Abstract
Background
Although cognitive impairment is a core feature of bipolar disorder (BD) there is no instrument of choice for the assessment of bipolar patients. The aim of this study is to assess cognitive performance using the Brief Assessment of Cognition in Affective Disorders (BAC-A), a comprehensive test battery developed specifically for BD, and determine its suitability to estimate global functioning.
Methods
The BAC-A was administered to 93 BD patients (M±S. E: 35.18±1.39 years) and 56 healthy controls (HC - M±S.E.: 36.17±1.91 years). The scores of the BAC-A were combined in eight summary scores: visuomotor, immediate affective and non-affective memory, verbal fluency, delayed affective and non-affective memory, inhibition, and problem solving. Post hoc analyses were performed on subtests of the summary scores found to be significantly different between BD patients and HC. Correlational analyses explored the association between the Global Assessment of Functioning (GAF) score and cognitive functioning.
Results
Compared to HC, BD patients showed a significant impairment in short-term non-affective memory and verbal fluency. Poorer performance in verbal memory and verbal fluency summary scores correlated positively with reduced GAF.
Conclusions
Our results are consistent with previous reports of verbal memory and verbal fluency impairment in BD. The deficits in short-term memory and semantic fluency may indicate inefficient learning strategies and/or difficulties in retrieving information. The BAC-A could be used to estimate global functioning in BD patients.
Keywords: cognitive, bipolar disorder, psychometrics, verbal memory, verbal fluency, BAC-A
Introduction
Bipolar disorder (BD) is a devastating mental illness with deleterious functional and social consequences for both the affected individuals and their families [1]. In most BD patients mood dysregulation is accompanied by significant cognitive impairment that persists during the euthymic and acute phases [2-5]. Indeed BD patients perform poorly on tests of visuomotor processing speed, verbal memory, sustained attention and executive functioning. Impairments of smaller effect size in visual and verbal memory, working memory, and sustained attention have also been reported [4-8]. Overall manic patients perform worse than depressed and remitted individuals on verbal memory, verbal fluency, and cognitive estimation tasks [9, 10]. A recent study comparing BD I and BD II patients to healthy volunteers showed that BD I patients display significant deficits in phonetic fluency, inhibition and set shifting and increased psychomotor speed during the performance of planning tasks. They did not, however, found any significant differences in cognitive performance between BD I and BD II patients [11].
The presence of verbal memory impairment across mood phases suggests that these deficits may be stable markers for BD [12]. Moreover, there is evidence suggesting that illness duration and symptom severity have a negative impact on memory performance and executive functioning [13-15]. In particular, a high number of mood episodes has been shown to be a predictor of poor performance in higher order cognitive domains [16, 17].
A methodological limitation of current cognitive studies in BD is related to the use of tests that differ in terms of content, duration, psychometric properties, and administration procedures. The Canadian Network for Mood and Anxiety Treatments (CANMAT) and the International Society for Bipolar Disorders (ISBD) have emphasized the need for instruments assessing cognitive domains found to be impaired in BD, such as verbal and working memory, psychomotor speed, executive function and attention [18]. However, to date, there is no established instrument of choice for the cognitive assessment of BD patients.
The Brief Assessment of Cognition in Affective Disorders (BAC-A) is a newly developed cognitive instrument for BD patients [19]. Compared to other batteries used in clinical research such as the Cambridge Neuropsychological Test Automated Battery (CANTAB), the BAC-A is a time-efficient, pencil-and-paper instrument that aims to assess cognitive deficits specific to BD. The BAC-A comprises eight tasks evaluating visuomotor abilities, working memory, learning and declarative memory, attention, verbal fluency, problem solving, affective interference and affective inhibition. A recent multi-site study has established that the BAC-A has strong psychometric properties in patients with BD Type I compared to HC [19]. In this study BD patients performed poorly in all subtests, and the affective auditory verbal learning test was found to discriminate the best between BD and HC. However, the version of the BAC-A used in Keefe et al.'s study did not include the Emotional Stroop test.
In summary, the suitability of the full version of the BAC-A for assessing cognitive functioning across subtypes of the bipolar illness remains to be explored, and knowledge on the relationship between the BAC-A scores and global functioning is limited. To address these research questions we administered the BAC-A in a population of adults with BD and healthy controls and investigated the relationship between BAC-A scores and the Global Assessment of Functioning (GAF) score.
