Abstract
More than half of older women who sustain a fragility fracture do not have osteoporosis by World Health Organization (WHO) bone mineral density (BMD) criteria; and, while BMD has been used to assess fracture risk for over 30 years, a range of other skeletal and nonskeletal clinical risk factors (CRFs) for fracture have been recognized. More than 30 assessment tools using CRFs have been developed, some predicting fracture risk and others low BMD alone. Recent systematic reviews have reported that many tools have not been validated against fracture incidence, and that the complexity of tools and the number of CRFs included do not ensure best performance with poor assessment of (internal or comparative) validity. Internationally, FRAX® is the most commonly recommended tool, in addition to QFracture in the UK, The Canadian Association of Radiologists and Osteoporosis Canada (CAROC) tool in Canada and Garvan in Australia. All tools estimate standard 10-year risk of major osteoporotic and 10-year risk of hip fracture: FRAX® is able to estimate fracture risk either with or without BMD, but CAROC and Garvan both require BMD and QFracture does not. The best evidence for the utility of these tools is in case finding but there may be future prospects for the use of 10-year fracture risk as a common currency with reference to the benefits of treatment, whether pharmacological or lifestyle. The use of this metric is important in supporting health economic analyses. However, further calibration studies will be needed to prove that the tools are robust and that their estimates can be used in supporting treatment decisions, independent of BMD.
Keywords: fracture risk assessment, FRAX®, Garvan, osteoporosis, postmenopausal women, QFracture
Introduction
The NIH Consensus Development Conference defined osteoporosis as ‘a skeletal disorder characterized by compromised bone strength predisposing to an increased risk of fracture’ [NIH Consensus Development Panel, 2001]. This definition incorporates three important factors:
low bone mineral density (BMD);
compromise to bone quality and strength;
fracture risk.
The measurement of BMD in screening for osteoporosis and assessing fracture risk has dominated practice for more than 20 years [World Health Organization, 1994] and BMD has provided a useful proxy measure for efficacy in clinical trials [Stevenson et al. 1990]. However, using these criteria, a third of 70-year-old and more than half of 80-year-old persons have osteoporosis [Kanis et al. 1994], although more than half of older women who fracture do not have osteoporosis by BMD criteria [Greenspan et al. 2001; Davis et al. 2011]. There are many other factors, including risk of falls and poor vision, which may increase fracture risk [NIH Consensus Development Panel, 1991; Lips, 1997]; and for a number of high-risk groups such as patients commencing glucocorticoids [Royal College of Physicians, 2002], androgen deprivation therapy [Higano, 2004], anti-oestrogens [Reid et al. 2008], or suffering from chronic kidney disease [Miller, 2014] BMD alone is not a good predictor of fracture risk.
Both skeletal factors (particularly BMD) and nonskeletal clinical risk factors (CRFs) predict fracture risk [Kanis et al. 2005] and both were evaluated in their prediction of fractures incidence over 4 years for 46,340 subjects. The resultant tools were subsequently validated in 11 cohorts, where 3360 hip fractures occurred in 230,486 subjects over 1,208,528 person-years. Using the gradient of hip fracture risk (GR, risk ratio/standard deviation [SD] change in risk score), BMD alone was a better predictor than CRFs (GR for BMD was 3.7/SD; for CRFs it was 2.1/SD). However, the GR was highest at 4.2/SD for CRFs combined with BMD [Kanis et al. 2007]. This work contributed to the development of the best known and most widely used fracture risk assessment tool (FRAX®) which was launched by the WHO in 2008 [McCloskey and Kanis, 2012]. However, many fracture risk assessment tools have now been developed including up to 31 different CRFs [Rubin et al. 2013].
The use of 10-year fracture risk, rather than diagnosis of osteoporosis using dual-energy X-ray absorptiometry (DXA), offers a common currency for fracture risk assessment. This was recently recognized by a committee of North American clinicians reporting to the National Bone Health Alliance, as they proposed a diagnosis of osteoporosis should be conferred by ‘hip fracture; osteopenia-associated vertebral, proximal humerus, pelvis, or some wrist fractures; or FRAX® scores with ⩾3% (hip) or 20% (major) 10-year fracture’ [Siris et al. 2014]. However, the value of fracture risk assessment is not universally recognized and various criticisms have been made, including its dependence on access to the internet, assumptions made by the tool about fracture risk, body mass index (BMI) and mortality across racial and ethnic groups, the exclusion of variables such as falls and the lack of adjustment for dose–response relationships [Silverman and Calderon, 2010].
