In comparison with DSA, three-dimensional fast black-blood MR imaging can provide an accurate depiction of moderate to severe carotid artery stenosis and plaque ulcers by directly allowing visualization of the vessel wall and lesion distribution.
Abstract
Purpose
To assess fast three-dimensional (3D) black-blood (BB) magnetic resonance (MR) imaging as a noninvasive alternative to intraarterial digital subtraction angiography (DSA) at quantifying moderate to severe carotid artery atherosclerotic disease.
Materials and Methods
Local ethics committee approval and written informed patient consent were obtained for this study. Sixty-five carotid arteries from 52 patients with at least 50% stenosis underwent 3D BB MR imaging and DSA. Quantitative measurements, including stenosis, lesion length, and the presence or absence of plaque ulceration, obtained with the two modalities were independently determined. Sensitivity and specificity, the intraclass correlation coefficient (ICC), Cohen κ, and Bland-Altman analysis were used to assess the agreement.
Results
Excellent agreement in measuring luminal stenosis was found between 3D BB MR imaging and DSA (ICC, 0.96; 95% confidence interval [CI]: 0.93, 0.97). Three-dimensional BB MR imaging was also found to have high sensitivity (91.7%), specificity (96.2%), and agreement (Cohen κ, 0.85; 95% CI: 0.66, 0.99) with DSA for detection of ulcers. Good agreement was found between lesion length measured by using 3D BB MR imaging and DSA (ICC, 0.75; 95% CI: 0.51, 0.84). However, lesion length measurements by using 3D BB MR imaging were, on average, 4.0 mm longer than those measured by using DSA (P < .001).
Conclusion
Three-dimensional BB MR imaging is a noninvasive and accurate way to quantify moderate to severe carotid artery atherosclerotic disease. With fast acquisition and large coverage, 3D BB MR imaging has the potential to become an alternative imaging approach in evaluating the severity of atherosclerosis.
© RSNA, 2014
Introduction
Luminal stenosis measured with digital subtraction angiography (DSA) remains the clinical standard for assessing the severity of carotid artery atherosclerosis (1). However, this imaging approach is known to have certain limitations, including invasiveness, an association with ionizing radiation exposure, and difficulty in visualizing potentially high-risk lesions primarily characterized by outward expansion of the vessel wall, as described by Glagov et al (2).
Black-blood (BB) magnetic resonance (MR) imaging techniques have been developed to accurately visualize and quantify both the lumen and the outer wall boundaries of large arteries (3–5). By selectively suppressing the signals coming from the artery lumen, BB MR imaging can delineate the structure of the arterial wall better than other techniques (6,7). Few studies in the literature have compared BB MR imaging with DSA (considered the standard) in measuring carotid artery lumen stenosis. A previous study showed that area stenosis calculated as per European Carotid Surgery Trial criteria by using two-dimensional (2D) BB MR imaging correlates well with stenosis measured by using DSA (8). However, limitations remain, including the following: (a) Inadequate coverage of the normal segments distal to the lesion makes the 2D BB MR imaging incapable of helping to quantifying the stenosis level by using criteria from the North American Symptomatic Carotid Endarterectomy Trial, and (b) the anisotropic acquisition of 2D BB MR imaging leads to increased partial volume effects and reduced reproducibility (9–11).
Three-dimensional (3D) BB MR imaging offers higher signal-to-noise ratio (SNR), improved resolution in the section direction, and helps achieve isotropic resolution, which can also improve measurement accuracy and reproducibility of vessel wall MR imaging by minimizing the section positioning and registration error in serial studies (11,12). Some techniques using motion-sensitized dephasing preparations (13–15) have been proposed to be used for 3D BB vessel wall images. In this study, we used the gradient-echo–based approach, as it provided the highest imaging efficiency with good blood suppression and large longitudinal coverage. This study sought to assess whether fast 3D BB MR imaging can be used as a noninvasive alternative to intraarterial DSA for quantifying moderate to severe carotid artery atherosclerotic disease.
Materials and Methods
The study protocol was approved by the local institutional review board, and informed consent was obtained from all patients.
Study Sample
Between April 2011 and May 2012, 55 patients (45 men, 10 women; mean age, 64.3 years ± 9.2 [standard deviation]; 87% with recent stroke or transient ischemic attack) who were scheduled for intraarterial DSA and with at least 50% carotid artery stenosis identified by using ultrasonography (US) were invited for additional 3D BB MR imaging. Patients were excluded if they had contraindications to MR imaging, had dementia or physical immobility, or had a medical inability to remain in the MR imaging unit for more than 10 minutes. All patients underwent 3D BB MR imaging within 3 days prior to DSA.
Digital Subtraction Angiography
A neurointerventionalist (J. Wan, with 10 years of experience with neurointervention) performed conventional intraarterial DSA studies by using a transfemoral artery approach and selective common carotid artery catheterization with a digital angiography unit (Innova 4100; GE Healthcare, Buc, France). Images of each bifurcation were obtained in both anterior-posterior and lateral projections. Additional projections were also added on an individual, as-needed basis to better visualize the carotid artery stenosis and ulcer. At each location, 7–8 mL of iodinated contrast medium (Iopamiro; Bracco Sine Pharmaceutical, Shanghai, China), 370 mg of iodine per milliliter, was injected at a flow rate of 5 mL/sec. No vasodilatation drugs were administered during the examination. The DSA was performed with a 40-cm field of view, a 1000 × 1000 matrix, and 0.4 × 0.4 mm2 spatial resolution.
