a.32761. [DOI] [PubMed] [Google Scholar]
- Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226–9. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A:185–92. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- Hu JC, Athanasiou KA. Structure and function of articular cartilage. In: An YH, Martin KL, editors. Handbook of Histology Methods for Bone and Cartilage. Totowa, NJ: Humana Press Inc; 2003. pp. 73–95. [Google Scholar]
- Huang AH, Farrell MJ, Kim M, Mauck RL. Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogel. European Cells & Materials. 2010;19:72–85. doi: 10.22203/ecm.v019a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AH, Stein A, Tuan RS, Mauck RL. Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue Eng Part A. 2009;15:3461–72. doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CYC, Hagar KL, Frost LE, Sun Y, Cheung HS. Effects of Cyclic Compressive Loading on Chondrogenesis of Rabbit Bone-Marrow Derived Mesenchymal Stem Cells. Stem Cells. 2004;22:313–23. doi: 10.1634/stemcells.22-3-313. [DOI] [PubMed] [Google Scholar]
- Hwang NS, Varghese S, Lee HJ, Zhang Z, Ye Z, Bae J, Cheng L, Elisseeff J. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proceedings of the National Academy of Sciences. 2008;105:20641–46. doi: 10.1073/pnas.0809680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang NS, Varghese S, Zhang Z, Elisseeff J. Chondrogenic Differentiation of Human Embryonic Stem Cell–Derived Cells in Arginine-Glycine-Aspartate–Modified Hydrogels. Tissue Engineering. 2006;12:2695–706. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- Hwang YS, Polak JM, Mantalaris A. In vitro direct chondrogenesis of murine embryonic stem cells by bypassing embryoid body formation. Stem Cells Dev. 2008;17:971–8. doi: 10.1089/scd.2007.0229. [DOI] [PubMed] [Google Scholar]
- Janjanin S, Li WJ, Morgan MT, Shanti RM, Tuan RS. Mold-shaped, nanofiber scaffold-based cartilage engineering using human mesenchymal stem cells and bioreactor. J Surg Res. 2008;149:47–56. doi: 10.1016/j.jss.2007.12.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes JM, van der Aa LJ, Hiemstra C, van Veen T, Dijkstra PJ, Zhong Z, Feijen J, van Blitterswijk CA, de Boer J. A Newly Developed Chemically Crosslinked Dextran–Poly(Ethylene Glycol) Hydrogel for Cartilage Tissue Engineering. Tissue Engineering Part A. 2009;16:565–73. doi: 10.1089/ten.TEA.2009.0173. [DOI] [PubMed] [Google Scholar]
- Jung Y, Bauer G, Nolta JA. Concise review: Induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. STEM CELLS. 2012;30:42–7. doi: 10.1002/stem.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Im GI. Combination of transforming growth factor-beta2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue-derived mesenchymal stem cells. Tissue Eng Part A. 2009;15:1543–51. doi: 10.1089/ten.tea.2008.0368. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Son MJ, Son MY, Seol B, Kim J, Park J, Kim JH, Kim YH, Park SA, Lee CH, Lee KS, Han YM, Chang JS, Cho YS. Generation of human induced pluripotent stem cells from osteoarthritis patient-derived synovial cells. Arthritis Rheum. 2011;63:3010–21. doi: 10.1002/art.30488. [DOI] [PubMed] [Google Scholar]
- Kisiday JD, Frisbie DD, McIlwraith CW, Grodzinsky AJ. Dynamic Compression Stimulates Proteoglycan Synthesis by Mesenchymal Stem Cells in the Absence of Chondrogenic Cytokines. Tissue Engineering Part A. 2009;15:2817–24. doi: 10.1089/ten.tea.2008.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koay EJ, Hoben GMB, Athanasiou KA. Tissue Engineering with Chondrogenically Differentiated Human Embryonic Stem Cells. Stem Cells. 2007;25:2183–90. doi: 10.1634/stemcells.2007-0105. [DOI] [PubMed] [Google Scholar]
- Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid Hematopoietic Recovery After Coinfusion of Autologous-Blood Stem Cells and Culture-Expanded Marrow Mesenchymal Stem Cells in Advanced Breast Cancer Patients Receiving High-Dose Chemotherapy. Journal of Clinical Oncology. 2000;18:307–16. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- Lai CH, Chen SC, Chiu LH, Yang CB, Tsai YH, Zuo CS, Chang WH, Lai WF. Effects of low-intensity pulsed ultrasound, dexamethasone/TGF-beta1 and/or BMP-2 on the transcriptional expression of genes in human mesenchymal stem cells: chondrogenic vs. osteogenic differentiation. Ultrasound Med Biol. 2010;36:1022–33. doi: 10.1016/j.ultrasmedbio.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Leddy HA, Awad HA, Guilak F. Molecular diffusion in tissue-engineered cartilage constructs: effects of scaffold material, time, and culture conditions. J Biomed Mater Res B Appl Biomater. 2004;70:397–406. doi: 10.1002/jbm.b.30053. [DOI] [PubMed] [Google Scholar]
- Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376:440–8. doi: 10.1016/S0140-6736(10)60668-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Watkins E, Burnsed OA, Schwartz Z, Boyan BD. Tailoring adipose stem cell trophic factor production with differentiation medium components to regenerate chondral defects. Tissue Eng Part A. 2013;19:1451–64. doi: 10.1089/ten.tea.2012.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Choi BH, Min BH, Park SR. Low-intensity ultrasound inhibits apoptosis and enhances viability of human mesenchymal stem cells in three-dimensional alginate culture during chondrogenic differentiation. Tissue Eng. 2007;13:1049–57. doi: 10.1089/ten.2006.0346. [DOI] [PubMed] [Google Scholar]
- Li J, Zhao Q, Wang E, Zhang C, Wang G, Yuan Q. Dynamic compression of rabbit adipose-derived stem cells transfected with insulin-like growth factor 1 in chitosan/gelatin scaffolds induces chondrogenesis and matrix biosynthesis. J Cell Physiol. 2012;227:2003–12. doi: 10.1002/jcp.22927. [DOI] [PubMed] [Google Scholar]
- Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(epsilon-caprolactone) scaffolds. J Biomed Mater Res A. 2003;67:1105–14. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- Li Z, Kupcsik L, Yao S-J, Alini M, Stoddart MJ. Mechanical load modulates chondrogenesis of human mesenchymal stem cells through the TGF-β pathway. Journal of Cellular and Molecular Medicine. 2010;14:1338–46. doi: 10.1111/j.1582-4934.2009.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang WH, Kienitz BL, Penick KJ, Welter JF, Zawodzinski TA, Baskaran H. Concentrated collagen-chondroitin sulfate scaffolds for tissue engineering applications. J Biomed Mater Res A. 2010;94:1050–60. doi: 10.1002/jbm.a.32774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little CJ, Bawolin NK, Chen X. Mechanical Properties of Natural Cartilage and Tissue-Engineered Constructs. Tissue Engineering Part B: Reviews. 2011;17:213–27. doi: 10.1089/ten.TEB.2010.0572. [DOI] [PubMed] [Google Scholar]
