Abstract
Biological agents for inflammatory bowel diseases (IBD) targeting tumor necrosis factor (TNF) have changed the way to treat IBD refractory to standard medications and allowed us to reach new therapeutic goals such as mucosal healing and deep remission. A better understanding of the components of the pathological processes that are a hallmark of IBD has led to the development of a new family of biological agents in Crohn’s disease and ulcerative colitis. Biosimilars, which are copy versions of currently licensed biological agents, will be soon available. The biosimilar of infliximab is as effective and as safe as its originator in rheumatologic conditions, while a new anti-TNF agent, namely golimumab, has been recently approved for refractory ulcerative colitis. Beyond TNF blockers, anti-adhesion molecules appear to be a potent drug class for IBD. Vedolizumab was recently approved for both Crohn’s disease and ulcerative colitis. Numerous other compounds are in the pipeline. Ustekinumab looks very promising for Crohn’s disease. Smad7 antisense oligonucleotide might enrich our armamentarium if preliminary data are confirmed in upcoming clinical trials. Herein, we review the efficacy and safety of new and emerging biological agents that are currently investigated in IBD clinical trials.
Keywords: anti-TNF agents, biologics, Crohn’s disease, inflammatory bowel diseases, ulcerative colitis
Introduction
Inflammatory bowel diseases (IBD), Crohn’s disease (CD) and ulcerative colitis (UC) are chronic, disabling and progressive diseases [Peyrin-Biroulet et al. 2009, 2012]. Most nonbiological drug therapies (aminosalicylates, steroids and immunomodulators) provide symptomatic improvement but fail to stop the underlying inflammatory process and do not change the disease course [Burger and Travis, 2011]. The advent of anti-tumor necrotizing factor-α (anti-TNF-α) agents (infliximab, adalimumab, certolizumab pegol) has dramatically changed the way we treat IBD by changing both disease course (fewer surgeries, less hospitalizations, better quality of life, steroid sparing, greater clinical remission and mucosal healing rates in both CD and UC) and patients’ life (quality of life and work productivity) [Rutgeerts et al. 2005; Feagan et al. 2008b]. However, the establishment of new goals in the management of IBD, such as mucosal healing and evolving strategies based on a tight monitoring and accelerated step-up care together with secondary failure to anti-TNF therapy (rate loss of response is 10–20% per year and withdrawal due to intolerance is frequent in the long term), underscored the need for new IBD drugs [Peyrin-Biroulet, 2008, 2013; Billioud et al. 2011]. Herein, we first review the biological agents that were recently approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for IBD and inhibiting (golimumab and biosimilars) or not (vedolizumab) tumor necrosis factor (TNF) before discussing the next generation of biological agents that may emerge from the pipeline.
Review criteria
An electronic search of publications in English on PubMed up to June 2014 was performed using the following keywords: ‘Crohn’s disease’, ‘ulcerative colitis’, ‘inflammatory bowel disease’, ‘treatment’, ‘biological therapy’, ‘cytokine’, ‘Tcell’, ‘adhesion’, ‘growth factors’, ‘biomolecules’ and ‘small molecules’. Also a hand search of abstracts from the yearly meetings of Digestive Disease Week and United European Gastroenterology Week between 2011 and 2014 was performed. In addition, clinical trials status was checked on http://www.clinicatrials.gov and http://www.clinicaltrialsregister.eu and new drug names were also searched and matched on google and on the website of the pharmaceutical companies developing new drugs [European Medicines Agency, 1995–2014; US National Institutes of Health, 2011].
Biological agents recently approved for IBD
Anti-TNF agents
The proinflammatory cytokine TNF plays a key role in chronic intestinal inflammation that causes IBD. Accordingly, most of the efficient biological agents developed so far in IBD aimed at neutralizing TNF. Until 2013, only infliximab and adalimumab were approved in Europe, while certolizumab pegol is also approved in USA, Switzerland and Russia [D’haens et al. 2011].
Golimumab
Golimumab is a subcutaneously administered fully human anti-TNF antibody. Golimumab is approved for the treatment of rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis [Kay et al. 2008; Kavanaugh et al. 2012]. In a phase II/III multicenter, randomized, placebo-controlled, induction study (PURSUIT-SC), anti-TNF-naïve patients with moderate-to-severe UC unresponsive to conventional treatment were randomly assigned to receive either placebo or two golimumab regimens given 2 weeks apart (200 mg followed by 100 mg, or 400 mg followed by 200 mg) [Sandborn et al. 2014a]. At week 6, both golimumab regimens induced significantly more clinical response (30% versus 51% and 55%, both p < 0.0001), clinical remission (6% versus 18% and 18%, both p < 0.0001) and mucosal healing (29% versus 42% and 45%, p = 0.001 and p < 0.0001) and improved quality of life (mean IBDQ: 14.8 ± 31.3 versus 27.0 ± 33.7 and 26.9 ± 34.3, both p < 0.0001) (Table 1). In the maintenance study (PURSUIT-M), patients in clinical response were treated with two regimens of golimumab (50 or 100 mg every 4 weeks) for 52 weeks. At week 54, patients treated with golimumab achieved significantly more continuous response (31% versus 47% and 50%, p = 0.01 and p < 0.001), remission (16% versus 23% and 28%, p = 0.12 and 0.004) and mucosal healing (27% versus 42% and 42%, p = 0.002 and 0.01) rates compared with those who received placebo [Sandborn et al. 2014b]. Golimumab was well tolerated with a safety profile consistent with other anti-TNFs. Antidrug antibodies (ADA) to golimumab formation have been reported in a few individuals, confirming the potential for immunogenicity of all TNF blockers [Choy et al. 2002; Zhou et al. 2007]. Similar to infliximab and adalimumab, golimumab was approved by both the FDA and the EMA for UC refractory to both steroids and azathioprine.
Table 1.
Characteristics of the main randomized controlled trials evaluating efficacy of monoclonal antibodies in patients with inflammatory bowel diseases.