Alternative fracture risk assessment tools
In their very timely systematic review of risk assessment tools, Rubin and colleagues [Rubin et al. 2013] comment that ‘Far from all [tools] have been validated in external studies [and] more of them have absence of methodological and transparent evidence’. They found 48 tools of which only 20 were externally validated and 6 had been tested more than once in a population-based setting with acceptable methodological quality. None of the tools performed consistently better than the others and they found no evidence that complex tools were superior to simple ones. However, the lack of head-to-head comparisons in similar populations makes it difficult to come to a firm conclusion on comparison. Another recent systematic review highlighted the lack of evaluation of performance and the failure to calibrate osteoporosis fracture risk assessment instruments across different risk categories and in populations separate from their development cohorts [Nayak et al. 2014]. Methods of development, validation and transparency relating to comparable risk assessment tools have also been critically reviewed, with only three fracture risk assessment tools identified which have been validated at least once in a population-based setting (Garvan, FRAX® and QFracture). There is clearly more work to be done in identifying which methods work the best in which populations [Collins and Michaelsson, 2012; Rubin et al. 2013].
Fracture risk assessment in practice
From case finding to decisions about treatment
Fracture risk assessment can be seen as the first of three stages in evaluating osteoporosis risk and treatment (see Figure 1) [Aspray, 2013]. First, CRFs may be used to assess fracture risk for case finding to identify those most likely to benefit from treatment. Subsequently, it is necessary to define those who warrant pharmacological therapies and subsequently prioritize the choice of different treatments informed by their effect (usually estimated as absolute fracture risk reduction). Historically, the randomized controlled trial has been the means of estimating these effects and criteria based on low BMD and age have often been used for study recruitment. However, fracture risk derived from CRFs (with or without BMD) has potential, but has not been validated in randomized controlled trials of pharmacological treatments for osteoporosis, and so BMD criteria have been incorporated into clinical guidelines. In practice, the adoption of fracture risk assessment has been variable, as outlined in the following national case studies. Various tools are used, with FRAX® adopted in most countries. However, while these approaches are based on some evidence, local preference and previous national assessment tool development have also had a considerable impact.
Figure 1.

The place of fracture risk assessment in providing a tool for treatment choice.
The United Kingdom
Rubin and colleagues highlight the fact that national guidelines do not generally include fracture risk assessment tools, although FRAX® [Kanis et al. 2008] and QFracture [Hippisley-Cox and Coupland, 2012] have been recommended by the National Institute for Health and Care Excellence (NICE) in England and Wales for the assessment of fragility fracture risk [Rabar et al. 2012]. Both tools use CRFs to estimate 10-year risk of major osteoporotic fracture and hip fracture. FRAX® uses data derived from cohort studies, whereas QFracture uses routine clinical data from British general practice surgeries on approximately 5 million British patients [Hippisley-Cox et al. 2007]. The main differences between FRAX® and QFracture have been summarized elsewhere [Aspray, 2013], which comprise:
FRAX® has been used in a number of countries; QFracture was developed in the UK;
the age range for FRAX® is 40–90 years and for QFracture 30–99 years;
both tools estimate 10-year fracture risk; QFracture also estimates an annualized risk;
FRAX® (but not QFracture) can give fracture risk based on CRFs with BMD;
QFracture uses a wider range of CRFs;
FRAX® uses binary data for all variables other than age, weight, height and BMD (e.g. glucocorticoid therapy/no glucocorticoid therapy, smoker/non-smoker), whereas QFracture includes ‘dose response’ for some variables (e.g. non/ex/light/heavy/moderate smoker).
Perhaps the most comprehensive attempt to implement a strategy for fracture risk assessment has been in England and Wales, where NICE developed its Short Clinical Guideline on Osteoporosis: Assessing the Risk of Fragility Fracture (Clinical Guideline 146, [Rabar et al. 2012]). The guideline is comprehensive in its recommendations on: who to assess, how to assess them and when to re-assess their fracture risk.
Who?
The guideline recommends fracture risk assessment in women aged over 65 years and men over 75 years. For patients aged over 50 years, the presence of CRFs should prompt an assessment of fracture risk. These CRFs comprise: previous fragility fracture, glucocorticoid treatment, a history of falls, a family history of hip fracture, other causes of secondary osteoporosis, low BMI (<18.5 kg/m2), smoking (>10 per day) or alcohol (>4 units per day). For those aged 40–50 years of age fracture risk assessment is only advocated for patients with major risk factors: glucocorticoid therapy, untreated premature menopause or previous fragility fracture.