- Marolt D, Augst A, Freed LE, Vepari C, Fajardo R, Patel N, Gray M, Farley M, Kaplan D, Vunjak-Novakovic G. Bone and cartilage tissue constructs grown using human bone marrow stromal cells, silk scaffolds and rotating bioreactors. Biomaterials. 2006;27:6138–49. doi: 10.1016/j.biomaterials.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Matsiko A, Levingstone TJ, O’Brien FJ, Gleeson JP. Addition of hyaluronic acid improves cellular infiltration and promotes early-stage chondrogenesis in a collagen-based scaffold for cartilage tissue engineering. J Mech Behav Biomed Mater. 2012;11:41–52. doi: 10.1016/j.jmbbm.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Mauck R, Byers B, Yuan X, Tuan R. Regulation of Cartilaginous ECM Gene Transcription by Chondrocytes and MSCs in 3D Culture in Response to Dynamic Loading. Biomechanics and Modeling in Mechanobiology. 2007;6:113–25. doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- McIlwraith CW, Frisbie DD, Rodkey WG, Kisiday JD, Werpy NM, Kawcak CE, Steadman JR. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy. 2011;27:1552–61. doi: 10.1016/j.arthro.2011.06.002. [DOI] [PubMed] [Google Scholar]
- McMahon L, Reid A, Campbell V, Prendergast P. Regulatory Effects of Mechanical Strain on the Chondrogenic Differentiation of MSCs in a Collagen-GAG Scaffold: Experimental and Computational Analysis. Annals of Biomedical Engineering. 2008;36:185–94. doi: 10.1007/s10439-007-9416-5. [DOI] [PubMed] [Google Scholar]
- Mehlhorn AT, Niemeyer P, Kaschte K, Muller L, Finkenzeller G, Hartl D, Sudkamp NP, Schmal H. Differential effects of BMP-2 and TGF-β1 on chondrogenic differentiation of adipose derived stem cells. Cell Proliferation. 2007;40:809–23. doi: 10.1111/j.1365-2184.2007.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EG, Buckley CT, Steward AJ, Kelly DJ. The effect of cyclic hydrostatic pressure on the functional development of cartilaginous tissues engineered using bone marrow derived mesenchymal stem cells. Journal of the Mechanical Behavior of Biomedical Materials. 2011;4:1257–65. doi: 10.1016/j.jmbbm.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Miyanishi K, Trindade MCD, Lindsey DP, Beaupré GS, Carter DR, Goodman SB, Schurman DJ, Smith RL. Effects of Hydrostatic Pressure and Transforming Growth Factor-β3 on Adult Human Mesenchymal Stem Cell Chondrogenesis In Vitro. Tissue Engineering. 2006;12:1419–28. doi: 10.1089/ten.2006.12.1419. [DOI] [PubMed] [Google Scholar]
- Mohan N, Nair PD, Tabata Y. A 3D biodegradable protein based matrix for cartilage tissue engineering and stem cell differentiation to cartilage. J Mater Sci Mater Med. 2009;20(Suppl 1):S49–60. doi: 10.1007/s10856-008-3481-7. [DOI] [PubMed] [Google Scholar]
- Mow VC, Ratcliffe A, Poole AR. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13:67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Lee SY, Reddi AH. Induction of chondrogenesis from human embryonic stem cells without embryoid body formation by bone morphogenetic protein 7 and transforming growth factor beta1. Arthritis Rheum. 2009;60:3686–92. doi: 10.1002/art.27229. [DOI] [PubMed] [Google Scholar]
- Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflammation & allergy drug targets. 2009;8:110–23. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- Nguyen LH, Kudva AK, Saxena NS, Roy K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials. 2011;32:6946–52. doi: 10.1016/j.biomaterials.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA. Matrix development in self-assembly of articular cartilage. PLoS ONE. 2008;3:e2795. doi: 10.1371/journal.pone.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa R, Mizuno S, Murphy GF, Orgill DP. The Effect of Hydrostatic Pressure on Three-Dimensional Chondroinduction of Human Adipose–Derived Stem Cells. Tissue Engineering Part A. 2009;15:2937–45. doi: 10.1089/ten.tea.2008.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiris Therapeutics. Therapeutics: Chondrogen. 2012 4/12/2012, http://www.osiristx.com/prod_chondrogen.php.