| First author and year (study name) | Molecule | Disease | Previous anti-TNF exposure | Duration (weeks) | Patients (n) | Remission (n [%]) |
|---|---|---|---|---|---|---|
| Induction of remission at week 4-8 | ||||||
| Targan et al. 1997 | Placebo | CD | 0% | 12 | 25 | 1 (4) |
| IFX 5 mg/kg | 0% | 27 | 13 (48) | |||
| IFX 10 mg/kg | 0% | 28 | 7 (25) | |||
| IFX 20 mg/kg | 0% | 28 | 7 (25) | |||
| Schreiber et al. 2005 | Placebo | CD | 22% | 12 | 73 | 6 (8) |
| CZP 100 mg | 24% | 74 | 17 (23) | |||
| CZP 200 mg | 39% | 72 | 14 (19) | |||
| CZP 400 mg | 44% | 73 | 15 (21) | |||
| Hanauer et al. 2006 (CLASSIC I) | Placebo | CD | 0% | 4 | 74 | 9 (12) |
| ADA 40/20 mg | 0% | 74 | 13 (18) | |||
| ADA 80/40 mg | 0% | 75 | 18 (24) | |||
| ADA 160/80 | 0% | 76 | 27 (36) | |||
| Sandborn et al. 2007 (GAIN) | Placebo | CD | 100% | 4 | 166 | 12 (7) |
| ADA 160/80 | 100% | 159 | 34 (21) | |||
| Rutgeerts et al. 2005 (ACT I) | Placebo | UC | 0% | 46 | 121 | 18 (15) |
| IFX 5 mg/kg | 0% | 121 | 47 (39) | |||
| IFX 10 mg/kg | 0% | 122 | 39 (32) | |||
| Rutgeerts et al. 2005 (ACT II) | Placebo | UC | 0% | 22 | 123 | 7 (6) |
| IFX 5 mg/kg | 0% | 121 | 41 (34) | |||
| IFX 10 mg/kg | 0% | 120 | 33 (27.5) | |||
| Reinisch et al. 2011 (ULTRA-1) | Placebo | UC | 0% | 8 | 130 | 12 (9) |
| ADA 80/40 mg | 0% | 130 | 13 (10) | |||
| ADA 160/80 | 0% | 130 | 24 (18.5) | |||
| Sandborn, 2012 (ULTRA-2)* | Placebo | UC | 41% | 52 | 246 | 23 (9%) |
| ADA 160/80 | 39% | 248 | 41 (16.5) | |||
| Sandborn et al. 2014a (PURSUIT-SC) | Placebo | UC | 0% | 6 | 251 | 16 (6) |
| GLB 200/100 | 0% | 253 | 45 (18) | |||
| GLB 400/200 | 0% | 257 | 46 (18) | |||
| Sandborn et al. 2005 (ENACT-1) | Placebo | CD | 38% | 10 | 181 | 55 (30%) |
| NZB 300 mg | 40% | 724 | 267 (37%) | |||
| Targan et al. 2007 (ENCORE) | Placebo | CD | 45% | 8 | 250 | 40 (16%) |
| NZB 300 mg | 50% | 259 | 68 (26%) | |||
| Feagan et al. 2013 (GEMINI 1) | Placebo | UC | 49% | 6 | 149 | 8 (5%) |
| VDZ 300 mg | 42% | 225 | 38 (17%) | |||
| Sandborn et al. 2013 (GEMINI 2) | Placebo | UC | 49% | 6 | 148 | 10 (6.8%) |
| VDZ 300 mg | 51% | 220 | 32 (14.5%) | |||
| Maintenance of remission at week 20-30 after open label induction | ||||||
| Hanauer et al. 1999 | Placebo | CD | 0% | 52 | 110 | 23 (21) |
| IFX 5 mg/kg | 0% | 113 | 44 (39) | |||
| IFX 10 mg/kg | 0% | 112 | 51 (46) | |||
| Colombel et al. 2007 (CHARM) | Placebo | CD | 48% | 52 | 170 | 29 (17) |
| ADA 40 mg eow | 49% | 172 | 69 (40) | |||
| ADA 40 mg weekly | 45% | 157 | 74 (47) | |||
| Sandborn et al. 2007 (PRECISE I)* | Placebo | CD | 26% | 26 | 328 | 59 (18) |
| CZP 400 mg | 30% | 331 | 96 (29) | |||
| Schreiber et al. 2007 (PRECISE II) | Placebo | CD | 24% | 20 | 210 | 60 (29) |
| CZP 400 mg | 24% | 215 | 103 (48) | |||
| Rutgeerts et al. 2005 (ACT I)* | Placebo | UC | 0% | 46 | 121 | 19 (16) |
| IFX 5 mg/kg | 0% | 121 | 41 (34) | |||
| IFX 10 mg/kg | 0% | 122 | 45 (37) | |||
| Rutgeerts et al. 2005 (ACT II)* | Placebo | UC | 0% | 22 | 123 | 13 (11) |
| IFX 5 mg/kg | 0% | 121 | 31 (26) | |||
| IFX 10 mg/kg | 0% | 120 | 43 (36) | |||
| Sandborn, 2012 (ULTRA-2)* | Placebo | UC | 41% | 52 | 246 | 21 (8.5) |
| ADA 40 mg eow | 39% | 248 | 43 (17) | |||
| Rutgeerts et al. 2014 (PURSUIT-M)** | Placebo | UC | 0% | 52 | 154 | 24 (16) |
| GLB 50 | 0% | 151 | 35 (23) | |||
| GLB 100 | 0% | 151 | 42 (28) | |||
| Sandborn et al. 2005 (ENACT-2) | Placebo | CD | 40% | 36 | 171 | 51 (30%) |
| NZB 300 mg every 4 weeks | 33% | 168 | 92 (55%) | |||
| Feagan et al. 2013 (GEMINI 1) | Placebo | UC | 41% | 52 | 126 | 20 (16%) |
| VDZ 300 mg every 4 weeks | 125 | 56 (45%) | ||||
| VDZ 300 mg every 8 weeks | 122 | 51 (42%) | ||||
| Sandborn et al. 2013 (GEMINI 2) | Placebo | UC | 60% | 52 | 153 | 33 (22%) |
| VDZ 300 mg every 4 weeks | 154 | 56 (36%) | ||||
| VDZ 300 mg every 8 weeks | 154 | 60 (39%) | ||||
Randomization was performed before induction.
At both week 30 and 54.
ADA, adalimumab; CD, Crohn’s disease; CZP, certolizumab pegol; GLB, golimumab; IFX, infliximab; NZB, natalizumab; TNF, tumor necrosis factor; UC, ulcerative colitis; VDZ, vedolizumab.
Biosimilars
The extensive use of biological agents is a major concern in terms of economic burden that led some national agencies to restrain their use overtime after achieving clinical remission [Farkas et al. 2013; Rinaudo-Gaujous et al. 2013]. Development of generics for small-molecule drugs has offered price reductions up to 80% compared with their branded counterparts [Malik, 2009]. A biosimilar is a copy version of an approved original biologic medicine whose data protection has expired [Weise et al. 2012]. However, while a generic medicine is an exact copy of a small-molecule drug, a biosimilar could significantly differ from the reference drug through changes in the manufacturing process, including type of expression system, growth conditions, purification process, formulation and storage conditions. The latter changes could therefore be responsible for clinically meaningful changes in their pharmaceutical quality, efficacy and safety especially their immunogenicity [Crommelin et al. 2003]. The EMA and FDA have recently published guidelines regarding the similarity between biosimilar and the reference product in terms of quality, purity, safety and efficacy [European Medicines Agency, 2013a; Food and Drug Administration, 2013]. Interestingly, even consecutive batches of originator products are never identical to each other and its manufacturing process evolves over time under similar guidance that is currently proposed for the evaluation of biosimilars [ICH, 2004]. Recently, an infliximab biosimilar (CT-P13, Inflectra®) has been evaluated in rheumatologic diseases as compared with the infliximab originator. In a phase I, randomized, controlled, parallel-group study, CT-P13 demonstrated similar pharmacokinetics, efficacy and safety than the originator [Park et al. 2013]. In a phase III, randomized, controlled, parallel-group trial in rheumatoid arthritis patients with active disease despite methotrexate treatment, CT-P13 demonstrated equivalent efficacy to infliximab at week 30, with a comparable pharmacokinetic and immunogenicity profile. CT-P13 was well tolerated, with a safety profile comparable to that of infliximab [Yoo et al. 2013]. Those results led the EMA Committee for Medicinal Products for Human Use to adopt a positive opinion, recommending the granting of a marketing authorization for the treatment of rheumatoid arthritis, adult CD, pediatric CD, UC, pediatric UC, ankylosing spondylitis, psoriatic arthritis and psoriasis [European Medicines Agency, 2013b]. A pharmacovigilance plan for Inflectra® will be implemented as part of the marketing authorization [Rinaudo-Gaujous et al. 2013]. However, immunogenicity remains an ongoing concern especially in patients switching from the originator to the biosimilar. Immune responses have been observed and linked to serious safety issues such as pure red cell aplasia caused by cross-reacting neutralizing antibodies against erythropoietin in patients treated with biosimilars of erythropoietin [Praditpornsilpa et al. 2011]. Another important concern lies on the opportunity for pharmacy, insurance companies and/or healthcare system to substitute the originator without the knowledge and/or approval of the physician [Weise et al. 2012]. This will require further investigation in IBD patients.
Anti-adhesion molecules
In IBD, the inflammatory process is characterized by leukocytic infiltration of the intestinal lamina propria [Lobaton et al. 2014]. Therefore, strategies targeting the recruitment of leukocytes from circulation into the site of inflammation could be a cornerstone to control the inflammatory cascade. This process involves several steps, including the capture of leukocytes by the endothelium by the interaction between L-selectin at the surface of leukocytes and their ligands (P- and E-selectins) on endothelial cells [Lawrence and Springer, 1991; Vestweber and Blanks, 1999]. Secondary adhesion molecules belonging to the integrin family then allow leukocytes to migrate through the vascular wall. The expression of selectins or integrins is activated by chemokines, which are released by T cells. In addition to selectins and adhesion molecules, leucocytes also interact with chemokines through specific chemokine receptors (CCR) [Lobaton et al. 2014].
