Abstract
Glioblastoma is the most common aggressive, highly glycolytic, and lethal brain tumor. In fact, it is among the most commonly diagnosed lethal malignancies, with thousands of new cases reported in the United States each year. Glioblastoma's lethality is derived from a number of factors including highly active pro-mitotic and pro-metastatic pathways. Two factors increasingly associated with the intracellular signaling and transcriptional machinery required for such changes are anaplastic lymphoma kinase (ALK) and the hepatocyte growth factor receptor (HGFR or, more commonly MET). Both receptors are members of the receptor tyrosine kinase (RTK) family, which has itself gained much attention for its role in modulating mitosis, migration, and survival in cancer cells. ALK was first described as a vital oncogene in lymphoma studies, but it has since been connected to many carcinomas, including non-small cell lung cancer and glioblastoma. As the receptor for HGF, MET has also been highly characterized and regulates numerous developmental and wound healing events which, when upregulated in cancer, can promote tumor progression. The wealth of information gathered over the last 30 years regarding these RTKs suggests three downstream cascades that depend upon activation of STAT3, Ras, and AKT. This review outlines the significance of ALK and MET as they relate to glioblastoma, explores the significance of STAT3, Ras, and AKT downstream of ALK/MET, and touches on the potential for new chemotherapeutics targeting ALK and MET to improve glioblastoma patient prognosis.
Keywords: Glioblastoma, ALK, MET, AKT
1. Background
Glioblastoma is one of the most aggressive, highly glycolytic, and deadly brain tumors. Approximately 9,000 new cases are diagnosed each year in the United States according to the Central Brain Tumor Registry of the United States (www.cbtrus.org). Among these lethal CNS neoplasms, glioblastoma (WHO classification of Grade IV) is the most common (Wen & Kesari 2008). The rarity of glioblastoma diagnosis is contrasted by its extreme lethality; patients have a median life expectancy following diagnosis and aggressive treatment of just 14 months (Wen & Kesari 2008).
Pathologically, glioblastoma presents as a highly heterogeneous tumor in regard to histological morphology and genetic profile. Intratumoral giant cells, sporadic necrotic loci, and a high mitotic index are all pathological hallmarks of glioblastoma (Benito et al. 2010). In addition, glioblastoma tumors present intense metabolic needs that are fed by increased neo-vascularization often directly induced by such tumors (Benito et al. 2010). Despite increased angiogenic potential in glioblastoma, CNS tumors can develop necrotic or apoptotic cores if mitosis outpaces neo-vascularization of the tumor. These areas can be identified both by observation of necrosing cells and surrounding pseudopalisading tumor cell formations. Ultimately, these pro-survival angiogenic features elaborate the tenacity of glioblastoma tumors.
Current standard of care therapy, which includes combination radiation and temozolomide (TMZ) treatment, extends survival on average to 14.6 months (Stupp et al. 2005). Despite such extreme glioblastoma related lethality, long-term life expectancy increases with each year a patient survives with the disease (Polley et al. 2011). This would seem to suggest that a therapy capable of extending a patient's life expectancy in the immediate future also significantly increases long-term survival. However, Polley and others cautioned that such survival findings as theirs were based on data from patients who had enrolled in clinical trials and thus were predisposed to have a better prognosis than patients with more severe (i.e. study ineligible) cases of glioblastoma (Polley et al. 2011). Certainly, this does not diminish the validity of current and new therapies that extend life for glioblastoma patients, but it highlights that glioblastoma remains incurably lethal despite aggressive funding and research. Regardless of whether or not a patient survives one, two, or even five years following a diagnosis of glioblastoma, the disease itself presents with numerous debilitating neurological deficits which are only exacerbated by standard of care therapies.
The remainder of this review focuses on two components of glioblastoma related tumorigenesis that may represent significant regulatory targets for glioblastoma: anaplastic lymphoma kinase (ALK) and the hepatocyte growth factor (HGF) receptor (HGFR or MET). It will explore early clinical observations related to these factors, examine the functional significance of their dysregulation in glioblastoma, and finish by discussing current and future therapies that may correct or inhibit ALK and MET functionalities in glioblastoma.
2. ALK & MET in Glioblastoma
2.1 Significance of ALK & MET to cancer
Cancer in general is precipitated by aberrant expression of pro-mitotic and pro-metastatic genes resulting in potentially lethal proliferation and invasion by affected cells. However, the very mitotic and metastatic factors that are the hallmarks of many adult cancers are normally expressed or up-regulated at various points during embryonic development and wound healing. With this in mind, recent research has worked to identify pathways selectively expressed in cancers, including glioblastoma, in order to better differentiate cancer cells from normal cells and also to aid in the design of novel, specific chemotherapies.
Much work has focused on the role of receptor tyrosine kinases (RTK) and their role in the initiation and progression of glioblastoma. This is unsurprising considering nearly 40-60% of all glioblastomas express increased levels of epidermal growth factor receptor (EGFR), an angiogenic agent (Houillier et al. 2006, Ohgaki et al. 2004, Shinojima et al. 2003, Szerlip et al. 2012). In these cases, and in those where EGFR expression may be unaltered, other pro-survival, proliferative, and/or vasculogenic pathways are deregulated, including: the PTEN/Akt/mTOR pathway, the TP53/MDM2/ARF pathway, and INK4a/RB1 pathway. The latter pathways are discussed in greater detail elsewhere (Ohgaki & Kleihues 2007, 2008), but the significance of PTEN, AKT, and mTOR will be discussed further. As fellow members of the RTK family, ALK and MET have gained extensive experimental attention over the last few decades.
2.1.1 ALK
ALK and its ligand, pleiotrophin (PTN), are highly expressed during embryonic development of the nervous system and are normally limited in adult tissues (Grzelinski et al. 2009). Aberrantly expressed ALK was first determined to be a contributing oncogenic factor in non-Hodgkin's lymphoma (Morris et al. 1994). Since the initial discovery of oncogenic ALK translocations in lymphoma, various ALK mutations have been described in multiple cancer lines including glioblastoma (Fujimoto et al. 1996, Pulford et al. 1997, Pulford et al. 2004a, Pulford et al. 2004b, Dirks et al. 2002). ALK and PTN are expressed at significantly higher levels in high grade brain tumors (glioblastoma and anaplastic oligodendrogliomas) when compared to normal brain tissue and low grade tumors (Stylianou et al. 2009). The binding of PTN to its extracellular domain on ALK mediates growth stimulation and antiapoptotic pathways.
Previously, the PTN gene has been targeted, resulting in suppression of tumor angiogenesis, growth, and metastases in a variety of tumors, including glioblastoma (Grzelinski et al. 2009). Additionally, there was decreased growth and increased apoptosis of glioblastoma xenografts in athymic nude mice with ribozyme-mediated targeting of ALK (Grzelinski et al. 2009). A recent presentation at the November 2011 Conference in San Francisco, “Molecular Targets and Cancer Therapeutics” suggested that ALK/MET activity was present in over half of the patient tumors analyzed (http://mct.aacrjournals.org/cgi/content/short/10/11_MeetingAbstracts/A42?rss=1).