How?
FRAX® (without BMD) or QFracture should be used to calculate 10-year predicted absolute fracture risk. However, we should recognize underestimation of fracture risk in the case of: multiple fractures, high alcohol intake, obesity, heavy smoking, high-dose oral glucocorticoid therapy and in some secondary causes of osteoporosis.
When?
Fracture risk should not be recalculated within 2 years unless the original risk was close to an intervention threshold for a proposed treatment or there has been a change in the person’s risk factors (e.g. fracture).
Although these guidelines are clear and implementable, NICE technology appraisals for treatments [National Institute for Health and Care Excellence, 2010, 2011a, 2011b] had previously rejected the use of 10-year fracture risk to support treatment decisions, stating that ‘recommendations about treatment should [not] be based on absolute risk as calculated using FRAX®’. Instead, the technology appraisals use a DXA scan diagnosis of osteoporosis (T-score less than −2.5) as their starting point for decisions about treatment (although in women aged 75 years or older, osteoporosis ‘may be assumed’ if the clinician thinks DXA scan is ‘inappropriate or unfeasible’). In addition to low BMD, CRFs analogous to but not the same as those used in FRAX® or QFracture have been included and the cost-effectiveness calculations prioritize more expensive treatment with lower BMD or multiple fractures. Implementation of the technology appraisals has been problematic and it is notable that they specifically do not apply to men, premenopausal women, patients on glucocorticoids or cases of secondary osteoporosis, due to conditions such as diabetes mellitus [Giangregorio et al. 2012], chronic liver disease or thyroid disorder.
In Scotland, NICE Guidance does not apply. The Scottish Intercollegiate Guidelines Network (SIGN) Osteoporosis Guideline was published in 2004 and makes a number of evidence-based recommendations, including [Scottish Intercollegiate Guidelines Network, 2004]:
patients who have suffered one or more fragility fractures should be priority targets for investigation and treatment of osteoporosis;
use of family history in assessing risk of osteoporosis should include maternal, paternal and sister history;
family history should include not only a given diagnosis of osteoporosis but also kyphosis and low trauma fracture after age 50;
smokers should be considered at greater risk of osteoporosis than nonsmokers, and advised to stop, for this and other reasons;
the possibility of osteoporosis should be considered in patients with specific conditions, i.e. anorexia nervosa, chronic liver disease, coeliac disease, hyperparathyroidism, inflammatory bowel disease, male hypogonadism, renal disease, rheumatoid arthritis, long-term glucocorticoid use and vitamin D deficiency.
However, SIGN concludes that available risk scores for osteoporosis are ‘not of satisfactory quality and have not been validated’. An update of the SIGN guidance is awaited.
Does DXA help?
There is inconsistent evidence that BMD significantly improves the performance of FRAX® [Kanis et al. 2005, 2007], with little reclassification of subjects from high to low risk or vice versa [Leslie et al. 2012]. However, it has been argued that DXA scanning does help refine estimates of fracture risk [Johansson et al. 2009; Leslie et al. 2010; Bow et al. 2011]. The NICE guideline on fracture risk assessment specifically suggests that BMD should be used in assessing fracture risk in younger people at risk of osteoporotic fracture. It should also be noted that QFracture cannot be interpreted in the context of BMD, so that, if fracture risk is to be presented in the context of BMD, FRAX® CRFs must be used.
The National Osteoporosis Guideline Group
Partly in response to difficulties in implementing NICE technology appraisals, further developments in the UK have included the work of the National Osteoporosis Guideline Group (NOGG), whose clinical guidelines apply to both men and women at high fracture risk [National Osteoporosis Guideline Group et al. 2008]. The essentials of NOGG are that:
a history of a fragility facture in a post-menopausal woman should prompt consideration for treatment;
- 10-year fracture risk should be estimated in
- men aged 50 years or more with at least one CRF (derived from FRAX®) or BMI less than 19 kg/m2;
- postmenopausal women without fracture but with at least one CRF (derived from FRAX®) or BMI less than 19 kg/m2.
The resultant 10-year fracture risk estimate can then be plotted against age (see Figure 2) and the output includes an area of risk (shaded amber) for which DXA scanning is advocated, to refine the risk estimate. The refined estimate should be re-plotted on the right-hand panel to prompt a decision about treatment: the green zone recommending lifestyle advice; red zone recommending that pharmacological intervention is considered. The threshold for treatment merely reflects the mean fracture risk associated with a history of fragility fracture at that age. Thus, there may be an argument for setting the threshold higher at younger ages and lower at older age or using a single threshold for all ages, as used by the North Americans [National Osteoporosis Foundation, 2010].