Carotid Artery MR Imaging Protocol
All patients were imaged with a 3.0-T whole-body MR unit (Philips Achieva TX; Best, the Netherlands) using an eight-channel phased-array carotid artery coil. After scout and localization images (5) were obtained, 3D BB MR images centered on the carotid artery bifurcation were acquired with the improved motion-sensitization driven equilibrium (16) prepared rapid gradient-echo sequence and the image mode 3D turbo field-echo sequence. Parameters included the following: repetition time msec/echo time msec, 9.3/4.4; flip angle, 6°; field of view, 250 (foot-to-head direction) × 160 (right-to-left direction) × 64 (anterior-to-posterior direction) mm3; acquisition matrix, 312 × 200 × 80; acquired resolution, 0.8 × 0.8 × 0.8 mm3; reconstruction resolution, 0.4 × 0.4 × 0.4 mm3; motion-sensitization driven equilibrium module: first moment of the motion-sensitized gradients, 1574 mTms2/m; echo time preparation, 22.5 msec; fat suppression selective partial inversion recovery; number of signals acquired, one; acquisition time, 2 minutes 42 seconds.
Image Review
The following variables were measured on DSA and corresponding MR images: (a) stenosis: defined according to the North American Symptomatic Carotid Endarterectomy Trial criteria (17,18), with the following equation: (1− LDmin/RD) · 100, where LDmin is minimum lumen diameter and RD is reference diameter and the minimum lumen diameter was defined as the narrowest diameter of the stenotic lesion, measured in the direction perpendicular to the artery, and the reference diameter was defined as the normal diameter distal to the carotid artery lesion; (b) lesion length: defined as the length of visible carotid artery plaque along the longitudinal direction of the artery; (c) ulcer: defined as lesions with an obvious cleft or irregularity at the luminal surface of the plaque. The lesion length was not measured in cases with total occlusion. The carotid artery lesions were evaluated within the range of 100 mm centered at the bifurcation on both DSA and MR images to avoid the SNR decrease at the periphery of the MR image caused by the limited coil coverage. For arteries with multiple lesions, only the lesion with the highest degree of stenosis was evaluated.
DSA and MR images were independently reviewed. One author (J. Wan) reviewed the DSA images based on the best projection to better visualize the carotid artery stenosis, lesion length, and ulcer using the stenosis analysis software at the workstation (AW4.4; GE Healthcare). Before MR image interpretation, the postprocessed images of the carotid arteries were generated by multiplanar reformation (MPR) and curved MPR to better visualize the advanced carotid artery lesion characters by one author (Y.C., with 5 years of experience in MR image review) who had no knowledge of the projection used for the DSA. Meanwhile, the image quality of reformatted images was assessed by using three grades: grade 1, excellent, high SNR without artifacts, well-defined vessel wall and lumen margins; grade 2, appropriate for diagnosis, marginal SNR, identifiable wall structures, but partially obscured lumen and vessel wall boundaries; and grade 3, inadequate for diagnosis, low SNR and obscured arterial wall or vessel boundaries. Arteries with image quality of grade 3 were excluded from final analysis. The reformatted carotid artery MR images were then analyzed separately by two reviewers (H.Z. and X.L. who each had 5–7 years of experience in MR imaging of the vessel wall) on the same workstation.
Statistical Analysis
Continuous variables were summarized as the mean ± standard deviation. Categorical variables were summarized by count (percentage). The performance of MR imaging in detection of total occlusion or ulcer was summarized by sensitivity, specificity, and Cohen κ using DSA as the standard. Ulcer detection performance was also summarized by positive predictive value, negative predictive value, and accuracy. Interreader agreement of binary variables was evaluated using Cohen κ. A value of κ greater than 0.80 was used to indicate a high level of agreement. For continuous variables, agreement between MR imaging and DSA, as well as between the two readers, was assessed using the approach of Bland and Altman (19). Scatterplots of the differences versus the means were made and qualitatively assessed for bias or systematic differences. Bias was assessed quantitatively by the mean difference ± standard error and tested using the paired t test. Precision was assessed using 95% limits of agreement (± 2 · standard deviation of differences). Last, overall agreement was summarized by using the one-way random intraclass correlation coefficient (ICC), which is the ratio of the between-subject variance over the total variance. ICC is interpreted as follows: an ICC of zero to 0.20, poor agreement; an ICC of 0.21–0.40, fair agreement; an ICC of 0.41–0.60, moderate agreement; an ICC of 0.61–0.80, good agreement; and an ICC of 0.81–1.00, excellent agreement (20). Sensitivity, specificity, positive predictive value, negative predictive value, accuracy, Cohen κ, and ICC were presented as estimate (95% confidence interval [CI]). The nonparametric bootstrap with the percentile method was used to compute the 95% CI for Cohen κ and the ICC to account for potential dependence between arteries from the same subjects (21). Generalized estimating equations were used to compute standard errors, P values and 95% CIs for the other analyses, with multiple arteries per subject (22). All statistical calculations were performed using R 2.14.1 (R Foundation for Statistical Computing, Vienna, Austria). Throughout, two-tailed tests were used with P less than .05 denoting a significant difference.