Natalizumab
Natalizumab is an IgG4 humanized monoclonal antibody that specifically antagonizes α4 integrin. Natalizumab was first developed in IBD showing efficacy for induction and maintenance of clinical remission in CD [Ghosh et al. 2003; Sandborn et al. 2005; Targan et al. 2007]. However, cases of progressive multifocal leukoencephalopathy (PML) due to JC virus reactivation in natalizumab-treated patients have pull up the further development of the drug [Kleinschmidt-Demasters and Tyler, 2005; Langer-Gould et al. 2005; Van Assche et al. 2005]. The blockade of α4 integrins not only interferes with α4β7 in cell adhesion molecule 1 (MAdCAM-1) interaction which is gut-specific, but also with the α4β1-vascular cell adhesion molecule 1 (VCAM-1) which is brain-specific and is needed to prevent, notably, JC virus from infecting the brain [Lobaton et al. 2014]. Natalizumab is FDA-approved for inducing and maintaining clinical response and remission in adult patients with moderate-to-severe CD after failure of anti-TNF inhibitors and only available in the USA [Food and Drug Administration, 2009].
Vedolizumab
Vedolizumab is a humanized monoclonal antibody that specifically antagonizes α4β7 integrin, by inhibiting its binding to the gut-specific intestinal mucosal address in MAdCAM-1 [Feagan et al. 2005]. Vedolizumab was effective in three phase II, randomized controlled trials in UC and CD [Feagan et al. 2005, 2008a; Parikh et al. 2012]. Two large, phase III, randomized controlled trials evaluated either induction or maintenance therapy: one in UC and one in CD [Feagan et al. 2013, Sandborn et al. 2013]. The GEMINI I trial included 374 UC patients in the induction study (300 mg intravenously at weeks 0 and 2) and 373 UC patients who had respond to induction therapy in the maintenance study (300 mg intravenously every 4 or 8 weeks) [Feagan et al. 2013]. Vedolizumab demonstrated its efficacy for inducing clinical response (25.5% versus 47%, p < 0.001), clinical remission (5% versus 17%, p = 0.001) and mucosal healing (25% versus 41%, p = 0.001) at week 6. Vedolizumab was also effective to induce durable clinical response (24% versus 57% and 52%, both p < 0.001), durable clinical remission (9% versus 20.5% and 24%, p = 0.008 and 0.001) and mucosal healing (20% versus 52% and 56%, p < 0.001 for both comparisons) at week 52. The GEMINI II trial with the same study design, included 368 CD patients in the induction study and 461 in the maintenance study [Sandborn et al. 2013]. Vedolizumab was effective for inducing clinical remission (7% versus 14.5%, p = 0.02) at week 6, but it did not reach statistical significance (26% versus 31%, p = 0.23) for clinical response [at least 100 points reduction in CD activity index (CDAI)]. Placebo effect, a long disease duration and previous exposure to anti-TNF therapy, have been proposed to explain these findings. Indeed, preliminary results from the GEMINI III trial that had included CD patients with previous anti-TNF failure (failure or intolerance) have suggested that remission may be achieved beyond the 6-week period of treatment. Indeed, clinical response (25% versus 47%, p < 0.0001) and remission (12% versus 27%, p = 0.001) rates were significantly higher in the active arm at week 10 although it was no statistically significant at week 6 for clinical remission [Sands et al. 2013]. In the maintenance study, vedolizumab was effective at inducing clinical response (30% versus 43.5% and 45.5%, p = 0.01 and 0.005) and clinical remission (22% versus 39% and 36%, p < 0.001 and p = 0.004) at week 52. Vedolizumab was well tolerated in patients with either UC or CD, with no cases of PML while more 3000 patients have been exposed to this drug. Vedolizumab was approved by the EMA for the treatment of adult patients with moderately to severely active UC or CD who have had an inadequate response with, lost response to, or were intolerant to either conventional therapy or a TNF-α antagonist. Vedolizumab was approved by the FDA for the treatment of adult patients with moderate-to-severe UC or CD when one or more standard therapies (corticosteroids, immunomodulators or TNF blocker medications) have not resulted in an adequate response.
Biological agents in the pipeline
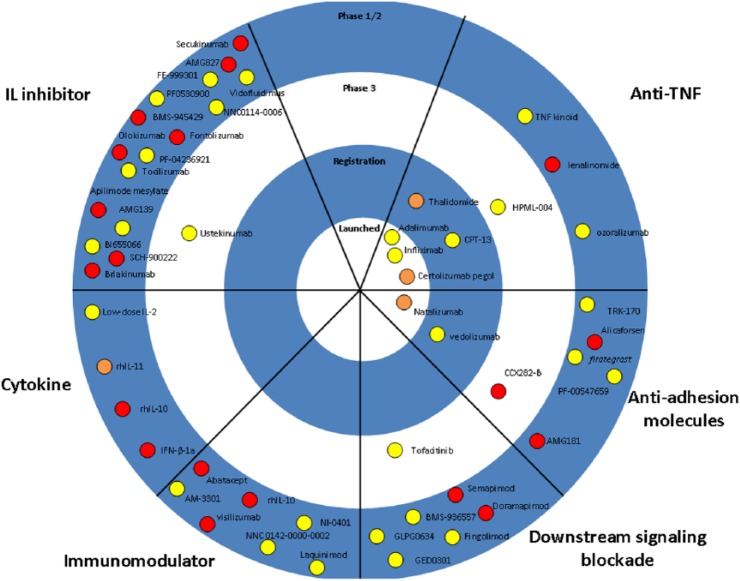
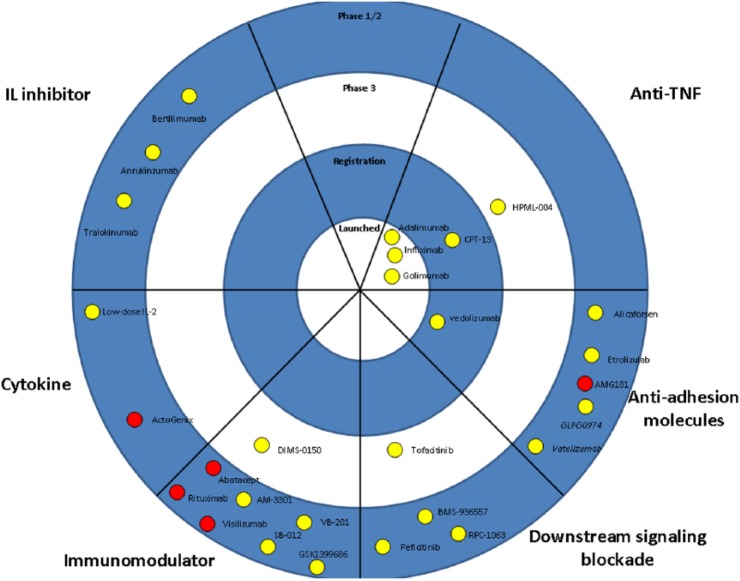
The majority of new molecules for IBD aim to target T-cell activation, adhesion molecules or pro-inflammatory cytokines. Table 2 and Figures 1 and 2 summarize all molecules under development for IBD.
Table 2.
Characteristics and dosage of monoclonal antibodies for inflammatory bowel disease.
| Drug | Type of monoclonal antibody | Therapeutic target | Half-life (days) | Route of administration | Induction phase |
Maintenance phase |
||
|---|---|---|---|---|---|---|---|---|
| Dosage | Interval | Dosage | Interval | |||||
| Infliximab | Chimeric IgG1 κ | TNF-α | 7.7–9.5 | IV | 5 mg/kg | W0-W2-W6 | 5 mg/kg | Every 8 weeks |
| Adalimumab | Human IgG1 κ | TNF-α | 10–20 | SC | 160 mg | W0 | 40 mg | Every 2 weeks |
| 80 mg | W2 | |||||||
| Certolizumab pegol | Humanized pegylated Fab IgG4 | TNF-α | 14 | SC | 400 mg | WO-W2-W4 | 400 mg | Every 4 weeks |
| Golimumab | Human IgG1 κ | TNF-α | 8-16 | SC | 200 mg | W0 | 50-100 mg | Every 4 weeks |
| 100 mg | W2 | |||||||
| CPT-13 | Chimeric IgG1 κ | TNF-α | 7.7–9.5 | IV | 5 mg/kg | W0-W2-W6 | 5 mg/kg | Every 8 weeks |
| Natalizumab | Humanized IgG4 | α4 integrin | 7-15 | IV | 300 mg | W0-W2-W8 | 300 mg | Every 4 weeks |
| Vedolizumab | Humanized IgG1 κ | α4β7 integrin | 15-22 | SC | 300 mg | W0-W2 | 300 mg | Every 4 weeks |
TNF, tumor necrosis factor; W, week.
Figure 1.