- Pelaez D, Huang CY, Cheung HS. Cyclic Compression Maintains Viability and Induces Chondrogenesis of Human Mesenchymal Stem Cells in Fibrin Gel Scaffolds. Stem Cells and Development. 2009;18:93–102. doi: 10.1089/scd.2008.0030. [DOI] [PubMed] [Google Scholar]
- Perrier E, Ronziere MC, Bareille R, Pinzano A, Mallein-Gerin F, Freyria AM. Analysis of collagen expression during chondrogenic induction of human bone marrow mesenchymal stem cells. Biotechnol Lett. 2011;33:2091–101. doi: 10.1007/s10529-011-0653-1. [DOI] [PubMed] [Google Scholar]
- Puetzer JL, Williams JM, Gillies A, Bernacki S, Loboa E. The Effects of Cyclic Hydrostatic Pressure on Viability and Chondrogenesis of Human Adipose and Bone Marrow Derived Mesenchymal Stem Cells in 3-D Agarose Constructs. Tissue Eng Part A. 2012;19:299–306. doi: 10.1089/ten.tea.2012.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Chen A, You H, Li K, Zhang D, Guo F. Proliferation and chondrogenic differentiation of CD105-positive enriched rat synovium-derived mesenchymal stem cells in three-dimensional porous scaffolds. Biomedical materials (Bristol, England) 2011;6:015006. doi: 10.1088/1748-6041/6/1/015006. [DOI] [PubMed] [Google Scholar]
- Safshekan F, Tafazzoli-Shadpour M, Shokrgozar MA, Haghighipour N, Mahdian R, Hemmati A. Intermittent Hydrostatic Pressure Enhances Growth Factor-Induced Chondroinduction of Human Adipose-Derived Mesenchymal Stem Cells. Artif Organs. 2012;36:1065–71. doi: 10.1111/j.1525-1594.2012.01507.x. [DOI] [PubMed] [Google Scholar]
- Sakao K, Takahashi KA, Arai Y, Inoue A, Tonomura H, Saito M, Yamamoto T, Kanamura N, Imanishi J, Mazda O, Kubo T. Induction of chondrogenic phenotype in synovium-derived progenitor cells by intermittent hydrostatic pressure. Osteoarthritis Cartilage. 2008;16:805–14. doi: 10.1016/j.joca.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Schatti O, Grad S, Goldhahn J, Salzmann G, Li Z, Alini M, Stoddart MJ. A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur Cell Mater. 2011;22:214–25. doi: 10.22203/ecm.v022a17. [DOI] [PubMed] [Google Scholar]
- Schinagl RM, Gurskis D, Chen AC, Sah RL. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. Journal of Orthopaedic Research. 1997;15:499–506. doi: 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]
- Schumann D, Kujat R, Zellner J, Angele MK, Nerlich M, Mayr E, Angele P. Treatment of human mesenchymal stem cells with pulsed low intensity ultrasound enhances the chondrogenic phenotype in vitro. Biorheology. 2006;43:431–43. [PubMed] [Google Scholar]
- Solorio LD, Vieregge EL, Dhami CD, Dang PN, Alsberg E. Engineered cartilage via self-assembled hMSC sheets with incorporated biodegradable gelatin microspheres releasing transforming growth factor-beta1. J Control Release. 2012;158:224–32. doi: 10.1016/j.jconrel.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NJ, Bryant SJ. The effects of intermittent dynamic loading on chondrogenic and osteogenic differentiation of human marrow stromal cells encapsulated in RGD-modified poly(ethylene glycol) hydrogels. Acta Biomater. 2011;7:3829–40. doi: 10.1016/j.actbio.2011.06.031. [DOI] [PubMed] [Google Scholar]
- Steward AJ, Wagner DR, Kelly DJ. The pericellular environment regulates cytoskeletal development and the differentiation of mesenchymal stem cells and determines their response to hydrostatic pressure. Eur Cell Mater. 2013;25:167–78. doi: 10.22203/ecm.v025a12. [DOI] [PubMed] [Google Scholar]