Another possible reason for the widespread connection between ALK and cancer may lie in the generalized transcriptional and protein level expression of ALK during embryonic development (Vernersson et al. 2006). Since ALK expression is partially responsible for developmental control of cellular proliferation, it may be that increased activation of ALK plays a part in tumor stem cell like proliferation in glioblastoma. In mice, increased expression of ALK in the developing CNS and PNS versus other tissues further highlights the likely importance of this RTK in CNS tumors like glioblastoma (Vernersson et al. 2006).
Pro-oncogenic ALK mutations may occur through a gain-of-function effect (i.e. constitutively expressed ALK) as suggested by two cases of mutation-related disruption of CNS development (de Pontual et al. 2011). In their paper, de Pontual and others make a strong case for ALK mediated abdominal and medullar tumor formation in two infants born with ALK germline translocation mutations. In both cases, de novo germline mutations were associated with both PNS and CNS tumor formation and, ultimately, death. Experimental observations also lend support to the theory of ALK mediated tumorigenesis in glioblastoma. Stylianou and others had developed both activating and inactivating antibodies aimed at the PLT binding domain on ALK that increased or decreased (respectively) the oncogenic activity of the ALK receptor in U87MG cells in vitro and in vivo (Stylianou et al. 2009).
2.1.2 MET
HGF and its target receptor, MET, are two factors that are generally detectable only in epithelial or mesenchymal cells during development and wound healing (Di Renzo et al. 1991, Sonnenberg et al. 1993). Both immune response and injury repair mechanisms depend heavily on HGF/MET activation (Michalopoulos & DeFrances 1997, Jin et al. 2003). Together, HGF and MET are essential to normal murine development and result in embryonic lethality when absent (Schmidt et al. 1995, Uehara et al. 1995, Bladt et al. 1995, Michalopoulos & DeFrances 1997, Jin et al. 2003). An excellent history of HGF/MET discovery and functional characterization can be found in Birchmeier and colleagues' review article (Birchmeier et al. 2003).
The first characterization of MET came from the identification of the human oncogene, tpr-met (often referred to as c-Met) (Cooper et al. 1984). Since then, MET and HGF expression have been correlated with glioblastoma prognosis (Arrieta et al. 2002). In Liu et al, it was found that patients who expressed a higher level of c-Met had a significantly shorter progression free survival time of 6.1 months, versus 11.5 months in those patients who expressed a lower level of the oncogene (Liu et al. 2011). It has also been suggested that autocrine MET tumors (i.e. tumor HGF targeting tumor MET) rather than paracrine MET tumors are more responsive to MET inhibitor therapy (Xie et al. 2012). Given that ligand independent activating mutations of ALK are known to contribute to CNS tumor formation, it is somewhat intriguing that ligand dependent activation of MET should drive MET mediated glioblastoma growth.
In recent years, activity of the epidermal growth factor receptor (EGFR) mediated PI3K/AKT/mTOR signal transduction cascade has gained significant attention. EGFR is the primary amplified RTK related to Ras/PI3K activation among glioblastoma tumors (2008, 2008, 2008, 2008, 2008, Verhaak et al. 2010). Amplifications of EGFR, which is itself a member of the RTK family, appear in 36% of glioblastoma samples with 1/3 of those cases exhibiting a specific EGFRvIII mutation (Wong et al. 1992, Rao et al. 2010, Rao et al. 2008). In part due to the known amplification of chromosome 7 (the chromosome on which MET is located) among glioblastoma tumors, it has been suggested that amplification of EGFR in glioblastoma could be related to aberrant MET expression (2008, Xie et al. 2012). Surprisingly, glioblastoma tumors expressing MET mutations only represent ∼5% of the total population (2008). However, the significant downstream effects of MET activation make it a promising target in glioblastoma treatment. The role of MET in regulating glioblastoma stem cells (GSCs) has also been a target of recent discussion. Joo and colleagues isolated glioblastoma cells that expressed a high level of MET and which were found to be located in perivascular regions (Joo et al. 2012). These cells were found to be tumorigenic and resistant to radiation, and the disruption of their signaling to GSCs was proven, both in vivo and in vitro, to significantly reduce the proliferative and invasive capabilities of glioblastoma (Joo et al. 2012).
In short, MET activation is essential to development, engaged following injury to assist with wound repair, and expressed widely in both developing and adult organisms. All of these characteristics allow for oncogenic transformation of normal cells to carcinomas through MET mutations. However, MET and ALK modifications broadly affect normal cellular processes (as do many RTKs) via numerous pathways. Many of these processes are only activated in highly mitotic cells, such as those found in developing embryos or in adult stem cell populations, and as such, play a significant role in cell survival and motility. Therefore, a careful review of these downstream effects is paramount to understanding the implications for ALK/MET-related oncogenesis and ALK/MET anti-cancer therapies.
2.2 Downstream effectors: STAT3, Ras, AKT
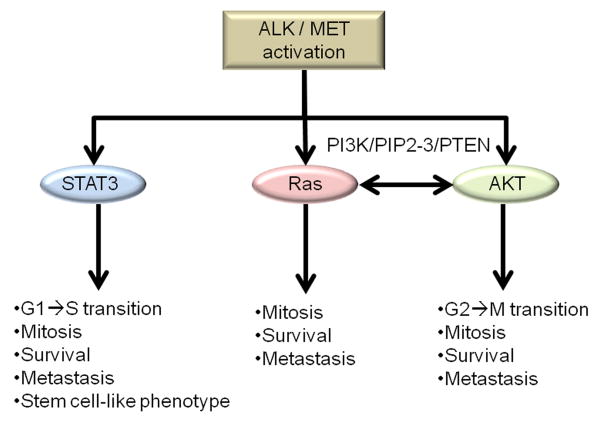
Ligand activation of RTKs triggers phosphorylation of intracellular tyrosine residues and a cascade of pro-survival and pro-mitotic effectors. Likewise, constitutive phosphorylation of the intracellular domains of RTKs also affects pro-oncogenic cascade induction. Not surprisingly, activation of ALK and MET (either ligand dependent or independent) also triggers these cascades. For most RTKs, including ALK and MET, there are three points of intracellular signal diversion which may mediate the transition from a normal cell to cancer cell (Figure 1). STAT (signal transducer and activation of transcription; STAT3, in particular), AKT (also known as protein kinase B), and Ras (‘rat sarcoma’; named from the first identified gene transcript in rat sarcomas) downstream signaling appear to be at once independently and interactively responsible for the oncogenic activities of both ALK and MET (Boufaied et al. 2010, Zhang et al. 2002, Chiarle et al. 2005, Porter & Vaillancourt 1998, De Luca et al. 2012). Though these three downstream components are highly dependent upon transduction factors such as Grb2, Gab1 and JAK, which respond more directly to RTK tyrosine phosphorylation, they represent intracellular turning points that individually promote oncogenic transition and tumor growth (Birchmeier et al. 2003). Interactions among these cascades do occur, and understanding those interactions therefore becomes vital to understanding the significance of ALK/MET oncogenic activities. To begin with, it is important to understand the individual effects of STAT3, Ras, and AKT.