The therapeutic pipeline in Crohn’s disease. Drugs are categorized based on the mechanism of action. Purple symbols indicate oral drugs.
rh, recombinant human; IL, interleukin; TNF, tumor necrosis factor.
Figure 2.
The therapeutic pipeline in ulcerative colitis. Drugs are categorized based on the mechanism of action. Purple symbols indicate oral drugs.
IL, interleukin; TNF, tumor necrosis factor.
Blockade of pro-inflammatory cytokines
TNF-α
A new approach for targeting TNF regardless of immunogenicity has been proposed through the generation of a polyclonal antibody response directly by the immune system of the patient. TNF-Kinoid (TNF-K) is an immunotherapeutic composed of recombinant human TNF conjugated to keyhole limpet hemocyanin as a carrier protein, inactivated and adjuvanted with ISA-51 emulsion. The administration of TNF-K prompts the production of neutralizing polyclonal antibodies against TNF [Delavallee et al. 2008]. In an open-label, phase I/II dose escalation trial, patients with moderate-to-severe CD, three doses of TNF-K were evaluated in 22 patients [Dewit et al. 2012]. No related serious adverse events were observed and all patients completed the trial. A few minor and transient local and systemic reactions were reported following immunization. Anti-TNF antibodies were induced and were variable in intensity, but persisted 3–4 months. No T-cell response specific to TNF was detected. Clinical response (at least 70 points reduction in CDAI) was observed in 66–78% of the patients whereas clinical remission (CDAI less than 150 points) was observed in 36–50% of patients. Preliminary analysis of a phase II study enrolling 60 patients with CD were disappointing. The final results of this trial are eagerly awaited.
HMPL-004, an Andrographis paniculata extract has shown its ability to reduce TNF and interleukin (IL)-1β, interferon (IFN)-γ and IL-22 expression and to prevent the development of experimental colitis by inhibiting T-cell proliferation and Th1/Th17 responses [Michelsen et al. 2013]. HMPL-004 is currently being evaluated in two phase III trials in CD and UC.
IL-12/23
IL-12 and IL-23 are pro-inflammatory cytokines sharing a common p40 subunit: IL-12 (p35 + p40) can induce Th1 differentiation whereas IL-23, together with transforming growth factor (TGF)-β and IL-6 can induce Th17 differentiation [Vignali and Kuchroo, 2012]. Ustekinumab is a monoclonal IgG1 antibody targeting the p40 subunit of IL-12/IL-23. Ustekinumab is approved for the treatment of psoriasis and psoriatic arthritis by the FDA and the EMA [Leonardi et al. 2008; Papp et al. 2008; Gottlieb et al. 2009].
The efficacy of ustekinumab was first investigated in a double-blind, cross-over trial with either subcutaneous or intravenous ustekinumab regimens in 104 moderate-to-severe CD patients [Sandborn et al. 2008]. Ustekinumab seemed effective for inducing a clinical response at weeks 4 and 6, but not at week 8. Interestingly, better results were observed in anti-TNF experienced patients. Ustekinumab induction and maintenance therapy was then evaluated in a large phase IIb induction and maintenance trial in moderate-to severe active CD refractory to anti-TNF agents [Sandborn et al. 2012a]. During induction therapy, 526 patients were randomly assigned to receive either placebo or three intravenous ustekinumab regimens (1, 3 or 6 mg/kg at week 0) At week 6, the clinical response (at least 100 points reduction in CDAI) was significantly increased in the three ustekinumab groups (23.5% versus 37%, 34% and 40%, p = 0.02, 0.06 and 0.005), while no differences were found regarding clinical remission (11% versus 16%, 12% and 12%, p = 0.20, 0.21 and 0.68). Of note, both response (17% versus 32%, 32% and 43.5%, p = 0.006, p = 0.007 and p < 0.001) and remission (11% versus 18%, 18% and 18%, p = 0.11, 0.08 and 0.07) rates increased at week 8. During the maintenance phase, 145 responders at week 6 were rerandomized to receive either placebo or subcutaneous ustekinumab 90 mg at weeks 8 and 16. At week 22, ustekinumab resulted in significantly higher clinical response (69% versus 42.5%, p < 0.001) and remission (42% versus 27%, p = 0.03) compared with placebo. No new safety signals were reported. Overall, this molecule looks promising in CD. A phase III trial is ongoing.
IL-13
The cytokine IL-13 belongs to the Th2 cytokine family and activates JAK/STAT pathway [Danese, 2012; Mannon and Reinisch, 2012]. Results from a phase-2 trial investigating QAX576 in fistulizing perianal CD are still pending [US National Institutes of Health, 2011]. Results from two phase II randomized controlled trials investigating safety and efficacy profile of two anti-IL-13 monoclonal antibodies in patients with moderate-to-severe active UC, have been recently made public. In the first, tralokinumab 300 mg every 2 weeks for 12 weeks showed no significant improvement in response, remission and mucosal healing rates [Danese et al. 2014]. However, a significant decrease of the total Mayo score at week 12 was reported. The second one has investigated anrunkinzumab (IMA-638) at three doses (200, 400 and 600 mg) versus placebo in 84 patients [Reinisch et al. 2014]. The primary endpoint was change from baseline in fecal calprotectin at week 14 and was not met for all the three studied doses. There was a trend for a decrease of the total Mayo score for the 200 and 400 mg arms. Another anti-IL-13 monoclonal antibody (QAX576) and a monoclonocal antibody against eotaxin-1, an eosinophil chemoattractant anti-eotaxin-1 monoclonal antibody (Bertilimumab) are currently being investigated in two phase II trials.
IL-6
The cytokine IL-6 is a contributor of Th-17 differentiation. Increased levels of IL-6 and soluble IL-6 receptor have been associated with a more severe form of IBD [Mudter and Neurath, 2007]. Tocilizumab, a fully humanized monoclonal antibody that blocks both membrane-bound and soluble IL-6 receptor that has been approved for the treatment of rheumatoid and juvenile arthritis, but had been stopped after a small pilot study in 36 patients with active CD [Ito et al. 2004; Singh et al. 2010]. A phase II placebo-controlled trial is ongoing to evaluate safety and efficacy of a subcutaneously administrated anti-IL-6 monoclonal antibody in patients with active CD (PF-04236921; the ANDANTE study) [US National Institutes of Health, 2011].
Anti-adhesion molecules
Etrolizumab is a fully humanized monoclonal antibody that selectively binds the β7 subunit of the heterodimeric integrins α4β7 and αEβ7. Similar to natalizumab and vedolizumab, etrolizumab was shown to be effective in a phase II, randomized controlled trial in patients with moderate-to-severe active UC [Vermeire et al. 2014]. In the EUCALYPTUS trial, 124 patients were assigned to etrolizumab 100 mg at weeks 0, 4 and 8 or etrolizumab 420 mg (loading dose) and then 300 mg at weeks 2, 4 and 8 or placebo. Clinical remission (Mayo score of ≤ 2 with no subscore > 1) was observed in 21% of patients in the etrolizumab 100 mg group and in 8% in the etrolizumab 300 mg group, as compared with none in the placebo group (p = 0.004 and 0.05, respectively). These very promising results await confirmation in ongoing phase III trials. Other anti-adhesion molecules are currently under investigation, including an anti-MadCAM-1 inhibitor in UC and CD (PF-00547659), an anti- α2β1 integrin (vatelizumab), and FFA-2, a G-protein-coupled receptor activated by short chain fatty acid and implicated in the regulation of neutrophils activation and migration (GLPG0974).
Blockade of the downstream signaling pathways mediated by cytokines
JAK inhibitors
The involvement of Janus kinase (JAK)1 and JAK3 in the transduction processes of the IL-2R and IL-6R (including IL-12 and IL-23) families of cytokines has made JAK inhibition a potential therapeutic target in IBD [Coskun et al. 2013].
Tofacitinib is an oral JAK inhibitor that inhibits JAK1, JAK2 and JAK3 with in vitro functional specificity for kinases 1 and 3, which can modulate the signaling of a large subset of proinflammatory cytokines such as IL-2, -4, -7, -9, -15 and -21 [Riese et al. 2010]. These cytokines are integral to lymphocyte activation, function, and proliferation.