Results
Image Quality
Images from three of 55 (5%) patients were excluded from analysis either because of inadequate image quality on the MR image from swallowing motion artifacts (n = 1) or excessive vascular tortuousness at the site of greatest narrowing (n = 2), which makes measuring stenosis on MPR images difficult. This yielded a total of 52 patients with 65 diseased arteries (13 of 52 patients had with bilateral disease) for the final analysis. Of the 65 sets of MR images of diseased arteries, 56 (86%) were graded as “excellent.”
Interreader Agreement on 3D BB MR Images
The MR image readers each identified 14 of 65 (22%) total occlusions with 100% interreader agreement. The κ value was 0.95 (95% CI: 0.84, 0.99) for identification of ulceration, and ICC was 0.99 (95% CI: 0.98, 0.99) for both stenosis and lesion length measurements (Table 1). There were small but significant mean differences between readers in measuring stenosis diameter (1.80 mm ± 0.91 vs 1.75 mm ± 0.89, P = .001) and reference diameter (6.87 mm ± 1.20 vs 6.77 mm ± 1.11, P = .009), but the mean difference was not significant in measuring luminal stenosis (73.5% ± 13.4 vs 73.9% ± 13.0, P = .122) (Table 1).
Table 1.
Interreader Agreement for 51 Arteries without Occlusion on 3D BB MR Images
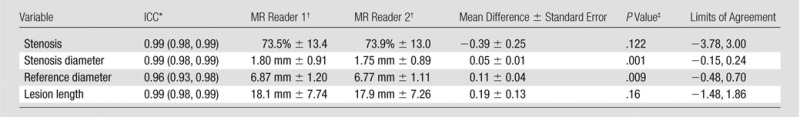
Note—Standard error refers to standard error of the mean difference.
Numbers in parentheses are the 95% CIs.
Data are means ± standard deviations.
For comparison of mean difference to zero.
Agreement of Stenosis and Other Quantitative Measurements between 3D BB MR Imaging and DSA
Of the 65 arteries, 11 (17%) had total occlusion by using DSA. The MR image readers detected all 11 occlusions with DSA (sensitivity, 100%; 95% CI: 71.5%, 100%) (Fig 1) but were unable to distinguish three pseudo-occlusions from total occlusions of the internal carotid arteries (specificity, 94.4%; 95% CI: 84%, 98.2%). The stenoses of those three were 98.3%, 97.9%, and 95.9% by using DSA.
Figure 1:
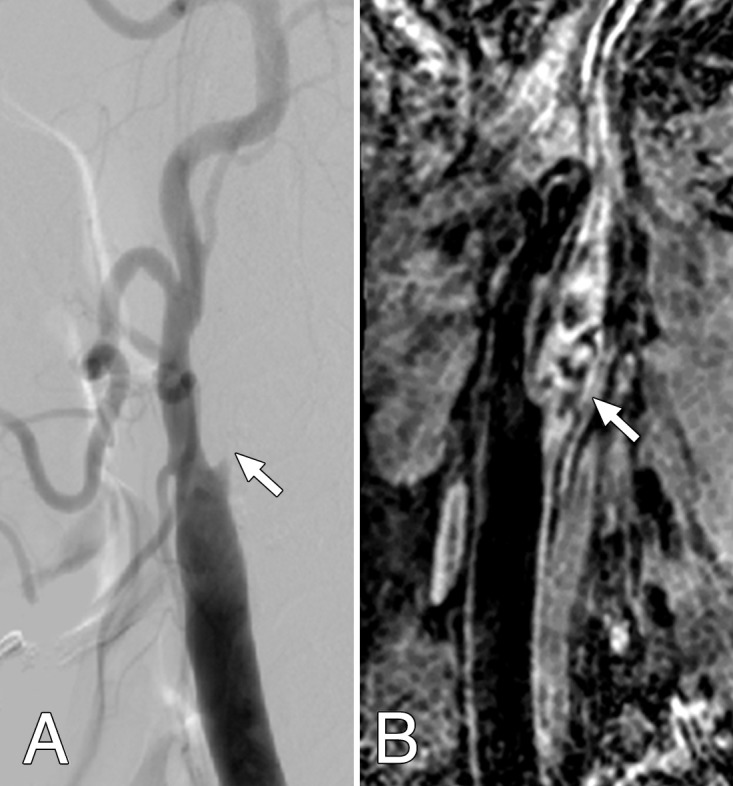
Both, A, Lateral projection DSA image and, B, oblique 3D BB MR image depicted artery occlusion in the internal carotid artery (arrow) in a 61-year-old man.