Figure 1. Downstream effectors: STAT3, RAS, AKT.
ALK/MET mediated phosphorylating activation of STAT3, Ras, and AKT result in expression of numerous distinct, yet interconnected pro-survival, pro-mitotic, and pro-metastatic pathways. While STAT3 acts directly as a transcription factor, Ras and AKT cascades begin at the membrane and are connected by the PI3K/PIPx/PTEN modality.
2.2.1 STAT3 transcriptional activator
STAT3 is one of the master transcriptional regulators responsible for a majority of downstream signaling events most commonly associated with aggressive carcinomas and poor patient prognosis (Cooper et al. 2012). Activation of ALK or MET directly stimulates STAT3 phosphorylation (Zhang et al. 2002, Zamo et al. 2002). Following phosphorylation, STAT proteins translocate to the nucleus where they act as transcriptional promoters for various genes, which themselves can promote cell survival (Aaronson & Horvath 2002) and anchorage independent growth (Zhang et al. 2002, Schaper et al. 1997). In ALK positive tumors, ALK-STAT3 activation promotes tumor cell outgrowth and directly prevents apoptosis, in part, by increasing Bcl-x(L) expression (Zamo et al. 2002).
Phosphorylated STAT3 can be found following prolonged (>24hr) activation of the ALK ligand binding site, but the level of phospho-STAT3 engendered by ligand-independent activation of ALK was exponentially higher. This discovery may point to a ligand independent role for ligand-independent oncogenesis constitutive activation of STAT3 by ALK and possibly MET. Recently, STAT3 mediated activation of the insulin like growth factor 1 (IGF-1) autocrine loop has enthralled clinicians and researchers (Gariboldi et al. 2010). Glioblastoma cell line experiments have identified a role for IGF directed pro-metastatic changes and temozolomide resistance (Hsieh et al. 2011). Phospho-STAT3 suppresses IGFBP-5, which normally suppresses IGF-1, with resultanting increases in IGF-1 leading to autocrine stimulation of antiapoptotic transcription factors such as hypoxia inducible factor (HIF)-1α. In turn, HIF-1α stimulates pro-survival and pro-metastatic effectors such as: toll like receptor 9, VEGF, IGF, and indirectly, Bcl-2 (Sinha et al. 2011, Gariboldi et al. 2010).
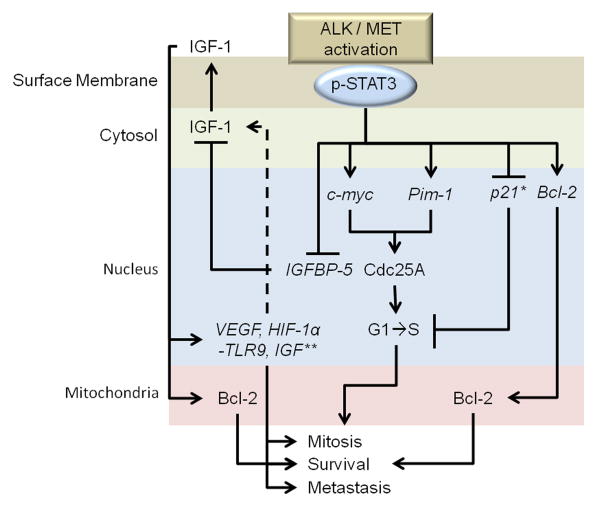
Finally, embryonic stem cell studies have determined a necessary role of STAT3 activation for preserving pluripotency among stem cell populations (Matsuda et al. 1999). In glioblastoma, stem cell populations are postulated to mediate drug and radiotherapy resistance. STAT3 directly binds to and promotes transcription of the Cdc25A gene, which controls cell cycle progression from G1 to S phase (Barre et al. 2005). In cytokine receptor activation, STAT3 activation also contributes to transcription of c-myc and Pim-1 genes to further contribute to both mitosis and survival (Shirogane et al. 1999). Finally, STAT3 also blocks p21 transcriptional activities that would otherwise impede cell cycle progression (Zhang et al. 2002). Thus the significance of STAT3 phosphorylation in tumor cells, including glioblastoma, likely depends on four key features: 1) activation of pro-survival pathways, 2) promotion of cell cycle progression, 3) induction of metastatic marker expression, and 4) by potentiating the development of stem-like cells in tumors. Figure 2 provides a summary of the discussed effects of STAT3 phosphorylation by ALK/MET.
Figure 2. STAT3 transcriptional activation.
Phospho-STAT3 dimerizes with other STAT proteins upon translocation to the nucleus where it potentiates transcription of: c-myc, Pim-1, p21, and IGFBP-5. Among other transcripts, production of Cdc25A by c-myc and Pim-1 directly contributes to the G1 to S phase transition. p-STAT3 inhibits IGFBP-5 to promote an IGF-1 autocrine loop. The IGF-1 autocrine loop thus contributes to production of cell growth signaling and contributes to increased: VEGF, HIF-1α, TLR9, IGF. *p21 activities are numerous, but here it suppresses mitosis and survival protein production; **IGF initiates numerous other pro-mitotic/survival/metastatic cascades
2.2.2 Ras: dual oncogenic pathways
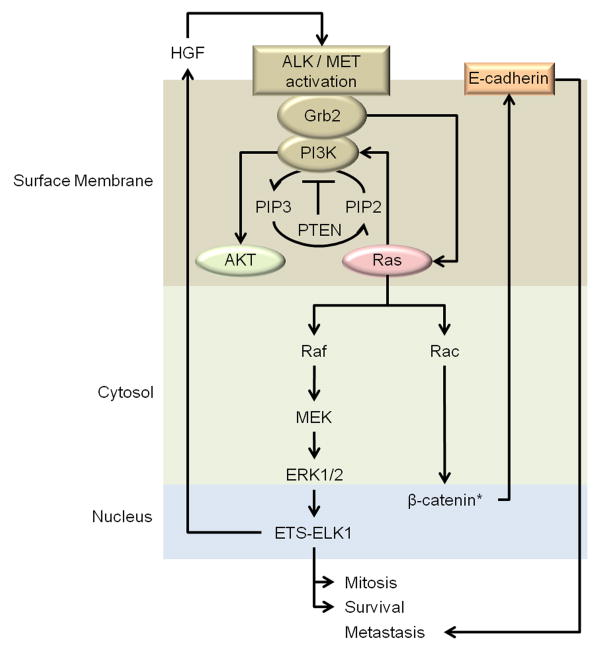
Ras, embedded in the cellular membrane proximally to ALK or MET, is quickly activated following receptor tyrosine phosphorylation (De Luca et al. 2012). Once activated, Ras may stimulate PI3K-mediated AKT activity as well as the RAF/MEK/ERK pathway which ultimately promotes activation of cell cycle progression factors (De Luca et al. 2012). A general diagram is found in Figure 3. Recently, it was determined that Ras mediated tumorigenesis in epidermal cells first stimulates elevated Rac1 activity (Samuel et al. 2011). Since Rac1 activity is dependent upon GTP, Ras mediated activation of downstream MEK and ERK is likewise dependent upon Rac1-GTP binding activities. Ultimately, interactions between Ras and Rac1 eventually result in increased β-catenin activity, which then leads to E-cadherin production (Gherardi et al. 2012). E-cadherin is thus capable of stabilizing cancer cells for migration and metastasis.