Methods
Participants
The BAC-A was administered to 90 euthymic BD outpatients (59 BD I, 28 BDII, 3 BD NOS, M±S.E. 35.18±1.39 years, 56 women/34men) and 5 6 healthy controls (HC - M±S.E.: 36.17±1.91 years, 36 women/20 men). 58 BD patients were on psychiatric medication (atypical antipsychotics, antidepressants, anticonvulsants, stimulants) at the time of assessment. Healthy participants were recruited via oral presentations and flyers. All participants underwent the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders Axis I (SCID I) to confirm or rule out the diagnosis of BD. Functional impairment was evaluated using the Global Assessment of Functioning (GAF) which is the Axis V of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision (DSM-IV-TR) [20]. Specific inclusion criteria for healthy controls were: no current or lifetime axis I psychiatric comorbidities, previous history of neurologic disorders including head injury with loss of consciousness for any period of time, pregnancy, family history of hereditary neurologic disorder, psychiatric disorder in first-degree relatives, use of any prescribed psychiatric medication in their lifetimes. Current mood phase was assessed using the Montgomery–Åsberg Depression Rating Scale (MADRS) and Young Mania Rating Scale (YMRS) (Table 1). Premorbid intellectual quotient (IQ) was estimated by the reading test of the Wide Range Achievement Test-4 (WRAT-4) [21] and the Wechsler Abbreviated Scale of Intelligence - IV (WASI-IV) [22]. The study protocol was approved by the Institutional Review Board and informed consent was obtained from all the participants.
Table 1. Demographic and clinical characteristic of the sample.
| Group | N (BD/HC) | Healthy Controls | Bipolar disorder | p/X2 |
|---|---|---|---|---|
| (Mean ± S.E) | ||||
| Gender Male/female, N | 93/56 | 20/36 | 34/56* | .38 |
| Age | 92/56 | 36.17±1.91 | 35.18±1.39 | .56 |
| Education (years) | 84/55 | 16.01±.35 | 14.33±.31 | .001 |
| WRAT Reading | 90/47 | 111.674±2.28 | 104.83±1.57 | .01 |
| WASI Vocabulary | 92/54 | 58.37±1.54 | 54.79±1.31 | .08 |
| WASI Matrix | 92/54 | 56.94±.92 | 55.91±.82 | .42 |
| Full Scale IQ (WASI) | 92/54 | 113.61±1.79 | 109.5±1.5 | .08 |
| YMRS | 39/16 | .88±.48 | 5.53±.86 | .00 |
| MADRS | 76/42 | 1.09±.7 | 14.84±1.27 | .00 |
| GAF | 79/41 | 89.31±1.4 | 62.90±1.4 | .00 |
| Lifetime number of depressive episodes | 61/54 | - | 13.39±2.76 | - |
| Lifetime number of manic episodes | 68/54 | - | 3.88±.88 | - |
Abbreviations: WRAT=Wide Range Achievement Test; WASI=Wechsler Abbreviated Scale: WASI; IQ=Intellectual Quotient; YMRS=Young Mania Rating Scale; Montgomery Åsberg Depression Rating Scale (MADRS); Global Assessment of Functioning (GAF).
3 missing values
BAC-A
The BAC-A is based on the Brief Assessment of Cognition in Schizophrenia (BAC-S). The BAC-S has been validated both linguistically and psychometrically in a number of psychiatric populations, including patients with schizophrenia and BD [23-27]. It has been shown to be as valid and sensitive as a traditional neuropsychological assessment and takes approximately 35 minutes to administer [28, 29]. Six of the 8 subtests of the BAC-A match those found in the BAC-S. These tests are the Token Motor Task, Symbol Coding, List Learning, Digit Sequencing Task, Category Instances (Animals) and Controlled Oral Word Association Test (F and S-words), Tower of London [29]. In addition to these 6 subtests the BAC-A comprises the Emotion Inhibition Test (a modified version of the Emotional Stroop task [30, 31]) and the affective auditory verbal learning test (Affective interference test). The latter task is similar to the Affective Auditory Verbal Learning Test (AAVLT) [32].