- Tao Y, Shih J, Sinacore M, Ryll T, Yusuf-Makagiansar H. Development and implementation of a perfusion-based high cell density cell banking process. Biotechnol Prog. 2011;27:824–9. doi: 10.1002/btpr.599. [DOI] [PubMed] [Google Scholar]
- Terraciano V, Hwang N, Moroni L, Park HB, Zhang Z, Mizrahi J, Seliktar D, Elisseeff J. Differential Response of Adult and Embryonic Mesenchymal Progenitor Cells to Mechanical Compression in Hydrogels. Stem Cells. 2007;25:2730–38. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- Thorpe S, Buckley C, Vinardell T, O’Brien F, Campbell V, Kelly D. The Response of Bone Marrow-Derived Mesenchymal Stem Cells to Dynamic Compression Following TGF-β3 Induced Chondrogenic Differentiation. Annals of Biomedical Engineering. 2010;38:2896–909. doi: 10.1007/s10439-010-0059-6. [DOI] [PubMed] [Google Scholar]
- Toh WS, Lee EH, Guo XM, Chan JK, Yeow CH, Choo AB, Cao T. Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials. 2010;31:6968–80. doi: 10.1016/j.biomaterials.2010.05.064. [DOI] [PubMed] [Google Scholar]
- Toh WS, Lim TC, Kurisawa M, Spector M. Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials. 2012;33:3835–45. doi: 10.1016/j.biomaterials.2012.01.065. [DOI] [PubMed] [Google Scholar]
- Vet-Stem. Press Releases. 2012 4/21/2012, http://www.vet-stem.com/pr_detail.php?id=19.
- Vinardell T, Rolfe RA, Buckley CT, Meyer EG, Ahearne M, Murphy P, Kelly DJ. Hydrostatic pressure acts to stabilise a chondrogenic phenotype in porcine joint tissue derived stem cells. Eur Cell Mater. 2012;23:121–32. doi: 10.22203/ecm.v023a09. discussion 33–4. [DOI] [PubMed] [Google Scholar]
- Wilke MM, Nydam DV, Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. Journal of Orthopaedic Research. 2007;25:913–25. doi: 10.1002/jor.20382. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Liu S, Woltjen K, Thomas B, Meng G, Hotta A, Takahashi K, Ellis J, Yamanaka S, Rancourt DE. Cartilage tissue engineering identifies abnormal human induced pluripotent stem cells. Scientific reports. 2013;3:19781–6. doi: 10.1038/srep01978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon IS, Chung CW, Sung JH, Cho HJ, Kim JS, Shim WS, Shim CK, Chung SJ, Kim DD. Proliferation and chondrogenic differentiation of human adipose-derived mesenchymal stem cells in porous hyaluronic acid scaffold. J Biosci Bioeng. 2011;112:402–8. doi: 10.1016/j.jbiosc.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Zhang K, Zhang Y, Yan S, Gong L, Wang J, Chen X, Cui L, Yin J. Repair of an articular cartilage defect using adipose-derived stem cells loaded on a polyelectrolyte complex scaffold based on poly(l-glutamic acid) and chitosan. Acta Biomater. 2013;9:7276–88. doi: 10.1016/j.actbio.2013.03.025. [DOI] [PubMed] [Google Scholar]
- Zscharnack M, Hepp P, Richter R, Aigner T, Schulz R, Somerson J, Josten C, Bader A, Marquass B. Repair of chronic osteochondral defects using predifferentiated mesenchymal stem cells in an ovine model. American Journal of Sports Medicine. 2010;38:1857–69. doi: 10.1177/0363546510365296. [DOI] [PubMed] [Google Scholar]
- zur Nieden NI, Kempka G, Rancourt DE, Ahr HJ. Induction of chondro-, osteo- and adipogenesis in embryonic stem cells by bone morphogenetic protein-2: effect of cofactors on differentiating lineages. BMC developmental biology. 2005;5:1. doi: 10.1186/1471-213X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