Figure 3. Ras: dual oncogenic pathways.
Ras activation further potentiates AKT activation by stimulating PI3K mediated phosphorylation of PIP2 to PIP3; this process is inhibited in the presence of functional PTEN. Ras/Rac activities work with AKT to further stabilize β-catenin activity and thus promote the pro-metastatic adherin product, E-cadherin. Ras/Raf activity triggers activation of the Raf/MEK/ERK cascade which culminates in, among others, increased transcriptional activity of the ETS-ELK-1 complex to further activate pro-mitotic and pro-survival pathways. Ras mediated ELK-1 activation results in an autocrine HGF/MET feedback loop which may further stabilize cell survival and metastatic potential. *Represents another point of interaction with AKT
The Ras/MEK/ERK pathway has been clearly mapped out largely due to its involvement in numerous cancers. Still, it is known that Ras activation which stimulates ERK, and subsequently Elk-1 transcription factor activation, directly contributes to HGF production and autocrine stimulation of MET (Goudar et al. 2005). In the case of cytokine stimulation of Ras/ERK pathways, a feedback loop which prevents excessive STAT activation through an intracellular Ras expression modulator (RasGAP), simultaneously appears to further activate Ras/ERK mediated cell survival activity (Chi et al. 2012, Chin et al. 2012, Cooper et al. 2012, Del Vecchio et al. 2012). The interplay between Ras and other members of the RTK downstream cascade remains underexplored. For instance, what activation level of Ras or its associate cascades identified by AKT and/or STAT3 activation, is necessary for oncogenic transformation? A fascinating study that outlined the genetic creation of astrocytomas by inserting constitutively active Ras and AKT genes into the glial fibrillary acidic protein (GFAP) sequence may help to understand this question. Holland and colleagues discovered that in this genetic induction model of glioblastoma, both Ras and AKT together were necessary for oncogenesis and glioblastoma tumor formation (Holland et al. 2000). Separately, the roles for Ras and AKT are fairly well known, but the interdependency between the two pathways is less so.
These findings highlight the potential for AKT or Ras specific inhibitors as well as more generalized RTK inhibitors. They also highlight the significance of the third RTK-mediated oncogenic pathway pivoting on AKT phosphorylation.
2.2.3 AKT in oncogenesis
Following discovery in the late 1970's, a virus derived oncogene, AKT8, was used to induce tumorigenesis in vivo (Staal & Hartley 1988). Humans express three isoforms: AKT1, AKT2, and AKT3, which are implicated in DNA repair mechanisms, growth and insulin metabolism respectively (Chen et al. 2001, Garofalo et al. 2003, Easton et al. 2005).
While the role of AKT in gliomas has been well elaborated previously (McDowell et al. 2011), it will be useful to briefly review the main mechanisms of AKT activity and downstream effects. AKT phosphorylation by RTKs begins with the phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2) to phosphatidylinositol (3,4,5)-triphosphate (PIP3) by phosphatidylinositol 3-Kinase (PI3K), which is itself directly activated following RTK tyrosine phosphorylation (Vignot et al. 2005, Larue & Bellacosa 2005). The transition from PIP2 to PIP3 is directly controlled by phosphatase and tensin homologue (PTEN). PTEN thus acts as a master switch for AKT activity intracellularly, a fact which makes the use of U87MG, SNB-19, and U373MG (the most popular cell lines for glioblastoma in vitro studies which differentially express PTEN activities) highly valuable to understanding AKT phosphorylation effects in tumorigenesis (McDowell et al. 2011). In general, PTEN deletions are found in ∼30% of gliomas making it an enticing target for future study (Li et al. 1997, Pulford et al. 1997, Ward et al. 1997).
AKT inhibits TSC2 mediated inhibition of the mammalian target of rapamycin, or mTOR, functionality (McDowell et al. 2011). When AKT activation leads to mTOR activation, translation of cell cycle progression markers as well as activation of pro-survival cascades favor tumor growth and progression (for a thorough review, see (Faivre et al. 2006) & (McDowell et al. 2011)). Rapamycin is known to inhibit cancer cell cycle progression and enhance sensitivity to radiation by blocking mTOR-mediated translational modifications of oncogenic gene products (Anandharaj et al. 2011). Experiments targeting mTOR suggest that blocking PTEN activity sensitizes cells to mTOR inhibition and prevents IGF-mediated pro-survival initiation (Vivanco & Sawyers 2002, Hagerstrand et al. 2010). Rapamycin mediated inhibition of survivin (a microtubule stabilizing protein associated with cell survival, mitosis, and metastasis) also inhibits AKT phosphorylation, significantly reducing viability of cancer cell lines (Anandharaj et al. 2011, Weiss et al. 2012). This suggests mTOR (and presumably AKT) controls expression of AKT phosphorylation mediators. Currently, everolimus (Novartis) is an orally available mTOR inhibitor with a high lipophilic index allowing penetration of the blood-brain barrier and with combination gefitinib, offer a clinically active combination in recurrent glioblastoma where EGFR and PI3K/AKT are co-activated (Kreisl et al. 2009). Additionally, survivin is of particular interest to cancer identification since only highly mitotically active cells and human tumors generally express this protein. High expression levels of survivin throughout development point back to a stem cell like phenotype that is increasingly important to glioblastoma study.
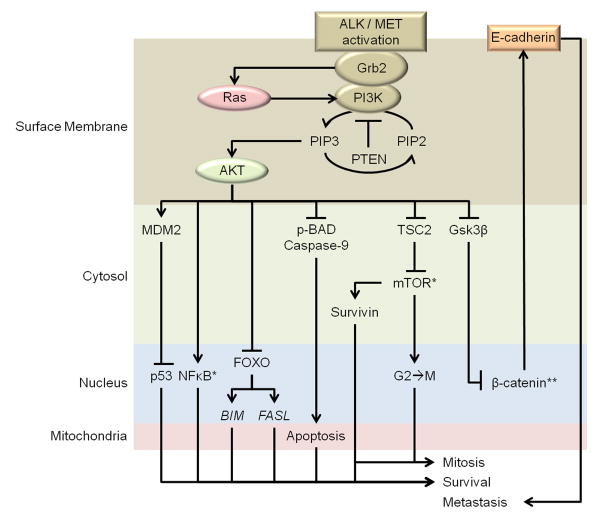
AKT blocks activation of a number of other factors as well. Indirectly, AKT inhibits p53 by enabling MDM2 phosphorylation (McDowell et al. 2011, Birchmeier et al. 2003). As a control mechanism for proliferation and anti-apoptotic survival, p53 is of great interest to glioblastoma studies (Bajbouj et al. 2012). Additionally, AKT mediated inhibition of FOXO transcription of BIM and FASL genes directly contributes to cell survival (Nakamura et al. 2000). Further stabilization of cellular survival comes in the form of anti-phospho-BAD activities as well as caspase inhibition by phospho-AKT (Birchmeier et al. 2003, McDowell et al. 2011). Finally, AKT mediated inhibition of Gsk3β, which forms a complex with other proteins to promote β-catenin degradation, results in increased production of E-cadherin which in turn promotes motility and metastasis (Gherardi et al. 2012). This is a particularly interesting effect as Ras activation also modulates β-catenin expression to promote E-cadherin production (Gherardi et al. 2012). Finally, AKT mediates activation of NFκB, which in turn mediates transcription of numerous pro-survival factors (Syed et al. 2008). However, whether NFκB activation is essential to AKT related glioblastoma progression is unclear (Zeng et al. 2002). An overview of the discussed AKT pathway can be found in Figure 4.