In a phase II, randomized controlled trial, patients with moderate-to-severe UC were randomized to receive four tofacitinib regimens or placebo twice daily for 8 weeks [Sandborn et al. 2012b]. Of the 194 randomized treated patients, a statistical difference in clinical response rates (decrease in Mayo score of at least 3 points and 30%; decrease in rectal bleeding subscore of at least 1 point or absolute subscore of 1 or 0) from placebo was only found in the 15 mg group with 78% as compared with 42% in the placebo group For clinical remission (Mayo score of ≤ 2 with no subscore > 1), the figures were 33% for 3 mg, 48% for 10 mg and 41% for 15 mg as compared with 10% of patients receiving placebo. For endoscopic remission (endoscopy subscore of 0), the figures were 18% for 3 mg, 30% for 10 mg and 27% for 15 mg as compared with 2% of patients receiving placebo.
Overall, it was well tolerated. The most commonly reported adverse events were influenza and nasopharyngitis (in six patients each). Two serious infection-related adverse events were observed in three patients in the 10 mg group (one anal abscess and one postoperative abscess). Also, the absolute neutrophil count was less than 1500 cells per cubic millimeter in three patients receiving tofacitinib. Therefore; the risk of opportunistic infection may be explained by cytopenia and the effects on immune cells related to JAK inhibition [Peyrin-Biroulet and Danese, 2013]. Although it was assumed that targeting JAK molecules which are only expressed in immune cells, could be associated with a good safety profile, a dose-dependent increase in low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol which is not completely understood.
Tofacitinib is currently approved by the FDA for rheumatoid arthritis in patients that failed to respond to methotrexate or who are intolerant to the drug [Food and Drug Administration, 2012]. By contrast, those concerns have led the EMA to state that the benefits of tofacitinib did not outweigh its risks in rheumatoid arthritis [European Medicines Agency, 2013c]. An ongoing phase III, induction and maintenance, randomized controlled trial aiming to assess safety and efficacy of tofacitinib in patients with UC is still recruiting. Two JAK1 inhibitors are currently evaluated in CD (GLPG0634) and UC (Peficitinib).
Laquinimod
Laquinimod is a small, synthetic, orally administered molecule that showed clinical efficacy in patients with multiple sclerosis [Comi et al. 2012]. Although its mode of action needs to be fully elucidated, it results in a T-cell shift into an anti-inflammatory phenotype and a decrease of proinflammatory cytokines [Varrin-Doyer et al. 2014]. In a phase IIa placebo-controlled, dose-finding study, patients were randomly assigned to laquinimod 0.5, 1, 1.5, or 2 mg/day or placebo for 8 weeks or placebo. In this study, the highest response (at least 100 points reduction in CDAI) and remission rates were observed at the lowest dosage of laquinimod (55% and 48%, respectively versus 32% and 16% in the placebo group) [D’haens et al. 2013]. Phase IIb/III trials are awaited.
Smad7 antisense oligonucleotide
In CD, a defective activity of the suppressive cytokine TGF-β1 is often observed, due to increased levels of Smad7, an intracellular protein that binds to the TGF-β1 receptor preventing the downstream TGF-β1-driven signaling [Montel-eone et al., 2001]. In a phase I, open-label, dose-escalation study of GED0301, a Smad7 antisense oligonucleotide, Monteleone and colleagues found a good safety profile and evidence of a significant decrease in the percentage of circulating IFN-γ-expressing cells. Looking at CCR9-positive cells (a homing receptor that allows T cells to migrate to nonlymphoid tissue such as the lamina propria), a significant decrease was observed in the percentages of circulating IFN-γ- and IL-17A-expressing cells with a nonsignificant increase in the percentage of Foxp3-expressing cells [Monteleone et al. 2012]. A phase III study conducted by Celgene is scheduled to be launched by the end of 2014.
Masitinib
Gastrointestinal mast cells are sentinels of the immune system located in the digestive mucosa and submucosa at the host–environment interface. MCs proliferation/activation could be observed in various conditions including IBD [Beunk et al. 2013]. Upon activation, mast cells release histamine, tryptase, membrane-derived lipid mediators and cytokines and growth factors including TNF, IL-4, IL-6, bFGF, VEGF and TGF-β [Wernersson and Pejler, 2014]. Masitinib (formerly AB1010) is a potent and selective tyrosine kinase inhibitor that targets c-kit receptor (expressed by mast cells), but also platelet-derived growth factor receptor-α/β, lymphocyte-specific kinase (Lck), Lck/Yes-related protein (LYn), and fibroblast growth factor receptor 3 (FGFR3) and the focal adhesion kinase (FAK) activation pathway [D’allard et al. 2013]. Masitinib has demonstrated efficacy and safety in an uncontrolled, open-label, randomized, dose-ranging, phase IIa trial in patients with active rheumatoid arthritis unresponsive to disease-modifying antirheumatic drugs [Tebib et al. 2009]. A phase II trial is ongoing in patients with active CD.
Other biological agents
Other biological agents that aim to block the downstream signaling pathways mediated by cytokines are mentioned in Table 3 and are currently evaluated in phase II or phase III trials: NNC 0142-0000-0002 (NKG2D), SB012 (GATA-3), VB-201 and DIMS0150 (TLRs) fingolimod and RPC1063 (sphingosine 1-phosphate 1 receptor) and GSK1399686 (ribosomal 50S subunit).
Table 3.
Current and novel biologics to the treatment of inflammatory bowel diseases.
| Drug | Manufacturer | Target | Admin | Development status |
||
|---|---|---|---|---|---|---|
| Crohn’s disease | Ulcerative colitis | |||||
| BLOCKADE PRO-INFLAMMATORY CYTOKINES | ||||||
| TNF | Infliximab | MSD | TNF | IV | Approved in EU and USA | Approved in EU and USA |
| Adalimumab | Abbvie | TNF | SC | Approved in EU and USA | Approved in EU and USA | |
| Certolizumab pegol | UCB Pharma. | TNF | SC | Approved in USA | – | |
| Golimumab | MSD | TNF | SC | – | Approved in EU and USA | |
| CT-P13 | Celltrion, Hospira | TNF | IV | Approved in EU | Approved in EU | |
| TNF-Kinoid | Neovacs | TNF | IV | Phase II (-) | – | |
| HMPL-004 (Andrographis paniculata extract) | Hutchison Medipharma Limited | TNF and IL-1β | Oral | Ongoing Phase III | Ongoing Phase III | |
| IL-12/IL-23 | Ustekinumab | Janssen | IL-12/IL-23 (p40 subunit) | IV/SC | Ongoing Phase III | – |
| AMG139 | Amgen | IL-23/IL-23R interaction | IV | Ongoing Phase II | – | |
| BI 655066 | Boehringer Ingelheim | IL-23 (p19 subunit) | SC | Ongoing Phase II | – | |