Figure 2.
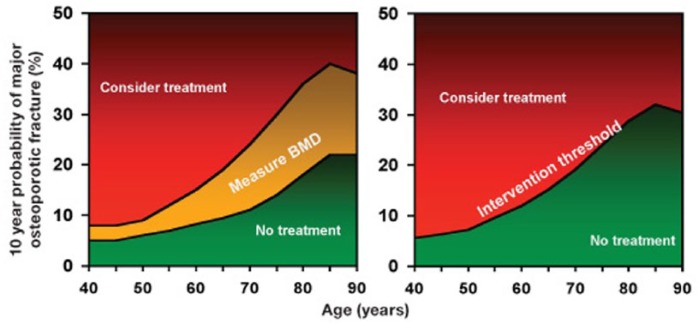
The National Osteoporosis Guideline Group (NOGG) interpretation of fracture risk.
Canada
In Canada, a similar process to that seen in England and Wales has been undertaken, with the Osteoporosis Canada 2010 Guidelines recommending two alternative fracture risk assessment tools [Papaioannou et al. 2010]: The Canadian Association of Radiologists and Osteoporosis Canada tool (CAROC) and FRAX®. However, CAROC incorporates age, sex, prior fragility fracture, and systemic glucocorticoid use, together with bone mineral density [Leslie et al. 2010]. The output for each tool gives a 10-year risk of major osteoporotic fracture, and is associated with thresholds of risk: low (<10%), intermediate (10-20%) or high (>10%). There are calibration data for CAROC and FRAX®, which show good results, when used in a Canadian population, and a low rate of reclassification between low, intermediate and high fracture risk groups [Leslie et al. 2011].
Australia
In Australia, FRAX® (with BMD) and Garvan are recommended by the Royal Australian College of General Practitioners [The Royal Australian College of General Practitioners, 2010]. The latter is a tool validated in an Australian population and incorporates gender, age, falls and BMD. Both tools incorporate DXA results and the guideline acknowledges that there has been no calibration of the results of the tests. However, more recent studies have shown a similar area under the curve (AUC) for these two methods in Australian [Nguyen et al. 2013], but not in New Zealand populations [Bolland et al. 2011], suggesting that calculators should be validated in local cohorts before clinical use.
USA
The National Osteoporosis Federation of America has included some recommendations on case finding and the role of fracture risk assessment in its comprehensive Clinician’s Guideline [National Osteoporosis Foundation, 2010]. Lists of risk factors, which should influence the clinician to consider osteoporosis and fracture risk, are tabulated. These include conditions, diseases and medications which contribute to osteoporosis and fracture risk as well as risk factors for falls and a summary of the FRAX® risk factors. However, the guide (rather ambitiously) recommends that all postmenopausal women and all men aged 50 years and older should be evaluated clinically for osteoporosis risk. This should include 10-year estimated fracture risk ‘where appropriate’, although details of when such an assessment is warranted are not clear. The guide also uses thresholds of 10-year fracture probability derived from health economic evaluations, recommending treatment for patients with a BMD in the osteopenic range, where hip fracture probability is ⩾3% or major osteoporosis-related fracture probability ⩾20% [Tosteson et al. 2008].
Europe
The variability in fracture risk assessment methodologies, their development, validation and implementation has been widely recognized. Here we have presented four national approaches which vary in their conclusions with regard to the best tools and thresholds for diagnosis. They have also used fracture risk assessment for case finding and making treatment decisions. The International Osteoporosis Foundation (IOF) and European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) have also reviewed the assessment and treatment of post-menopausal osteoporosis [Kanis et al. 2013]. Within their review of fracture risk and case-finding strategies, the potential of FRAX® to assess fracture risk, develop treatment thresholds and guide access to densitometry are considered in some detail as well as briefer coverage of the Garvan and QFracture tools, highlighting the fact that these both include falls as risk factors. A further innovation of guideline development has taken place in collaboration with IOF–ECTS, who have issued guidelines for the management of glucocorticoid-induced osteoporosis, which can be incorporated into the FRAX® output. For example, lower dose therapy (equivalent to prednisolone <2.5 mg/day) is associated with a 35% lower risk and higher dose (prednisolone >7.5 mg/day) associated with a 20% greater risk of hip fracture [Lekamwasam et al. 2012].