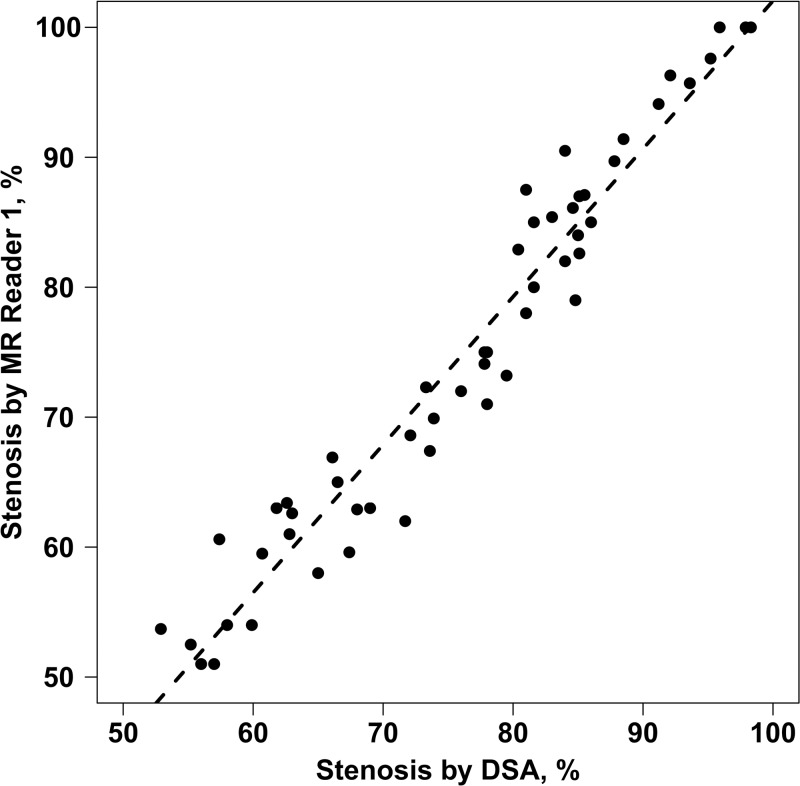
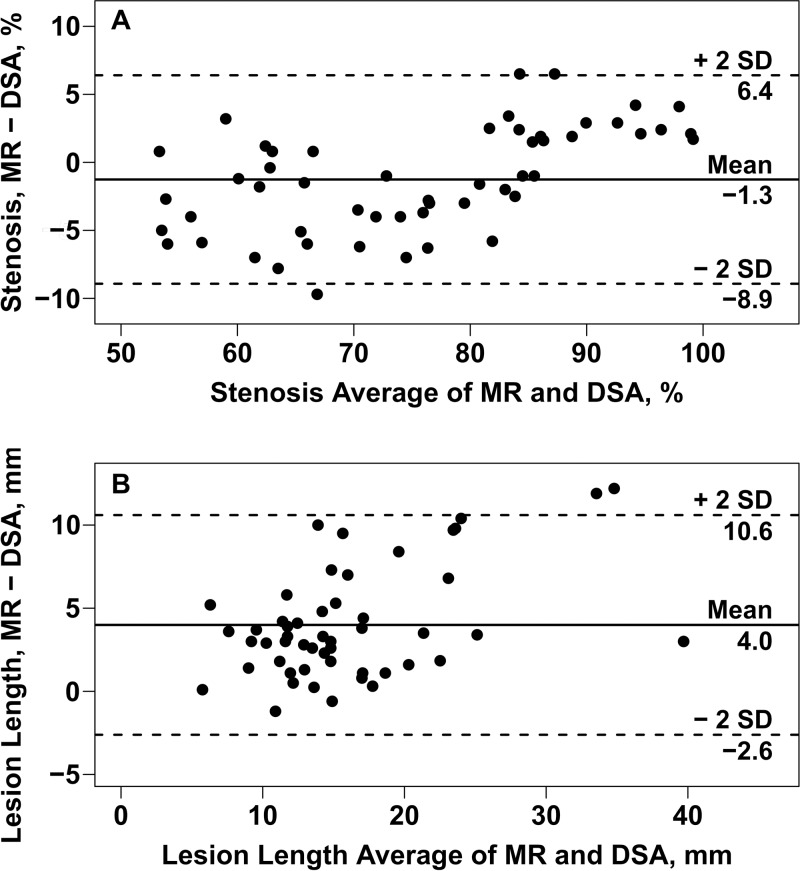
Agreement of the quantitative measurements between 3D BB MR imaging and DSA was evaluated in the 54 arteries without occlusion on DSA images, and the results of this analysis are summarized in Table 2. Only the results for MR reader 1 are shown because of strong interreader agreement. There was excellent agreement in stenosis measurement between 3D BB MR imaging and DSA (ICC, 0.96; 95% CI: 0.93, 0.97) (Fig 2). However, on average the MR stenosis measurements were 1.26% smaller than DSA (P = .007), with limits of agreement of −8.92% to 6.40% (Table 2). Bland-Altman plots (Fig 3, A) suggested that the bias between 3D BB MR imaging and DSA differed when stenosis was greater than or less than 85%. The bias was −2.6% ± 0.4 (P < .001) when stenosis was less than 85% and 2.5% ± 0.4 (P < .001) when stenosis was greater than 85%. When this bias pattern was adjusted during a post hoc analysis, the limits of agreement became ± 6.3%.
Table 2.
Summary of Agreement between DSA and 3D BB MR Imaging for 54 arteries
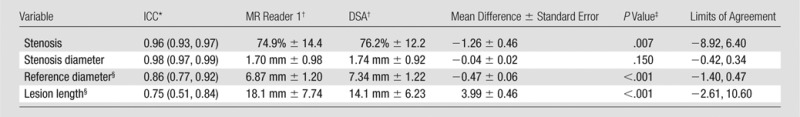
Note.—Eleven arteries with total occlusion depicted on DSA images were excluded. Standard error refers to standard error of the mean difference. Comparison between MR reader 2 and DSA was not shown because of a strong similarity with results for MR reader 1.
Numbers in parentheses are 95% CIs.
Data are means ± standard deviations.
For comparison of mean difference to zero.
Three additional arteries with total occlusion by MR were excluded.
Figure 2:
Graph shows detection of stenosis by MR reader 1 versus that by using DSA. Dashed line = regression line. There was a significant correlation between 3D BB MR imaging and DSA (ICC, 0.96; 95% CI: 0.93, 0.97).