Figure 4. AKT in oncogenesis.
ALK/MET activation results in activation of Ras and AKT. AKT activation inhibits various tonic inhibitors of mitotic and survival cascades including: p53 (downstream of AKT-MDM2 activation), FOXO (promotes transcription of pro-apoptotic genes like BIM and FASL), Caspase-9, Bad, TSC2 (inactivates pro-translational properties of mTOR), and Gsk3β (an integral component of the complex responsible for β-catenin degradation). AKT also activates pro-survival gene transcription through NFκB and, via mTOR disinhibition, potentiates expression of pro-mitotic factors like Survivin, which is highly expressed in glioblastoma. *NFκB & mTOR direct numerous other pro-survival activities not shown here; **Represents another point of interaction with Ras.
3. ALK & MET in Relation to Current Therapies
TL-4601, a farnesylated dibenzodiazepine which inhibits the Ras/MEK signaling cascade, has been evaluated in a phase II clinical trial (Mason et al. 2011). Unfortunately, TL-4601 failed to significantly affect tumor progression at the doses given in patients and only transiently suppressed Ras-ERK MAPK signaling compared to the more marked reductions seen in laboratory studies (Campbell et al. 2010, Boufaied et al. 2010). Crizotinib (PF-02341066) is an orally available ATP-competitive selective inhibitor of ALK and MET tyrosine kinases that inhibits tyrosine phosphorylation on these receptors at nanomolar concentrations (McDermott et al. 2008, Christensen et al. 2007). Pharmacokinetic-pharmacodynamic studies have indicated a near complete inhibition of MET activation in mouse glioblastoma tumors; indeed, near total inhibition may be required for successful targeting of MET (Yamazaki et al. 2008). Despite limited widespread use, crizotinib has been presented to positively affect non-small cell lung carcinoma metastasized to the brain of at least one patient (Costa et al. 2011). Additionally, this patient presented with fewer contraindications while on crizotinib versus previous treatments.
4. Future Directions for ALK & MET Targeted Therapies
4.1 Challenges to ALK/MET manipulation
Numerous bioactive agents have been developed in recent years to inhibit HGF/MET activity with the effect of blocking brain tumor progression (Welsh et al. 2009, Lal et al. 2009, Guessous et al. 2010, Crosswell et al. 2009, Cecchi et al. 2010). In all cases, inhibition of these RTKs reduces mitotic and metastatic profiles for numerous cancer cells. However, the very benefits imparted by RTK inhibitors, may pose significant risks to patients by blocking normal wound repair mechanisms (Ward et al. 1997). For example, STAT3 activation, as found downstream of ALK or MET activation, is involved in normal wound healing of the lung during infections (Pechkovsky et al. 2012). Combining ALK/MET inhibitors with current chemotherapies which diminish immune function would necessitate close observation to prevent severe pulmonary infections and scarring. Additionally, since ALK activity is integral to smooth muscle wound healing pathways (Ward et al. 1997), administration of ALK inhibitors may affect post-operative healing following surgical resections and could increase vascular pathology in glioblastoma patients with poor cardiovascular health.
Unfortunately, RTK inhibitors (like Bevacizumab) which target the RTK VEGF receptor, appear to exacerbate treatment by promoting both ischemic and hemorrhagic stroke in treated patients (Fraum et al. 2011). Cediranib was hailed as a potent anti-angiogenic agent that targeted the VEGF receptor and effectively halted new vessel growth in glioblastoma (Batchelor et al. 2010). One challenge of some anti-angiogenic inhibitors, like cediranib, is that it does not target ALK or MET. Thus, it is unclear how strong of a role ALK or MET plays in angiogenesis. Similarly, rapamycin, which blocks mTOR dependent protein synthesis, was expected to interfere with AKT mediated oncogenesis, but actually resulted in increased phospho-AKT levels and drug resistant tumor cell survival (Fan et al. 2010). Until trials are undertaken to explore the effectiveness of ALK/MET inhibition in human glioblastoma cases, it will be impossible to predict contraindications and complications.
4.2 Multi-drug therapy
The anti-proliferative potential for ALK/MET related inhibitors are exciting, yet their potential for inducing tumor regression is unknown. It is likely that any such drugs will excel at inhibiting tumor growth as well as metastatic potential. Still, blocking STAT3, AKT, and Ras downstream cascades would reduce pro-survival markers as discussed earlier. For instance, rapamycin co-treatment with a PTEN inhibitor blocks IGF mediated cell survival (Faivre et al. 2006, Anandharaj et al. 2011). Since PTEN activity is required for AKT activation, perhaps co-treatment of rapamycin with an ALK or MET inhibitor would enhance the activities of both drugs.
A few studies have undertaken to show similar effects with TMZ. Co-treatment of tumors with TMZ and AMG-102 (an antibody against HGF) significantly enhances in vitro apoptosis and in vivo tumor regression in animal models (Jun et al. 2007). Whether such effects will be mirrored in tumors expressing activating mutations of ALK or MET receptors remains to be seen. Nevertheless, the significance of extracellular repression of MET activation combined with TMZ cannot be understated. Blocking effectors downstream of ALK and MET in combination with more generalized ALK and MET inhibitors may also enhance therapy. For instance, administration of rapamycin in conjunction with gefitinib, an EGFR inhibitor, greatly increases the effectiveness of gefitinib while also overcoming the pro-survival AKT phosphorylation inducible by rapamycin alone (Fan et al. 2010, Goudar et al. 2005). The complex interactions of the broadly activating effectors in the RTK intracellular cascade both enhance the potential for ALK and MET inhibitors and also highlight the limitations of mono-therapies targeting these receptors. Indeed, the complexities of glioblastoma itself indicate the need for a complex approach to treatment.
4.3 Targeting metabolic pathways by ALK/MET inhibitors
Many known oncogenic signaling pathways involved in gliomagenesis have strong influences on tumor cell metabolism and promote the switch from oxidative phosphorylation to aerobic glycolysis for ATP generation (Wolf, etal). Glioblastoma cells, which have a higher ratio of lactate to pyruvate, require glutamate dehydrogenase (GDH) to survive impairments of glucose metabolism or Akt signaling. Akt indirectly regulates GDH through its effects on glucose metabolism where the inhibition of Akt signaling facilitates glycolysis and increases GDH activity and the over expression of Akt suppresses it (Wolf, et al). Therefore, future studies should investigate the impact of inhibiting specific enzymes in the metabolic network as a whole to include fatty acids, nucleotides, and amino acid metabolism by ALK/c-Met inhibitors. Results obtained from these studies will help us to understand the role of glucose metabolism, glutamine metabolism, and oncogenic signaling in glioblastoma cells. Exploiting compensatory pathways of glutamine metabolism can improve the efficacy of glioblastoma treatments that impair glucose utilization.
