Abstract
In preclinical research on pain and analgesia, noxious stimuli can stimulate expression of some behaviors (e.g. withdrawal reflexes) and depress others (e.g. feeding, locomotion, and positively reinforced operant responding). Tolerance to morphine antinociception is a robust and reliable phenomenon in preclinical assays of pain-stimulated behavior, but development of morphine tolerance in assays of pain-depressed behavior has not been studied. This study compared morphine antinociceptive tolerance in parallel assays of pain-stimulated and pain-depressed behavior in male Sprague-Dawley rats. Intraperitoneal injection of dilute lactic acid served as a noxious stimulus to stimulate a stretching response in one group of rats and to depress operant responding for electrical brain stimulation (intracranial self-stimulation; ICSS) in another group of rats. Antinociception produced by morphine (1.0 mg/kg) was determined after a regimen of chronic treatment with either saline or morphine in separate subgroups of rats in each procedure. In rats receiving chronic saline, acid alone stimulated a stretching response and depressed ICSS, and both acid effects were blocked by 1.0 mg/kg morphine. Rats receiving chronic morphine displayed hyperalgesic responses to the acid noxious stimulus in both procedures. Complete tolerance developed to morphine antinociception in the assay of acid-stimulated stretching, but morphine retained full antinociceptive effectiveness in the assay of acid-depressed ICSS. These results suggest that morphine antinociception in an assay of pain-depressed behavior is relatively resistant to tolerance. More broadly, these results suggest that antinociceptive tolerance can develop at different rates or to different degrees for different measures of antinociception.
Keywords: analgesia, antinociception, morphine, tolerance, intracranial self-stimulation
1. Introduction
Preclinical assays of nociception play a key role in research on both the neurobiology of pain and the development of novel analgesics. Sensitivity of these procedures to antinociceptive effects of mu opioid analgesics like morphine is important for claims of translational relevance, because opioids are among the most effective analgesics for pain treatment in humans (Max, 2003). In addition, these procedures are often used to investigate variables that influence expression of opioid antinociception and that might also modulate opioid analgesia. For example, a common finding in many preclinical procedures is the development of tolerance to opioid antinociception after regimens of repeated opioid treatment (Fernandes et al., 1977; Williams et al., 2013). This antinociceptive tolerance is typically viewed as an undesirable effect, and a large literature has been devoted to strategies for reducing opioid antinociceptive tolerance with the underlying rationale that reduction of tolerance would improve clinical utility (Garzon et al., 2008; Ueda and Ueda, 2009). However, there is weaker evidence from clinical studies to suggest that tolerance is a significant obstacle to the use of mu agonists to treat pain (Foley, 1995; Rosenblum et al., 2008). Although analgesic tolerance can occur, pain can be effectively managed in many patients with little or no change in opioid dose over time, and dose escalation is often attributed to factors other than pharmacodynamic tolerance, such as disease progression. Moreover, tolerance to opioid side effects such as sedation, nausea/emesis, and respiratory depression can improve the safety and tolerability of mu agonists for the treatment of pain (Benyamin et al., 2008; Labianca et al., 2012).
These observations suggest a potential discordance between the preclinical phenomenon of opioid antinociceptive tolerance and the clinical phenomenon of opioid analgesic tolerance. One potential basis for this discordance could be related to the dependent measures of pain and analgesia in preclinical vs. clinical studies. In human clinical contexts, the principal measure of pain is a verbal report, such as a visual analog scale (Hawker et al., 2011; Rauh et al., 2013; Schmitter et al., 2013). Different dependent measures are required in preclinical animal studies. For example, we have described “pain-stimulated behaviors” and “pain-depressed behaviors” as two categories of pain-related behavior in animals (Negus et al., 2006; Stevenson et al., 2006). Pain-stimulated behaviors are behaviors that increase in rate, frequency or intensity after delivery of a noxious stimulus, and common examples include tail withdrawal response from noxious thermal stimuli or writhing/stretching responses after intraperitoneal administration of irritants such as dilute acid. Conversely, pain-depressed behaviors are behaviors that decrease in rate, frequency or intensity after delivery of a noxious stimulus, and examples include pain-related reductions in feeding, locomotion, or rates of positively reinforced operant responding. One possibility is that tolerance develops at different rates or to different degrees for different measures of antinociception and/or analgesia.
To address this issue, the primary goal of the present study was to compare the development and expression of morphine tolerance in parallel assays of (1) a pain-stimulated behavior (stimulation of a stretching response), and (2) a pain-depressed behavior [depression of operant responding maintained by electrical brain stimulation in an assay of intracranial self-stimulation (ICSS)], elicited by a common noxious stimulus (intraperitoneal administration of dilute lactic acid) (Negus and Altarifi, 2013; Negus, 2013). We have shown previously that morphine produces dose-dependent and equipotent antinociception in both assays (Pereira Do Carmo et al., 2009; Altarifi et al., in press). The goal of this study was to assess the degree to which morphine tolerance might also be similar across these two assays.