Affective interference [19]
In this task participants are given four trials containing 10 non-affective words (fruits and vegetables) and 10 affective words (e.g. “cancer,” “triumphant,” “enraged”). After a 20-minute delay, recognition memory is tested by presenting the initial 20 words (10 emotional and 10 fruits and vegetables) along with 20 words that had not been presented earlier. This task evaluates components of immediate and delayed affective and non-affective memory.
Emotion Inhibition Test
Participants are presented with sheets of papers with 4 columns of words of either neutral (e.g. method, cow) or affective polarity (e.g. afraid, happy, stress) in colored (red, blue, green and yellow) or black ink. They are then instructed to either read the words (Word naming) or the color of the words (Color naming) going down the columns. Participants are given 30 seconds to read as many words as they can on each page. The goal of this task is determine the individual's ability to suppress irrelevant stimuli and read a word whose meaning identifies a different color from the color of presentation of the word (Interference). The Affective interference index is calculated by subtracting the number of correct responses to emotional words during the Color naming condition from the number of correct responses to emotional words during the Word naming condition. The Affective interference index was adjusted for the number of correctly identified neutral words during the “Word” and “Color naming” conditions. The following formula was used: (Emotional Word Naming divided by Word Naming) minus (Emotional Color Naming divided by Color naming).
The scores of the BAC-A were standardized by creating z-scores (M±SD: 0±1) using the entire sample of patients and controls and then summarized in eight cognitive domains: visuomotor (number of correct responses on the Symbol coding and Token Motor tasks), short-term affective memory (number of correctly recalled words during the affective learning trials of the Affective Interference test), short-term non-affective memory (number of correct words during the non-affective learning trials of the List Learning and Affective Interference tests and number of correct answers on the Digit Sequencing task), delayed affective memory (number of correct words during the delayed free recall of affective words of the Affective Interference test), delayed non-affective memory (number of correct words during the delayed free recall of non-affective words of the Affective Interference test), fluency (number of correct responses on the Category and Controlled Oral Word Association tests), inhibition (Affective interference index of the Emotion Inhibition Test) and problem solving (number of correct responses on the Tower of London test). The total score of the BAC-A was calculated by summing the standardized scores of the eight cognitive domains.
Statistical analysis
Statistical analyses were performed using SPSS Statistics (IBM - version 21) and SAS v. 9.3 (SAS Institute Inc., Cary, N.C.). Demographic characteristics and cognitive scores of the BAC-A of BD patients and healthy controls were compared using χ2 and univariate analyses. SAS PROC MEANS and PROC FREQ were used to screen the data. Tests of normality, linearity, multicollinearity and homogeneity of covariance assumptions were performed. The cognitive profile of BD patients and HC was compared using a profile analysis – a multivariate alternative to repeated measure ANOVAs - with Age included as a covariate. This type of analysis accounts for correlations between variables (in this case the BAC-A scores) and is treated as an individual test. Thus, multiple comparison corrections are not needed. Post-hoc t-test analyses were performed to compare individual BAC subscale scores between BD and HC using an FDR-corrected statistical threshold (p=0.0064). A logistic regression model adjusted for age was used to predict diagnostic group from the total score of the BAC-A. Pearson's bivariate correlational analyses were performed to examine the strength of the relationship between the summary scores of the BAC-A and the GAF scores.
Results
Demographics and clinical description
Demographics and clinical features for BD and HC are reported in Table 1. There was no significant difference in age and gender between the two groups. However, educational attainment and pre-morbid IQ estimated by the Reading component of the Wide Range Achievement Test (WRAT) were significantly reduced in BD patients compared to HC.
Summary scores of the BAC-A
As illustrated in Figure 1 the profile analysis of the summary scores of the BAC-A revealed a significant group effect across cognitive domains (Wilk's Lambda λ = 0.87, F(7,145) = 5.21, p=0.02)), with significantly reduced performance in BD patients on short-term non-affective memory (p = .02, η2 =.05) and verbal fluency (p = .03, η2 = .04). The standardized BAC-A total score was comparable across groups (HC – M (SD) 1.15 (3.90); BD – M (SD) = -0.62 (4.54). The full logistic regression model based on the BAC-A total score did not fit the data (χ2= 5.704, p = 0.057). There was a trend toward a negative association between the BAC-A total score and BD (b = -0.97, p = 0.02) with odds ratio of 0.907 (95% CI = [0.832,0.989]) meaning that for every one unit increase in BAC-A total score, the odds of belonging to the BD group decreased by a factor of 0.907.