| IL-6 | PF-04236921 | Pfizer | IL-6 | SC | Ongoing phase I/II | – |
| IL-13 | Tralokinumab | AstraZeneca | IL-13 | SC | – | Phase II (-) |
| Anrukinzumab | Pfizer | IL-13 receptor | IV | – | Phase II (-) | |
| QAX576 | Novartis Pharmaceuticals | IL-13 | IV | Ongoing phase II | – | |
| Bertilimumab | Immune Pharmaceuticals | Eotaxin-1 | IV | – | Ongoing phase II | |
| IL-17 | Vidofluidimus | 4SC AG | IL-17 release | Oral | Phase II (+) | Phase II (+) |
| IL-21 | ATR-107 (PF0530900) | Pfizer | IL-21 receptor | IV/SC | Ongoing phase I | – |
| NNC0114-0006 | Novo Nordisk A/S | IL-21 | IV | Ongoing phase II | – | |
| BLOCKADE OF THE DOWNSTREAM SIGNALLING PATHWAYS MEDIATED BY CYTOKINE | ||||||
| JAK/STAT pathway | Tofacitinib | Pfizer | JAK1, 2 and 3 | Oral | Ongoing phase III | Ongoing phase III |
| Peficitinib (JNJ-54781532) | Janssen | JAK1 | Oral | – | Ongoing phase II | |
| GLPG0634 | Galapagos NV | JAK1 | Oral | Ongoing phase II | – | |
| TGF-β | GED0301 | Giuliani | Smad7 antisense oligonucleotide | Oral | Phase I (+) | – |
| IP-10 antagonists | BMS-936557 | Bristol-Myers Squibb | IP-10 | IV | Ongoing phase II | Phase II (±) |
| Tyrosine kinase receptor | Masitinib (AB1010) | AB science | c-kit, PDGFR α/β; Lck, Lyn, FGFR-3; FAK | Ongoing phase II | – | |
| ANTI-ADHESION MOLECULES | ||||||
| Natalizumab | Tysabri, Biogen Idec | α4 | IV | Approved in USA | – | |
| Vedolizumab | Millennium Pharma . | α4β7 | IV | Phase III (±) | Phase III (+) | |
| Etrolizumab | Genentech | β7 | IV/SC | – | Phase II (+) | |
| PF-00547659 | Pfizer | MadCAM-1 | IV/SC | Ongoing phase II | Ongoing phase II | |
| AJM300 | Ajinomoto | α4 | Oral | – | Phase II (+) | |
| Alicaforsen | ISIS Pharma. | ICAM-1 | Oral/intrarectal | Phase II (-) | Phase II (+) | |
| Vatelizumab | Sanofi | α2β1 integrin | SC | – | Ongoing phase II | |
| firategrast (SB-683699) (formerly T-0047) | GlaxoSmithKline | α4 | Oral | Ongoing phase II | – | |
| GLPG0974 | Galapagos NV | FFA-2 | Oral | – | Ongoing phase II | |
| TRK-170 | Toray Industries, Inc. | β7 | Oral | Ongoing phase II | – | |
| ADMINISTRATION OF ANTI-INFLAMMATORY CYTOKINE | ||||||
| IL-2 | Low dose IL-2 | ILTOO Pharma | IL-2 | SC | Ongoing phase II | Ongoing phase II |
| BLOCKADE OF T-CELL STIMULATION AND INDUCTION OF APOPTOSIS | ||||||
| SB012 | Sterna Biologicals GmbH & Co. KG | GATA-3 | Intrarectal | – | Ongoing phase I/II | |
| VB-201 | VBL Therapeutics | TLR2 dependent innate cell activation | Oral | – | Ongoing phase II | |
| GSK1399686 | GSK | Ribosomal 50S subunit | Oral | – | Ongoing phase II | |
| Laquinimod | Teva Pharmaceutical Industries | ? | Oral | Phase II (+) | – | |
| NNC 0142-0000-0002 | Novo Nordisk A/S | NKG2D | Ongoing phase II | – | ||
| DIMS0150 | InDex Pharmaceuticals | TLR9 | Intrarectal | – | Ongoing phase III | |
| OTHER MECHANISM | ||||||
| Fingolimod | Mitsubishi Tanabe Pharma Corporation | sphingosine 1-phosphate 1 receptor | Oral | Ongoing phase I | – | |
| RPC1063 | Receptos, Inc. | sphingosine 1-phosphate 1 receptor | Oral | – | Ongoing phase II | |
| GSK1399686 | GSK | Ribosomal 50S subunit | Oral | – | Ongoing phase 2 | |
FFA-2, free fatty acid receptor-2; ICAM-1, InterCellular Adhesion Molecule-1; infliximab; Ig, immunoglobulin; IL, interleukin; IP-10, interferon-γ-inducible protein-10; IV, intravenous; JAK, Janus kinase; Lck, lymphocyte-specific kinase; Lyn, Lck/Yes-related protein; MadCAM-1, mucosal address in cell adhesion molecule 1; NZB, natalizumab; NKG2D, natural killer group 2, member D; PDGFR α/β, platelet-derived growth factor receptor-α/β; SC, subcutaneous; TNF, tumor necrosis factor; TGF, transforming growth factor; TLR, Toll-like receptor.
Conclusion
Despite decades of intensive research, the pathogenesis of IBD is still not completely understood. Furthermore, animal models are relevant to explore signaling pathways and the potential effect of a large panel of candidate drugs. However, they may not predict the safety and efficacy of the latter IBD drug through the bench to bedside transition. The development of TNF-blocking agents has been launched in the 1990s based on the pivotal role of TNF in the pathogenesis of IBD. Subsequently, several biological agents targeting other cytokines, such as IL-17 and IL-10 have failed. Also, an agent that appeared relatively safe in preclinical studies may raise some safety concerns when applied in humans. This has been highlighted by experience with vizilizumab, an anti-CD3 monoclonal antibody that induces cytokine release syndrome and significant systemic consequences [Sandborn et al. 2010].
Interestingly, the drugs developed so far and that were successful in IBD, namely anti-TNF agents (infliximab, adalimumab, golimumab, certolizumab pegol), aimed to reduce the burden of local and systemic inflammation, but their systemic effect may be associated with an increased risk of infections. The development of new approaches that could provide the targeted delivery of a drug to the gut could increase clinical efficacy and limit potential adverse events [Lautenschlager et al. 2013]. In this regard, the gut-specificity of vedolizumab may be associated with improved safety profile even though more data are needed. Post-marketing studies will specifically address this question. Other biologics, such as ustekinumab appear promising. Numerous compounds in the pipeline will offer soon new therapeutic options for these incurable diseases. Overall, beyond TNF blockers, anti-adhesion molecules appear to be the most promising drug class in IBD, while Smad7 antisense oligonucleotide might open new therapeutic avenues for CD patients. Pending results of these trials, it is recommended to optimize available drugs in clinical practice [Asthana et al. 2014].
Footnotes
Author contributions: AA wrote the first draft; LPB edited the first draft and supervised the work.
Conflict of interest statement: AA has received payment for lectures from Biocodex and MSD and travel accommodation from Abbvie, MSD and Biocodex. LP-B has received consulting fees from Merck, Abbott, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, Shire, Therakos, Pharmacosmos, Pilège, BMS, UCB-pharma, Hospira, Celltrion, Takeda, Boerhinger-Ingelheim and Lilly; and lecture fees from Merck, Abbott, Janssen, Ferring, Norgine, Tillots, Vifor, Therakos and HAC-pharma.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Aurelien Amiot, Assistance Publique-Hôpitaux de Paris, Paris Est Creteil University, Henri Mondor Hospital, Department of Gastroenterology and EA-EC2M3, Creteil, France.
Laurent Peyrin-Biroulet, Inserm U954 and Department of Hepato-Gastroenterology, University Hospital of Nancy-Brabois, Université de Lorraine, Allée du Morvan, 54511 Vandoeuvre-lès-Nancy, France.