2. Materials and Methods
2.1. Subjects
Twenty-four male Sprague–Dawley rats (Harlan, Frederick, Maryland, USA) weighing 310–350 g at the time of surgery were used. Rats were individually housed and maintained on a 12 h light/dark cycle, with lights on from 06:00 to 18:00 h. Rats had free access to food and water except during testing. Animal maintenance and research were in compliance with National Institutes of Health guidelines on care and use of animals in research, and all animal-use protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
2.2. Assay of intracranial self-stimulation (ICSS)
ICSS has proven to be a useful behavioral assay for preclinical research on expression and treatment of pain-depressed behavior, and the rationale and methods for use of ICSS to study pain-depressed behavior are discussed extensively elsewhere (Negus and Altarifi, 2013; Negus, 2013). For the present study, 13 rats were anesthetized with isoflurane gas (2.5–3% in oxygen; Webster Veterinary, Phoenix, Arizona, USA) for implantation of stainless steel electrodes (Plastics One, Roanoke, Virginia, USA). The cathode of each electrode was 0.25mm in diameter and covered with polyamide insulation except at the flattened tip, whereas the anode was 0.125mm in diameter and uninsulated. The cathode was implanted in the left medial forebrain bundle at the level of the lateral hypothalamus (2.8mm posterior to bregma, 1.7mm lateral from midsaggital suture, and 7.8mm below dura). The anode was wrapped around one of three skull screws to serve as the ground, and the skull screws and electrode assembly were secured to the skull with orthodontic resin. The subjects were allowed to recover for at least 7 days before commencing ICSS training.
2.2.1. Apparatus
Experiments were conducted in sound-attenuating boxes that contained modular acrylic test chambers (29.2 × 30.5 × 24.1 cm) equipped with a response lever (4.5 cm wide, extended 2.0 cm through the center of one wall, 3 cm off the floor), stimulus lights (three lights colored red, yellow, and green, positioned 7.6 cm directly above the response lever), a 2W white house light, and an ICSS stimulator (Med Associates, St. Albans, Vermont, USA). Electrodes were connected to the stimulator with bipolar cables routed through a swivel connector (Model SL2C, Plastics One). The stimulator was controlled by a computer and software that also controlled all the programming parameters and data collection (Med Associates).
2.2.2. Behavioral procedure
After initial shaping of lever-press responding, rats were trained under a fixed-ratio 1 (FR 1) schedule of brain stimulation using procedures similar to those described previously (Altarifi and Negus, 2011; Altarifi et al., 2012; Altarifi et al., 2013). During experimental sessions, each lever press resulted in the delivery of a 0.5-s train of square wave cathodal pulses (0.1 ms pulse duration), and stimulation was accompanied by the illumination of the stimulus lights over the lever. Responses during the 0.5 s stimulation period did not earn additional stimulation. During initial training sessions lasting 30–60 min, the frequency of stimulation was held constant at 158 Hz, and the stimulation intensity for each rat was adjusted gradually to the lowest value that would sustain a high rate of reinforcement (>30 stimulations/min). Once this criterion was met, frequency manipulations were introduced. Sessions involving frequency manipulations consisted of sequential 10-min components. During each component, a descending series of 10 current frequencies (158–56 Hz in 0.05 log increments) was presented, with a 60 s trial at each frequency. A frequency trial was initiated by a 5 s time-out followed by a 5 s ‘priming’ phase, during which subjects received five non-contingent stimulations with a 0.5 s interval between each stimulation. This non-contingent stimulation was then followed by a 50 s ‘response’ phase, during which responding produced electrical stimulation under the FR 1 schedule. Training continued with presentation of up to three sequential components per day, and the current intensity was again adjusted at this stage of training until rats reliably responded for the first three to four frequency trials of all components for at least three consecutive days. This intensity was then held constant for the remainder of the study.
2.2.3. Testing
Once training was completed, tests sessions were initiated. Behavioral tests were conducted and injections administered daily between 12:00 pm and 2:00 pm. Initially, all rats received a single injection of 1.8% lactic acid to confirm sensitivity to acid-induced depression of ICSS prior to further testing. Next, “pre-drug baseline” sessions were conducted over a period of three consecutive days to establish baseline ICSS performance before administration of any dose of morphine. Each pre-drug baseline session consisted of three ICSS components as described above. Rats were then divided into two groups that received either repeated morphine (N=6) or repeated vehicle (N=7) for seven consecutive days. Rats receiving repeated morphine were treated with 3.2 mg/kg/day on days 1 and 2, 5.6 mg/kg/day on days 3 and 4, and 10 mg/kg/day on days 5, 6, and 7. The control group received daily vehicle (saline) injections. Three ICSS components were conducted before each daily injection, and two additional ICSS components were conducted beginning 30 min after each injection. On days 8, 10, 12, and 14, all animals in both groups were tested with a sequence of four treatments: (1) morphine vehicle + acid vehicle, (2) morphine vehicle + 1.8% lactic acid, (3) 1.0 mg/kg morphine + acid vehicle, or (4) 1.0 mg/kg morphine + 1.8% lactic acid. Treatment order was counterbalanced across rats using a Latin-square design. On each test day, ICSS was evaluated during three baseline components, followed immediately by subcutaneous treatment with 1.0 mg/kg morphine or its vehicle, after which subjects were returned to their home cages. After 30 min, subjects were treated intraperitoneally with 1.8% lactic acid or its vehicle and returned to the ICSS chambers for two ICSS test components. Immediately after testing, subjects in the chronic morphine group received a supplemental injection of morphine (either 9 or 10 mg/kg) to maintain the total daily dose of 10 mg/kg/day. In addition, on the non-test days (i.e. Days 9, 11, and 13), animals were maintained on 10 mg/kg/day morphine or vehicle, and ICSS components were conducted before and after injections as on Days 1-7. Table 1 summarizes all the treatments over the two-week chronic experiment.
Table 1.