5. Conclusions
For too long, patients have endured the aggressively lethal tumors of glioblastoma with little to no hope of survival. Finding new therapies to block the mitotic, metastatic, and pro-survival pathways activated in glioblastoma is therefore vital. ALK and MET activated pathways, among other RTKs, present promising targets for such inhibition. By inactivating pro-survival and proliferative transcriptional and translational activities induced by ALK and MET activation, new therapies stand a stronger chance of blocking tumor growth and progression in glioblastoma patients. However, the three-part cascade of STAT3, AKT, and Ras activation also presents numerous difficulties in modulating treatments. The interplay among cascade components both pre- and post-transcriptionally, as well as the intrinsically self-propagating nature of those components will require careful manipulation. Thus, multi-target therapies utilizing standard chemotherapuetics, like TMZ, and novel drugs must be combined with ALK/MET inhibitors in future studies. Future studies should investigate the impact of ALK/MET inhibitors on metabolic network as a whole including fatty acid, nucleotide and amino acid metabolism.
Finally, the varied presentation of tumors among patients also calls for further developments in targeted chemotherapy for glioblastoma tumors. Early efforts to target the most commonly altered genes (PDGFR, VEGF, EGFR, etc.) have met with frustratingly little success despite widespread presentation among patient tumors. While ALK/MET aberrations are far less common than EGFR mutations, it may be that these receptors play a larger role in mediating the effects of more commonly mutated RTK receptor activities in glioblastoma. RTKs in general remain promising therapeutic targets, but recent clinical trial setbacks and poorly understood RTK-RTK intracellular signaling dynamics will necessitate increased bench-side and bedside study.
Highlights.
Glioblastoma is a devastating, deadly prognosis for thousands each year.
ALK & MET pathways are integral to tumor survival, growth, and metastasis.
STAT3, Ras, and AKT are 3 key downstream effectors of ALK & MET.
New drugs targeting ALK & MET offer great promise for glioblastoma patients.
Acknowledgments
Completion of this project was made possible by funding from the National Institutes of Health (NIH) and National Institute of Neurological Disorders and Stroke (NINDS): (NS31622, NS-38146, NS-57811, and NS-41088), the State of South Carolina Spinal Cord Injury Research Project (SCSCIRF), Pfizer, Inc., and the Jerry Zucker Fund for Brain Tumor Research of the MUSC Foundation.
References
- Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson DS, Horvath CM. A road map for those who don't know JAK-STAT. Science. 2002;296:1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- Anandharaj A, Cinghu S, Park WY. Rapamycin-mediated mTOR inhibition attenuates survivin and sensitizes glioblastoma cells to radiation therapy. Acta biochimica et biophysica Sinica. 2011;43:292–300. doi: 10.1093/abbs/gmr012. [DOI] [PubMed] [Google Scholar]
- Arrieta O, Garcia E, Guevara P, Garcia-Navarrete R, Ondarza R, Rembao D, Sotelo J. Hepatocyte growth factor is associated with poor prognosis of malignant gliomas and is a predictor for recurrence of meningioma. Cancer. 2002;94:3210–3218. doi: 10.1002/cncr.10594. [DOI] [PubMed] [Google Scholar]
- Bajbouj K, Mawrin C, Hartig R, Schulze-Luehrmann J, Wilisch-Neumann A, Roessner A, Schneider-Stock R. P53-dependent antiproliferative and pro-apoptotic effects of trichostatin A (TSA) in glioblastoma cells. Journal of neuro-oncology. 2012;107:503–516. doi: 10.1007/s11060-011-0791-2. [DOI] [PubMed] [Google Scholar]
- Barre B, Vigneron A, Coqueret O. The STAT3 transcription factor is a target for the Myc and riboblastoma proteins on the Cdc25A promoter. The Journal of biological chemistry. 2005;280:15673–15681. doi: 10.1074/jbc.M413203200. [DOI] [PubMed] [Google Scholar]
- Batchelor TT, Duda DG, di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito R, Gil-Benso R, Quilis V, Perez M, Gregori-Romero M, Roldan P, Gonzalez-Darder J, Cerda-Nicolas M, Lopez-Gines C. Primary glioblastomas with and without EGFR amplification: relationship to genetic alterations and clinicopathological features. Neuropathology : official journal of the Japanese Society of Neuropathology. 2010;30:392–400. doi: 10.1111/j.1440-1789.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nature reviews. Molecular cell biology. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Boufaied N, Wioland MA, Falardeau P, Gourdeau H. TLN-4601, a novel anticancer agent, inhibits Ras signaling post Ras prenylation and before MEK activation. Anti-cancer drugs. 2010;21:543–552. doi: 10.1097/CAD.0b013e328337f373. [DOI] [PubMed] [Google Scholar]
- Campbell PM, Boufaied N, Fiordalisi JJ, Cox AD, Falardeau P, Der CJ, Gourdeau H. TLN-4601 suppresses growth and induces apoptosis of pancreatic carcinoma cells through inhibition of Ras-ERK MAPK signaling. Journal of molecular signaling. 2010;5:18. doi: 10.1186/1750-2187-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi F, Rabe DC, Bottaro DP. Targeting the HGF/Met signalling pathway in cancer. European journal of cancer. 2010;46:1260–1270. doi: 10.1016/j.ejca.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WS, Xu PZ, Gottlob K, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes & development. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi AS, Batchelor TT, Kwak EL, Clark JW, Wang DL, Wilner KD, Louis DN, Iafrate AJ. Rapid radiographic and clinical improvement after treatment of a MET-amplified recurrent glioblastoma with a mesenchymal-epithelial transition inhibitor. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:e30–33. doi: 10.1200/JCO.2011.38.4586. [DOI] [PubMed] [Google Scholar]
- Chiarle R, Simmons WJ, Cai H, Dhall G, Zamo A, Raz R, Karras JG, Levy DE, Inghirami G. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nature medicine. 2005;11:623–629. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- Chin LP, Soo RA, Soong R, Ou SH. Targeting ROS1 with Anaplastic Lymphoma Kinase Inhibitors: A Promising Therapeutic Strategy for a Newly Defined Molecular Subset of Non-Small-Cell Lung Cancer. J Thorac Oncol. 2012;7:1625–1630. doi: 10.1097/JTO.0b013e31826baf83. [DOI] [PubMed] [Google Scholar]
- Christensen JG, Zou HY, Arango ME, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Molecular cancer therapeutics. 2007;6:3314–3322. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, Vande Woude GF. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984;311:29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- Cooper LA, Gutman DA, Chisolm C, et al. The Tumor Microenvironment Strongly Impacts Master Transcriptional Regulators and Gene Expression Class of Glioblastoma. The American journal of pathology. 2012 doi: 10.1016/j.ajpath.2012.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W, Wilner KD. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:e443–445. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- Crosswell HE, Dasgupta A, Alvarado CS, Watt T, Christensen JG, De P, Durden DL, Findley HW. PHA665752, a small-molecule inhibitor of c-Met, inhibits hepatocyte growth factor-stimulated migration and proliferation of c-Met-positive neuroblastoma cells. BMC cancer. 2009;9:411. doi: 10.1186/1471-2407-9-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Maiello MR, D'Alessio A, Pergameno M, Normanno N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert opinion on therapeutic targets. 2012 doi: 10.1517/14728222.2011.639361. [DOI] [PubMed] [Google Scholar]