Figure 1.
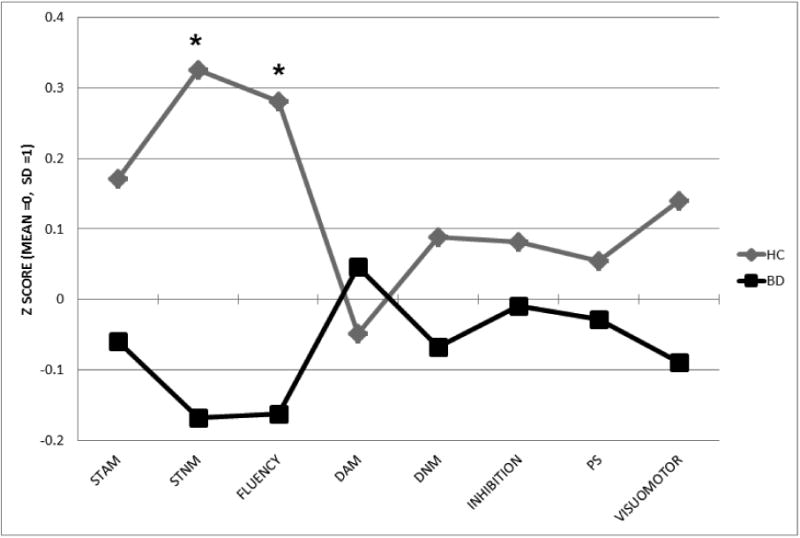
Composite z-scores of the BAC-A scale. Abbreviations: STAM = short-term affective memory, STNM = short-term non-affective memory, fluency = verbal fluency, DAM = delayed affective memory, DNM = delayed non-affective memory, PS = problem solving in bipolar patients (BD) and healthy controls (HC). *p<.05
Subtests of the BAC-A
Secondary profile analyses on subtests of the short-term non-affective memory and verbal fluency summary scores showed that the performance of BD patients on all learning trials of the List Learning test, and non-affective learning trials 3 and 4 of the Affective Interference test was impaired when compared to that of HC (Wilk's Lambda λ=0.88, F(9,145)=9.36, p=0.002). In terms of verbal fluency (Wilk's Lambda λ=0.98, F(2,145)=6.96, p=0.009), BD patients generated fewer animal names than HC (p =0.005, η2=.06). Performance on phonemic fluency tasks (F and S-words) was comparable across groups (Table 1 and Figure 1S).
Correlations between cognitive functioning and clinical characteristics
Correlational analyses showed that the short-term non-affective memory summary score correlated positively with GAF scores (r = .26, p<.01). The short-term affective memory summary score correlated positively with the GAF score (r = 0.183, p<.05) (Table 1S and Figure 2). The coefficients of correlation between the GAF and the other BAC-A summary scores were not statistically significant.
Figure 2.