Clinical implications
The availability of a 10-year fracture risk estimate offers a new currency for osteoporosis and a means to target fracture risk reduction, which aids communication with patients, as it is more intuitive than the WHO diagnostic criteria for osteoporosis. The clinical significance of a femoral neck T-score of −2.5 differs between men and women, young and old and those with or without CRF comorbidities. Whereas 10-year risk of hip fracture means the same thing for all and has the potential to be estimated for any interventions, whether targeting bone or falls prevention, using pharmacological or lifestyle modifications. However, although clinical trials have used fracture incidence to measure clinical outcomes, we are a long way from understanding the potential benefit of treatment using fracture risk. Calibration studies are few and focus on the natural history of fracture and not the relation of fracture risk estimates to fracture incidence in response to interventions.
BMD (and biochemical markers of bone turnover) remain important biomarkers and are attractive outcomes for studies to compare treatment effects, as the least significant change is easily established, allowing the design of trials which can be ‘powered’ to deliver predetermined clinically and statistically significant benefit [Baim et al. 2005; Baxter et al. 2013]. However, the clinical role of DXA needs clarification and the feasibility of fracture risk estimation using QFracture or FRAX® without access to BMD requires some evidence. Vertebral fractures are a significant source of morbidity [Francis et al. 2008] and an independent predictor of fracture risk [McCloskey et al. 2008] but only femoral neck BMD contributes to FRAX®, so the adjustment of fracture risk estimates in patients for spinal BMD is a welcome development, which may help in future clinical practice [Leslie et al. 2011], However, spinal osteoporosis should be considered as a separate clinical entity, with vertebral osteoporotic fractures associated with at least a threefold increased risk of further vertebral fracture [Lunt et al. 2003].
The future
The 1994 WHO densitometry criteria for the diagnosis of osteoporosis have been extremely influential in forming our ideas on clinical osteoporosis, but CRFs can improve our understanding of fracture risk. High fracture risk is associated with the greatest benefit from treatment [McCloskey et al. 2009] and a clinical trial assessing the effect of bisphosphonates on fracture incidence is currently underway, following 11,580 older women for 5 years. Treatment decisions are based on estimated fracture probability, using FRAX® (with BMD). While fracture incidence is the primary outcome, this will be supplemented by an economic analysis to assess the cost-effectiveness of screening [Shepstone et al. 2012]. Such a study will help to fill gaps in our knowledge of the relationship between estimated fracture risk, fracture incidence and the impact of pharmacological therapy on both.
We need to know more about the performance of available fracture risk assessment tools across populations. There are few calibration studies, so we cannot say how consistent most tools are at predicting fracture incidence or how they compare with one another when used in the same population. There is particularly poor evidence for patients who are frail, those who fall, in the oldest old and in care home residents, as well as for those treated with glucocorticoids and the need for future research in these areas has been identified [Rabar et al. 2012]. For some patients, fracture risk assessment may fail to consider competing risks of mortality [Leslie et al. 2013]; whereas for others BMD may contribute to an underestimate of fracture risk, as seen with glucocorticoids, anti-oestrogens and anti-androgens [Neubecker et al. 2011; Hadji et al. 2012; Lekamwasam et al. 2012; Rizzoli et al. 2012]. BMD remains an effective way to diagnose osteoporosis, with more than 30 years’ experience of the tool and a wealth of evidence from randomised clinical trials [NIH Consensus Development Panel, 1984]. However, CRFs are valuable in case finding and are most useful at a population level, where mass screening using DXA is not going to be cost-effective. In principle, estimated fracture risk should be a better predictor than BMD, as it targets a hard clinical outcome (fracture) and it can be used both to compare treatments and inform health economic analyses. The utility of fracture risk derived from CRFs (particularly without DXA) still requires more research evidence, so we still need DXA to supporting decisions to treat unless and until a new evidence base is created.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author declares no conflict of interest in preparing this article.