Summary table representing the daily treatments for each group in the study.
| Day | Chronic vehicle group | Chronic Morphine Group | |
|---|---|---|---|
| Daily treatment | Daily treatment | Supplemental treatment | |
| 1-2 | Saline | 3.2 morphine | N/A |
| 3-4 | Saline | 5.6 morphine | N/A |
| 5-7 | Saline | 10 morphine | N/A |
| 8 | Test | Test | 9-10 morphine |
| 9 | Saline | 10 morphine | N/A |
| 10 | Test | Test | 9-10 morphine |
| 11 | Saline | 10 morphine | N/A |
| 12 | Test | Test | 9-10 morphine |
| 13 | Saline | 10 morphine | N/A |
| 14 | Test | Test | N/A |
Morphine doses are shown in mg/kg. “Test” indicates a treatment with (1) morphine vehicle + acid vehicle, (2) morphine vehicle + 1.8% lactic acid, (3) 1.0 mg/kg morphine + acid vehicle, or (4) 1.0 mg/kg morphine + 1.8% lactic acid. Subjects in the chronic morphine group also received a supplemental treatment of morphine at the end of the test session on that day to maintain the total dose at 10 mg/kg/day. The chronic vehicle group did not receive any supplemental injections on test days.
2.2.4. Data analysis
The primary dependent measure was the total number of stimulations delivered across all 10 frequency trials of each component. The first ICSS component each day was considered to be a warm-up component, and data were discarded. Baseline ICSS in each subject was determined by averaging the number of stimulations per component during the second and third components across the three pre-drug baseline days before chronic treatment was initiated (6 total components). Baseline ICSS values in the chronic saline and morphine groups were compared by t-test. Data collected during chronic treatment and testing were then normalized to these baselines using the equation % Baseline Stimulations per Component = (Stimulations per Test Component /Baseline) × 100. Statistical analysis focused on data from the test components on Days 8, 10, 12 and 14. Data from the two test components on each test day were averaged within each rat and then across rats within a given treatment. Results were compared by two-way ANOVA, with acute treatment as a within-subject factor (1.0 mg/kg morphine or vehicle + 1.8% acid or vehicle), and chronic treatment as a between-subjects factor (chronic morphine or vehicle). A significant ANOVA was followed by the Bonferroni post-hoc test, and the criterion for significance was set a priori at P < 0.05.
To provide higher resolution analysis of ICSS performance at each brain stimulation frequency, an additional dependent variable was the reinforcement rate in stimulations/trial during each frequency trial. To normalize these raw data, reinforcement rates from each trial in each rat were converted to Percent Maximum Control Rate (%MCR) for that rat. The maximum control rate was determined for each rat during the pre-drug baseline sessions at the beginning of the experiment. As mentioned above, the first component from these sessions was considered to be an acclimation component, and data were discarded. The maximum control rate was defined as the mean of the maximal rates observed during any frequency trial of the second and third components of the three pre-drug baseline sessions (six total pre-drug baseline components). Subsequently, %MCR for each trial throughout the rest of the experiment was calculated as (Reinforcement Rate During a Frequency Trial ÷ Maximum Control Rate) × 100. Graphs show mean frequency-rate curves, with brain stimulation frequency on the abscissa, and ICSS rate expressed as %MCR on the ordinate.
Frequency-rate curves from test sessions were submitted for analysis. Within each chronic treatment group, ICSS test data were averaged across rats for each acute treatment, and treatment effects were compared by two-way ANOVA, with acute treatment as a within-subjects factor and ICSS frequency as a second within-subjects factor. A significant ANOVA was followed by a Holm-Sidak post hoc test, and the criterion for significance was set at P < 0.05.
2.3. Assay of acid-stimulated stretching
To evaluate stretching behavior, rats were placed into acrylic test chambers (31.0 × 20.1 × 20.0 cm) for 30-min observation periods. A stretch was operationally defined as a contraction of the abdomen followed by extension of the hind limbs, and the number of stretches during the observation period was counted. Initially, all rats were evaluated for 30 min after a single injection of 1.8% lactic acid to confirm sensitivity to acid-stimulated stretching prior to further testing. Subsequently, rats were divided into two groups that received either repeated morphine (N=6) or repeated vehicle (N=5) for 7 consecutive days. As in the assay of acid-depressed ICSS, rats receiving repeated morphine were treated with 3.2 mg/kg/day on days 1 and 2, 5.6 mg/kg/day on days 3 and 4, and 10 mg/kg/day on days 5, 6, and 7, whereas the control group received daily vehicle (saline) injections. To mimic handling conditions in ICSS rats, subjects were placed into a clean acrylic chamber for 30 mins before each daily injection, returned to their home cage for 30 min after each daily injection, and then placed back into the acrylic chamber for another 30 min. On days 8, 10, 12, and 14, all rats in both groups were tested with the same sequence of treatments that was tested in ICSS rats: (1) morphine vehicle + acid vehicle, (2) morphine vehicle + 1.8% lactic acid, (3) 1.0 mg/kg morphine + acid vehicle, or (4) 1.0 mg/kg morphine + 1.8% lactic acid. Treatment order was counterbalanced across rats using a Latin-square design. On each test day, rats were placed into the acrylic observation chamber for 30 min, followed immediately by subcutaneous treatment with 1.0 mg/kg morphine or its vehicle, and subjects were then returned to their home cages. After 30 min, subjects were treated intraperitoneally with 1.8% lactic acid or its vehicle and returned to the chamber for observation of stretching. Immediately after testing, subjects in the chronic morphine group received a supplemental injection of morphine to maintain the total daily dose of 10 mg/kg/day. In addition, on the non-treatment days (i.e. Days 9, 11, and 13), rats were maintained on 10 mg/kg/day morphine or vehicle and exposed to the acrylic chamber before and after injections as on Days 1-7.