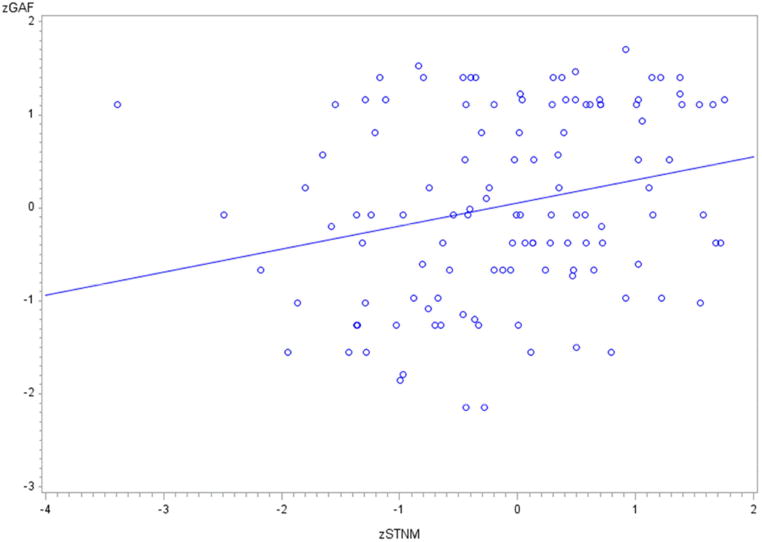
Scatterplot showing the relationship between standardized scores of short-term non-affective memory (zSTNM) and scores of Global Assessment of Functioning (GAF)
Discussion
The current study examined the cognitive functioning of BD patients and HC using the BAC-A, a comprehensive cognitive battery with strong psychometric properties specifically designed for BD [19]. Compared to HC our BD patients demonstrated impaired short-term non-affective memory and verbal fluency impairment. These results are consistent with Bora et al.'s meta-analytical review of 12 cognitive studies that found impairments of large effect size in verbal memory and word generation in BD [33]. However, in contrast to our findings, current literature points towards deficits in both immediate and delayed recall [4, 8]. The divergent findings may be due to the clinical characteristics of our sample. For instance, the higher level of memory performance among BD patients may be explained by the fact that the BD participants were euthymic, medicated at the time of the assessment and had experienced a higher number of depressive episodes than manic episodes. Indeed, a study showed that manic patients present with greater impairments in verbal memory, verbal fluency and cognitive estimation skills when compared with depressed and remitted patients [9]. Another study found that relative to hypomanic, depressive and euthymic BD patients, manic patients present with extensive deficits in immediate and delayed verbal and visual memory (measured by the California Verbal Learning Test – CVLT and the logical memory and visual reproduction subtests of the Wechsler Memory Scale) [34]. Furthermore, our clinical sample was heterogeneous and included patients with BD Types I, II and NOS.
It is noteworthy that BD patients produced fewer animal words than HC. Deficits in verbal fluency have been consistently reported in the literature on BD and psychotic disorders [35]. Verbal fluency has been linked to executive dysfunction [63] and inefficient information retrieval techniques [64][34]. Further, findings of a neuropsychological study in older individuals suggested that while phonological fluency tasks require strategic planning, category fluency tasks are affected by the participant's verbal abilities [36]. Given the participants' preserved vocabulary scores on the WASI, the present results do not, however, appear to support the latter hypothesis. A thorough cognitive assessment is not only essential for diagnostic purposes but is also crucial to develop an appropriate cognitive and functional rehabilitation plan. In our sample higher short-term verbal memory abilities were associated with greater global functioning and reduced severity of manic and depressive symptoms. This result is consistent with a recent study showing that verbal fluency and verbal memory are positive predictors of occupational and psychosocial functioning as measured by the GAF score [8].
The exploration of the demographic differences between HC and BD participants showed a reduced mean IQ score and lower education levels in BD patients compared to HC. This finding is intriguing as, to date, only a small number of studies has explored the link between IQ, education and mental illness. A study in Swedish conscripts aged 43-54 years found that a low premorbid IQ was associated with increased risk of developing psychotic illnesses, but not BD [37]. Another study in male inpatients suffering from either BD or unipolar depression showed that while patients with major depression exhibited a lower IQ -approximately 2-3 points below the mean IQ of HC – BD patients had an IQ comparable to HC. Notably, the depressed group had completed higher levels of education than the BD group [38]. These findings suggest that the neurocognitive development of BD patients may differ from that of depressed patients. However, since we used the WRAT and the WASI to estimate premorbid IQ and previous studies used non-standardized tests of intellectual functioning such as the Børge Priens Prøve (BPP) [38] these results may not be directly comparable.
A strength of the BAC-A battery is certainly its time efficiency and focus on cognitive domains that have been recommended by the ISBDD and CANMAT to be assessed in BD patients such as psychomotor accuracy (e.g. Token Motor Test), executive functions (e.g. verbal fluency, Tower of London test), verbal memory (e.g. List learning) and working memory (Digit sequencing task). Furthermore, our findings show that the BAC-A battery detects impairments in short-term verbal memory and verbal fluency and may be able to assist in establishing the individual's overall functioning.