Figure 3:
Bland-Altman plots of, A, the difference in stenosis, and, B, lesion length measurements between MR reader 1 and DSA versus the means. Solid horizontal lines = mean differences (overall bias); dashed lines = 95% limits of agreement. There was a small but significant overall downward bias in the MR-based stenosis measurements (P = .007). The bias was −2.6% ± 0.4 (P < .001) when stenosis was less than 85% and 2.5% ± 0.4 (P < .001) when stenosis was greater than 85% (A).
Lesion length measurements obtained by using 3D BB MR imaging and DSA had good agreement (ICC, 0.75; 95% CI: 0.51, 0.84) but were significantly larger on average when they were obtained with 3D BB MR imaging (18.1 mm ± 7.74 vs 14.1 mm ± 6.23, P < .001), with limits of agreement of −2.61 to 10.60 mm (Table 2; Figs 3, B, 4).
Figure 4:
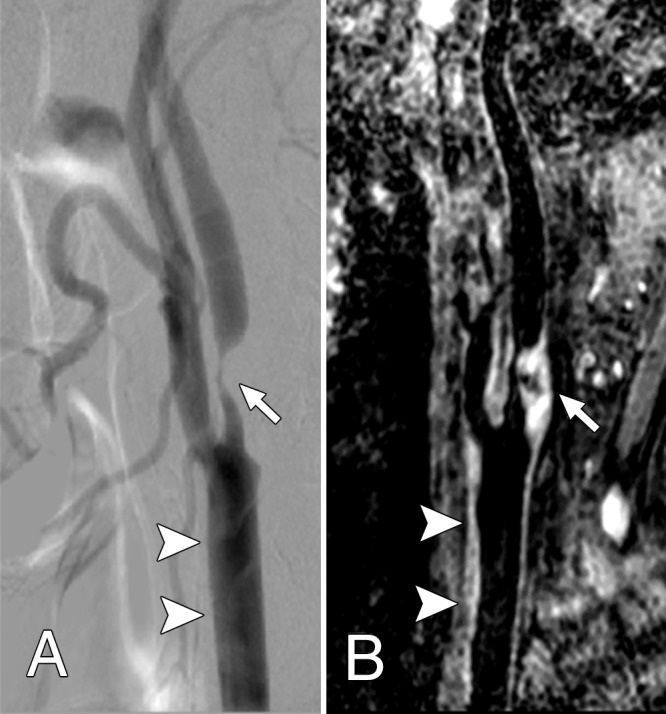
Both, A, Lateral projection DSA image and, B, oblique 3D BB MR image, show advanced atheroscleroitc disease (arrow), which caused severe internal carotid artery stenosis. The common carotid artery wall thickening (arrowheads) was visualized on, B, the 3D BB MR image, but presented as normal on, A, the DSA image.
Agreement of Ulcer Identification between 3D BB MR Imaging and DSA
On DSA images, of the 65 arteries, 12 (18%) had ulcers. The two MR image readers each identified the same 11 of 12 ulcers detected on DSA images, yielding a sensitivity of 91.7% (Fig 5, Table 3). They had two and three false-positive results each for a specificity of 96.2% and 94.3%, respectively. Of the total 13 ulcers identified by MR reader 1 and the 14 ulcers identified by MR reader 2, 11 were true-positive results by using DSA (positive predictive value, 84.6% and 78.6%, respectively). One ulcer identified by using DSA was missed by both readers (negative predictive value, 98.1% and 98.0%, respectively). Overall agreement in identification of ulceration between 3D BB MR imaging and DSA was high for both readers, with agreement in 62 (95%) and 61 (94%) arteries, respectively, leading to κ of 0.85 (95% CI: 0.66, 0.99) and κ of 0.81 (95% CI: 0.59, 0.96), respectively (Table 3).
Figure 5:
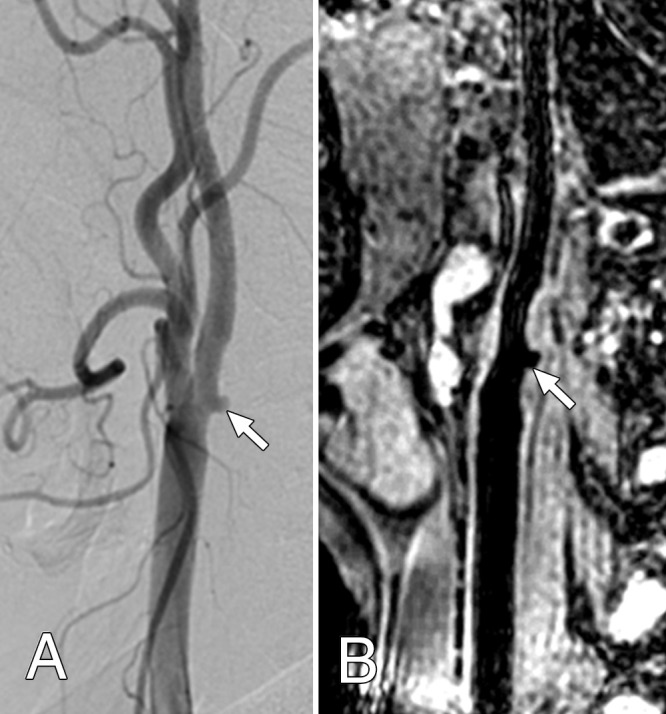
Both, A, Lateral projection DSA image and, B, oblique 3D BB MR image, show the ulceration (arrow) of the carotid artery plaque surface at the bifurcation.