- de Pontual L, Kettaneh D, Gordon CT, et al. Germline gain-of-function mutations of ALK disrupt central nervous system development. Human mutation. 2011;32:272–276. doi: 10.1002/humu.21442. [DOI] [PubMed] [Google Scholar]
- Del Vecchio CA, Li G, Wong AJ. Targeting EGF receptor variant III: tumor-specific peptide vaccination for malignant gliomas. Expert review of vaccines. 2012;11:133–144. doi: 10.1586/erv.11.177. [DOI] [PubMed] [Google Scholar]
- Dirks WG, Fahnrich S, Lis Y, Becker E, MacLeod RA, Drexler HG. Expression and functional analysis of the anaplastic lymphoma kinase (ALK) gene in tumor cell lines. International journal of cancer. Journal international du cancer. 2002;100:49–56. doi: 10.1002/ijc.10435. [DOI] [PubMed] [Google Scholar]
- Easton RM, Cho H, Roovers K, et al. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Molecular and cellular biology. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nature reviews. Drug discovery. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- Fan QW, Cheng C, Hackett C, et al. Akt and autophagy cooperate to promote survival of drug-resistant glioma. Science signaling. 2010;3:ra81. doi: 10.1126/scisignal.2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraum TJ, Kreisl TN, Sul J, Fine HA, Iwamoto FM. Ischemic stroke and intracranial hemorrhage in glioma patients on antiangiogenic therapy. Journal of neuro-oncology. 2011;105:281–289. doi: 10.1007/s11060-011-0579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto J, Shiota M, Iwahara T, Seki N, Satoh H, Mori S, Yamamoto T. Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t(2;5) Proceedings of the National Academy of Sciences of the United States of America. 1996;93:4181–4186. doi: 10.1073/pnas.93.9.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariboldi MB, Ravizza R, Monti E. The IGFR1 inhibitor NVP-AEW541 disrupts a pro-survival and pro-angiogenic IGF-STAT3-HIF1 pathway in human glioblastoma cells. Biochemical pharmacology. 2010;80:455–462. doi: 10.1016/j.bcp.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Garofalo RS, Orena SJ, Rafidi K, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. The Journal of clinical investigation. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nature reviews. Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- Goudar RK, Shi Q, Hjelmeland MD, et al. Combination therapy of inhibitors of epidermal growth factor receptor/vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. Molecular cancer therapeutics. 2005;4:101–112. [PubMed] [Google Scholar]
- Grzelinski M, Steinberg F, Martens T, Czubayko F, Lamszus K, Aigner A. Enhanced antitumorigenic effects in glioblastoma on double targeting of pleiotrophin and its receptor ALK. Neoplasia. 2009;11:145–156. doi: 10.1593/neo.81040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guessous F, Zhang Y, diPierro C, Marcinkiewicz L, Sarkaria J, Schiff D, Buchanan S, Abounader R. An orally bioavailable c-Met kinase inhibitor potently inhibits brain tumor malignancy and growth. Anti-cancer agents in medicinal chemistry. 2010;10:28–35. doi: 10.2174/1871520611009010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerstrand D, Lindh MB, Pena C, Garcia-Echeverria C, Nister M, Hofmann F, Ostman A. PI3K/PTEN/Akt pathway status affects the sensitivity of high-grade glioma cell cultures to the insulin-like growth factor-1 receptor inhibitor NVP-AEW541. Neuro-oncology. 2010;12:967–975. doi: 10.1093/neuonc/noq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nature genetics. 2000;25:55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- Houillier C, Lejeune J, Benouaich-Amiel A, et al. Prognostic impact of molecular markers in a series of 220 primary glioblastomas. Cancer. 2006;106:2218–2223. doi: 10.1002/cncr.21819. [DOI] [PubMed] [Google Scholar]
- Hsieh A, Ellsworth R, Hsieh D. Hedgehog/GLI1 regulates IGF dependent malignant behaviors in glioma stem cells. Journal of cellular physiology. 2011;226:1118–1127. doi: 10.1002/jcp.22433. [DOI] [PubMed] [Google Scholar]
- Jin H, Yang R, Li W, Ogasawara AK, Schwall R, Eberhard DA, Zheng Z, Kahn D, Paoni NF. Early treatment with hepatocyte growth factor improves cardiac function in experimental heart failure induced by myocardial infarction. The Journal of pharmacology and experimental therapeutics. 2003;304:654–660. doi: 10.1124/jpet.102.041772. [DOI] [PubMed] [Google Scholar]
- Joo KM, Jin J, Kim E, et al. MET signaling regulates glioblastoma stem cells. Cancer Res. 2012;72:3828–3838. doi: 10.1158/0008-5472.CAN-11-3760. [DOI] [PubMed] [Google Scholar]
- Jun HT, Sun J, Rex K, Radinsky R, Kendall R, Coxon A, Burgess TL. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:6735–6742. doi: 10.1158/1078-0432.CCR-06-2969. [DOI] [PubMed] [Google Scholar]
- Kreisl TN, Lassman AB, Mischel PS, Rosen N, Scher HI, Teruya-Feldstein J, Shaffer D, Lis E, Abrey LE. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM) J Neurooncol. 2009;92:99–105. doi: 10.1007/s11060-008-9741-z. [DOI] [PubMed] [Google Scholar]
- Lal B, Goodwin CR, Sang Y, Foss CA, Cornet K, Muzamil S, Pomper MG, Kim J, Laterra J. EGFRvIII and c-Met pathway inhibitors synergize against PTEN-null/EGFRvIII+ glioblastoma xenografts. Molecular cancer therapeutics. 2009;8:1751–1760. doi: 10.1158/1535-7163.MCT-09-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3' kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Liu W, Fu Y, Xu S, Ding F, Zhao G, Zhang K, Du C, Pang B, Pang Q. c-Met expression is associated with time to recurrence in patients with glioblastoma multiforme. J Clin Neurosci. 2011;18:119–121. doi: 10.1016/j.jocn.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Mason WP, Belanger K, Nicholas G, Vallieres I, Mathieu D, Kavan P, Desjardins A, Omuro A, Reymond D. A phase II study of the Ras-MAPK signaling pathway inhibitor TLN-4601 in patients with glioblastoma at first progression. Journal of neuro-oncology. 2011 doi: 10.1007/s11060-011-0747-6. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, Yokota T. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. The EMBO journal. 1999;18:4261–4269. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott U, Iafrate AJ, Gray NS, et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer research. 2008;68:3389–3395. doi: 10.1158/0008-5472.CAN-07-6186. [DOI] [PubMed] [Google Scholar]