A potential limitation of the BAC-A is the absence of measures of reaction times for psychomotor and executive function tasks. Indeed while accuracy levels indicate whether the individual attends to relevant stimuli and is able to discard unnecessary stimuli, reaction times provide insight into the patients' motor and cognitive speed [39]. Measures of accuracy and cognitive speed could, for instance, help determine if disruptions occur at the early stages of the sensory processing or are rather due to impaired high order functions such as attention and inhibition. Furthermore, the ratio between reaction times and response accuracy would have provided information on how efficiently patients planned their moves on the Tower of London task. One could also argue that the analyses of the 8 subdomains of the BAC-A were not optimal because they are not subscales and are single items. Thus, our methodological approach may have blurred the concepts of “subscale” and “test”. Further, since these subscales have not been psychometrically validated, the reliability of each subscale measure may be limited. In conclusion, owing to its brevity and sensitivity to domains that have been shown to be impaired in BD, the BAC-A has the potential to become an important tool for evaluating cognitive functioning in BD and may enable predicting long-term global functioning in adults with BD. Despite promising findings, a psychometric validation on a larger and more heterogeneous sample, and assessments enabling test-retest reliability measurement are recommended.
Supplementary Material
Table 1S: Coefficients of correlation between BAC-A summary scores and Global Assessment of Functioning (GAF) scores. Abbreviations: STAM = short-term affective memory, STNM = short-term non-affective memory, fluency = verbal fluency, DAM = delayed affective memory, DNM = delayed non-affective memory, PS = problem solving (PS), and attention in bipolar patients (BD) and healthy controls (HC). *p<.05, **p<.01
Figure 1S: Performance of BD patients relative to HC on subtests of the short-term non-affective memory (STNM) and verbal fluency of the BAC-A scale in bipolar patients (BD) and healthy controls (HC). *<.05. **p.001.
Highlights.
We used the BAC-A, a BD-specific cognitive battery with strong psychometric properties
BD patients exhibited deficits in short-term memory and verbal fluency
Reduced cognitive functioning was associated with reduced global functioning
BD patients recalled affective words better than non-affective words
Acknowledgments
Role of funding source: This work was partly supported by the Stanley Medical Research Institute, NIH grant MH 085667 (JCS), and by the Pat Rutherford Jr. Chair in Psychiatry (UTHealth).
Dr. Sanches has served on the speakers' bureau for Astra Zeneca and has received research support from Janssen. This work was partly supported by 1R01MH085667 and Pat Rutherford, Jr Chair in Psychiatry at UTHealth.
Dr Keefe currently or in the past 3 years has received investigator-initiated research funding support from the Brain Plasticity, Inc., Department of Veteran's Affair, Feinstein Institute for Medical Research, GlaxoSmithKline, National Institute of Mental Health, Novartis, PsychoGenics, Research Foundation for Mental Hygiene, Inc., and the Singapore National Medical Research Council. He currently or in the past 3 years has received honoraria, served as a consultant, or advisory board member for Abbvie, Akebia, Amgen, Astellas, Asubio, AviNeuro/ChemRar, BiolineRx, Biogen Idec, Biomarin, Boehringer-Ingelheim, Eli Lilly, EnVivo, GW Pharmaceuticals, Helicon, Lundbeck, Merck, Minerva Neurosciences, Inc., Mitsubishi, Novartis, Otsuka, Pfizer, Roche, Shire, Sunovion, Takeda, Targacept. Dr. Keefe receives royalties from the BACS and BAC-A testing batteries and the MATRICS Battery (Symbol Coding). He is also a shareholder in NeuroCog Trials, Inc. and Sengenix.
Professor J. C. Soares has received grants/research support from Forrest, BMS, Merck, Stanley Medical Research Institute, NIH 69774 and has been a speaker for Pfizer and Abbott.
Footnotes
Contributors: IB wrote the first draft of the manuscript; RK, MS and JCS designed the study and managed data collection; IB, RS and CG analysed the data; RK, MS and JCS revised and approved the final version of the manuscript.
Conflict of interest: Drs Bauer, Suchting and Green have no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Isabelle E. Bauer, University of Texas Health Science Center at Houston, Department of Psychiatry and Behavioral Sciences, 77054 Houston, TX, United States
Richard S. E. Keefe, Division of Medical Psychology, Duke University, Medical Centre, 27710 Durham, NC, United States
Marsal Sanches, University of Texas Health Science Center at Houston, Department of Psychiatry and Behavioral Sciences, Houston, TX, United States.
Robert Suchting, University of Texas Health Science Center at Houston, Department of Psychiatry and Behavioral Sciences, 77054 Houston, TX, United States.