Table 3.
Ulcer Detection in 65 Arteries with 3D BB MR Imaging Using DSA as Reference
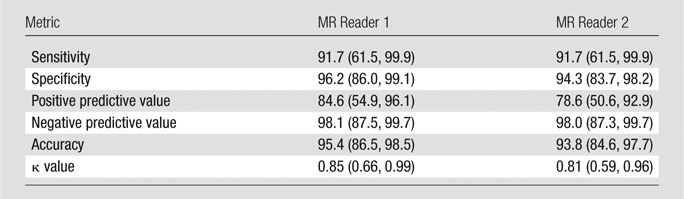
Note.— All data, except for κ values, are percentages. Numbers in parentheses are 95% CIs.
Discussion
To our knowledge, this is the first attempt to validate the 3D BB MR imaging–based stenosis measurements against DSA in patients with carotid artery advanced atherosclerotic lesions. In the artery-by-artery analysis, compared with DSA, the improved motion-sensitization driven equilibrium–based 3D BB MR imaging provided high agreement with DSA for stenosis measurements and ulcer detection in patients with greater than 50% carotid artery stenosis where MR image quality was adequate. In addition, 3D BB MR imaging depicted a longer lesion length than DSA by its capability to delineate the vessel wall morphology and plaque distribution. This study shows the usefulness of 3D BB MR imaging in noninvasive evaluation of the severity of carotid artery atherosclerotic disease.
The agreement in measuring luminal stenosis by using North American Symptomatic Carotid Endarterectomy Trial criteria in our study was higher than the previously reported agreement (ICC, 0.83; 95% CI: 0.74, 0.89) between 2D BB MR imaging and DSA by using European Carotid Surgery Trial criteria (8). Furthermore, 3D BB MR imaging had the capability to cover the carotid artery bifurcation in less than 3 minutes, which is a larger range within a clinically acceptable image time than 2D. In this study, post hoc analysis after inspecting the Bland-Altman plots suggests that 3D BB MR imaging tends to lead to a slightly underestimated stenosis on average when DSA stenosis is 50%–85%, but tends to lead to overestimation when DSA stenosis is greater than 85%. Similarly, 2D BB MR imaging tends to lead to an overestimation of the stenosis (70%–79%) by using DSA (8). Previous observational studies using MR angiography reported overestimations that have included a wide range of carotid artery stenosis, including a significant number of lesions with moderate to severe stenosis (23,24). Multiple factors may contribute to the stenosis measurement bias, including spatial resolution, sequence type (25), and postprocessing algorithms (26). However, as we found in this study, the improved motion-sensitization driven equilibrium–based 3D BB MR approach has potential advantages, such that flow artifacts should be of lesser concern and steno-occlusive lesions are directly visualized.
In this study, on average, 3D BB MR–based lesion lengths were found to be nearly 4.0 mm larger than the corresponding DSA measurements. Our findings are in line with measurements in previous studies (27). The vessel walls near the stenotic lesion appear to be thickened by using 3D BB MR imaging but seem normal by using DSA (Fig 4), suggesting that the plaque can extend, either in a wedge-shaped fashion or due to expansion (2). Researchers in previous studies have found that, in cases of carotid artery endarterectomy, the end of the plaque is more distal than was expected on the basis of the preoperative DSA results (28,29). With 3D BB MR imaging, both the inner and outer wall of the artery are directly visualized, making this imaging technique a potentially more accurate approach to visualize the distal extent of the plaque. Overall utility of 3D BB MR imaging as an adjunct to DSA for carotid artery imaging in patients undergoing revascularization allows for better assessment of proximal and distal anatomy for the purposes of planning endovascular stent placement.
In this study, we found that curved MPR along the lumen by using isotropic 3D BB MR imaging allowed for direct visualization of the details on the plaque surface. Although 3D BB MR imaging showed a good accuracy for detection of plaque ulcer when compared with DSA, it also led to misidentification of ulcerations on a small group of arteries. False-positive results due to tiny juxtaluminal calcifications may obscure the lumen surface and consequently the detection of the ulcers (30). Great care must be taken to examine the surface of plaque for calcific irregularities or possible ulcers on BB MR images. Nevertheless, bright-blood sequences, such as time-of-flight or gadolinium-enhanced MR angiography, are helpful to accurately characterize the luminal boundary (31).
A limitation of our study was that we only compared the preselected MPR images of 3D BB MR imaging with the limited 2D DSA projections. Although we carefully researched and minimized potential sources of bias before making our measurements, the discrepancy of viewing angles of the carotid artery lesions would inevitably hamper the agreement analysis. Repeated resectioning of the 3D image data with multiple views may be helpful for assessment of stenosis and ulceration. Another limitation was that 3D BB MR imaging was only validated in a small sample of selected patients with moderate to severe carotid artery atherosclerotic lesions on the basis of screening Doppler US from a single institution. A larger sample with a wider range of stenosis should be examined before 3D BB MR to demonstrate this method as a reliable clinical tool.
Three-dimensional BB MR imaging may be of most benefit when used in conjunction with other MR imaging sequences for identification of a high-risk vulnerable plaque (32). The future work of 3D BB MR imaging aimed at the advanced carotid artery atherosclerotic lesions is sure to focus on defining plaque morphology to better stratify patients according their risk for either endarterectomy or stent procedure.