2.3.1. Data analysis
The primary dependent variable was the number of stretches observed during the 30-min observation period after treatments on test days 8, 10, 12 and 14. Data for each treatment were averaged across rats and compared by two-way ANOVA, with acute treatment as a within-subjects factor (1.0 mg/kg morphine or vehicle + 1.8% acid or vehicle), and chronic treatment as a between-subjects factor (chronic morphine or vehicle). A significant ANOVA was followed by the Bonferroni post-hoc test, and the criterion for significance was set a priori at P < 0.05.
2.4. Drugs
Morphine sulfate was provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, Maryland, USA) and prepared in sterile saline for subcutaneous injection. Lactic acid was purchased from Sigma Aldrich (St. Louis, MO) and diluted in sterile water for intraperitoneal injection. All injections were delivered in a volume of 1.0 ml/kg.
3. Results
3.1. Assay of acid-stimulated stretching
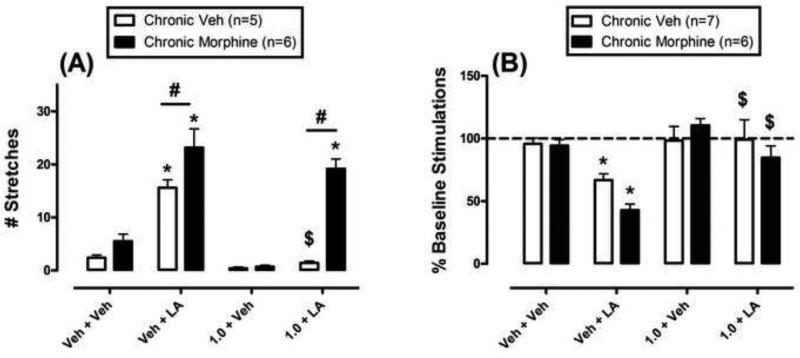
Fig. 1A shows effects of acute morphine/vehicle + acid/vehicle in rats treated chronically with either vehicle or morphine. In the chronic vehicle group (open bars), 1.8% lactic acid stimulated a stretching response, and 1.0 mg/kg morphine blocked acid-stimulated stretching while having no effect on stretching in the absence of the noxious stimulus. In the chronic morphine group, behavioral tests were conducted approximately 24hr after the most recent morphine injection. Under these conditions, 1.8% lactic acid still stimulated a stretching response, and the number of stretches was significantly greater in the chronic morphine group than in the chronic vehicle group. Moreover, tolerance developed to the antinociceptive effects of 1.0 mg/kg morphine, such that morphine no longer produced a significant decrease in acid-stimulated stretching. In addition, stretching after treatment with 1.0 mg/kg morphine + acid was significantly greater in the chronic morphine group than in the chronic vehicle group.
Fig. 1.
Effects of different acute treatments on stretching (A) and ICSS (B) in rats treated chronically with vehicle (open bars) or morphine (filled bars). A) Abscissa: acute treatment of 1.0 mg/kg morphine or vehicle + 1.8% lactic acid or vehicle. Ordinate: number of stretches during 30-min observation period. Two-way ANOVA showed that there was a significant main effect of acute treatment [F=45.4; P<0.001], significant main effect of chronic group [F=34.1; P<0.001], and significant treatment × chronic group interaction [F=9.7; P<0.001]. B) Abscissa: acute treatment of 1.0 mg/kg morphine or vehicle + 1.8% lactic acid or vehicle. Ordinate: percent baseline number of stimulations per component. Two-way ANOVA showed that there was a significant main effect of acute treatment [F=11.8; P<0.001], no significant main effect of chronic group [F=1.2; P=0.281], and no significant treatment × chronic group interaction [F=1.6; P=0.214]. * Asterisks indicate treatments that were significantly different from vehicle + vehicle within the same group. $ Dollar signs indicate a significant antinociceptive effect of morphine relative to the Veh+LA treatment within the same group. # Number signs indicate significance between chronic vehicle and chronic morphine groups after the same acute treatment.
3.2. Assay of acid-depressed ICSS
During baseline sessions, the mean ± S.E.M. total numbers of stimulations for the chronic vehicle and morphine groups were 339.8 ± 37.8 and 312.9 ± 57.9 stimulations per component, respectively (t=0.34, not significantly different). Fig. 1B shows the effects of different acute treatments on ICSS in rats treated chronically with vehicle or morphine. In the chronic vehicle group, 1.8% lactic acid significantly depressed ICSS, and 1.0 mg/kg morphine blocked acid-induced depression of ICSS while having no effect on ICSS in the absence of the noxious stimulus. In the chronic morphine group, 1.8% lactic acid also depressed ICSS. Although mean rates of ICSS were lower after acid in the chronic morphine group than in the chronic vehicle group, this difference was not statistically significant (but see below). Tolerance did not develop to morphine antinociception in the chronic morphine group. Thus, as in the chronic vehicle group, 1.0 mg/kg morphine blocked acid-induced depression of ICSS at a dose that did not significantly alter % Baseline Stimulations in the absence of the noxious stimulus. ICSS after 1.0 mg/kg morphine + acid was not different in the chronic vehicle and chronic morphine groups.