References
- Asthana A., Sparrow M., Peyrin-Biroulet L. (2014) Optimizing conventional medical therapies in inflammatory bowel disease. Cur Drug Targets September 14 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Billioud V., Sandborn W., Peyrin-Biroulet L. (2011). Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol 106: 674–684. [DOI] [PubMed] [Google Scholar]
- Beunk L., Verwoerd A., Van Overveld F., Rijkers G. (2013) Role of mast cells in mucosal diseases: current concepts and strategies for treatment. Expert Rev Clin Immunol 9: 53–63. [DOI] [PubMed] [Google Scholar]
- Burger D., Travis S. (2011) Conventional medical management of inflammatory bowel disease. Gastroenterology 140: 1827–1837 e1822. [DOI] [PubMed] [Google Scholar]
- Choy E., Hazleman B., Smith M., Moss K., Lisi L., Scott D., et al. (2002) Efficacy of a novel pegylated humanized anti-TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double-blinded, randomized, dose-escalating trial. Rheumatology (Oxford) 41: 1133–1137. [DOI] [PubMed] [Google Scholar]
- Colombel J.F., Sandborn W.J., Rutgeerts P., Enns R., Hanauer S.B., Panaccione R., et al. (2007) Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. http://www.ncbi.nlm.nih.gov/pubmed/17241859 Gastroenterology 132: 52-65. [DOI] [PubMed] [Google Scholar]
- Comi G., Jeffery D., Kappos L., Montalban X., Boyko A., Rocca M., et al. (2012) Placebo-controlled trial of oral laquinimod for multiple sclerosis. N Engl J Med 366: 1000–1009. [DOI] [PubMed] [Google Scholar]
- Coskun M., Salem M., Pedersen J., Nielsen O. (2013) Involvement of Jak/Stat signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res 76: 1–8. [DOI] [PubMed] [Google Scholar]
- Crommelin D., Storm G., Verrijk R., De Leede L., Jiskoot W., Hennink W. (2003) Shifting paradigms: biopharmaceuticals versus low molecular weight drugs. Int J Pharm 266: 3–16. [DOI] [PubMed] [Google Scholar]
- D’allard D., Gay J., Descarpentries C., Frisan E., Adam K., Verdier F., et al. (2013) Tyrosine kinase inhibitors induce down-regulation of c-kit by targeting the ATP pocket. PLoS One 8: e60961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’haens G., Colombel J., Sandborn W., Rutgeerts P., Feagan B. (2013) Safety and efficacy of laquinimod in inducing clinical and biochemical improvement in active Crohn’s disease: results of an exploratory trial. Gastroenterology 144: S21. [Google Scholar]
- D’haens G., Panaccione R., Higgins P., Vermeire S., Gassull M., Chowers Y., et al. (2011) The London position of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol 106: 199–212. [DOI] [PubMed] [Google Scholar]
- Danese S. (2012) New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 61: 918–932. [DOI] [PubMed] [Google Scholar]
- Danese S., Rudzinski J., Brandt W., Dupas J., Peyrin-Biroulet L., Bouhnik Y., et al. (2014) Tralokinumab (CAT-354), an interleukin 13 antibody, in moderate to severe ulcerative colitis: a phase 2a randomized placebo-controlled study. Gastroenterology 146: S149. [Google Scholar]
- Delavallee L., Le Buanec H., Bessis N., Assier E., Denys A., Bizzini B., et al. (2008) Early and long-lasting protection from arthritis in tumour necrosis factor alpha (TNFalpha) transgenic mice vaccinated against TNFalpha. Ann Rheum Dis 67: 1332–1338. [DOI] [PubMed] [Google Scholar]
- Dewit O., Hebuterne X., Dupas J., Howaldt S., Bures J., Schreiber S., et al. (2012) Results of a phase II, randomized, double blind, controlled trial of the efficacy of active therapeutic immunization with TNF-Kinoid in patients with moderate to severe Crohn’s disease with secondary resistance to TNFα antagonist. Gastroenterology 142: S-567-S-568. [Google Scholar]
- European Medicines Agency (2013) Guideline on Similar Biological Medicinal Products Containing Biotechnology-Derived Proteins as Active Substance: Non-Clinical and Clinical Issues. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/06/WC500144124.pdf.
- European Medicines Agency (2013) Inflectra – Summary of Opinion (Initial Authorisation). Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002778/WC500144831.pdf.
- European Medicines Agency (2013) Refusal of the Marketing Authorisation for Xeljanz (Tofacitinib). Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002542/WC500146629.pdf.
- European Medicines Agency (1995–2014) Clinical trials register. http://www.clinicaltrialsregister.eu (accessed June 2014).
- Farkas K., Lakatos P., Nagy F., Szepes Z., Miheller P., Papp M., et al. (2013) Predictors of relapse in patients with ulcerative colitis in remission after one-year of infliximab therapy. Scand J Gastroenterol 48: 1394–1398. [DOI] [PubMed] [Google Scholar]
- Feagan B., Greenberg G., Wild G., Fedorak R., Pare P., Mcdonald J., et al. (2005) Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med 352: 2499–2507. [DOI] [PubMed] [Google Scholar]
- Feagan B., Greenberg G., Wild G., Fedorak R., Pare P., Mcdonald J., et al. (2008a) Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol 6: 1370–1377. [DOI] [PubMed] [Google Scholar]
- Feagan B., Panaccione R., Sandborn W., D’haens G., Schreiber S., Rutgeerts P., et al. (2008b) Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn’s disease: results from the CHARM study. Gastroenterology 135: 1493–1499. [DOI] [PubMed] [Google Scholar]
- Feagan B., Rutgeerts P., Sands B., Hanauer S., Colombel J., Sandborn W., et al. (2013) Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 369: 699–710. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Goldin E., Gordon F., Malchow H., Rask-Madsen J., Rutgeerts P., et al. (2003) Natalizumab for active Crohn’s disease. N Engl J Med 348: 24–32. [DOI] [PubMed] [Google Scholar]
- Gottlieb A., Menter A., Mendelsohn A., Shen Y., Li S., Guzzo C., et al. (2009) Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet 373: 633–640. [DOI] [PubMed] [Google Scholar]
- Hanauer S.B., Feagan B.G., Lichtenstein G.R., Mayer L.F., Schreiber S., Colombel J.F., et al. (2002) Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 359: 1541–1549. [DOI] [PubMed] [Google Scholar]
- Hanauer S.B., Sandborn W.J., Rutgeerts P., Fedorak R.N., Lukas M., MacIntosh D., et al. (2006) Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 130: 323–333. [DOI] [PubMed] [Google Scholar]
- ICH (2004) Comparability of biotechnological/biological products subject to changes in their manufacturing process. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q5E/Step4/Q5E_Guideline.pdf [Google Scholar]
- Ito H., Takazoe M., Fukuda Y., Hibi T., Kusugami K., Andoh A., et al. (2004) A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology 126: 989–996; discussion 947. [DOI] [PubMed] [Google Scholar]
- Kavanaugh A., Van Der Heijde D., Mcinnes I., Mease P., Krueger G., Gladman D., et al. (2012) Golimumab in psoriatic arthritis: one-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial. Arthritis Rheum 64: 2504–2517. [DOI] [PubMed] [Google Scholar]
- Kay J., Matteson E., Dasgupta B., Nash P., Durez P., Hall S., et al. (2008) Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum 58: 964–975. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-Demasters B., Tyler K. (2005) Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med 353: 369–374. [DOI] [PubMed] [Google Scholar]
- Langer-Gould A., Atlas S., Green A., Bollen A., Pelletier D. (2005) Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 353: 375–381. [DOI] [PubMed] [Google Scholar]
- Lautenschlager C., Schmidt C., Fischer D., Stallmach A. (2013) Drug delivery strategies in the therapy of inflammatory bowel disease. Adv Drug Deliv Rev, in press. [DOI] [PubMed] [Google Scholar]
- Lawrence M., Springer T. (1991) Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell 65: 859–873. [DOI] [PubMed] [Google Scholar]
- Leonardi C., Kimball A., Papp K., Yeilding N., Guzzo C., Wang Y., et al. (2008) Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 371: 1665–1674. [DOI] [PubMed] [Google Scholar]
- Lobaton T., Vermeire S., Van Assche G., Rutgeerts P. (2014) Review article: Anti-adhesion therapies for inflammatory bowel disease. Aliment Pharmacol Ther 39: 579–594. [DOI] [PubMed] [Google Scholar]
- Malik N. (2009) Controlling the cost of innovative cancer therapeutics. Nat Rev Clin Oncol 6: 550–552. [DOI] [PubMed] [Google Scholar]
- Mannon P., Reinisch W. (2012) Interleukin 13 and its role in gut defence and inflammation. Gut 61: 1765–1773. [DOI] [PubMed] [Google Scholar]
- Michelsen K., Wong M., Ko B., Thomas L., Dhall D., Targan S. (2013) HMPL-004 (Andrographis paniculata extract) prevents development of murine colitis by inhibiting T-cell proliferation and Th1/Th17 responses. Inflamm Bowel Dis 19: 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone G., Fantini M., Onali S., Zorzi F., Sancesario G., Bernardini S., et al. (2012) Phase I clinical trial of Smad7 knockdown using antisense oligonucleotide in patients with active Crohn’s disease. Mol Ther 20: 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone G., Kumberova A., Croft N., Mckenzie C., Steer H., Macdonald T. (2001) Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest 108: 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudter J., Neurath M. (2007) Il-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis 13: 1016–1023. [DOI] [PubMed] [Google Scholar]