Charles E. Green, University of Texas Health Science Center at Houston, Department of Psychiatry and Behavioral Sciences, 77054 Houston, TX, United States
Jair C. Soares, University of Texas Health Science Center at Houston, Department of Psychiatry and Behavioral Sciences, 77054 Houston, TX, United States
References
- 1.Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. The Lancet. 2013;381(9878):1672–1682. doi: 10.1016/S0140-6736(13)60857-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.APA. Practice guideline for the treatment of patients with bipolar disorder (revision) Am J Psychiatry. 2002:159. [PubMed] [Google Scholar]
- 3.MacQueen GM, Young LT, Joffe RT. A review of psychosocial outcome in patients with bipolar disorder. Acta Psychiatrica Scandinavica. 2001;103(3):163–170. doi: 10.1034/j.1600-0447.2001.00059.x. [DOI] [PubMed] [Google Scholar]
- 4.Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of affective disorders. 2009;113(1):1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Quraishi S, Frangou S. Neuropsychology of bipolar disorder: a review. Journal of affective disorders. 2002;72(3):209. doi: 10.1016/s0165-0327(02)00091-5. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg TE, et al. Contrasts between patients with affective disorders and patients with schizophrenia on a neuropsychological test battery. Am J Psychiatry. 1993;150(9):1355–62. doi: 10.1176/ajp.150.9.1355. [DOI] [PubMed] [Google Scholar]
- 7.Albus M, et al. Contrasts in neuropsychological test profile between patients with first-eoisode schizophienia and first-episohe affective disorders. Acta Psychiatrica Scandinavica. 1996;94(2):87–93. doi: 10.1111/j.1600-0447.1996.tb09830.x. [DOI] [PubMed] [Google Scholar]
- 8.Martínez-Arán A, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. American Journal of Psychiatry. 2004;161(2):262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- 9.Dixon T, et al. Effect of symptoms on executive function in bipolar illness. Psychological medicine. 2004;34(5):811–821. doi: 10.1017/s0033291703001570. [DOI] [PubMed] [Google Scholar]
- 10.Aminoff SR, et al. Neurocognitive features in subgroups of bipolar disorder. Bipolar disorders. 2013;15(3):272–283. doi: 10.1111/bdi.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pålsson E, et al. Neurocognitive function in bipolar disorder: a comparison between bipolar I and II disorder and matched controls. BMC psychiatry. 2013;13(1):165. doi: 10.1186/1471-244X-13-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gualtieri CT, Johnson LG. Comparative neurocognitive effects of 5 psychotropic anticonvulsants and lithium. Medscape General Medicine. 2006;8(3):46. [PMC free article] [PubMed] [Google Scholar]
- 13.Cavanagh J, et al. Case—control study of neurocognitive function in euthymic patients with bipolar disorder: an association with mania. The British Journal of Psychiatry. 2002;180(4):320–326. doi: 10.1192/bjp.180.4.320. [DOI] [PubMed] [Google Scholar]
- 14.Martínez-Arán A, et al. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar disorders. 2004;6(3):224–232. doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- 15.Bearden CE, et al. Patterns of memory impairment in bipolar disorder and unipolar major depression. Psychiatry Research. 2006;142(2):139–150. doi: 10.1016/j.psychres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Camelo EV, et al. Attention impairment in bipolar disorder: a systematic review. Psychology & Neuroscience. 2013;6(3):299–309. [Google Scholar]
- 17.Levy B, et al. The duration of inpatient admission predicts cognitive functioning at discharge in patients with bipolar disorder. Comprehensive psychiatry. 2009;50(4):322–326. doi: 10.1016/j.comppsych.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yatham LN, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2009. Bipolar disorders. 2009;11(3):225–255. doi: 10.1111/j.1399-5618.2009.00672.x. [DOI] [PubMed] [Google Scholar]
- 19.Keefe R, et al. The brief assessment of cognition in affective disorders (BAC-A):performance of patients with bipolar depression and healthy controls. Journal of Affective Disorders. doi: 10.1016/j.jad.2014.05.002. In press. [DOI] [PubMed] [Google Scholar]
- 20.Association AP. DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision. American Psychiatric Association: 2000. [Google Scholar]
- 21.Wilkinson GS, Robertson G. Wide Range Achievement Test (WRAT4) Psychological Assessment Resources, Lutz. 2006 [Google Scholar]