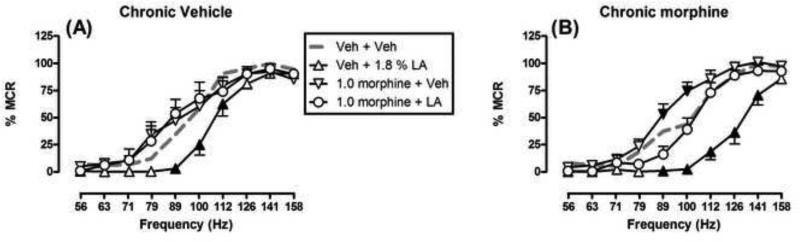
Fig. 2 shows the full rate-frequency curves after acute treatments in each group of ICSS rats. In both groups, brain stimulation maintained a frequency-dependent increase in ICSS rates, and 1.8% lactic acid produced significant depression of ICSS compared to vehicle + vehicle. However, acid depressed ICSS across a broader range of brain stimulation frequencies in the chronic morphine group (89-141 Hz) than in the chronic vehicle group (89-112 Hz). These data provide evidence for a greater nociceptive effect of acid in the chronic morphine group. Also in both groups, 1.0 mg/kg morphine completely blocked acid-induced depression of ICSS, such that rates of ICSS after 1.0 mg/kg morphine + acid were not different than rates after vehicle + vehicle at any brain stimulation frequency. When morphine was administered alone, it did not significantly alter ICSS in the chronic vehicle group; however, in the chronic morphine group, 1.0 mg/kg morphine did significantly increase ICSS rates at two frequencies (89-100 Hz).
Fig. 2.
Effects of different acute treatments on ICSS frequency-rate curves in rats treated chronically with chronic vehicle (A) or chronic morphine (B). Abscissae: Frequency of electrical brain stimulation in Hz (log scale). Ordinates: ICSS rate expressed as percent maximum control rate (%MCR). ANOVA results were as follows: Chronic vehicle: Significant main effect of frequency [F(9,54)=184.5; P<0.001], no significant main effect of treatment [F(3,18)=3.1; P=0.055], but significant treatment × frequency interaction [F(27,126)=2.4; P<0.001]. Repeated morphine: Significant main effect of frequency [F(9,45)=142.9; P<0.001], significant main effect of treatment [F(3,15)=22.8; P<0.001], and significant treatment × frequency interaction [F(27,135)=5.8; P<0.001]. Filled points show frequencies at which a treatment produced effects significantly different from vehicle + vehicle.
4. Discussion
The goal of this study was to compare tolerance to morphine antinociception in parallel assays of acid-stimulated stretching and acid-depressed ICSS. There were two main findings. First, intraperitoneal injection of dilute lactic acid produced both a stimulation of stretching and a depression of ICSS, and these acid effects were greater in the chronic morphine groups than in the chronic saline groups. Second, morphine at a dose of 1.0 mg/kg was effective to block acid-induced stimulation of stretching and depression of ICSS in the chronic vehicle groups. However, chronic morphine produced tolerance to morphine antinociception in the assay of acid-stimulated stretching but not in the assay of acid-depressed ICSS. These results suggest that morphine antinociception is relatively resistant to tolerance in the assay of acid-depressed ICSS.
The nociceptive effects of intraperitoneal lactic acid in this study agree with previous findings from our laboratory (Pereira Do Carmo et al., 2009; Negus et al., 2010). For example, we reported previously that intraperitoneal lactic acid is equipotent to stimulate a stretching response and to depress ICSS in rats (Pereira Do Carmo et al., 2009). Similarly, the antinociceptive effects of morphine observed here also agree with previous studies using morphine and other mu opioid analgesics (Pereira Do Carmo et al., 2009; Negus and Altarifi, 2013; Negus, 2013; Altarifi et al., in press). In particular, we have shown previously that morphine is equipotent to block both acid-stimulated stretching and acid-induced depression of ICSS in rats, with complete antinociception in both procedures at a morphine dose of 1.0 mg/kg (Pereira Do Carmo et al., 2009; Altarifi et al., 2014).
Chronic morphine enhanced the nociceptive effects of intraperitoneal acid. This agrees with previous reports of “opioid-induced hyperalgesia” following regimens of opioid exposure in assays of acid-stimulated stretching in mice (Li et al., 2014) and in other preclinical assays of pain-stimulated behavior (Dong et al., 2006; Ross et al., 2012; Wei and Wei, 2012). Hyperalgesia after repeated treatment with morphine or other mu opioid agonists has also been reported in humans (De Conno et al., 1991; Chu et al., 2008; Lee et al., 2011). The reliability and clinical relevance of opioid-induced hyperalgesia remain controversial (Ishii et al., 2013; Eisenberg et al., 2014); however, results of the present study suggest that preclinical evidence for opioid-induced hyperalgesia may also extend to measures of pain-related depression of behavior.
Opioid-induced hyperalgesia is often associated with opioid antinociceptive tolerance and may contribute to expression of opioid tolerance (Chu et al., 2008). Consistent with prior reports of this association, the regimen of repeated morphine treatment used in this study produced both an enhancement of acid-stimulated stretching and tolerance to the decrease in acid-stimulated stretching produced by 1.0 mg/kg morphine. This is consistent with previous publications showing tolerance to morphine antinociception in assays of acid-stimulated stretching in rats (Fernandes et al., 1977; Feng et al., 1994) and mice (Su et al., 2000), and it also agrees with evidence for morphine antinociceptive tolerance in other assays of pain-stimulated behavior, such as tail-flick and hot-plate procedures (Dong et al., 2006; Lilius et al., 2009; Lin et al., 2011; Ahmadi et al., 2014). However, dissociations between opioid-induced hyperalgesia and tolerance have also been reported in both preclinical and clinical studies (Juni et al., 2006; Chu et al., 2012), and the present results in the assay of acid-depressed ICSS provide another example of this dissociation. Although repeated morphine treatment enhanced the ICSS-depressing effects of i.p. acid, morphine retained its efficacy to block acid-induced depression of ICSS. Thus, this study provides an example of morphine-induced hyperalgesia without morphine antinociceptive tolerance.