- Papp K., Langley R., Lebwohl M., Krueger G., Szapary P., Yeilding N., et al. (2008) Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 371: 1675–1684. [DOI] [PubMed] [Google Scholar]
- Parikh A., Leach T., Wyant T., Scholz C., Sankoh S., Mould D., et al. (2012) Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis 18: 1470–1479. [DOI] [PubMed] [Google Scholar]
- Park W., Hrycaj P., Jeka S., Kovalenko V., Lysenko G., Miranda P., et al. (2013) A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: The PLANETAS Study. Ann Rheum Dis 72: 1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrin-Biroulet L. (2008) Crohn’s disease: beyond antagonists of tumor necrosis factor. Lancet 372: 67–81. [DOI] [PubMed] [Google Scholar]
- Peyrin-Biroulet L, Cieza A, Sandborn WJ, Coenen M, Chowers Y, Hibi T., et al. (2012) Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. International Programme to Develop New Indexes for Crohn’s Disease (IPNIC) group. Gut 61: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrin-Biroulet L., Loftus E., Jr, Colombel J., Sandborn W. (2009) The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol 105: 289–297. [DOI] [PubMed] [Google Scholar]
- Peyrin-Biroulet L. (2013) Disease-modifying anti-inflammatory bowel disease drugs (DMAIDs): the missing term in the literature. Am J Gastroenterol 108: 859–860. [DOI] [PubMed] [Google Scholar]
- Peyrin-Biroulet L., Danese S. (2013) Tofacitinib: Janus bifrons in ulcerative colitis treatment. Gastroenterology 144: 1136–1138. [DOI] [PubMed] [Google Scholar]
- Praditpornsilpa K., Tiranathanagul K., Kupatawintu P., Jootar S., Intragumtornchai T., Tungsanga K., et al. (2011) Biosimilar recombinant human erythropoietin induces the production of neutralizing antibodies. Kidney Int 80: 88–92. [DOI] [PubMed] [Google Scholar]
- Reinisch W., Panes J., Page K., Khurana S., Hua F., Comer G., et al. (2014) Discrepancy between fecal biomarkers and their intestinal gene expression in ulcerative colitis: results from an anti-IL-13 antibody study. J Crohns Colitis 8: S283. [Google Scholar]
- Riese R., Krishnaswami S., Kremer J. (2010) Inhibition of Jak kinases in patients with rheumatoid arthritis: scientific rationale and clinical outcomes. Best Pract Res Clin Rheumatol 24: 513–526. [DOI] [PubMed] [Google Scholar]
- Rinaudo-Gaujous M., Paul S., Tedesco E., Genin C., Roblin X., Peyrin-Biroulet L. (2013) Review article: Biosimilars are the next generation of drugs for liver and gastrointestinal diseases. Aliment Pharmacol Ther 38: 914–924. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P., Sandborn W., Feagan B., Reinisch W., Olson A., Johanns J., et al. (2005) Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 353: 2462–2476. [DOI] [PubMed] [Google Scholar]
- Sandborn W., Colombel J., Enns R., Feagan B., Hanauer S., Lawrance I., et al. (2005) Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med 353: 1912–1925. [DOI] [PubMed] [Google Scholar]
- Sandborn W., Colombel J., Frankel M., Hommes D., Lowder J., Mayer L., et al. (2010) Anti-CD3 antibody visilizumab is not effective in patients with intravenous corticosteroid-refractory ulcerative colitis. Gut 59: 1485–1492. [DOI] [PubMed] [Google Scholar]
- Sandborn W., Feagan B., Fedorak R., Scherl E., Fleisher M., Katz S., et al. (2008) A randomized trial of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology 135: 1130–1141. [DOI] [PubMed] [Google Scholar]
- Sandborn W., Feagan B., Marano C., Zhang H., Strauss R., Johanns J., et al. (2014a) Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 146: 85–95; quiz e14–85. [DOI] [PubMed] [Google Scholar]
- Sandborn W., Feagan B., Marano C., Zhang H., Strauss R., Johanns J., et al. (2014b) Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 146: 96–109 e101. [DOI] [PubMed] [Google Scholar]
- Sandborn W., Feagan B., Rutgeerts P., Hanauer S., Colombel J., Sands B., et al. (2013) Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 369: 711–721. [DOI] [PubMed] [Google Scholar]
- Sandborn W., Gasink C., Gao L., Blank M., Johanns J., Guzzo C., et al. (2012a) Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med 367: 1519–1528. [DOI] [PubMed] [Google Scholar]
- Sandborn W., Ghosh S., Panes J., Vranic I., Su C., Rousell S., et al. (2012b) Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 367: 616–624. [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., Feagan B.G., Marano C., Zhang H., Strauss R., Johanns J., et al. (2014) Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 146: 96–109. [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., Rutgeerts P., Enns R., Hanauer S.B., Colombel J.F., Panaccione R., et al. (2007) Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med 146: 829–838. [DOI] [PubMed] [Google Scholar]
- Sandborn W.J., van Assche G., Reinisch W., Colombel J.F., D’Haens G., Wolf D.C., et al. (2012) Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 142: 257–265. [DOI] [PubMed] [Google Scholar]
- Sands B., Feagan B., Rutgeerts P., Colombel J., Sandborn W., Sy R., et al. (2013) Vedolizumab induction therapy for patients with Crohn’s disease and prior anti-tumour necrosis factor antagonist failure: a randomised, placebo-controlled, double-blind, multicentre trial. J Crohns Colitis 7: S5–S6. [Google Scholar]
- Schreiber S., Khaliq-Kareemi M., Lawrance I.C., Thomsen O.Ø., Hanauer S.B., McColm J., et al. (2007) Maintenance therapy with certolizumab pegol for Crohn’s disease. http://www.ncbi.nlm.nih.gov/pubmed/17634459 N Engl J Med 357: 239–250. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Rutgeerts P., Fedorak R.N., Khaliq-Kareemi M., Kamm M.A., Boivin M., et al. (2005) A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterology http://www.ncbi.nlm.nih.gov/pubmed/?term=schreiber+2005+certolizumab 129: 807–818. [DOI] [PubMed] [Google Scholar]
- Singh J., Beg S., Lopez-Olivo M. (2010) Tocilizumab for rheumatoid arthritis. Cochrane Database Syst Rev CD008331. [DOI] [PubMed] [Google Scholar]
- Targan S., Feagan B., Fedorak R., Lashner B., Panaccione R., Present D., et al. (2007) Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology 132: 1672–1683. [DOI] [PubMed] [Google Scholar]
- Targan S.R., Hanauer S.B., van Deventer S.J., Mayer L., Present D.H., Braakman T., et al. (1997) A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med 337: 1029–1035. [DOI] [PubMed] [Google Scholar]
- Tebib J., Mariette X., Bourgeois P., Flipo R., Gaudin P., Le Loet X., et al. (2009) Masitinib in the treatment of active rheumatoid arthritis: results of a multicentre, open-label, dose-ranging, phase 2a study. Arthritis Res Ther 11: R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration (2009) Natalizumab: Highlights of Prescribing Information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/125104s106lbl.pdf
- US Food and Drug Administration (2012) Advisory Committee Meeting. Tofacitinib for the Treatment of Rheumatoid Arthritis. NDA 203214. Briefing Document. Available at: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/arthritisadvisorycommittee/ucm302960.pdf.
- US Food and Drug Administration (2013) Draft Guidance on Biosimilar Product Development. Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/default.html.
- US National Institutes of Health (2011) Clinicaltrials.Gov. http://www.clinicaltrials.gov (accessed June 2014).
- Van Assche G., Van Ranst M., Sciot R., Dubois B., Vermeire S., Noman M., et al. (2005) Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med 353: 362–368. [DOI] [PubMed] [Google Scholar]
- Varrin-Doyer M., Zamvil S., Schulze-Topphoff U. (2014) Laquinimod, an up-and-coming immunomodulatory agent for treatment of multiple sclerosis. Exp Neurol, in press: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeire S., O’byrne S., Keir M., Williams M., Lu T., Mansfield J., et al. (2014) Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet, in press. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Blanks J. (1999) Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev 79: 181–213. [DOI] [PubMed] [Google Scholar]
- Vignali D., Kuchroo V. (2012) IL-12 family cytokines: immunological playmakers. Nat Immunol 13: 722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise M., Bielsky M., De Smet K., Ehmann F., Ekman N., Giezen T., et al. (2012) Biosimilars: what clinicians should know. Blood 120: 5111–5117. [DOI] [PubMed] [Google Scholar]
- Wernersson S., Pejler G. (2014) Mast cell secretory granules: armed for battle. Nat Rev Immunol 14: 478–494. [DOI] [PubMed] [Google Scholar]
- Yoo D., Hrycaj P., Miranda P., Ramiterre E., Piotrowski M., Shevchuk S., et al. (2013) A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: The PLANETRA Study. Ann Rheum Dis 72: 1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Jang H., Fleischmann R., Bouman-Thio E., Xu Z., Marini J., et al. (2007) Pharmacokinetics and safety of golimumab, a fully human anti-TNF-alpha monoclonal antibody, in subjects with rheumatoid arthritis. J Clin Pharmacol 47: 383–396. [DOI] [PubMed] [Google Scholar]