References
- Aspray T. (2013) New horizons in fracture risk assessment. Age Ageing 42: 548–554. [DOI] [PubMed] [Google Scholar]
- Baim S., Wilson C., Lewiecki E., Luckey M., Downs R., Jr, Lentle B. (2005) Precision assessment and radiation safety for dual-energy X-ray absorptiometry: position paper of the International Society for Clinical Densitometry. J Clin Densitom 8: 371–378. [DOI] [PubMed] [Google Scholar]
- Baxter I., Rogers A., Eastell R., Peel N. (2013) Evaluation of urinary N-telopeptide of type I collagen measurements in the management of osteoporosis in clinical practice. Osteoporos Int 24: 941–947. [DOI] [PubMed] [Google Scholar]
- Bolland M., Siu A., Mason B., Horne A., Ames R., Grey A., et al. (2011) Evaluation of the FRAX and Garvan fracture risk calculators in older women. J Bone Miner Res 26: 420–427. [DOI] [PubMed] [Google Scholar]
- Bow C., Tsang S., Loong C., Soong C., Yeung S., Kung A. (2011) Bone mineral density enhances use of clinical risk factors in predicting ten-year risk of osteoporotic fractures in Chinese men: the Hong Kong Osteoporosis Study. Osteoporos Int 22: 2799–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins G., Michaelsson K. (2012) Fracture risk assessment: state of the art, methodologically unsound, or poorly reported? Curr Osteoporos Rep 10: 199–207. [DOI] [PubMed] [Google Scholar]
- Davis S., Kirby C., Weekes A., Lanzafame A., Piterman L. (2011) Simplifying screening for osteoporosis in Australian primary care: the Prospective Screening for Osteoporosis; Australian Primary Care Evaluation of Clinical Tests (Prospect) Study. Menopause 18: 53–59. [DOI] [PubMed] [Google Scholar]
- Francis R., Aspray T., Hide G., Sutcliffe A., Wilkinson P. (2008) Back pain in osteoporotic vertebral fractures. Osteoporos Int 19: 895–903. [DOI] [PubMed] [Google Scholar]
- Giangregorio L., Leslie W., Lix L., Johansson H., Oden A., McCloskey E., et al. (2012) FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res 27: 301–308. [DOI] [PubMed] [Google Scholar]
- Greenspan S., Von Stetten E., Emond S., Jones L., Parker R. (2001) Instant vertebral assessment: a noninvasive dual X-ray absorptiometry technique to avoid misclassification and clinical mismanagement of osteoporosis. J Clin Densitom 4: 373–380. [DOI] [PubMed] [Google Scholar]
- Hadji P., Hartenfels M., Kyvernitakis J., Hars O., Baumann K., Kalder M. (2012) Recommendations for antiresorptive therapy in postmenopausal patients with breast cancer: Marburg AIBL Guideline Evaluation Study (MAGES). Breast Cancer Res Treat 133: 1089–1096. [DOI] [PubMed] [Google Scholar]
- Higano C. (2004) Understanding treatments for bone loss and bone metastases in patients with prostate cancer: a practical review and guide for the clinician. Urol Clin North Am 31: 331–352. [DOI] [PubMed] [Google Scholar]
- Hippisley-Cox J., Coupland C. (2012) Derivation and Validation of Updated Qfracture Algorithm to Predict Risk of Osteoporotic Fracture in Primary Care in the United Kingdom: Prospective Open Cohort Study. BMJ 344: e3427. [DOI] [PubMed] [Google Scholar]
- Hippisley-Cox J., Coupland C., Vinogradova Y., Robson J., May M., Brindle P. (2007) Derivation and validation of Qrisk, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ 335: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson H., Kanis J., Oden A., Johnell O., McCloskey E. (2009) BMD, clinical risk factors and their combination for hip fracture prevention. Osteoporos Int 20: 1675–1682. [DOI] [PubMed] [Google Scholar]
- Kanis J., Borgstrom F., De Laet C., Johansson H., Johnell O., Jonsson B., et al. (2005) Assessment of fracture risk. Osteoporos Int 16: 581–589. [DOI] [PubMed] [Google Scholar]
- Kanis J., Johnell O., Oden A., Johansson H., McCloskey E. (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis J., McCloskey E., Johansson H., Cooper C., Rizzoli R., Reginster J., et al. (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24: 23–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis J., Melton L., III, Christiansen C., Johnston C., Khaltaev N. (1994) The diagnosis of osteoporosis. J Bone Miner Res 9: 1137–1141. [DOI] [PubMed] [Google Scholar]
- Kanis J., Oden A., Johnell O., Johansson H., De Laet C., Brown J., et al. (2007) The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int 18: 1033–1046. [DOI] [PubMed] [Google Scholar]