In conclusion, in comparison with DSA, 3D fast BB MR imaging can provide an accurate depiction of moderate to severe carotid artery stenosis and plaque ulcer by directly allowing visualization of vessel wall and lesion distribution. With the benefit of noninvasive approach and isotropic resolution, 3D BB MR imaging can be a promising alternative imaging approach to DSA in evaluating the advanced atherosclerosis at the carotid artery bifurcation.
Advances in Knowledge
■ In comparison with digital subtraction angiography (DSA), three-dimensional (3D) black-blood (BB) MR imaging showed an excellent agreement with an intraclass correlation coefficient of 0.96 in stenosis measurement at advanced carotid artery atherosclerosis disease, by using criteria from the North American Symptomatic Carotid Endarterectomy Trial.
■ Three-dimensional BB MR imaging was also found to have high sensitivity (91.7%), specificity (96.2%), and agreement (Cohen κ = 0.85) with DSA for detection of carotid artery plaque ulcers.
■ Lesion length measurements by using 3D BB MR imaging were, on average, 4.0 mm longer than those measured by using DSA in patients with advanced atherosclerotic lesions.
Implication for Patient Care
■ Three-dimensional fast BB MR imaging can provide an accurate depiction of moderate to severe carotid artery stenosis and plaque ulcer by directly allowing visualization of the vessel wall and lesion distribution; this technique has the potential to become an alternative imaging approach to DSA in evaluating advanced atherosclerosis at the carotid artery bifurcation.
Received November 20, 2013; revision requested January 21, 2014; final revision received July 18; final version accepted August 12.
Supported by the Shanghai Leading Academic Discipline Project (grant S30203) and National Natural Science Foundation of China (grants 81271575 and 81271536).
Funding: This research was supported by the National Institutes of Health (grants HL 56874 and HL 103609).
Disclosures of Conflicts of Interest: H.Z. Activities related to the present article: institution received grants from Shanghai Leading Academic Discipline Project (530203) and National Natural Science Foundation of China (81271575, 81271536). Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. J. Wang Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: employed by Philips Research North America. Other relationships: disclosed no relevant relationships. X.L. Activities related to the present article: institution received grants from Shanghai Leading Academic Discipline Project (530203) and National Natural Science Foundation of China (81271575, 81271536). Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. X.Z. disclosed no relevant relationships. D.S.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received grants or has grants pending for analysis of unrelated data from GE Healthcare, Philips Healthcare, and Society of Interventional Radiology Foundation. Other relationships: disclosed no relevant relationships. Y.C. Activities related to the present article: institution received grants from Shanghai Leading Academic Discipline Project (530203) and National Natural Science Foundation of China (81271575, 81271536). Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. J. Wan disclosed no relevant relationships. C.Y. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received a grant from Philips Medical and was paid as a consultant by Bristol Myers Squibb and Philips Healthcare; has patents issued (US 7,353,117 B2; US 7,627,359 B2; US 7,340,083 B2; US 7,315,756 B2). Other relationships: disclosed no relevant relationships. J.X. Activities related to the present article: institution received grants from Shanghai Leading Academic Discipline Project (530203) and National Natural Science Foundation of China (81271575, 81271536). Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships.
Abbreviations:
- DSA
- digital subtraction angiography
- BB
- black blood
- CI
- confidence interval
- ICC
- intraclass correlation coefficient
- MPR
- multiplanar reformation
- SNR
- signal-to-noise ratio
- 3D
- three-dimensional
- 2D
- two-dimensional
References
- 1.Moore WS, Barnett HJ, Beebe HG, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Circulation 1995;91(2):566–579. [DOI] [PubMed] [Google Scholar]
- 2.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 1987;316(22):1371–1375. [DOI] [PubMed] [Google Scholar]
- 3.Yuan C, Beach KW, Smith LH, Jr, Hatsukami TS. Measurement of atherosclerotic carotid plaque size in vivo using high resolution magnetic resonance imaging. Circulation 1998;98(24):2666–2671. [DOI] [PubMed] [Google Scholar]
- 4.Saam T, Ferguson MS, Yarnykh VL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol 2005;25(1):234–239. [DOI] [PubMed] [Google Scholar]
- 5.Li F, Yarnykh VL, Hatsukami TS, et al. Scan-rescan reproducibility of carotid atherosclerotic plaque morphology and tissue composition measurements using multicontrast MRI at 3T. J Magn Reson Imaging 2010;31(1):168–176. [DOI] [PubMed] [Google Scholar]
- 6.Edelman RR, Mattle HP, Wallner B, et al. Extracranial carotid arteries: evaluation with “black blood” MR angiography. Radiology 1990;177(1):45–50. [DOI] [PubMed] [Google Scholar]
- 7.Edelman RR, Chien D, Kim D. Fast selective black blood MR imaging. Radiology 1991;181(3):655–660. [DOI] [PubMed] [Google Scholar]