- McDowell KA, Riggins GJ, Gallia GL. Targeting the AKT pathway in glioblastoma. Current pharmaceutical design. 2011;17:2411–2420. doi: 10.2174/138161211797249224. [DOI] [PubMed] [Google Scholar]
- Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Molecular and cellular biology. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer research. 2004;64:6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. The American journal of pathology. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechkovsky DV, Prele CM, Wong J, et al. STAT3-Mediated Signaling Dysregulates Lung Fibroblast-Myofibroblast Activation and Differentiation in UIP/IPF. The American journal of pathology. 2012 doi: 10.1016/j.ajpath.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Polley MY, Lamborn KR, Chang SM, Butowski N, Clarke JL, Prados M. Conditional probability of survival in patients with newly diagnosed glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4175–4180. doi: 10.1200/JCO.2010.32.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AC, Vaillancourt RR. Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene. 1998;17:1343–1352. doi: 10.1038/sj.onc.1202171. [DOI] [PubMed] [Google Scholar]
- Pulford K, Lamant L, Espinos E, Jiang Q, Xue L, Turturro F, Delsol G, Morris SW. The emerging normal and disease-related roles of anaplastic lymphoma kinase. Cellular and molecular life sciences : CMLS. 2004a;61:2939–2953. doi: 10.1007/s00018-004-4275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulford K, Morris SW, Turturro F. Anaplastic lymphoma kinase proteins in growth control and cancer. Journal of cellular physiology. 2004b;199:330–358. doi: 10.1002/jcp.10472. [DOI] [PubMed] [Google Scholar]
- Rao SK, Edwards J, Joshi AD, Siu IM, Riggins GJ. A survey of glioblastoma genomic amplifications and deletions. Journal of neuro-oncology. 2010;96:169–179. doi: 10.1007/s11060-009-9959-4. [DOI] [PubMed] [Google Scholar]
- Samuel MS, Lourenco FC, Olson MF. K-Ras mediated murine epidermal tumorigenesis is dependent upon and associated with elevated Rac1 activity. PloS one. 2011;6:e17143. doi: 10.1371/journal.pone.0017143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- Shinojima N, Tada K, Shiraishi S, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer research. 2003;63:6962–6970. [PubMed] [Google Scholar]
- Shirogane T, Fukada T, Muller JM, Shima DT, Hibi M, Hirano T. Synergistic roles for Pim-1 and c-Myc in STAT3-mediated cell cycle progression and antiapoptosis. Immunity. 1999;11:709–719. doi: 10.1016/s1074-7613(00)80145-4. [DOI] [PubMed] [Google Scholar]
- Sinha S, Koul N, Dixit D, Sharma V, Sen E. IGF-1 induced HIF-1alpha-TLR9 cross talk regulates inflammatory responses in glioma. Cellular signalling. 2011;23:1869–1875. doi: 10.1016/j.cellsig.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Sonnenberg E, Meyer D, Weidner KM, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. The Journal of cell biology. 1993;123:223–235. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal SP, Hartley JW. Thymic lymphoma induction by the AKT8 murine retrovirus. The Journal of experimental medicine. 1988;167:1259–1264. doi: 10.1084/jem.167.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Stylianou DC, Auf der Maur A, Kodack DP, Henke RT, Hohn S, Toretsky JA, Riegel AT, Wellstein A. Effect of single-chain antibody targeting of the ligand-binding domain in the anaplastic lymphoma kinase receptor. Oncogene. 2009;28:3296–3306. doi: 10.1038/onc.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed DN, Afaq F, Sarfaraz S, Khan N, Kedlaya R, Setaluri V, Mukhtar H. Delphinidin inhibits cell proliferation and invasion via modulation of Met receptor phosphorylation. Toxicology and applied pharmacology. 2008;231:52–60. doi: 10.1016/j.taap.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szerlip NJ, Pedraza A, Chakravarty D, et al. Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3041–3046. doi: 10.1073/pnas.1114033109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernersson E, Khoo NK, Henriksson ML, Roos G, Palmer RH, Hallberg B. Characterization of the expression of the ALK receptor tyrosine kinase in mice. Gene expression patterns : GEP. 2006;6:448–461. doi: 10.1016/j.modgep.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2005;16:525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nature reviews. Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Ward MR, Agrotis A, Kanellakis P, Dilley R, Jennings G, Bobik A. Inhibition of protein tyrosine kinases attenuates increases in expression of transforming growth factor-beta isoforms and their receptors following arterial injury. Arteriosclerosis, thrombosis, and vascular biology. 1997;17:2461–2470. doi: 10.1161/01.atv.17.11.2461. [DOI] [PubMed] [Google Scholar]
- Weiss A, Brill B, Borghouts C, Delis N, Mack L, Groner B. Survivin inhibition by an interacting recombinant peptide, derived from the human ferritin heavy chain, impedes tumor cell growth. Journal of cancer research and clinical oncology. 2012 doi: 10.1007/s00432-012-1195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JW, Mahadevan D, Ellsworth R, Cooke L, Bearss D, Stea B. The c-Met receptor tyrosine kinase inhibitor MP470 radiosensitizes glioblastoma cells. Radiation oncology. 2009;4:69. doi: 10.1186/1748-717X-4-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen PY, Kesari S. Malignant gliomas in adults. The New England journal of medicine. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- Wong AJ, Ruppert JM, Bigner SH, Grzeschik CH, Humphrey PA, Bigner DS, Vogelstein B. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:2965–2969. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Bradley R, Kang L, et al. Hepatocyte growth factor (HGF) autocrine activation predicts sensitivity to MET inhibition in glioblastoma. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:570–575. doi: 10.1073/pnas.1119059109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Skaptason J, Romero D, Lee JH, Zou HY, Christensen JG, Koup JR, Smith BJ, Koudriakova T. Pharmacokinetic-pharmacodynamic modeling of biomarker response and tumor growth inhibition to an orally available cMet kinase inhibitor in human tumor xenograft mouse models. Drug metabolism and disposition: the biological fate of chemicals. 2008;36:1267–1274. doi: 10.1124/dmd.107.019711. [DOI] [PubMed] [Google Scholar]
- Zamo A, Chiarle R, Piva R, Howes J, Fan Y, Chilosi M, Levy DE, Inghirami G. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene. 2002;21:1038–1047. doi: 10.1038/sj.onc.1205152. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Chen S, You Z, Yang F, Carey TE, Saims D, Wang CY. Hepatocyte growth factor inhibits anoikis in head and neck squamous cell carcinoma cells by activation of ERK and Akt signaling independent of NFkappa B. The Journal of biological chemistry. 2002;277:25203–25208. doi: 10.1074/jbc.M201598200. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Wang LM, Jove R, Vande Woude GF. Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene. 2002;21:217–226. doi: 10.1038/sj.onc.1205004. [DOI] [PubMed] [Google Scholar]