- Lekamwasam S., Adachi J., Agnusdei D., Bilezikian J., Boonen S., Borgstrom F., et al. (2012) A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int 23: 2257–2276. [DOI] [PubMed] [Google Scholar]
- Leslie W., Berger C., Langsetmo L., Lix L., Adachi J., Hanley D., et al. (2011) Construction and validation of a simplified fracture risk assessment tool for Canadian women and men: results from the Camos and Manitoba cohorts. Osteoporos Int 22: 1873–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie W., Lix L., Johansson H., Oden A., McCloskey E., Kanis J., et al. (2010) Independent clinical validation of a Canadian FRAX tool: fracture prediction and model calibration. J Bone Miner Res 25: 2350–2358. [DOI] [PubMed] [Google Scholar]
- Leslie W., Lix L. and Manitoba Bone Density Program (2010) Simplified 10-year absolute fracture risk assessment: a comparison of men and women. J Clin Densitom 13: 141–146. [DOI] [PubMed] [Google Scholar]
- Leslie W., Lix L. and Manitoba Bone Density Program (2011) Absolute fracture risk assessment using lumbar spine and femoral neck bone density measurements: derivation and validation of a hybrid system. J Bone Miner Res 26: 460–467. [DOI] [PubMed] [Google Scholar]
- Leslie W., Lix L., Wu X. and Manitoba Bone Density Program (2013) Competing mortality and fracture risk assessment. Osteoporos Int 24: 681–688. [DOI] [PubMed] [Google Scholar]
- Leslie W., Morin S., Lix L., Johansson H., Oden A., McCloskey E., et al. (2012) Fracture risk assessment without bone density measurement in routine clinical practice. Osteoporos Int 23: 75–85. [DOI] [PubMed] [Google Scholar]
- Lips P. (1997) Epidemiology and predictors of fractures associated with osteoporosis. Am J Med 103: 3S–8S; discussion 8S-11S. [DOI] [PubMed] [Google Scholar]
- Lunt M., O’Neill T., Felsenberg D., Reeve J., Kanis J., Cooper C., et al. (2003) Characteristics of a prevalent vertebral deformity predict subsequent vertebral fracture: results from the European Prospective Osteoporosis Study (EPOS). Bone 33: 505–513. [DOI] [PubMed] [Google Scholar]
- McCloskey E., Kanis J. (2012) FRAX updates 2012. Curr Opin Rheumatol 24: 554–560. [DOI] [PubMed] [Google Scholar]
- McCloskey E., Johansson H., Oden A., Vasireddy S., Kayan K., Pande K., et al. (2009) Ten-year fracture probability identifies women who will benefit from clodronate therapy - additional results from a double-blind, placebo-controlled randomised study. Osteoporos Int 20: 811–817. [DOI] [PubMed] [Google Scholar]
- McCloskey E., Vasireddy S., Threlkeld J., Eastaugh J., Parry A., Bonnet N., et al. (2008) Vertebral fracture assessment (VFA) with a densitometer predicts future fractures in elderly women unselected for osteoporosis. J Bone Miner Res 23: 1561–1568. [DOI] [PubMed] [Google Scholar]
- Miller P. (2014) Chronic kidney disease and osteoporosis: evaluation and management. Bonekey Rep 3: 542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (2010) Osteoporotic Fractures - Denosumab (NICE Technology Appraisal Guidance 204). London: National Institute for Health and Clinical Excellence. [Google Scholar]
- National Institute for Health and Care Excellence (2011a) Alendronate, Etidronate, Risedronate, Raloxifene and Strontium Ranelate for the Primary Prevention of Osteoporotic Fragility Fractures in Postmenopausal Women (NICE Technology Appraisal Guidance 160). London: National Institute for Health and Clinical Excellence. [Google Scholar]
- National Institute for Health and Care Excellence. (2011b) Alendronate, Etidronate, Risedronate, Raloxifene, Strontium Ranelate and Teriparatide for the Secondary Prevention of Osteoporotic Fragility Fractures in Postmenopausal Women (NICE Technology Appraisal Guidance 161). London: National Institute for Health and Clinical Excellence. [Google Scholar]
- National Osteoporosis Foundation (2010) Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation. [Google Scholar]
- National Osteoporosis Guideline Group on Behalf of the Bone Research Society, B.G.S., British Orthopaedic Association, British Society of Rheumatology, National Osteoporosis Society, Osteoporosis 2000, Osteoporosis Dorset, Primary Care Rheumatology Society and Society for Endocrinology (2008) Osteoporosis. Clinical Guideline for Prevention and Treatment. Sheffield: University of Sheffield Press. [Google Scholar]