- 8.U-King-Im JM, Trivedi RA, Sala E, et al. Evaluation of carotid stenosis with axial high-resolution black-blood MR imaging. Eur Radiol 2004;14(7):1154–1161. [DOI] [PubMed] [Google Scholar]
- 9.Steinman DA, Rutt BK. On the nature and reduction of plaque-mimicking flow artifacts in black blood MRI of the carotid bifurcation. Magn Reson Med 1998;39(4):635–641. [DOI] [PubMed] [Google Scholar]
- 10.Thomas JB, Antiga L, Che SL, et al. Variation in the carotid bifurcation geometry of young versus older adults: implications for geometric risk of atherosclerosis. Stroke 2005;36(11):2450–2456. [DOI] [PubMed] [Google Scholar]
- 11.Antiga L, Wasserman BA, Steinman DA. On the overestimation of early wall thickening at the carotid bulb by black blood MRI, with implications for coronary and vulnerable plaque imaging. Magn Reson Med 2008;60(5):1020–1028. [DOI] [PubMed] [Google Scholar]
- 12.Balu N, Kerwin WS, Chu B, Liu F, Yuan C. Serial MRI of carotid plaque burden: influence of subject repositioning on measurement precision. Magn Reson Med 2007;57(3):592–599. [DOI] [PubMed] [Google Scholar]
- 13.Koktzoglou I, Li D. Diffusion-prepared segmented steady-state free precession: Application to 3D black-blood cardiovascular magnetic resonance of the thoracic aorta and carotid artery walls. J Cardiovasc Magn Reson 2007;9(1):33–42. [DOI] [PubMed] [Google Scholar]
- 14.Fan Z, Zhang Z, Chung YC, et al. Carotid arterial wall MRI at 3T using 3D variable-flip-angle turbo spin-echo (TSE) with flow-sensitive dephasing (FSD). J Magn Reson Imaging 2010;31(3):645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balu N, Yarnykh VL, Chu B, Wang J, Hatsukami T, Yuan C. Carotid plaque assessment using fast 3D isotropic resolution black-blood MRI. Magn Reson Med 2011;65(3):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Yarnykh VL, Yuan C. Enhanced image quality in black-blood MRI using the improved motion-sensitized driven-equilibrium (iMSDE) sequence. J Magn Reson Imaging 2010;31(5):1256–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.North American Symptomatic Carotid Endarterectomy Trial . Methods, patient characteristics, and progress. Stroke 1991;22(6):711–720. [DOI] [PubMed] [Google Scholar]
- 18.North American Symptomatic Carotid Endarterectomy Trial Collaborators . Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325(7):445–453. [DOI] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res 1999;8(2):135–160. [DOI] [PubMed] [Google Scholar]
- 20.Curvo-Semedo L, Lambregts DM, Maas M, et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy—conventional MR volumetry versus diffusion-weighted MR imaging. Radiology 2011;260(3):734–743. [DOI] [PubMed] [Google Scholar]
- 21.Davidson AC, Hinkley DV. Bootstrap methods and their application (Cambridge Series in Statistical and Probabilistic Mathematics). Cambridge, England: Cambridge University Press, 1997. [Google Scholar]
- 22.Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of longitudinal data (Oxford Statistical Science Series). 2nd ed. Oxford, England: Oxford University Press, 2002. [Google Scholar]
- 23.Nederkoorn PJ, Elgersma OE, Mali WP, Eikelboom BC, Kappelle LJ, van der Graaf Y. Overestimation of carotid artery stenosis with magnetic resonance angiography compared with digital subtraction angiography. J Vasc Surg 2002;36(4):806–813. [PubMed] [Google Scholar]
- 24.Nonent M, Ben Salem D, Serfaty JM, et al. Overestimation of moderate carotid stenosis assessed by both Doppler US and contrast enhanced 3D-MR angiography in the CARMEDAS study. J Neuroradiol 2011;38(3):148–155. [DOI] [PubMed] [Google Scholar]
- 25.U-King-Im JM, Trivedi RA, Cross JJ, et al. Measuring carotid stenosis on contrast-enhanced magnetic resonance angiography: diagnostic performance and reproducibility of 3 different methods. Stroke 2004;35(9):2083–2088. [DOI] [PubMed] [Google Scholar]
- 26.Runck F, Steiner RP, Bautz WA, Lell MM. MR imaging: influence of imaging technique and postprocessing on measurement of internal carotid artery stenosis. AJNR Am J Neuroradiol 2008;29(9):1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida K, Endo H, Sadamasa N, et al. Evaluation of carotid artery atherosclerotic plaque distribution by using long-axis high-resolution black-blood magnetic resonance imaging. J Neurosurg 2008;109(6):1042–1048. [DOI] [PubMed] [Google Scholar]
- 28.Randoux B, Marro B, Koskas F, et al. Carotid artery stenosis: prospective comparison of CT, three-dimensional gadolinium-enhanced MR, and conventional angiography. Radiology 2001;220(1):179–185. [DOI] [PubMed] [Google Scholar]
- 29.Benes V, Netuka D, Mandys V, et al. Comparison between degree of carotid stenosis observed at angiography and in histological examination. Acta Neurochir (Wien) 2004;146(7):671–677. [DOI] [PubMed] [Google Scholar]
- 30.Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002;106(11):1368–1373. [DOI] [PubMed] [Google Scholar]
- 31.Mitsumori LM, Hatsukami TS, Ferguson MS, Kerwin WS, Cai J, Yuan C. In vivo accuracy of multisequence MR imaging for identifying unstable fibrous caps in advanced human carotid plaques. J Magn Reson Imaging 2003;17(4):410–420. [DOI] [PubMed] [Google Scholar]
- 32.Zhao X, Underhill HR, Yuan C, et al. Minimization of MR contrast weightings for the comprehensive evaluation of carotid atherosclerotic disease. Invest Radiol 2010;45(1):36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]