- 22.Wechsler D. Wechsler adult intelligence scale–Fourth Edition (WAIS–IV) San Antonio, TX: NCS Pearson; 2008. [Google Scholar]
- 23.Kuswanto CN, Sum MY, Sim K. Neurocognitive functioning in schizophrenia and bipolar disorder: clarifying concepts of diagnostic dichotomy vs. continuum. Frontiers in psychiatry. 2013:4162. doi: 10.3389/fpsyt.2013.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salgado JV, et al. Sensitivity and applicability of the Brazilian version of the Brief Assessment of Cognition in Schizophrenia (BACS) Dement Neuropsychol. 2007;1(3):260–5. doi: 10.1590/S1980-57642008DN10300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segarra N, et al. Spanish validation of the Brief Assessment in Cognition in Schizophrenia (BACS) in patients with schizophrenia and healthy controls. European Psychiatry. 2011;26(2):69–73. doi: 10.1016/j.eurpsy.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Cuesta MJ, et al. Brief cognitive assessment instruments in schizophrenia and bipolar patients, and healthy control subjects: A comparison study between the Brief Cognitive Assessment Tool for Schizophrenia (B-CATS) and the Screen for Cognitive Impairment in Psychiatry (SCIP) Schizophrenia research. 2011;130(1):137–142. doi: 10.1016/j.schres.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 27.Hill SK, et al. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. American Journal of Psychiatry. 2013;170(11):1275–1284. doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velligan DI, et al. A brief cognitive assessment for use with schizophrenia patients in community clinics. Schizophrenia research. 2004;71(2):273–283. doi: 10.1016/j.schres.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 29.Keefe R, et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophrenia Research. 2004;68:2–3. 283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Williams JMG, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological bulletin. 1996;120(1):3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- 31.LaMonica HM, et al. Differential effects of emotional information on interference task performance across the life span. Frontiers in aging neuroscience. 2010;2:141. doi: 10.3389/fnagi.2010.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder KA, Harrison DW. The affective auditory verbal learning test. Archives of Clinical Neuropsychology. 1997;12(5):477–482. [PubMed] [Google Scholar]
- 33.Bora E, et al. Meta-analytic review of neurocognition in bipolar II disorder. Acta psychiatrica scandinavica. 2011;123(3):165–174. doi: 10.1111/j.1600-0447.2010.01638.x. [DOI] [PubMed] [Google Scholar]
- 34.Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biological psychiatry. 2000;48(7):674–684. doi: 10.1016/s0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- 35.Rossell SL. Category fluency performance in patients with schizophrenia and bipolar disorder: The influence of affective categories. Schizophrenia Research. 2006;82(2–3):135–138. doi: 10.1016/j.schres.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Shao Z, et al. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Frontiers in psychology. 2014:5. doi: 10.3389/fpsyg.2014.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zammit S, et al. A longitudinal study of premorbid IQ score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Archives of general psychiatry. 2004;61(4):354–360. doi: 10.1001/archpsyc.61.4.354. [DOI] [PubMed] [Google Scholar]
- 38.Sørensen HJ, et al. Premorbid intelligence and educational level in bipolar and unipolar disorders: A Danish draft board study. Journal of Affective Disorders. 2012;136(3):1188–1191. doi: 10.1016/j.jad.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Salthouse TA, Hedden T. Interpreting reaction time measures in between-group comparisons. Journal of Clinical and Experimental Neuropsychology. 2002;24(7):858–872. doi: 10.1076/jcen.24.7.858.8392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1S: Coefficients of correlation between BAC-A summary scores and Global Assessment of Functioning (GAF) scores. Abbreviations: STAM = short-term affective memory, STNM = short-term non-affective memory, fluency = verbal fluency, DAM = delayed affective memory, DNM = delayed non-affective memory, PS = problem solving (PS), and attention in bipolar patients (BD) and healthy controls (HC). *p<.05, **p<.01
Figure 1S: Performance of BD patients relative to HC on subtests of the short-term non-affective memory (STNM) and verbal fluency of the BAC-A scale in bipolar patients (BD) and healthy controls (HC). *<.05. **p.001.