Previous studies have documented different rates and degrees of tolerance development to different mu agonist effects. In preclinical studies, for example, tolerance develops more readily to morphine antinociception in conventional assays of pain-stimulated behavior than to other morphine effects such as constipation or respiratory depression (Ling et al., 1989; Paronis and Woods, 1997; Ross et al., 2008). The present study extends this general observation to identify differential development of tolerance to different measures of morphine antinociception. In addition, the resistance of morphine antinociception to tolerance in the assay of acid-depressed ICSS may be related to clinical findings that opioids often maintain analgesic efficacy both in human laboratory studies of experimental pain (Cooper et al., 2012) and in clinical treatment of chronic and severe pain (Cowan et al., 2001; Watson, 2012). Although opioid dose escalation is often necessary to maintain analgesic effectiveness of opioids (Collett, 1998), this loss of analgesia is often related to factors other than pharmacological tolerance, such as disease progression (Portenoy, 1994; Portenoy and Savage, 1997; Rosenblum et al., 2008). The translational relevance of preclinical assays of pain-depressed behavior for prediction of acute and chronic drug effects on pain in humans will benefit from further studies. For example, as one strategy to address this issue in preclinical research, future studies will examine the degree to which resistance to morphine antinociceptive tolerance in this assay of relatively acute pain-depressed behavior will extend to assays of more sustained pain-depressed behavior (Leitl et al., 2014).
Mechanisms that underlie resistance of morphine antinociception to tolerance in the assay of acid-depressed ICSS will require further study. Previous studies of morphine effects on ICSS in the absence of noxious stimulation may provide leads for future research on these mechanisms. For example, ICSS is one procedure that can be used to assess abuse potential of drugs, and drug-induced facilitation of low ICSS rates maintained by low brain stimulation frequencies or intensities is often interpreted as an abuse-related effect (Negus and Miller, 2014). Morphine and other mu opioid agonists are acknowledged drugs of abuse that can facilitate ICSS, but this facilitation is determined in part by histories of opioid exposure (Lorens and Mitchell, 1973; Altarifi and Negus, 2011; Altarifi et al., 2012; Altarifi et al., 2013). Thus, in opioid-naïve rats, morphine produces little or no facilitation of ICSS, and the primary effect of morphine is to depress ICSS. However, after various regimens of repeated opioid exposure, tolerance develops to the rate-decreasing effects of morphine, but rate-increasing effects of morphine are preserved or enhanced. This pattern was evident in the present study when 1.0 mg/kg morphine was administered without acid after chronic treatment with vehicle or morphine. In the chronic-vehicle group, rats were opioid-naïve, and 1.0 mg/kg morphine did not facilitate ICSS relative to the vehicle + vehicle control. Conversely, in the rats treated with chronic morphine, subsequent treatment with 1.0 mg/kg morphine did produce significant but weak facilitation of ICSS. One possible mechanism for this change in opioid effects is that repeated morphine may produce desensitization of mu receptors in brain stem regions that mediate rate-decreasing effects but not in ventral forebrain regions that mediate rate-increasing effects (Broekkamp et al., 1976; Sim et al., 1996). Morphine-induced antinociception in the assay of acid-depressed ICSS may be resistant to tolerance because it is also mediated through these desensitization-resistant receptors in ventral forebrain regions such as nucleus accumbens, Consistent with this possibility, morphine blocks not only acid-induced depression of ICSS, but also acid-induced depression of dopamine release in nucleus accumbens (Leitl et al., 2014). Regardless of precise mechanisms, though, the present results suggest that noxious stimulation can produce multiple changes in behavior, that morphine can modulate expression of these pain-related behaviors, and that tolerance to morphine effects on one type of pain-related behavior may not be accompanied by tolerance to morphine effects on other pain-related behaviors.