- Nayak S., Edwards D., Saleh A., Greenspan S. (2014) Performance of risk assessment instruments for predicting osteoporotic fracture risk: a systematic review. Osteoporos Int 25: 23–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubecker K., Adams-Huet B., Farukhi I., Delapena R., Gruntmanis U. (2011) Predictors of fracture risk and bone mineral density in men with prostate cancer on androgen deprivation therapy. J Osteoporos 2011: 924595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T., Center J., Eisman J. (2013) Individualized fracture risk assessment: progresses and challenges. Curr Opin Rheumatol 25: 532–541. [DOI] [PubMed] [Google Scholar]
- NIH Consensus Development Panel (1984) Osteoporosis (National Institutes of Health Consensus Development Conference Statement, 5). London: National Institutes of Health. [PubMed] [Google Scholar]
- NIH Consensus Development Panel (1991) Consensus Development Conference: Prophylaxis and Treatment of Osteoporosis. Osteoporos Int 1: 114-117. [PubMed] [Google Scholar]
- NIH Consensus Development Panel (2001) NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7–29, 2000: Highlights of the Conference. South Med J 94: 569-573. [PubMed] [Google Scholar]
- Papaioannou A., Morin S., Cheung A., Atkinson S., Brown J., Feldman S., et al. (2010) 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. DOI: 10.1503/cmaj.100771 Available at: http://www.cmaj.ca/content/early/2010/10/12/cmaj.100771.full.pdf+html?ijkey=edc6c6048e7d4acdc41368fe3f1e622bf5a2deac&keytype2=tf_ipsecsha (accessed 5 December 2014). [DOI] [PMC free article] [PubMed]
- Rabar S., Lau R., O’Flynn N., Li L., Barry P. and Guideline Development Group (2012) Risk assessment of fragility fractures: summary of NICE guidance. BMJ 345: e3698. [DOI] [PubMed] [Google Scholar]
- Reid D., Doughty J., Eastell R., Heys S., Howell A., McCloskey E., et al. (2008) Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK expert group. Cancer Treat Rev 34(Suppl. 1): S3–18. [DOI] [PubMed] [Google Scholar]
- Rizzoli R., Body J., De Censi A., Reginster J., Piscitelli P., Brandi M., et al. (2012) Guidance for the prevention of bone loss and fractures in postmenopausal women treated with aromatase inhibitors for breast cancer: an ESCEO position paper. Osteoporos Int 23: 2567–2576. [DOI] [PubMed] [Google Scholar]
- Royal College of Physicians (2002) Glucocorticoid-Induced Osteoporosis: A Concise Guide to Prevention and Treatment. London: Royal College of Physicians. [Google Scholar]
- Rubin K., Friis-Holmberg T., Hermann A., Abrahamsen B., Brixen K. (2013) Risk assessment tools to identify women with increased risk of osteoporotic fracture. complexity or simplicity? A systematic review. J Bone Miner Res, in press. [DOI] [PubMed] [Google Scholar]
- Scottish Intercollegiate Guidelines Network (2004) Management of Osteoporosis- Section 2: Risk Factors for Osteoporosis. Edinburgh: Healthcare Improvement Scotland. [Google Scholar]
- Shepstone L., Fordham R., Lenaghan E., Harvey I., Cooper C., Gittoes N., et al. (2012) A pragmatic randomised controlled trial of the effectiveness and cost-effectiveness of screening older women for the prevention of fractures: rationale, design and methods for the SCOOP study. Osteoporos Int 23: 2507–2515. [DOI] [PubMed] [Google Scholar]
- Silverman S., Calderon A. (2010) The utility and limitations of FRAX: a US perspective. Curr Osteoporos Rep 8: 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siris E., Adler R., Bilezikian J., Bolognese M., Dawson-Hughes B., Favus M., et al. (2014) The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int 25: 1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson J., Cust M., Gangar K., Hillard T., Lees B., Whitehead M. (1990) Effects of transdermal versus oral hormone replacement therapy on bone density in spine and proximal femur in postmenopausal women. Lancet 336: 265–269. [DOI] [PubMed] [Google Scholar]
- The Royal Australian College of General Practitioners (2010) Clinical Guideline for the Prevention and Treatment of Osteoporosis in Postmenopausal Women and Older Men. South Melbourne: The Royal Australian College of General Practitioners. [Google Scholar]
- Tosteson A., Melton L., III, Dawson-Hughes B., Baim S., Favus M., Khosla S., et al. (2008) Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int 19: 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (1994) Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis (WHO Study Group Report). Geneva: World Health Organization. [PubMed] [Google Scholar]