Acknowledgments
This research was supported by NIH grant R01 NS070715 and by training support from the Jordan University of Science and Technology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmadi S, Golbaghi H, Azizbeigi R, Esmailzadeh N. N-methyl-D-aspartate receptors involved in morphine-induced hyperalgesia in sensitized mice. Eur J Pharmacol. 2014;737:85–90. doi: 10.1016/j.ejphar.2014.04.048. [DOI] [PubMed] [Google Scholar]
- Altarifi A, Negus SS, Rice KC. Effects of Mu Opioid Receptor Agonists in Assays of Acute Pain-Stimulated and Pain-Depressed Behavior in Rats: Role of Mu Agonist Efficacy and Noxious Stimulus intensity. J Pharmacol Exp Ther. 2014 doi: 10.1124/jpet.114.219873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Miller LL, Negus SS. Role of mu-opioid receptor reserve and mu-agonist efficacy as determinants of the effects of mu-agonists on intracranial self-stimulation in rats. Behav Pharmacol. 2012;23(7):678–692. doi: 10.1097/FBP.0b013e328358593c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Negus SS. Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatmenttime, repeated treatment, and rate dependence. Behav Pharmacol. 2011;22(7):663–673. doi: 10.1097/FBP.0b013e32834aff54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Rice KC, Negus SS. Abuse-related effects of mu-opioid analgesics in an assay of intracranial self-stimulation in rats: modulation by chronic morphine exposure. Behav Pharmacol. 2013;24(5-6):459–470. doi: 10.1097/FBP.0b013e328364c0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Giaser SE, Vallejo R. Opioid compilations and side effects. Pain Physician. 2008;11(2 Suppl):S105–120. [PubMed] [Google Scholar]
- Broekkamp CL, Van den Bogaard JH, Heijnen HJ, Rops RH, Cools AR, Van Rossum JM. Separation of inhibiting and stimulating effects of morphine on self-stimulation behaviour by intracerebral microinjections. Eur J Pharmacol. 1976;36(2):443–446. doi: 10.1016/0014-2999(76)90099-6. [DOI] [PubMed] [Google Scholar]
- Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24(6):479–496. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- Chu LF, D'Arcy N, Brady C, Zamora AK, Young CA, Kim JE, Clemenson AM, Angst MS, Clark JD. Analgesic tolerance without demonstrable opioid-induced hyperaigesia: a double-blinded, randomized, placebo-controlled trial of sustained-release morphine for treatment of chronic nonradicular low-back pain. Pain. 2012;153(8):1583–1592. doi: 10.1016/j.pain.2012.02.028. [DOI] [PubMed] [Google Scholar]
- Collett BJ. Opioid tolerance: the clinical perspective. Br J Anaesth. 1998;81(1):58–68. doi: 10.1093/bja/81.1.58. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Sullivan MA, Vosburg SK, Manubay JM, Haney M, Foltin RW, Evans SM, Kowalczyk WJ, Saccone PA, Comer SD. Effects of repeated oxycodone administration on its analgesic and subjective effects in normal, healthy volunteers. Behav Pharmacol. 2012;23(3):271–279. doi: 10.1097/FBP.0b013e3283536d6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan DT, Allan LG, Libretto SE, Griffiths P. Opioid drugs: a comparative survey of therapeutic and “street” use. Pain Med. 2001;2(3):193–203. doi: 10.1046/j.1526-4637.2001.01026.x. [DOI] [PubMed] [Google Scholar]
- De Conno F, Caraceni A, Martini C, Spoldi E, Salvetti M, Ventafridda V. Hyperalgesia and myoclonus with intrathecal infusion of high-dose morphine. Pain. 1991;47(3):337–339. doi: 10.1016/0304-3959(91)90225-M. [DOI] [PubMed] [Google Scholar]
- Dong Z, Mao R, Han H, Cao J, Xu L. Morphine withdrawal modifies antinociceptive effects of acute morphine in rats. Biochem Biophys Res Commun. 2006;346(2):578–582. doi: 10.1016/j.bbrc.2006.05.151. [DOI] [PubMed] [Google Scholar]
- Eisenberg E, Suzan E, Pud D. Opioid-induced Hyperaigesia (OiH): A Real Clinical Problem or Just an Experimental Phenomenon? J Pain Symptom Manage. 2014 doi: 10.1016/j.jpainsymman.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Feng YZ, Tseng YT, Jaw SP, Hoskins B, Ho IK. Tolerance development to butorphanol: comparison with morphine. Pharmacol Biochem Behav. 1994;49(3):649–655. doi: 10.1016/0091-3057(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Kluwe S, Coper H. The development of tolerance to morphine in the rat. Psychopharmacology (Berl) 1977;54(2):197–201. doi: 10.1007/BF00426780. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Kluwe S, Coper H. Quantitative assessment of tolerance to and dependence on morphine in mice. Naunyn Schmiedebergs Arch Pharmacol. 1977;297(1):53–60. doi: 10.1007/BF00508810. [DOI] [PubMed] [Google Scholar]
- Foley KM. Misconceptions and controversies regarding the use of opioids in cancer pain. Anticancer Drugs. 1995;6(Suppl 3):4–13. doi: 10.1097/00001813-199504003-00002. [DOI] [PubMed] [Google Scholar]
- Garzon J, Rodriguez-Munoz M, Sanchez-Blazquez P. Do pharmacologicai approaches that prevent opioid tolerance target different elements in the same regulatory machinery? Curr Drug Abuse Rev. 2008;1(2):222–238. doi: 10.2174/1874473710801020222. [DOI] [PubMed] [Google Scholar]
- Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGiii Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S240–252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- Ishii H, Petrenko AB, Kohno T, Baba H. No evidence for the development of acute analgesic tolerance during and hyperaigesia after prolonged remifentanil administration in mice. Mol Pain. 2013:9–11. doi: 10.1186/1744-8069-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni A, Klein G, Kest B. Morphine hyperaigesia in mice is unrelated to opioid activity, analgesia, or tolerance: evidence for multipie diverse hyperalgesic systems. Brain Res. 2006;1070(1):35–44. doi: 10.1016/j.brainres.2005.11.054. [DOI] [PubMed] [Google Scholar]
- Labianca R, Sarzi-Puttini P, Zuccaro SM, Cherubino P, Vellucci R, Fornasari D. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin Drug investig. 2012;32(Suppl 1):53–63. doi: 10.2165/11630080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145–161. [PubMed] [Google Scholar]
- Leitl MD, Onvani S, Bowers MS, Cheng K, Rice KC, Carlezon WA, Jr., Banks ML, Negus SS. Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous kappa-opioids. Neuropsychopharmacology. 2014;39(3):614–624. doi: 10.1038/npp.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Potter DN, Cheng K, Rice KC, Carlezon WA, Jr., Negus SS. Sustained pain-related depression of behavior: effects of intraplantar formalin and complete freund's adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers in rats. Mol Pain. 2014:10–62. doi: 10.1186/1744-8069-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Tang M, Li H, Huang X, Chen L, Zhai H. Effects of ginsenosides on opioid-induced hyperalgesia in mice. Neuroreport. 2014;25(10):749–752. doi: 10.1097/WNR.0000000000000166. [DOI] [PubMed] [Google Scholar]
- Lilius TO, Rauhaia PV, Kambur O, Kalso EA. Modulation of morphine-induced antinociception in acute and chronic opioid treatment by ibudilast. Anesthesioiogy. 2009;111(6):1356–1364. doi: 10.1097/ALN.0b013e3181bdfa11. [DOI] [PubMed] [Google Scholar]
- Lin JA, Chen JH, Lee YW, Lin CS, Hsieh MH, Chang CC, Wong CS, Chen JJ, Yeh GC, Lin FY, Chen TL. Biphasic effect of curcumin on morphine tolerance: a preliminary evidence from cytokine/chemokine protein array analysis. Evid Based Complement Alternat Med. 2011:452153. doi: 10.1093/ecam/neq018. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling GS, Paul D, Simantov R, Pasternak GW. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci. 1989;45(18):1627–1636. doi: 10.1016/0024-3205(89)90272-5. [DOI] [PubMed] [Google Scholar]
- Lorens SA, Mitchell CL. Influence of morphine on laterai hypothalamic self-stimulation in the rat. Psychopharmacologia. 1973;32(3):271–277. doi: 10.1007/BF00422149. [DOI] [PubMed] [Google Scholar]
- Max MB. How to move pain and symptom research from the margin to the mainstream. J Pain. 2003;4(7):355–360. doi: 10.1016/s1526-5900(03)00719-3. [DOI] [PubMed] [Google Scholar]
- Negus S, S., Altarifi AA. American Chemical Society. Washington, DC: 2013. Mu, delta and kappa opioid agonist effects in novel assays of pain-depressed behavior, in: H. Ko and S. Husbands. Research and Development of Opioid-Related Ligands. pp. 163–176. [Google Scholar]
- Negus SS, Bilsky EJ, Pereira Do Carmo G, Stevenson GW. Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia. In: Szallasi A, editor. Methods in Molecuiar Biology:Analgesia. Humana Press; New York: 2010. pp. 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Expression and treatment of pain-related behavioral depression. Lab Anim (NY) 2013;42(8):292–300. doi: 10.1038/laban.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Miller LL. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev. 2014;66(3):869–917. doi: 10.1124/pr.112.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther. 2006;319(2):507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Woods JH. Ventilation in morphine-maintained rhesus monkeys. II: Tolerance to the antinociceptive but not the ventilatory effects of morphine. J Pharmacol Exp Ther. 1997;282(1):355–362. [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain. 2009;144(1-2):170–177. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portenoy RK. Tolerance to opioid analgesics: clinical aspects. Cancer Surv. 1994;21:49–65. [PubMed] [Google Scholar]
- Portenoy RK, Savage SR. Clinical realities and economic considerations: special therapeutic issues in intrathecal therapy--tolerance and addiction. J Pain Symptom Manage. 1997;14(3 Suppl):S27–35. doi: 10.1016/s0885-3924(97)00168-1. [DOI] [PubMed] [Google Scholar]
- Rauh KH, Andersen RS, Rosenberg J. [Visual analogue scale for measuring post-operative pain]. Ugeskr Laeger. 2013;175(24):1712–1716. [PubMed] [Google Scholar]
- Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16(5):405–416. doi: 10.1037/a0013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross GR, Gabra BH, Dewey WL, Akbarali HI. Morphine tolerance in the mouse ileum and coion. J Pharmacol Exp Ther. 2008;327(2):561–572. doi: 10.1124/jpet.108.143438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross GR, Gade AR, Dewey WL, Akbarali HI. Opioid-induced hypernociception is associated with hyperexcitability and altered tetrodotoxin-resistant Na+ channel function of dorsal root ganglia. Am J Physiol Cell Physiol. 2012;302(8):C1152–1161. doi: 10.1152/ajpcell.00171.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitter M, List T, Wirz S. [The assessment of pain intensity using one-dimensional scales]. Z Evid Fortbild Qual Gesundhwes. 2013;107(4-5):279–284. doi: 10.1016/j.zefq.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Dworkin SI, Childers SR. Effects of chronic morphine administration on mu opioid receptor-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci. 1996;16(8):2684–2692. doi: 10.1523/JNEUROSCI.16-08-02684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson GW, Bilsky EJ, Negus SS. Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. J Pain. 2006;7(6):408–416. doi: 10.1016/j.jpain.2006.01.447. [DOI] [PubMed] [Google Scholar]
- Su RB, Li J, Gao K, Pei G, Qin BY. Influence of idazoxan on analgesia, tolerance, and physical dependence of morphine in mice and rats in vivo. Acta Pharmacol Sin. 2000;21(11):1011–1015. [PubMed] [Google Scholar]
- Ueda H, Ueda M. Mechanisms underlying morphine analgesic tolerance and dependence. Front Biosci (Landmark Ed) 2009;14:5260–5272. doi: 10.2741/3596. [DOI] [PubMed] [Google Scholar]
- Watson CP. Opioids in chronic noncancer pain: more faces from the crowd. Pain Res Manag. 2012;17(4):263–275. doi: 10.1155/2012/495781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Wei W. Role of gabapentin in preventing fentanyl- and morphine-withdrawal-induced hyperalgesia in rats. J Anesth. 2012;26(2):236–241. doi: 10.1007/s00540-011-1272-7. [DOI] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ. Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65(1):223–254. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]